Abstract
Aims
The direct renin inhibitor, aliskiren, is known to reduce plasma renin activity (PRA), but whether the efficacy of aliskiren varies based on an individual’s baseline PRA in patients hospitalized for heart failure (HF) is presently unknown. We characterized the prognostic value of PRA and determined if this risk is modifiable with use of aliskiren.
Methods and results
This pre-specified neurohormonal substudy of ASTRONAUT analysed all patients hospitalized for HF with ejection fraction (EF) ≤40% with available baseline PRA data (n = 1306, 80.9%). Risk associated with baseline PRA and short-term changes in PRA from baseline to 1 month was modelled with respect to 12-month clinical events. Median baseline PRA was 3.0 (interquartile range 0.6–16.4) ng/mL/h. Aliskiren significantly reduced PRA early after treatment initiation through 12-month follow-up compared with placebo (P < 0.001). The lowest baseline PRA quartile (<0.6 ng/mL/h) was independently predictive of lower all-cause mortality [adjusted hazard ratio (HR) 0.50, 95% confidence interval (CI) 0.31–0.81] and the composite of cardiovascular mortality and HF hospitalization (adjusted HR 0.57, 95% CI 0.40–0.79). Delta log-normalized PRA (from baseline to 1 month) was not predictive of either primary endpoint at 12 months (P ≥ 0.43). The prognostic value of baseline PRA and short-term changes in PRA did not vary by randomization to aliskiren or placebo (interaction P ≥ 0.13).
Conclusions
Plasma renin activity is reduced early and durably by aliskiren, but this did not translate into improved clinical outcomes in ASTRONAUT. Baseline PRA or short-term reduction in PRA do not identify a subgroup who may preferentially benefit from direct renin inhibition.
Keywords: Clinical outcomes, Heart failure, Neurohormones, Renin
Introduction
Secondary perturbations of the neurohormonal axis have traditionally been the major target for drug development in heart failure (HF). Renin, a 37kDa enzyme released from the kidney’s juxtaglomerular apparatus, orchestrates the renin–angiotensin–aldosterone system (RAAS), alters systemic vasomotor tone, and may contribute to progression of HF. Several studies have suggested that high levels of plasma renin activity (PRA) correlate with adverse cardiovascular (CV) outcomes,1-5 however therapeutic trials6,7 have failed to demonstrate clinical benefits with direct renin inhibition in patients with HF. Limited mechanistic data, including whether PRA was adequately suppressed with aliskiren, are available to better understand the lack of success of incremental RAAS modulation in this population. This pre-specified analysis of neurohormonal data from ASTRONAUT (Aliskiren Trial on Acute Heart Failure Outcomes) dissects the complex interplay between measured neurohormones, their response to aliskiren, and clinical outcomes. We specifically aimed to: (i) characterize the patient-level characteristics that are associated with PRA; (ii) track the effects of aliskiren on PRA levels over time; (iii) assess the prognostic utility of baseline and short-term changes in PRA on post-discharge clinical outcomes; and (iv) determine whether baseline or short-term changes in PRA identify a subgroup who preferentially benefit from direct renin inhibition.
Methods
ASTRONAUT: patient selection and study flow
The study design and primary results of the ASTRONAUT trial have been previously described.8 In brief, the ASTRONAUT trial was an international, prospective, double-blind, randomized placebo-controlled study investigating the effect of aliskiren, a direct renin inhibitor, on clinical outcomes in haemodynamically stable patients hospitalized for HF. Patients were randomized a median of 5 days after admission. The trial studied patients who were 18 years or older, with left ventricular ejection fraction (EF) of ≤40%, who had elevated natriuretic peptide levels [B-type natriuretic peptide (BNP) ≥400pg/mL or N-terminal pro-B-type natriuretic peptide (NT-proBNP) ≥1600pg/mL] at admission, with signs and symptoms of fluid overload. The investigation conforms with the principles outlined in the Declaration of Helsinki.
Plasma renin activity
Our primary variable of interest was log-transformed PRA. PRA was measured by radioimmunoassay using the GammaCoat Plasma Renin Activity Assay (DiaSorin Inc., Stillwater, MN, USA) and analysed in a central core laboratory (Quest Diagnostics, Valencia, CA USA). PRA was determined by quantification of angiotensin I in the presence of certain enzyme inhibitors. The dynamic range for this assay is 0.1 ng/mL/h to 50 ng/mL/h, intra-assay variation is 4.6–10%, and inter-assay variation is 5.6–7.6%. Laboratory personnel handling these samples were blinded to treatment assignment. All biomarker samples were obtained in the morning in a fasting state with patients in seated positions with their legs extended. To minimize the influence of outliers, the sponsor set the extremes of PRA recorded in the database to set values: <0.2 (set to 0.1) and >30 (set to 45). PRA was measured at the following time-points: baseline (time of randomization), 1 month, 6 months, 9 months, and 12 months.
Plasma aldosterone
Plasma aldosterone was only available in a select sample of patients. Plasma aldosterone was measured using the Coat-a-Count assay (Siemens Healthcare Diagnostics, Tarrytown, NY, USA) and analyzed in a central core laboratory (CRL-USA Lenexa, KS, USA). Aldosterone-to-renin ratio was calculated at baseline, 6 months, and 12 months, and expressed as ng/dL per ng/mL/h.
Study endpoints and definitions
The two primary endpoints for the present analysis were all-cause mortality (ACM) and a composite of CV mortality and HF hospitalization within 12-month follow-up. Select secondary endpoints were cause-specific CV mortality, all-cause rehospitalization, myocardial infarction, stroke, and individual components of the primary composite endpoint. All clinical endpoints were independently adjudicated by a clinical events committee at Brigham and Women’s Hospital (Boston, MA USA) in a blinded manner. Select safety endpoints were mild hyperkalaemia (serum potassium ≥5.5 and <6.0 mmol/L), severe hyperkalaemia (serum potassium ≥6.0 mmol/L), and worsening renal function [decrease in estimated glomerular filtration rate (eGFR) to <30 mL/min/1.73 m2]. These definitions were based on the maximum potassium level at any time-point within the first year and the minimum eGFR recorded within the first year. eGFR was calculated using the Modification of Diet in Renal Disease formula.
Statistical analyses
We included all patients in both treatment arms with available baseline PRA levels. Although aliskiren is known to influence PRA baseline PRA was measured at time of randomization, prior to aliskiren exposure. The primary variable was analysed as quartiles and as a continuous function, since specific parameters for ‘normal’ values in this cohort have not been defined. Continuous variables were expressed as mean ± standard deviation (SD) if normally distributed and median [interquartile range (IQR)] if not normally distributed. Categorical variables were expressed as a number (percentage). Demographic characteristics, medical history, vital signs, laboratory data, and baseline medication therapies were compared across quartiles of baseline PRA levels using chi-square and analysis of variance tests.
Kaplan–Meier curves by baseline PRA quartiles were constructed for both primary endpoints and compared using log-rank tests. Outcomes were assessed using univariable and multivariable Cox proportional hazard models to calculate hazard ratios (HR) and 95% confidence intervals (CI) for the primary predictor. Proportional hazards and linearity assumptions were met for log-normalized PRA as a predictor of both primary endpoints. For the quartile-based analysis, the reference group was quartile 4. For the continuous analysis, HR was calculated per one SD decrease in log-normalized PRA. Interaction analyses were performed by aliskiren/placebo (treatment status) and diabetes mellitus. 9 We also identified the ‘optimal’ cut-point for risk prediction, by conducting a series of univariable Cox regression models with various cut-points and identifying the minimal point estimate of hazard for each of the primary endpoints. Non-linear associations between log-normalized PRA and the two primary endpoints were tested and characterized by applying restricted cubic spline terms with three knots.
Multivariate models account for 38 covariates determined at the time of randomization: age, sex, race, geographic region, ischemic HF aetiology, New York Heart Association functional class, left ventricular EF, systolic blood pressure, heart rate, weight, body mass index, NT-proBNP, serum sodium, serum haemoglobin, serum troponin, serum creatinine, serum blood urea nitrogen, aliskiren randomization, medical history (hypertension, coronary artery disease, atrial fibrillation, diabetes, hyperlipidaemia, chronic obstructive pulmonary disease, chronic kidney disease, prior percutaneous coronary intervention, prior coronary bypass arterial graft surgery, prior stroke or transient ischaemic attack, prior myocardial infarction, prior HF hospitalization), and baseline therapies [loop diuretics, angiotensin-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB), β-blockers, mineralocorticoid receptor antagonist (MRA), digoxin, implantable cardioverter-defibrillator, cardiac resynchronization therapy, and/or pacemaker].
The association between PRA at randomization and plasma aldosterone level was tested. The response of plasma aldosterone to aliskiren therapy was assessed through 12-month follow-up. To assess the prognostic effects of short-term changes in PRA, the difference between the 1-month and baseline log-normalized PRA levels was calculated for each patient. The 1-month time-point was selected to minimize patient attrition. Baseline log-normalized PRA, delta log-normalized PRA, aliskiren, and the delta log-normalized PRA by aliskiren treatment interaction were included in left-truncated analyses in predicting the two co-primary endpoints. In addition, intra-class correlation (ICC) analyses were used to assess the amount of change in PRA across the follow-up time-frame relative to the variability at each time-point.
Data imputation or correction for multiple statistical testing was not performed. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Of the overall ASTRONAUT trial cohort (n = 1615), 1306 patients (80.9%) had available PRA levels at the time of randomization. The median PRA level in ASTRONAUT was 3.0 (IQR 0.6–16.4) ng/mL/h (see Supplementary material online, Figure S1). Patients were divided into quartiles based on baseline PRA levels with the following ranges: Q1 (n = 317; <0.6 ng/mL/h); Q2 (n = 332; 0.6-2.9 ng/mL/h); Q3 (n = 332; 3.0–16.3 ng/mL/h); Q4 (n = 325; ≥16.4 ng/mL/h).
Patient-level variation in plasma renin activity
Patients in the lowest quartile of PRA (<0.6 ng/mL/h) were more likely to be female and non-white and have higher rates of hypertension, but less likely to have chronic kidney disease or coronary artery disease compared to patients in the other quartiles (P ≤ 0.04 for all comparisons; Table 1). There was marked variability in mean PRA levels at randomization across geographic regions ranging from North America (n = 105; 3.9 ng/mL/h) to Western Europe (n = 298; 13.8 ng/mL/h; P < 0.001). At randomization, these patients had higher median systolic blood pressures, serum sodium levels, NT-pro-BNP levels, and eGFR values (P ≤ 0.02 for all comparisons). A greater proportion of patients in the lowest quartile of PRA were receiving β-blockers, while fewer were receiving MRAs and digoxin at the time of randomization (P ≤ 0.008 for all comparisons). While 42.3% of patients in the lowest quartile of PRA were co-treated with ACEi/ARB and MRA, 53.5% received dual RAAS therapy in the highest quartile of PRA (P = 0.03). Patients in the lowest PRA quartile were also less likely to have implantable cardioverter-defibrillator or cardiac resynchronization therapy than the other quartiles (P ≤ 0.006 for all comparisons). Overall, patients in the lower PRA quartiles experienced slightly shorter total hospital length of stays compared to the highest quartile (P = 0.008; Table 1).
Table 1.
Baseline characteristics by quartiles of plasma renin activity
| Quartiles of PRA (ng/mL/h) | P-value | ||||
|---|---|---|---|---|---|
| Q1 (<0.6) n=317 |
Q2 (0.6–2.9) n=332 |
Q3 (3.0–16.3) n=332 |
Q4 (≥16.4) n=325 |
||
| Aliskiren (%) PRA (ng/mL/h) | 175 (55.2) | 154 (46.4) | 161 (48.5) | 166 (51.1) | 0.20 |
| Mean ± SD | 0.2±0.1 | 1.5±0.6 | 7.8±3.9 | 35.9±10.9 | |
| Median, IQR | 0.2 (0.1–0.4) | 1.3 (0.9–1.9) | 6.8 (4.5–10.6) | 45 (25.4–45) | |
| Demographics | |||||
| Age (years), median (IQR) | 65 (56–74) | 65 (56–74) | 66 (55–74) | 64 | 0.08 |
| Male (n, %) | 225 (71) | 260 (78.3) | 259 (78) | (56–71) 269 (82.8) | 0.004 |
| Race (n, %) | <0.001 | ||||
| White | 202 (63.7) | 221 (66.6) | 240 (72.3) | 236 (72.6) | |
| Black | 31 (9.8) | 16 (4.8) | 14 (4.2) | 4 (1.2) | |
| Asian | 68 (21.5) | 84 (25.3) | 73 (22) | 76 (23.4) | |
| Other | 16 (5) | 11 (3.3) | 5 (1.5) | 9 (2.8) | |
| Region (n, %) North America | 41 (12.9) | 34 (10.2) | 23 (6.9) | 6 (1.8) | <0.001 |
| Latin America | 35 (11) | 28 (8.4) | 26 (7.8) | 17 (5.2) | |
| Western Europe | 55 (17.4) | 71 (21.4) | 77 (23.2) | 90 (27.7) | |
| Eastern Europe | 101 (31.9) | 93 (28) | 104 (31.3) | 114 (35.1) | |
| Asia/Pacific | 85 (26.8) | 106 (31.9) | 102 (30.7) | 98 (30.2) | |
| Time intervals (days), median (IQR) Time from admission to randomization | 4 (2–6) | 4 (2–6) | 5 (2–7) | 5 (2–8) | <0 . 001 |
| Hospital length of stay | 8 (4–12) | 8 (5–12) | 8 (5–12) | 9 (5–14) | 0.008 |
| Medical history | |||||
| Ischaemic HF etiology (n, %) | 193 (60.9) | 216 (65.3) | 223 (67.2) | 213 (65.5) | 0.36 |
| NYHA class III/IV (n, %) | 188 (59.3) | 216 (65.1) | 225 (67.8) | 209 (64.3) | 0.21 |
| Prior HF hospitalization (n, %) | 205 (64.7) | 211 (63.6) | 241 (72.6) | 233 (71.7) | 0.02 |
| LV EF (%), mean±SD | 28.3±7.4 | 27.8±7.7 | 27.4±7.3 | 27.4±7.1 | 0.38 |
| Hypertension (n, %) | 268 (84.5) | 252 (75.9) | 238 (71.7) | 219 (67.4) | <0 . 001 |
| Hyperlipidaemia (n, %) | 73 (23.9) | 75 (23.7) | 110 (35.4) | 80 (26.3) | 0.005 |
| Diabetes mellitus (n, %) | 125 (39.4) | 143 (43.1) | 136 (41) | 145 (44.6) | 0.47 |
| Coronary artery disease (n, %) | 152 (47.9) | 182 (54.8) | 200 (60.2) | 180 (55.4) | 0.01 |
| Atrial fibrillation/flutter (n, %) | 132 (41.6) | 147 (44.3) | 141 (42.5) | 128 (39.4) | 0.45 |
| Chronic kidney disease (n, %) | 50 (15.8) | 74 (22.3) | 68 (20.5) | 81 (24.9) | 0.04 |
| Chronic obstructive pulmonary disease (n, %) | 58 (18.3) | 68 (20.5) | 54 (16.3) | 78 (24) | 0.08 |
| Prior PCI (n, %) | 51 (16.1) | 67 (20.2) | 65 (19.6) | 78 (24) | 0.07 |
| Prior CABG (n, %) | 36 (11.4) | 56 (16.9) | 79 (23.8) | 50 (15.4) | <0.001 |
| Prior stroke (n, %) | 32 (10.1) | 40 (12) | 29 (8.7) | 27 (8.3) | 0.40 |
| Prior transient ischaemic attack (n, %) | 9 (2.8) | 14 (4.2) | 10 (3) | 10 (3.1) | 0.60 |
| Prior myocardial infarction (n, %) | 111 (35) | 137 (41.3) | 160 (48.2) | 147 (45.2) | 0.002 |
| Vital signs | |||||
| Weight (kg), median (IQR) | 76.3 (64.3–90) | 73.3 (62–86.1) | 75 (62–85.7) | 75 (64–89.6) | 0.28 |
| BMI (kg/m2), median (IQR) | 26.5 (23.3–30.6) | 25.8 (22.7–29.7) | 25.7 (22.7–29.6) | 26.2 (22.8–30.5) | 0.32 |
| SBP (mmHg), median (IQR) | 125 (118–138) | 121 (115–130) | 120 (112–130) | 119 (111–125) | <0.001 |
| Heart rate (b.p.m.), median (IQR) | 75 (65–87) | 76 (68–88) | 77 (67–89) | 77 (69–87) | 0.36 |
| Electrocardiogram | |||||
| Atrial fibrillation/flutter (n, %) | 103 (33.4) | 109 (33.3) | 100 (30.4) | 103 (32.1) | 0.84 |
| QRS duration (ms), median (IQR) | 102 (88–140) | 108 (90–130) | 110 (92–143) | 120 (93–152) | 0.003 |
| Laboratory data at randomization | |||||
| Haemoglobin (g/dL), median (IQR) | 13.6 (12.2–14.8) | 13.7 (12.4–15.1) | 13.6 (12.2–14.9) | 142 (12.5–15.5) | 0.009 |
| NT-proBNP admission (pg/mL), median (IQR)* | 4697 (3000–8967) | 4591 (2799–8675) | 3973 (2671−7962) | 3586.5 (2389–6966) | 0.02 |
| NT-proBNP at randomization (pg/mL), median (IQR)* | 3141.5 (1742–6414) | 2870.5 (1700–5374.5) | 2631 (1369–5562) | 2384 (1398–4423) | <0.001 |
| Troponin I (ng/mL), median (IQR) | 0 (0–0.1) | 0 (0–0.1) | 0 (0–0.1) | 0 (0–0.1) | 0.13 |
| Sodium (mmol/L), median (IQR) | 140 (138–142) | 140 (138–141) | 139 (137–141) | 137 (134–139) | <0.001 |
| Creatinine (μmol/L), median (IQR) | 93 (78.1–107.8) | 96.1 (79.6–113.5) | 100.7 (83.2–120.5) | 99 (82.4–116.6) | 0.002 |
| BUN (mmol/L), median (IQR) | 7 (5.7–9.2) | 8.2 (6.3–11.1) | 8.7 (6.7–11.5) | 9.3 (7.4–12.5) | <0 . 001 |
| eGFR (mL/min/1.73m2), median (IQR) | 67.2 (55.8–78.7) | 63.7 (53–81.9) | 62.2 (50–75.8) | 63.5 (52–78.6) | 0.02 |
| Baseline therapies | |||||
| Diuretics (n, %) | 302 (95.3) | 314 (94.6) | 326 (98.2) | 312 (96) | 0.16 |
| ACEi or ARB (n, %) | 275 (86.8) | 275 (82.8) | 268 (80.7) | 275 (84.6) | 0.14 |
| β-blocker (n, %) | 282 (89) | 276 (83.1) | 276 (83.1) | 253 (77.8) | 0.003 |
| MRA (n, %) | 155 (48.9) | 180 (54.2) | 190 (57.2) | 208 (64) | 0.003 |
| ACEi/ARB + MRA (n, %) | 134 (42.3) | 146 (44) | 149 (44.9) | 174 (53.5) | 0.03 |
| Digoxin (n, %) | 107 (33.8) | 125 (37.7) | 133 (40.1) | 151 (46.5) | 0.008 |
| ICD (n, %) | 26 (8.2) | 37 (11.1) | 56 (16.9) | 80 (24.6) | <0 . 001 |
| CRT (n, %) | 12 (3.8) | 17 (5.1) | 26 (7.8) | 33 (10.2) | 0.006 |
| Permanent pacemaker (n, %) | 28 (8.8) | 36 (10.8) | 38 (11.4) | 40 (12.3) | 0.50 |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; CRT, cardiac resynchronization therapy; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; LV, left ventricular; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PRA, plasma renin activity; SBP, systolic blood pressure; SD, standard deviation.
Data available for 641 patients at admission and 1305 patients at baseline (randomization).
Response to aliskiren
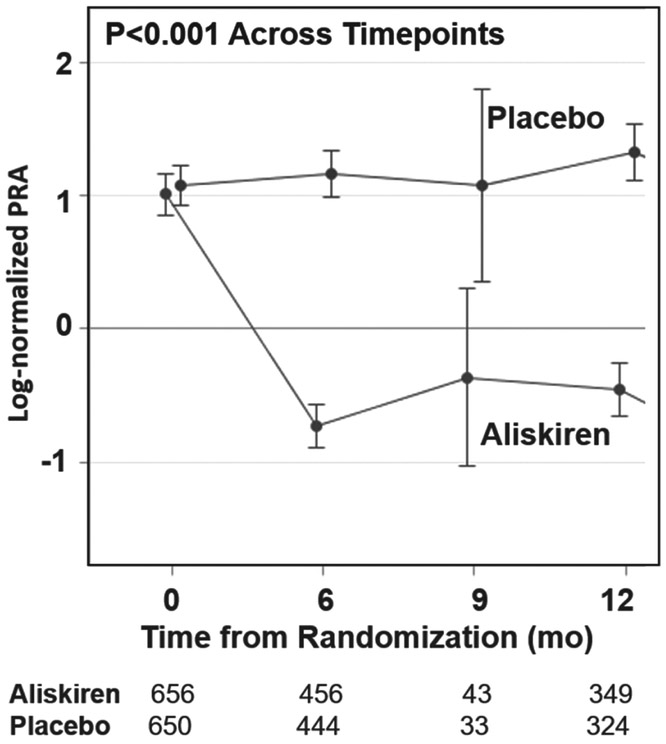
Aliskiren significantly reduced PRA early after treatment initiation compared with placebo, and continued to suppress PRA through 12-month follow-up (P < 0.001; Figure 1). PRA levels slightly increased from randomization through 12-month follow-up. In patients treated with aliskiren, after initial large reductions in PRA levels from randomization to 1 month, there was a slight regression to randomization levels from 1-month to 12-month follow-up. The effect of aliskiren on PRA levels over time does not appear to vary by geographic region (region-by-visit interaction P ≥ 0.21 for both time intervals).
Figure 1.
Log-normalized plasma renin activity (PRA) by treatment arm through 12-month follow-up. Log-normalized PRA levels (and 95% confidence interval bars) over time in the placebo group and in response to aliskiren
Prognostic value of plasma renin activity
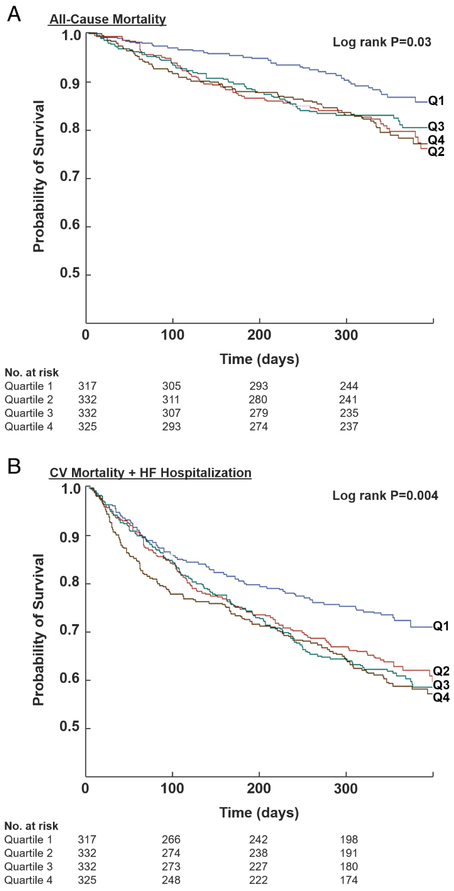
At 12-month follow-up, rates of ACM and the composite of CV mortality and HF hospitalization were significantly lower in the lowest PRA quartile compared with the other three quartiles (P ≤ 0.03 for both primary endpoints; Table 2 and Figure 2). After accounting for baseline risk predictors, compared to the highest quartile, the lowest quartile of PRA at randomization (which corresponded to PRA <0.6 ng/mL/h) was independently predictive of improved ACM (adjusted HR 0.50, 95% CI 0.31–0.81; P = 0.005) and the composite endpoint (adjusted HR 0.57, 95% CI 0.40–0.79; P < 0.001). Lower log-normalized PRA (per 1 SD), when treated as a continuous function, was independently associated with a decrease in risk of ACM (adjusted HR 0.80, 95% CI 0.67–0.95; P = 0.01) and the composite endpoint (adjusted HR 0.83, 95% CI 0.74–0.94; P = 0.003) (Table 3). The relationship between log-normalized PRA and the co-primary endpoints is visually displayed in Figure S2 (see Supplementary material online) using adjusted restricted cubic splines. When individual cut-points were analysed, the lowest point estimate of hazard function was associated with a PRA <0.7 (as compared to PRA ≥0.7: HR 0.60 for ACM and HR 0.65 for the composite endpoint). Interaction terms with PRA (as a continuous function) and aliskiren treatment randomization (interaction P ≥ 0.13 for both primary endpoints) and history of diabetes mellitus (interaction P ≥ 0.16 for both primary endpoints) were negative, and thus were not included in the final multivariable models. As such, the treatment effects of aliskiren (vs. placebo) on the co-primary endpoints did not vary by baseline PRA. Rates of hyperkalaemia and worsening renal function were not significantly different across baseline PRA levels (Table 2).
Table 2.
Event rates by quartiles of plasma renin activity
| Quartiles of PRA (ng/mL/h) | P-value | ||||
|---|---|---|---|---|---|
| Q1 (<0.6) n=317 |
Q2 (0.6–2.9) n=332 |
Q3 (3.0–16.3) n=332 |
Q4 (≥16.4) n=325 |
||
| 30-day events (n, %) | |||||
| All-cause mortality | 3 (0.9) | 3 (0.9) | 8 (2.4) | 6 (1.8) | 0.32 |
| All-cause rehospitalization | 33 (10.4) | 48 (14.5) | 37 (11.1) | 51 (15.7) | 0.14 |
| Rehospitalization for HF | 13 (4.1) | 19 (5.7) | 13 (3.9) | 28 (8.6) | 0.04 |
| 6-month events (n, %) | |||||
| All-cause mortality | 16 (5) | 45 (13.6) | 38 (11.4) | 39 (12) | 0.003 |
| CV mortality or rehospitalization for HF | 64 (20.2) | 88 (26.5) | 89 (26.8) | 92 (28.3) | 0.09 |
| CV mortality | 14 (4.4) | 43 (13) | 36 (10.8) | 38 (11.7) | 0.002 |
| All-cause rehospitalization | 106 (33.4) | 132 (39.8) | 125 (37.7) | 127 (39.1) | 0.37 |
| Rehospitalization for HF | 53 (16.7) | 63 (19) | 65 (19.6) | 73 (22.5) | 0.33 |
| 12-month events (n, %) | |||||
| All-cause mortality | 39 (12.3) | 66 (19.9) | 59 (17.8) | 66 (20.3) | 0.03 |
| CV mortality or rehospitalization for HF | 87 (27.4) | 124 (37.3) | 129 (38.9) | 131 (40.3) | 0.003 |
| CV mortality | 32 (10.1) | 61 (18.4) | 54 (16.3) | 62 (19.1) | 0.008 |
| Worsening HF | 8 (2.5) | 25 (7.5) | 23 (6.9) | 31 (9.5) | 0.004 |
| Sudden cardiac death | 14 (4.4) | 22 (6.6) | 19 (5.7) | 17 (5.2) | 0.65 |
| Presumed sudden death | 3 (0.9) | 2 (0.6) | 1 (0.3) | 3 (0.9) | 0.72 |
| All-cause rehospitalization | 135 (42.6) | 163 (49.1) | 152 (45.8) | 165 (50.8) | 0.20 |
| Rehospitalization for HF | 69 (21.8) | 87 (26.2) | 95 (28.6) | 102 (31.4) | 0.04 |
| Myocardial infarction | 12 (3.8) | 12 (3.6) | 13 (3.9) | 8 (2.5) | 0.73 |
| Stroke | 5 (1.6) | 13 (3.9) | 11 (3.3) | 5 (1.5) | 0.13 |
| Select safety endpoints (n, %) | |||||
| Hyperkalaemia | 0.70 | ||||
| None | 265 (86.3) | 264 (82.2) | 266 (84.2) | 258 (83.0) | |
| Mild* | 28 (9.1) | 38 (11.8) | 28 (8.9) | 33 (10.6) | |
| Severe † | 14 (4.6) | 19 (5.9) | 22 (7.0) | 20 (6.4) | |
| Worsening renal failure ‡ | 20 (6.5) | 29 (9.0) | 34 (10.8) | 31 (10.0) | 0.28 |
Laboratory data used to assess safety endpoints represent maximum post-baseline potassium and minimum post-baseline eGFR values.
CV, cardiovascular; eGFR, estimated glomerular filtration rate; HF, heart failure; MDRD, modification of Diet in Renal Disease; PRA, plasma renin activity.
Mild hyperkalaemia defined as serum potassium ≥.5 and <6.0 mmol/L.
Severe hyperkalaemia defined as serum potassium ≥6.0 mmol/L.
Worsening renal failure defined as a decrease in eGFR levels (calculated based on the abbreviated MDRD formula) to <30 mL/min/1.73m2.
Figure 2.
Kaplan–Meier curves for all-cause mortality (A) and cardiovascular (CV) mortality or rehospitalization for heart failure (HF) (B) at 12-month follow-up by quartiles of baseline plasma renin activity. Times to events were compared using log-rank tests
Table 3.
Risk models for plasma renin activity (analysed as quartiles and as a continuous function) predicting the co-primary endpoints
| Unadjusted HR (95% CI) |
Adjusted* HR (95% CI) |
P-value | |
|---|---|---|---|
| All-cause mortality | |||
| Quartile-based | |||
| Q1 | 0 . 57 (0.38–0.85) | 0 . 50 (0.31–0.81) | 0 . 005 |
| Q2 | 0 . 97 (0.69–1.37) | 0 . 80 (0.54–1.18) | 0 . 26 |
| Q3 | 0 . 87 (0.61–1.23) | 0 . 81 (0.55–1.19) | 0 . 28 |
| Q4 | Reference | Reference | P (overall) =0.15 |
| Continuous | |||
| PRA (log-normalized) | 0 . 85 (0.74–0.97) | 0 . 80 (0.67–0.95) | 0 . 01 |
| Aliskiren treatment by PRA interaction term | 0 . 13 | ||
| CV mortality and rehospitalization for HF | |||
| Quartile-based | |||
| Q1 | 0 . 67 (0.51–0.88) | 0 . 57 (0.40–0.79) | <0 . 001 |
| Q2 | 0 . 89 (0.69–1.13) | 0 . 80 (0.60–1.06) | 0 . 11 |
| Q3 | 0 . 93 (0.73–1.19) | 0 . 86 (0.66–1.14) | 0 . 29 |
| Q4 | Reference | Reference | P (overall) =0.03 |
| Continuous | |||
| PRA (log-normalized) | 0 . 86 (0.78–0.95) | 0 . 83 (0.74–0.94) | 0 . 003 |
| Aliskiren treatment by PRA interaction term | 0 . 23 | ||
Data represent HR and 95% CI for risk of the primary co-endpoints. For quartile-based analyses, cut-offs for each quartile are included in Tables 1 and 2, and quartile 4 was used as the reference group. For continuous analyses, HR were estimated for 1 standard deviation decrease in log-normalized PRA. The proportional hazards and linearity assumptions were met for log-normalized PRA as a predictor of both co-primary endpoints. There was little evidence that quadratic polynomial improves on the linear model fit. Interactions with PRA (as a continuous function) and aliskiren treatment randomization and history of diabetes were negative, and thus were not included in the final multivariable models.
CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; PRA, plasma renin activity.
Adjusted for age, sex, race, geographic region, body mass index, ischemic HF aetiology, New York Heart Association class III/IV, ejection fraction, systolic blood pressure, heart rate, N-terminal pro-B-type natriuretic peptide level, serum sodium, blood urea nitrogen, serum creatinine, QRS duration, aliskiren treatment randomization, past medical history (prior HF hospitalization, coronary artery disease, atrial fibrillation, diabetes, chronic obstructive pulmonary disease), and baseline therapies (angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, β-blocker, mineralocorticoid receptor antagonist, digoxin, implantable cardioverter-defibrillator, cardiac resynchronization therapy).
Change in plasma renin activity
Complete data through 1-month follow-up were available in 1029 patients and the spread of log-normalized delta PRA values maintained a normal distribution. The median change in log-normalized PRA from randomization to 1-month follow-up was 0.68 (−2.20 to 0.45). In left-truncated analysis, delta log-normalized PRA was not predictive of 12-month ACM (HR 1.03, 95% CI 0.89–1.19, P = 0.72 for aliskiren arm and HR 0.96, 95% CI 0.85–1.10, P = 0.57 for placebo arm) and the composite endpoint (HR 1.02, 95% CI 0.92–1.12, P = 0.76 for aliskiren arm and HR 0.97, 95% CI 0.89–1.05, P = 0.43 for placebo arm) after accounting for baseline log-normalized PRA level. The lack of prognostic value of delta log-normalized PRA did not vary by randomization to aliskiren or placebo (interaction P ≥ 0.39). After initial changes to 1-month post-randomization (ICC term 0.51), log-normalized PRA levels remained relatively stable through 12-month follow-up. Baseline log-normalized PRA quartiles maintained separation and stability from 1 month to 6 months (ICC term 0.65) and from 6 months to 12 months (ICC term 0.71), after accounting for the treatment interaction term (see Supplementary material online, Figure S3).
Plasma aldosterone
Plasma aldosterone levels were available in a subset of ASTRONAUT patients (n = 613; 38.0%). At baseline, PRA levels modestly correlated with plasma aldosterone (r = 0.31). Aliskiren diminished post-discharge increases in plasma aldosterone, which continued to increase in the placebo arm up to 12 months post-randomization (see Supplementary material online, Figure S4). However, aliskiren’s effects on plasma aldosterone appeared to be more modest than its direct effects on PRA, such that the aldosterone-to-renin ratio was significantly increased by aliskiren compared with placebo at 6-month and 12-month follow-up (P < 0.001 at both time intervals).
Discussion
In this large neurohormonal substudy of a phase III clinical trial of patients hospitalized for HF and reduced EF, we highlight a number of important findings: (i) PRA levels >0.7 ng/mL/h prior to hospital discharge identify a high-risk cohort for subsequent mortality and CV events; (ii) PRA is reduced early and durably by aliskiren; and (iii) PRA at the time of randomization or short-term reductions in PRA do not identify a subgroup which may preferentially benefit from direct renin inhibition.
ASTRONAUT is well-suited to study neurohormonal status. The present analysis is one the largest neurohormonal substudies among patients hospitalized for HF with reduced EF, and PRA-based analysis was pre-specified in the study protocol. All enrolled patients were stabilized after initial haemodynamic and congestive perturbations, which may represent a relative ‘steady-state’. Serial measurements of PRA were available through 12 months post-discharge, tracking neurohormonal status well beyond the initial ‘vulnerable phase’. Finally, the study is uniquely positioned to inform whether aliskiren influences PRA trajectory and clinical outcomes in patients well treated with background neurohormonal blockers.
Renin—the ‘gatekeeper’ neurohormone
The RAAS system has historically been an important target of pharmacotherapies in chronic HF. Renin, involved in the rate-limiting step of this complex axis, is upregulated in response to a decrease in baroreceptor stretch from low arterial blood pressure, a decrease in sodium chloride flux into the juxtaglomerular apparatus, and activation of the sympathetic nervous system, and is downregulated by angiotensin II through feedback inhibition. Renin levels are known to increase in patients treated with RAAS modulating drugs, the so-called ‘renin escape’, 10 raising interest in pharmacologically blocking renin in patients with HF.11
Variation in plasma renin activity in a global trial
Average baseline levels of PRA in this cohort (3.0ng/mL/h) were lower than other recent in-hospital trial-based experiences (~5.0 ng/mL/h), 3 potentially reflecting the relative clinical stability of ASTRONAUT patients. We further demonstrate marked regional variation in PRA levels measured during hospitalization for HF. In addition to region-based variation in protocol execution, drug compliance, and background therapies, our data provide evidence of variation in neurohormonal status across geographic regions in a global HF program. Differences in PRA across geographic regions may reflect variation in diet, race/ethnic composition, environmental exposures, and background disease severity and neurohormonal antagonist use. Despite this regional heterogeneity, aliskiren appears to reduce PRA similarly across geographic regions. In light of recent trial experiences of potential regional departures from trial protocols,12 these data are reassuring of consistent drug exposure and effects across regions.
Prognostic significance of plasma renin activity
Unlike other markers of the neurohormonal axis, 13 PRA appears to be steadily elevated in placebo-treated patients even late after the ‘vulnerable phase’ of hospitalization for HF. Early separations in PRA levels during hospitalization appear to persist up to 1 year post-discharge. As such, PRA may represent a stable biomarker of neurohormonal status. High PRA measured shortly prior to discharge appears to identify a high-risk cohort for subsequent CV events. The risk associated with elevated PRA persisted after accounting for imbalances in region and background neurohormonal therapies.
Why was direct renin inhibition unsuccessful in heart failure?
Although PRA appears to be an independent marker of risk and aliskiren (at a dose of 150 mg to 300 mg once daily) reliably reduces PRA levels, direct renin inhibition did not translate to improved post-discharge clinical outcomes in ASTRONAUT. As such, this experience represents another cautionary example in drug development in which lowering of a target surrogate marker by an investigational therapy fails to reliably predict treatment effects with respect to definitive clinical endpoints. There are several potential explanations for this apparent disconnect. First, PRA levels were relatively low in this stabilized HF cohort with a large fraction of patients with baseline values at the lower reporting limit, potentially compromising our ability to demonstrate treatment benefit with aliskiren. Furthermore, PRA was subject to significant inter-individual variability and single time-point estimates of PRA during and soon after hospitalization may be altered by shifts in volume and haemodynamics, and transitions in medications, limiting its reliability. Second, PRA may represent an important marker of HF disease severity at a population level, but this conferred risk may not be modifiable despite reductions in measured levels for each individual patient. Renin is differentially absorbed in coronary and cardiac tissue beds, locally catalyzing angiotensin production.14 As such, there may be inter-individual variation in plasma vs. local renin activity. Third, downstream compensatory mechanisms may overcome upstream direct renin inhibition over time. For instance, tissue chymase may be an important non-renin-dependent mediator of angiotensin II production in the failing heart. These mechanisms may lend to non-linear associations between renin, angiotensin intermediates, and aldosterone in HF.15 Indeed, in our study, aliskiren appeared to reduce PRA levels more robustly than aldosterone levels. This magnitude of angiotensin and aldosterone modulation with add-on direct renin inhibition may not be sufficient to modify target pathways (including renal blood flow)16 and subsequent clinical outcomes. Finally, the potential benefits from direct renin inhibition may be counterbalanced by adverse safety signals, especially in certain high-risk subgroups such as patients with diabetes.9,17 Further modulation of the RAAS system may not confer incremental clinical benefit (ceiling effect in RAAS inhibition),7 but may only augment risk for adverse effects such as hypotension, hyperkalaemia, and worsening renal function. However, in prior experiences of RAAS inhibition, the treatment-related occurrence of these adverse effects did not negate the overall survival benefit conferred by these agents in carefully monitored patients.18 As such, excess risks of adverse effects alone are unlikely to account for all the lack of success of direct renin inhibition. Novel strategies to optimize RAAS modulation in a safe and effective manner in this population are necessary.19 The recent clinical testing of adjunctive potassium binders20-22 and potentially safer non-steroidal MRAs 23 has shown initial promise.
Study limitations
Despite the retrospective nature of the study design, these analyses were pre-specified and the measurement of neurohormones was protocol-driven. PRA and aldosterone levels were only available in a subset of patients and serial neurohormonal marker sampling and follow-up for endpoint analyses were limited to 12 months. Although PRA was subject to significant regional variation, the assay was handled in a central laboratory. Despite efforts to standardize collection, PRA measurement may be prone to significant diurnal variation and biological variability, and may vary substantially based on timing and posture of sampling and ambient temperature of storage.24 These intervening factors may limit the reliability and reproducibility of these neurohormonal measurements during hospitalization,25 and strict standardization of blood sampling conditions remains challenging in a global, multicenter trial. Residual measured and unmeasured confounding may influence these findings. Our findings may not be generalizable to patients with chronic stable HF, patients with preserved EF (although elevations in renin levels may be comparable to that observed in patients with reduced EF26), and those outside the immediate inclusion and exclusion criteria of the ASTRONAUT trial.
Conclusions
Plasma renin activity levels were relatively low and were subject to significant patient-level and regional variation in this cohort of patients stabilized after hospitalization for HF. Despite this, PRA represents an independent marker of disease severity, and elevated levels during hospitalization for HF with reduced EF predict subsequent risk for mortality and rehospitalization. The direct renin inhibitor, aliskiren, successfully reduced PRA in a broad range of hospitalized patients with HF in a global phase III trial. Baseline PRA or short-term changes in PRA did not identify a subgroup of patients who preferentially respond to direct renin inhibition. Taken together, successful therapeutic modulation of PRA was achieved with aliskiren in the ASTRONAUT trial, but this did not appear to translate into improved clinical outcomes. Further confirmatory studies are needed to clarify the role of PRA in risk stratification during this period of stabilization after hospitalization for HF with reduced EF. The dynamic nature of the plasma angiotensin peptide system is only recently becoming recognized, and more complete metabolic profiling of key angiotensin intermediates may be required to better understand the therapeutic effects of RAAS inhibitors.15 Future drug development27 will need to identify potentially safer strategies to attenuate secondary neurohormonal perturbations or re-focus attention on primary cardiac abnormalities. The potential for precision CV approaches in the application of novel or existing therapeutics in patients with HF requires further testing.
Supplementary Material
Figure S1. Distribution and descriptive statistics of plasma renin activity.
Figure S2. Restricted cubic spline regression-derived point estimates and 95% confidence intervals of hazard ratios for log-normalized plasma renin activity and the two co-primary endpoints: all-cause mortality and the composite of cardiovascular mortality and heart failure hospitalization.
Figure S3. Log-normalized plasma renin activity (PRA) during follow-up based on baseline quartiles of PRA.
Figure S4. Plasma aldosterone levels by treatment arm through 12-month follow-up.
Acknowledgments
Funding
Financial and material support for the ASTRONAUT trial was provided by Novartis Pharma AG (Basel, Switzerland). Haris Subacius conducted all final analyses for this report with funding from the Center for Cardiovascular Innovation, Northwestern University Feinberg School of Medicine, Chicago, IL, USA, and takes responsibility for the integrity of the data.
Conflict of interest: M.V. is supported by the NHLBI T32 post-doctoral training grant (T32HL007604). G.C.F. reports significant consulting for Novartis, and modest consulting for Amgen, Janssen, Novartis, Medtronic, St Jude Medical; holds the Eliot Corday Chair of Cardiovascular Medicine at UCLA and is also supported by the Ahmanson Foundation (Los Angeles, CA, USA). S.D.S. has received grant funding, consultant fees, and travel support from Novartis. S.J.G. is supported by the NHLBI T32 postdoctoral training grant (T32HL069749-14). A.P.M. has served on committees of clinical studies sponsored by Amgen, Bayer, Abbott Vascular, Cardiorentis, Johnson & Johnson, and Novartis Pharma AG. M.B. has served as a consultant for AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, AWD Dresden, Berlin-Chemie, MSD, Novartis, Pfizer, Sanofi-Aventis, and Servier. F.Z. has received grant funding from Novartis, BG Medicine, and Roche Diagnostics; served on a board for Boston Scientific; and served as a consultant for Novartis, Takeda, AstraZeneca, Boehringer-Ingelheim, GE Healthcare, Relypsa, Servier, Boston Scientific, Bayer, Johnson & Johnson, and ResMed. J.B. has received research support from the NIH and European Union; and has been a consultant for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Novartis, Relypsa, ZS Pharma, Medtronic, Merck, and CVRx. M.G. has been a consultant for Abbott Laboratories, Astellas, AstraZeneca, Bayer HealthCare AG, CorThera, Cytokinetics, DebioPharm SA, Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Johnson &Johnson, Medtronic, Merck, Novartis Pharma AG, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Takeda Pharmaceutical and Trevena Therapeutics. All other authors have no conflicts to declare.
Footnotes
Supplementary Information
Additional Supporting Information may be found in the online version of this article:
Clinical Trial Registration ClinicalTrials.gov Unique Identifier:
References
- 1.Masson S, Solomon S, Angelici L, Latini R, Anand IS, Prescott M, Maggioni AP, Tognoni G, Cohn JN; Val-HeFT Investigators. Elevated plasma renin activity predicts adverse outcome in chronic heart failure, independently of pharmacologic therapy: data from the Valsartan Heart Failure Trial (Val-HeFT). J Card Fail 2010;16:964–970. [DOI] [PubMed] [Google Scholar]
- 2.Tomaschitz A, Pilz S, Ritz E, Morganti A, Grammer T, Amrein K, Boehm BO, Marz W. Associations of plasma renin with 10-year cardiovascular mortality, sudden cardiac death, and death due to heart failure. Eur Heart J 2011;32:2642–2649. [DOI] [PubMed] [Google Scholar]
- 3.Mentz RJ, Stevens SR, DeVore AD, Lala A, Vader JM, AbouEzzeddine OF, Khazanie P, Redfield MM, Stevenson LW, O’Connor CM, Goldsmith SR, Bart BA, Anstrom KJ, Hernandez AF, Braunwald E, Felker GM. Decongestion strategies and renin–angiotensin–aldosterone system activation in acute heart failure. JACC Heart Fail 2015;3:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vergaro G, Emdin M, Iervasi A, Zyw L, Gabutti A, Poletti R, Mammini C, Giannoni Fontana M, Passino C. Prognostic value of plasma renin activity in heart failure. Am J Cardiol 2011;108:246–251. [DOI] [PubMed] [Google Scholar]
- 5.Volpe M, Francia P, Tocci G, Rubattu S, Cangianiello S, Elena Rao MA, Trimarco B Condorelli M. Prediction of long-term survival in chronic heart failure by multiple biomarker assessment: a 15-year prospective follow-up study. Clin Cardiol 2010;33:700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Bohm M, Greene SJ, Fonarow GC, Lewis EF, Zannad F, Solomon SD, Baschiera F, Botha J, Hua TA, Gimpelewicz CR, Jaumont X, Lesogor A, Maggioni AP; ASTRONAUT Investigators and Coordinators. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA 2013;309:1125–1135. [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJ, Krum H, Abraham WT, Dickstein K, Kober LV, Desai AS, Solomon SD, Greenlaw N, Ali MA, Chiang Y, Shao Q, Tarnesby G, Massie BM; ATMOSPHERE Committees Investigators. Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N Engl J Med 2016;374:1521–1532. [DOI] [PubMed] [Google Scholar]
- 8.Gheorghiade M, Albaghdadi M, Zannad F, Fonarow GC, Bohm M, Gimpelewicz C, Botha J, Moores S, Lewis EF, Rattunde H, Maggioni A; ASTRONAUT Investigators and Study Coordinators. Rationale and design of the multicentre, randomized, double-blind, placebo-controlled Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT). Eur J Heart Fail 2011;13:100–106. [DOI] [PubMed] [Google Scholar]
- 9.Maggioni AP, Greene SJ, Fonarow GC, Bohm M, Zannad F, Solomon SD, Lewis EF, Baschiera F, Hua TA, Gimpelewicz CR, Lesogor A, Gheorghiade M; ASTRONAUT Investigators and Coordinators. Effect of aliskiren on post-discharge outcomes among diabetic and non-diabetic patients hospitalized for heart failure: insights from the ASTRONAUT trial. Eur Heart J 2013;34:3117–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueda T, Kawakami R, Nishida T, Onoue K, Soeda T, Okayama S, Takeda Y, Watanabe M, Kawata H, Uemura S, Saito Y. Plasma renin activity is a strong and independent prognostic indicator in patients with acute decompensated heart failure treated with renin–angiotensin system inhibitors. Circ J 2015;79:1307–1314. [DOI] [PubMed] [Google Scholar]
- 11.Volpe M, Battistoni A, Chin D, Rubattu S, Tocci G. Renin as a biomarker of cardiovascular disease in clinical practice. Nutr Metab Cardiovasc Dis 2012;22:312–317. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner J, Lewis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer N, McKinlay S, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Pang PS, Ambrosy AP, Lan G, Schmidt P, Filippatos G, Konstam M, Swedberg K, Cook T, Traver B, Maggioni A, Burnett J, Grinfeld L, Udelson J, Zannad F. A comprehensive, longitudinal description of the in-hospital and post-discharge clinical, laboratory, and neurohormonal course of patients with heart failure who die or are re-hospitalized within 90 days: analysis from the EVEREST trial. Heart Fail Rev 2012;17:485–509. [DOI] [PubMed] [Google Scholar]
- 14.Muller DN, Fischli W, Clozel JP, Hilgers KF, Bohlender J, Menard J, Busjahn A, Ganten D, Luft FC. Local angiotensin II generation in the rat heart: role of renin uptake. Circ Res 1998;82:13–20. [DOI] [PubMed] [Google Scholar]
- 15.Basu R, Poglitsch M, Yogasundaram H, Thomas J, Rowe BH, Oudit GY. Roles of angiotensin peptides and recombinant human ACE2 in heart failure. J Am Coll Cardiol 2017;69:805–819. [DOI] [PubMed] [Google Scholar]
- 16.Schroten NF, Damman K, Hemmelder MH, Voors AA, Navis G, Gaillard CA, van Veldhuisen DJ, Van Gilst WH, Hillege HL. Effect of additive renin inhibition with aliskiren on renal blood flow in patients with chronic heart failure and renal dysfunction (Additive Renin Inhibition with Aliskiren on renal blood flow and Neurohormonal Activation in patients with Chronic Heart Failure and Renal Dysfunction). Am Heart J 2015;169:693–701.e3. [DOI] [PubMed] [Google Scholar]
- 17.Parving HH, Brenner BM, McMurray JJV, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012;367:2204–2213. [DOI] [PubMed] [Google Scholar]
- 18.Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, van Veldhuisen DJ, Shi H, Messig M, Vincent J, Girerd N, Bakris G, Pitt B, Zannad F. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014;7:51–58. [DOI] [PubMed] [Google Scholar]
- 19.Sarwar CM, Papadimitriou L, Pitt B, Pina I, Zannad F, Anker SD, Gheorghiade M, Butler J. Hyperkalemia in heart failure. J Am Coll Cardiol 2016;68:1575–1589. [DOI] [PubMed] [Google Scholar]
- 20.Pitt B, Bakris GL. New potassium binders for the treatment of hyperkalemia: current data and opportunities for the future. Hypertension 2015;66:731–738. [DOI] [PubMed] [Google Scholar]
- 21.Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, Qunibi W, Pergola P, Singh B. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015;372:222–231. [DOI] [PubMed] [Google Scholar]
- 22.Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, Wittes J, Christ-Schmidt H, Berman L, Pitt B; OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015;372:211–221. [DOI] [PubMed] [Google Scholar]
- 23.Filippatos G, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, Maggioni AP, Ponikowski P, Voors AA, Zannad F, Kim SY, Nowack C, Palombo G, Kolkhof P, Kimmeskamp-Kirschbaum N, Pieper A, Pitt B. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J 2016;37:2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilz S, Kienreich K, Gaksch M, Grubler M, Verheyen N, Bersuch LA, Schmid J, Drechsler C, Ritz E, Moosbrugger A, Stepan V, Pieber TR, Meinitzer A, Marz W, Tomaschitz A. Aldosterone to active renin ratio as screening test for primary aldosteronism: reproducibility and influence of orthostasis and salt loading. Horm Metab Res 2014;46:427–432. [DOI] [PubMed] [Google Scholar]
- 25.Kerstens MN, Kobold AC, Volmer M, Koerts J, Sluiter WJ, Dullaart RP. Reference values for aldosterone-renin ratios in normotensive individuals and effect of changes in dietary sodium consumption. Clin Chem 2011;57:1607–1611. [DOI] [PubMed] [Google Scholar]
- 26.Bishu K, Deswal A, Chen HH, LeWinter MM, Lewis GD, Semigran MJ, Borlaug BA, McNulty S, Hernandez AF, Braunwald E, Redfield MM. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J 2012;164:763–770.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaduganathan M, Butler J, Pitt B, Gheorghiade M. Contemporary drug development in heart failure: call for hemodynamically neutral therapies. Circ Heart Fail 2015;8:826–831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution and descriptive statistics of plasma renin activity.
Figure S2. Restricted cubic spline regression-derived point estimates and 95% confidence intervals of hazard ratios for log-normalized plasma renin activity and the two co-primary endpoints: all-cause mortality and the composite of cardiovascular mortality and heart failure hospitalization.
Figure S3. Log-normalized plasma renin activity (PRA) during follow-up based on baseline quartiles of PRA.
Figure S4. Plasma aldosterone levels by treatment arm through 12-month follow-up.