Novel therapies for heart failure (HF) are increasingly being tested in larger, global populations.1 This trend, in large part, may be driven by logistics of global trial conduct and the ability to more expediently enrol patients from certain geographic regions.2 Consequently, these trial-level patterns may decrease relative participation from countries where patient enrolment is more difficult or expensive. Given key differences in patient-level factors (e.g. race, diet, co-morbidities, socio-economic factors) and health care practices (e.g. baseline use of standard medical and device therapies) across geographic regions, there is potential that study results may not be generalizable to areas of the world under-represented in the trial.3 Moreover, increased regional heterogeneity may encumber site oversight and quality control, impacting the ability of the trial to include appropriately selected patients and demonstrate treatment benefit or harm, as exemplified in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) trial experience.4 To date, there has been limited quantitative appraisal of these aggregate trial-level patterns in geographic representation. Given the potential implications on future HF trial planning, we conducted a comprehensive systematic review of global region of enrolment in all HF trials published over the last 16 years in any peer-reviewed medical journal.
We identified phase II-IV HF clinical trials with a sample size >100 (since smaller studies are by design single centre and unlikely to enrol global populations) published between January 2001 and December 2016. We employed two separate search queries: (i) PubMed/MEDLINE using the following unique terms: publication year, ‘heart failure’, ‘trial*’, and ‘randomized’; and (ii) ClinicalTrials.gov employing the following limits: adult (18years and older), interventional, phase II-IV, ‘heart failure’.
Each trial was categorized by the regions of patient enrolment, as follows: (i) exclusively conducted in North America (NA); (ii) exclusively conducted in Western Europe (WE); (iii) exclusively conducted in Asia; (iv) exclusively conducted outside NA, WE, or Asia; and (v) mixed/multiregional. Trial sponsor was identified using the ClinicalTrials.gov entry (if registered), or was otherwise extracted from the primary publication.
We screened 4524 studies, of which 624 were phase II–IV trials of adult HF patients, and 326 trials included >100 patients. Twenty-one trials did not include sufficient details regarding site or region of enrolment and were omitted. Thus, this analysis included 305 unique trials studying 244 255 patients enrolled from 19 352 total sites. Among these trials, sites of enrolment were located exclusively in NA in 33.4%, exclusively in WE in 30.2%, exclusively in Asia in 5.9%, in multiple regions in 24.3%, and exclusively outside NA, WE, or Asia in 6.2%.
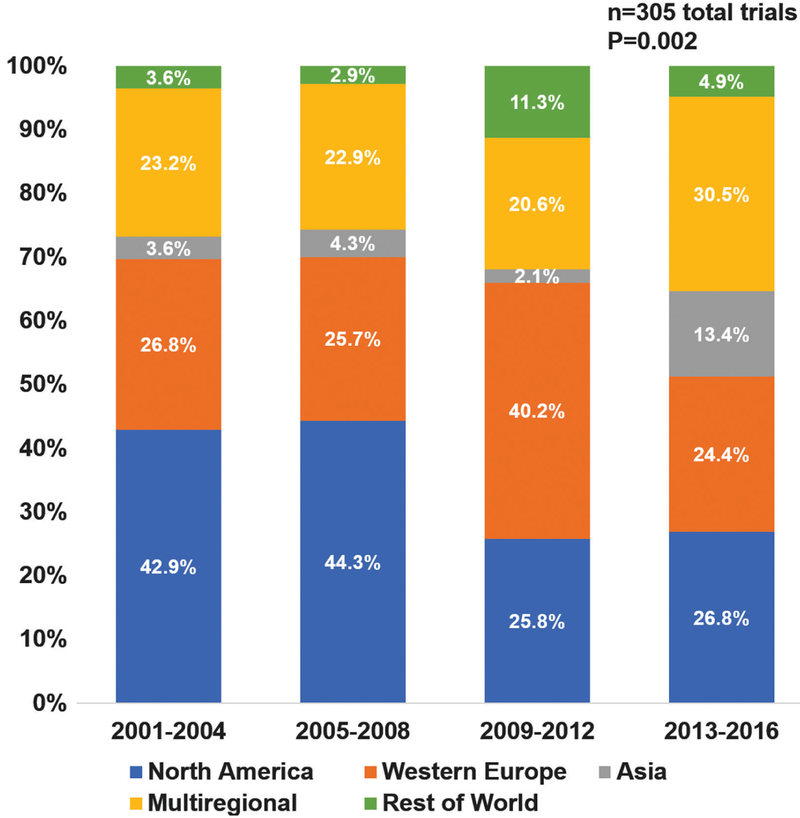
Significant changes in global enrolment patterns were observed over time from 2001–2004 to 2013–2016: the proportion of trials conducted in NA decreased from 42.9% to 26.8%, while the proportion conducted in Asia (3.6% to 1 3.4%) and multiple regions (23.2% to 30.5%) increased (P =0.002; Figure 1). Of the 74 trials conducted across multiple regions, 62 (83.8%) had available data regarding distribution of sites. In these multiregional trials, the proportion of sites located in NA decreased from 38.5% to 30.4%, sites located in WE decreased from 43.2% to 38.2%, while sites located outside NA and WE increased from 18.3% to 31.4% from 2001–2004 to 2013–2016 (P < 0.001).
Figure 1.
Proportion of heart failure clinical trials by primary region(s) of enrolment over the 16-year study period
Overall, 61.3% of trials studied HF with reduced ejection fraction, 4.3% evaluated HF with preserved ejection fraction, and the remaining 34.4% enrolled patients with HF regardless of ejection fraction. Most multiregional trials studied HF with reduced ejection fraction, while trials conducted in Asia or other regions of the world more commonly evaluated HF with preserved ejection fraction or HF regardless of ejection fraction (P < 0.001; Table 1). There was marked variation in sources of funding across regions: multiregional trials were predominantly industry-supported, NA trials were commonly supported by industry and government sources, and trials conducted in WE or in Asia were often supported by universities or independent organizations (P < 0.001). Among studies restricted to particular regions, trials conducted in NA included a median of 20 sites, trials in WE included seven sites, trials in Asia included six sites, and trials conducted outside these regions included only one site. Median site enrolment rates were lower for trials conducted in NA or across multiple regions compared with that in other geographic regions (P < 0.001 ; Table 1).
Table 1.
Key trial operating characteristics based on primary region(s) of enrolment
| Trial characteristics | Multiregional (n =74, 24.3%) |
North America (n = 102, 33.4%) |
Western Europe (n = 92,30.2% |
Asia (n = 18, 5.9%) |
Outside North America, Western Europe, and Asia (n =19, 6.2%) |
P-value |
|---|---|---|---|---|---|---|
| Year published | 0.002 | |||||
| 2001 –2004 | 13 (17.6) | 24 (23.5) | 15 (16.3) | 2 (11.1) | 2 (10.5) | |
| 2005–2008 | 16 (21.6) | 31 (30.4) | 18 (19.6) | 3 (16.7) | 2 (10.5) | |
| 2009–2012 | 20 (27) | 25 (24.5) | 39 (42.4) | 2 (11.1) | 11 (57.9) | |
| 2013–2016 | 25 (33.8) | 22 (21.6) | 20 (21.7) | 11 (61.1) | 4 (21.1) | |
| Heart failure classification | <0.001 | |||||
| Heart failure with reduced ejection fraction | 64 (86.5) | 59 (57.8) | 51 (55.4) | 6 (33.3) | 7 (36.8) | |
| Heart failure with preserved ejection fraction | 3 (4.1) | 3 (2.9) | 4 (4.3) | 2 (11.1) | 1 (5.3) | |
| Heart failure regardless of ejection fraction | 7 (9.5) | 40 (39.2) | 37 (40.2) | 10 (55.6) | 11 (57.9) | |
| Heart failure classification | 0.39 | |||||
| Chronic | 62 (83.8) | 88 (86.3) | 82 (89.1) | 16 (88.9) | 19 (100) | |
| Acute | 12 (16.2) | 14 (13.7) | 10 (10.9) | 2 (11.1) | 0 (0) | |
| Trial phase | <0.001 | |||||
| Unclassified | 21 (28.4) | 70 (68.6) | 62 (67.4) | 14 (77.8) | 14 (73.7) | |
| Phase II | 17 (23) | 11 (10.8) | 11 (12) | 0 (0) | 2 (10.5) | |
| Phase III | 30 (40.5) | 15 (14.7) | 8 (8.7) | 3 (16.7) | 3 (15.8) | |
| Phase IV | 6 (8.1) | 6 (5.9) | 11 (12) | 1 (5.6) | 0 (0) | |
| Intervention | <0.001 | |||||
| Device | 13 (17.6) | 19 (18.6) | 9 (9.8) | 0 (0) | 0 (0) | |
| Drug | 57 (77) | 29 (28.4) | 37 (40.2) | 12 (66.7) | 3 (1 5.8) | |
| Other | 2 (2.7) | 44 (43.1) | 38 (41.3) | 6 (33.3) | 12 (63.2) | |
| Procedure | 1 (1.4) | 6 (5.9) | 2 (2.2) | 0 (0) | 2 (10.5) | |
| Surgery | 1 (1.4) | 2 (2) | 2 (2.2) | 0 (0) | 1 (5.3) | |
| Testing/imaging | 0 (0) | 2 (2) | 4 (4.3) | 0 (0) | 1 (5.3) | |
| Sponsor a | <0.001 | |||||
| Government | 5 (6.8) | 34 (33.3) | 18 (19.6) | 3 (16.7) | 7 (36.8) | |
| University/Organization | 2 (2.7) | 19 (18.6) | 38 (41.3) | 7 (38.9) | 7 (36.8) | |
| Industry | 65 (87.8) | 46 (45.1) | 23 (25) | 3 (16.7) | 3 (15.8) | |
| Unclear | 2 (2.7) | 3 (2.9) | 13 (14.1) | 5 (27.8) | 2 (10.5) | |
| Trial size, median (interquartile range) | ||||||
| Patients | 1005 (456–2331) | 271 (192–420) | 200 (136–354) | 199.5 (110–306) | 223 (120–364) | <0.001 |
| Sites | 106 (54–233) | 20 (2–40) | 7 (2–18) | 6 (1–16) | 1 (1–3) | <0.001 |
| Countries | 11 (8–20) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | <0.001 |
| Duration (years) | 2.34 (1.7–3.4) | 2.25 (1.55–3.4) | 1.83 (1.08–2.92) | 2.25 (0.92–3) | 2.54 (1.58–5) | 0.41 |
| Enrolment rate (patient/site/month) | 0.31 (0.2–0.52) | 0.67 (0.29–3.32) | 1.65 (0.47–3.62) | 1.99 (0.76–2.94) | 3.69 (1.82–5.7) | <0.001 |
| Primary endpoint | <0.001 | |||||
| Mortality | 47 (63.5) | 33 (32.4) | 37 (40.2) | 5 (27.8) | 11 (57.9) | |
| Non-mortality clinical outcomes | 12 (16.2) | 55 (53.9) | 36 (39.1) | 10 (55.6) | 5 (26.3) | |
| Laboratory or imaging outcomes | 15 (20.3) | 14 (13.7) | 19 (20.7) | 3 (16.7) | 3 (15.8) |
Continuous variables were compared across nominal categories using the Kruskal-Wallis test and categorical variables were compared using the χ2 test.
Based on funding source designation in ClinicalTrials.gov entry, when available.
This comprehensive systematic review of over 300 contemporary phase II-IV HF trials supports a pattern of rapid globalization that appears to be especially apparent over the last decade. Increased globalization has been coupled with a relative decrease in patient enrolment from NA. Trials conducted exclusively in NA demonstrated poor enrolment efficiency, and thus required a greater number of sites to achieve enrolment targets. We further demonstrate that NA sites participate infrequently in trials of certain HF populations, namely HF with preserved ejection fraction.
In aggregate, these trial-level observations have important implications to future resource investment, trial planning, and regulation of global products. First, there is an urgent need to bolster NA research infrastructure to more efficiently and effectively test emerging HF therapeutics. The conduct of trials confined to other global regions will further decrease site-based exposure and experience in NA. Indeed, due to sluggish early enrolment by NA sites in TOPCAT, enrolment was driven by sites in Russia and Georgia.5 Several strategies have been proposed to improve engagement and participation of NA sites.6 Re-examination of incentives for NA site investigators and funding support for site maintenance may improve these adverse enrolment patterns. Given the increasing costs of trial conduct, enrolment may be more realistically tied to non-monetary incentives (for instance, institutional promotion or authorship on trial publications). In addition, trials may leverage local research networks of well-established sites to facilitate enrolment, while limiting upfront site initiation costs. Sponsors may also consider developing a pre-trial registry7 to monitor site performance and patient volume prior to trial launch to guide selection of high-performing sites. Although this approach would incur upfront resources, downstream cost savings may be achieved through more efficient enrolment by fewer sites.
Second, all global regions should continue to participate in trials testing therapies for HF with preserved ejection fraction, an entity that currently lacks disease-modifying therapeutics and is subject to heterogeneity in case identification.8 Rigorous screening and application of trial eligibility criteria are critical across geographic regions. The overall low rate of engagement in these trials across regions, but especially in NA and WE, is concerning.
Third, regulatory bodies, including the US Food and Drug Administration, will need to navigate differential global representation in emerging HF trials for purposes of assessing the validity of study results to their respective patients. Indeed, in certain cases, regulatory bodies may require an enrolment quota from a particular region to facilitate meaningful testing of regional consistency in treatment effects.9 Although these regulations may add cost and complexity to overall trial programmes, regional benchmarks for enrolment may be necessary to ensure adequate representativeness and generalizability of trial results.
This contemporary look at geographic representation in HF trials highlights concerning patterns of decreased trial participation and enrolment efficiency in NA and WE. These data may inform efforts to strengthen research infrastructure across regions to support the contemporary HF trial enterprise. Globalization in clinical trials is not fundamentally problematic and may carry certain advantages given the growing worldwide prevalence of HF. However, unbalanced enrolment with disproportionate representation from only few regions introduces challenges in interpreting the results and their clinical relevance in regions with significantly lower enrolment.
Acknowledgments
Conflict of interest: M.V. is supported by the NHLBI T32 postdoctoral training grant (T32HL007604). A.S.T. is supported by the Abraham J. & Phyllis Katz Foundation (Atlanta, GA) and NIH/NIA grant AG051633. S.J.G. is supported by the NHLBI T32 postdoctoral training grant (T32HL069749-14) and a Heart Failure Society of America/Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis. J.B. has received research support from the NIH and European Union; and has been a consultant for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CVRx, Janssen, Luit-pold Pharmaceuticals, Medtronic, Merck, Novartis, Relypsa, Vifor Pharma, and ZS Pharma. All other authors have no conflicts to declare.
References
- 1.Samman Tahhan A, Vaduganathan M, Kelkar A, Georgiopoulou VV, Kalogeropoulos AP, Greene SJ, Fonarow GC, Gheorghiade M, Butler J. Trends in heart failure clinical trials from 2001–2012. J Card Fail 2016;22: 171–179. [DOI] [PubMed] [Google Scholar]
- 2.Butler J, Subacius H, Vaduganathan M, Fonarow GC, Ambrosy AP, Konstam MA, Maggioni A, Mentz RJ, Swedberg K, Zannad F, Gheorghiade M; EVEREST Investigators. Relationship between clinical trial site enrollment with participant characteristics, protocol completion, and outcomes: insights from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) trial. J Am Coll Cardiol 2013;61: 571–579. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira JP, Girerd N, Rossignol P, Zannad F Geographic differences in heart failure trials. Eur J Heart Fail 2015;17:893–905. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 131:34–42. [DOI] [PubMed] [Google Scholar]
- 5.Bristow MR, Enciso JS, Gersh BJ, Grady C, Rice MM, Singh S, Sopko G, Boineau R, Rosenberg Y, Greenberg BH. Detection and management of geographic disparities in the TOPCAT trial: lessons learned and derivative recommendations. JACC Basic Transl Sci 2016; 1: 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler J, Fonarow GC, O’Connor C, Adams K, Bonow RO, Cody RJ, Collins SP, Dunn-mon P, Dinh W, Fiuzat M, Georgiopoulou VV, Grant S, Kim SY, Kupfer S, Lefkowitz M, Mentz RJ, Misselwitz F, Pitt B, Roessig L, Schelbert E, Shah M, Solomon S, Stockbridge N, Yancy C, Gheorghiade M. Improving cardiovascular clinical trials conduct in the United States: recommendation from clinicians, researchers, sponsors, and regulators. Am Heart J 2015;169: 305–314. [DOI] [PubMed] [Google Scholar]
- 7.Greene SJ, Shah AN, Butler J, Ambrosy AP, Anker SD, Chioncel O, Collins SP, Dinh W, Dunnmon PM, Fonarow GC, Lam CS, Mentz RJ, Pieske B, Roessig L, Rosano GM, Sato N, Vaduganathan M, Gheorghiade M. Designing effective drug and device development programs for hospitalized heart failure: a proposal for pretrial registries. Am Heart J 2014;168: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iorio A, Pozzi A, Senni M. Addressing the heterogeneity of heart failure in future randomized trials. Curr Heart Fail Rep 2017;14: 197–202. [DOI] [PubMed] [Google Scholar]
- 9.Vaduganathan M, Butler J, Gheorghiade M. Transforming drug development in heart failure: navigating the regulatory crossroads. Circ Heart Fail 2016;9:e003192. [DOI] [PubMed] [Google Scholar]