Abstract
Translational data on chimeric antigen receptor (CAR) T-cell trials indicate that the presence of naïve T cells in the premanufacture product is important to clinical response and persistence. In anticipation of developing CAR trials for other tumors, we investigated the T-cell distribution from children with solid tumors and lymphomas at diagnosis and after every cycle of chemotherapy. We found that patients with T cells enriched for naïve and stem central memory cells expanded well in vitro, but the majority of tumor types showed chemotherapy-related depletion of early lineage cells with a corresponding decline in successful ex vivo stimulation response. Unexpectedly, many pediatric patients with solid tumors had low numbers of naïve T cells prior to any therapy. These data indicate the ex vivo manufacture of CAR T cells may need to be customized based on the nature of T cells available in each disease type.
INTRODUCTION
Adoptive cellular therapy has become a promising therapy for hematologic malignancies expressing CD19 (1). There is intense interest in expanding this therapy concept not only to other hematologic cancers (targeting CD22, CD 123, FLT3, and others) but also to solid tumors. There is a difference in efficacy of chimeric antigen receptor (CAR) T cells targeting CD19 in lymphoma versus acute lymphoblastic leukemia (ALL) or chronic lymphocytic leukemia (CLL), as complete remission rates for tisagenlecleucel in pediatric ALL are >80% but for adult lymphoma are ~50% (2–6). There are two main areas of variance: the nature of the tumor and the nature of the T cells collected from the patient. The vector, cell manufacturing, CAR structure, infusion cell number, and supportive care are otherwise the same for the tisagenlecleucel product (7). Although the tumor environment is unquestionably important, and will be of critical importance for moving CAR T cells into solid tumors, the quality of the collected T cells for manufacture has also been noted to be critical. The presence of naïve and early memory T cells in the premanufacture product was found to correlate with a biomarker of successful CAR performance in pediatric ALL (8). The presence of CD27+CD45RO−CD8+ T cells (consistent with a naïve or early memory phenotype) in the premanufacture product of patients with CLL also correlated with eventual clinical response to CAR T-cell therapy (9). In pediatrics, the majority of patients treated with CAR T cells targeting CD19 have been after allogeneic transplant (2). In those cases, the T cells collected represent immunologic recovery from healthy, non-chemotherapy-exposed stem cells, which are likely fundamentally different than those from chronically chemotherapy-exposed patients. This will not be the case for solid tumors, where allogeneic transplant has no role and chemotherapy regimens are notable for their intensity (10).
Case series of pediatric patients demonstrated lower absolute T-cell counts in children with ALL both at diagnosis and throughout therapy (11). Work by Mackall and colleagues in the 1990s demonstrated that chemotherapy, especially cyclophosphamide, depleted lymphocytes in 16 children with various cancers, including brain tumors (12). The depletion was more pronounced in the CD4+CD45RA+ group, suggesting that naïve or early memory T cells were targeted by therapy. Even after quantitative recovery, there was evidence of poor function using mixed lymphocyte reaction assays and an increased susceptibility to activation-induced cell death in response to mitogens (12–16). The authors noted at the time that naïve T cells were expected to be quiescent, and thus chemotherapy must be targeting these cells despite the expectation that chemotherapy is more effective against proliferating or replicating cell targets. The importance of the phenotype of the collected cells as well as of the cells at time of infusion of adoptive cell therapies is a subject of intense investigation, as we may be able to wield significant influence over the phenotype with specific methods of ex vivo cell manufacture (5, 17).
We undertook a prospective and longitudinal analysis of T-cell expansion ability and surface phenotype after each cycle of chemotherapy in pediatric patients with 10 different diagnoses. We found that patients with standard-risk ALL had the highest percentage of samples that passed an in vitro stimulation test used in our previous work to characterize CAR T-cell potential (8). Using this group as a baseline comparator, we found most solid tumors to have a lower percentage to pass stimulation/expansion tests. We also repeated our prior finding that T cells from patients with non-Hodgkin lymphoma (NHL) have poor expansion potential, observing the same is true for Hodgkin lymphoma [or Hodgkin disease (HD)]. Analysis of the change in T-cell subsets over time showed a significant correlation in the decline of naïve and stem central memory (SCM) T-cell subsets with poor expansion, and that these subsets decline over time with cumulative chemotherapy cycles of varying composition. We investigated the ability of IL7 and IL15 to improve expansion, and found this was true for all tumor types, though the nature of which T-cell subsets expanded varied based on initial response. This study is the largest ever of pediatric patients to examine in this degree of detail the T-cell composition of peripheral blood at diagnosis and through therapy, and reveals unexpected variance in T-cell distribution among tumor types and after chemotherapy.
RESULTS
Patient Characteristics
One hundred ninety-five patients with newly diagnosed malignancies were enrolled as part of a clinical trial under an approved Children’s Hospital of Philadelphia Institutional Review Board (IRB) protocol (Table 1). Patient age at time of enrollment ranged from 1 month to 21 years. Disease groups included ALL, stratified as National Cancer Institute standard risk (SR ALL; n = 40) or high risk and very high risk (HR ALL; n = 37), NHL (n = 15), HD (n = 23), acute myeloid leukemia (AML; n = 7), T leukemia or lymphoblastic lymphoma (T LL/Ly; n = 9), rhabdomyosarcoma (n = 12), Ewing sarcoma (n = 13), osteosarcoma (n = 9), neuroblastoma (n = 11), and Wilms tumor (n = 19). Blood samples were obtained at diagnosis (prior to chemotherapy) and just prior to each cycle of chemotherapy with regular labs (1,404 individual blood samples). The decision to collect on the day of starting each subsequent cycle of therapy was to reflect the time at which centers are targeting for T-cell collections (i.e., count recovery before the next administration of disease-targeted therapy).
Table 1.
Patient characteristics
| Disease | Age (years) | Number |
|---|---|---|
| ALL | 5.8 ± 4.7 | 77 |
| NHL | 11.5 ± 5.6 | 15 |
| HD | 15 ± 2.6 | 23 |
| T-LL/Ly | 11.1 ± 6 | 9 |
| AML | 6.5 ± 5.7 | 7 |
| NB | 1.9 ± 1.5 | 11 |
| Wilms | 3.5 ± 3 | 19 |
| Ewings | 11 ± 5.9 | 13 |
| Rhabdo | 7.6 ± 5.3 | 12 |
| Osteo | 12.1 ± 4.9 | 9 |
NOTE: One hundred ninety-five patients were included in our prospective analysis. Age represented as means with standard deviation.
Abbreviations: ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin lymphoma; HD, Hodgkin disease; T-LL/Ly, T-cell leukemia/lymphoma; AML, acute myeloid leukemia; NB, neuroblastoma; Wilms, Wilms tumor; Ewings, Ewing sarcoma; Rhabdo, rhabdomyosarcoma; Osteo, osteosarcoma.
T-cell Expansion Potential Varies by Disease Group and Cycle of Therapy
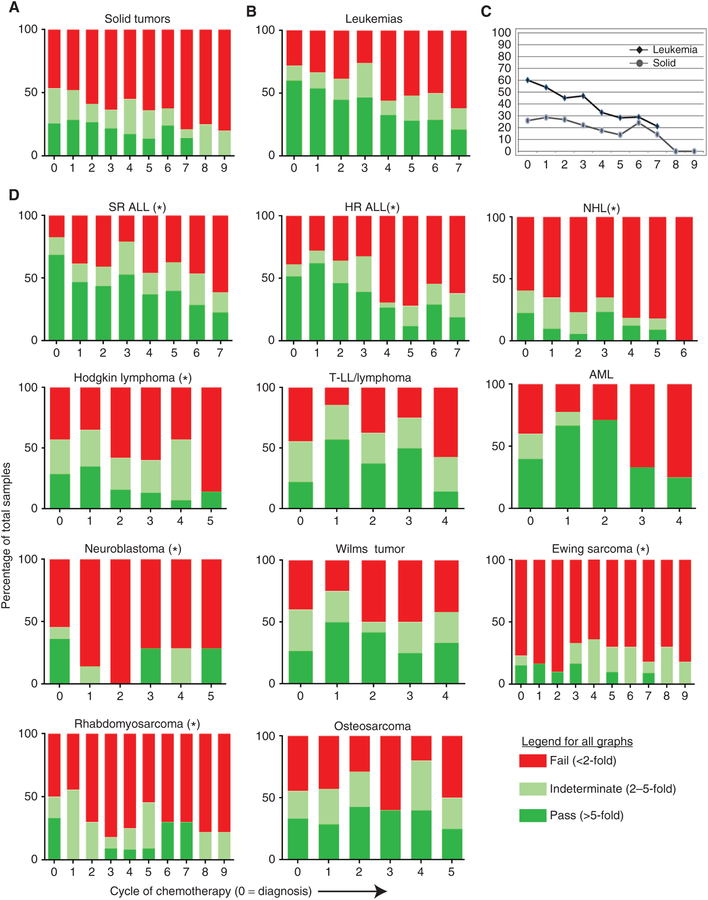
Peripheral blood was collected from each enrolled patient at diagnosis and after each cycle of chemotherapy, with cycles defined by the protocol on which the children were treated. T cells were isolated and stimulated using beads coated with CD3 and CD28 agonist antibodies, as used in our GMP cell-manufacturing process (18), and cell expansion was measured over time as previously described (8). As we reported previously, a threshold of >5-fold expansion during stimulation was associated with a high likelihood of successful clinical expansion. We applied the same threshold to stratify our analysis into samples that “pass” expansion (>5-fold), those that “fail” expansion (<2-fold), and those that are indeterminate (2–5-fold). Patients with SR ALL had the highest initial pass rate at diagnosis (69%), which trended downward over time (Fig. 1A–D). HR ALL did not have a statistically lower pass rate at diagnosis (52%), but pass rates trended down more quickly than those in SR ALL, consistent with our prior report. Both HD (29%) and NHL (23%) had lower pass rates than SR ALL at diagnosis (69%), and both trended even worse over time. In contrast, AML (40%) and T LL/Ly (22%) had lower initial pass rates but did not see a significant downward trend until after cycle 3 or 4 of therapy. In solid tumors, rhabdomyosarcoma (33%) and neuroblastoma (36%) had the highest initial pass rates, though both were statistically significantly lower than that of SR ALL. In contrast, Ewing sarcoma (15.3%) was low at diagnosis and fell to very low levels of pass rates after only one or two cycles of therapy, and then remained low. Wilms tumor (27%) and osteosarcoma (33%) held their pass rates stable over time (see Supplementary Table S1 for summary of statistical comparisons).
Figure 1.
Percentage of peripheral blood samples that demonstrated >5-fold in vitro expansion. A, Samples from patients with solid tumors (Wilms tumor, neuroblastoma, osteosarcoma, Ewing sarcoma, rhabdomyosarcoma) display low pass rates with CD3/CD28 stimulation, and this declines over time (X axis is cycle of chemotherapy, with 0 = diagnosis). B, Samples from patients with lymphoblastic or myeloid leukemia show high pass rates but also decline over time with each chemotherapy cycle. C, Line graph showing data from A/B with pass and indeterminate added together. D, Each individual disease type with pass rates over time. Asterisk indicates a significant decline over time (increasing number of chemotherapy cycles) by regression analysis (P < 0.05). N for each disease group is as in Table 1. See Supplementary Table S1 for summary of statistics for each disease compared with SR ALL.
T-cell Expansion Potential Directly Correlates with Naïve and SCM Percentages Regardless of Disease Type
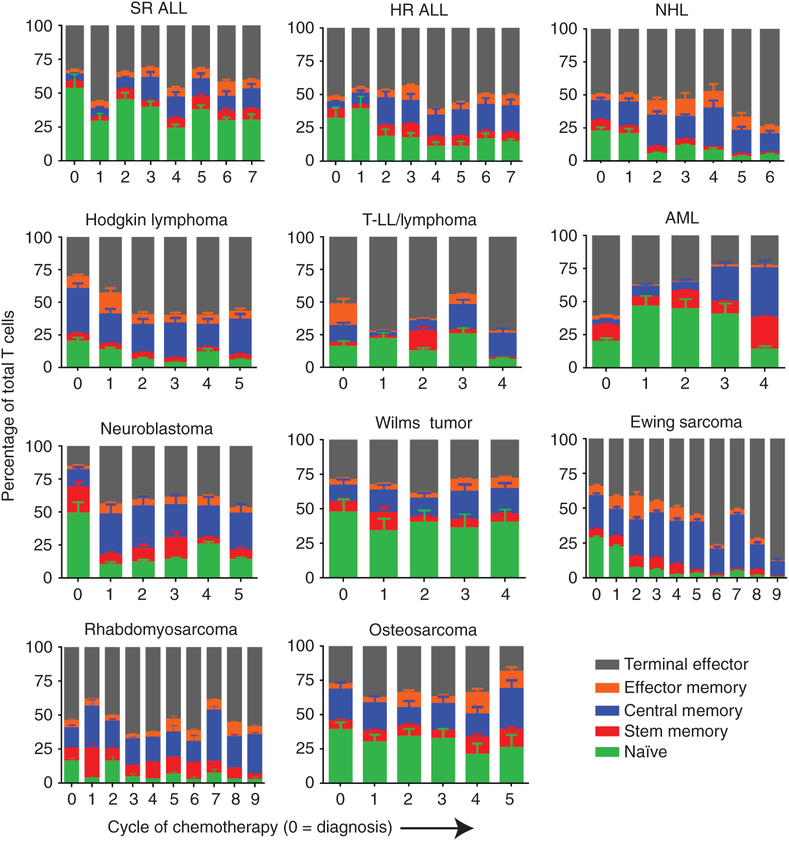
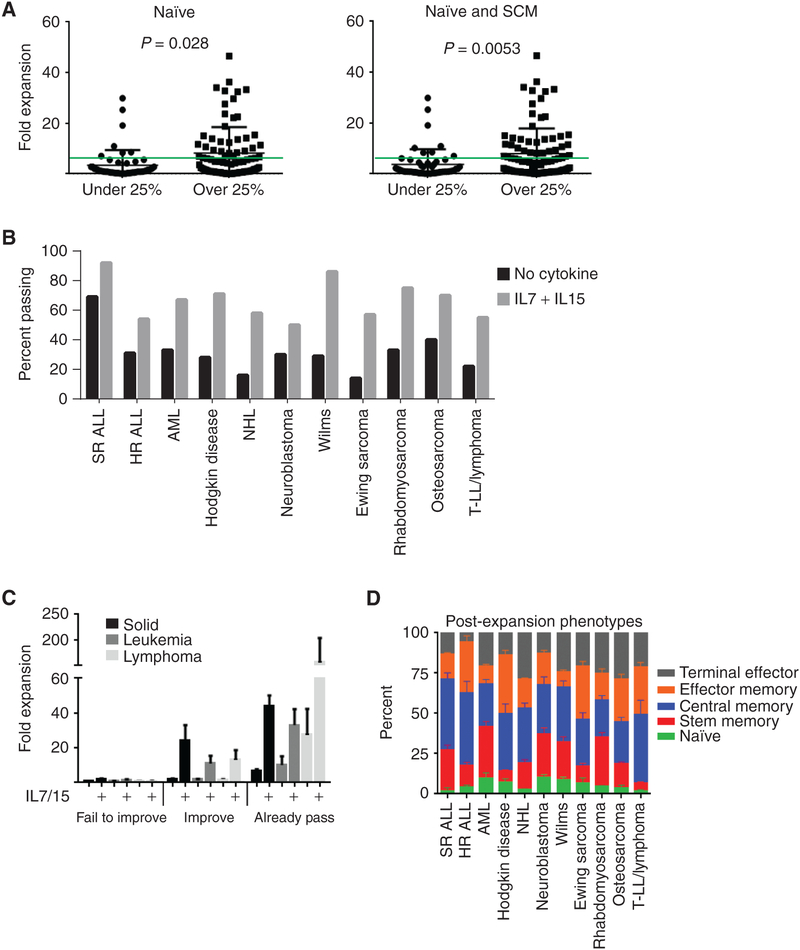
We examined the relationship between T-cell subsets and expansion potential to evaluate if the decline in expansion correlated with decline in any particular T-cell subset. For comparison, studies on healthy children find a naïve T-cell percentage of~80% for the first several years of life, ~70% between 2 and 6 years, ~60% between 6 and 12 years, and declining to ~50% by 18 years of age (19). Once again, patients with SR ALL had the highest naïve (53%) percentage of any tumor type, though this is lower than average in healthy children in this age range. Using SR ALL as a baseline, every other diagnosis had significantly fewer naïve cells at diagnosis (P < 0.01). The percentage of naïve cells fluctuated but never fell below 25% in SR ALL. In contrast, naïve cells declined significantly over time in HR ALL, HD, NHL, Ewing sarcoma, rhabdomyosarcoma, and neuroblastoma (Fig. 2). There was no significant decline in naïve T-cell percentage in T LL/Ly or AML, though post cycle 4 is very low compared with prechemotherapy. Patients with Wilms tumor, AML, and osteosarcoma had no significant decline in naïve percentage. There were some disease-specific changes in other T-cell subsets, mostly an increase in central memory (CM) or terminal effector (TE) cells, which are noted in Supplementary Table S2. We also observed higher SCM percentages (from 1% to 24%) in these children than were reported in normal adults (0.5%–1%). CD4 and CD8 ratios were calculated, and no significant trends could be identified (Supplementary Table S3). When combining all patients, there is no correlation between absolute lymphocyte count or absolute T-cell count and expansion potential (Supplementary Fig. S1A and S1B). Based on SR ALL, we chose a threshold of 25% or higher naïve cells or naïve plus SCM and looked for predictive value of passing the stimulation test. Both were statistically significantly associated with passing expansion, but this cutoff had relatively disappointing sensitivity (0.44–0.51) and specificity (0.11–0.20) when applied prospectively (Fig. 3A). Empiric adjustment found no better threshold when accounting for repeat statistical measurement.
Figure 2.
T-cell phenotypes of cells harvested from the peripheral blood of patients undergoing chemotherapy. Each disease from Fig. 1 is represented with percentages of each of the five subsets of T cells described. The X axis represents each cycle of chemotherapy (0 is prechemotherapy). Error bars, SD of each subset. The pattern of naïve T-cell percentage largely tracks with expansion potential. SR ALL, AML, Wilms tumor, and osteosarcoma are notable for the stable percentage of naïve T cells compared with other diseases. The definitions of the T-cell phenotypes are described in the Methods. See Supplementary Table S2 for statistical summary of significant trends.
Figure 3.
Effect of naïve or SCM percentage and cytokines on expansion potential. A, There is a significant difference in expansion potential when using 25% naïve T cells (P = 0.028) or 25% naïve plus SCM (P = 0.0053) as a cutoff. The green line represents 5-fold expansion. Samples are from time point 0 (diagnosis). B, Addition of IL7 and IL15 to the expansion procedure results in a higher percentage of patient samples surpassing 5-fold expansion in all tumor types (n = 79 patients in total). C, Prechemotherapy/diagnostic samples were separated into three groups based on initial pass rate and response to the addition of IL7 and IL15. Supplementary cytokines did not rescue some samples (fail to improve), rescued others (improve), and always increased the cell number in samples that passed initially without them (already pass). D, Diagnostic/prechemotherapy samples after bead expansion demonstrate a majority of CM T cells in most disease types, with a possible inverse relationship between SCM and effector memory (EM) cells that is not statistically significant. Please see the Methods for the phenotypic definitions of each subset.
Effect of IL7 and IL15 on Expansion Rates and T-cell Phenotypes
We have previously reported that the addition of IL7 and IL15 improved the success rate of expansion in patients with NHL (8). We tested the addition of IL7 and IL15 to the expansion with CD3/CD28 beads and noted an increase in the number of samples that expanded greater than 5-fold in each disease type (Fig. 3B). We were unable to test all patient samples due to cell number limitations, so this represents a subset of patients from Fig. 1 (79 of 195). We broke down the effects on expansion by initial response and response to cytokines into three groups (Fig. 3C): fail to expand >5-fold with or without cytokines (“fail to improve”), fail without cytokines but improve to >5-fold with cytokines (“improve”), and samples that had already expanded >5-fold without cytokines (“already pass”). There were no samples that passed without cytokines and then failed with the addition of cytokines. We observed the post-bead expansion phenotypes for each disease type without cytokines at the prechemotherapy/diagnostic time point (Fig. 3D) and found few statistically significant variations, though this is biased to those samples in which there were surviving cells to analyze. Furthermore, we plotted the change in this post-expansion phenotype with the addition of IL7 and IL15 (Supplementary Fig. S2). Universally, IL7 and IL15 increase the final post-expansion percentage of SCM T cells, and in samples that had previously failed this is at the expense of terminal effector T cells. However, in samples from both solid tumors and leukemias that already passed expansion, the addition of cytokines also increased the number of terminal effector T cells. We were unable to assess the “already passed” lymphoma subgroup due to a lack of samples that passed without cytokines.
DISCUSSION
Here, we significantly extend our previous work on the expansion potential of T cells from pediatric patients with cancer at diagnosis and throughout therapy. We detail the depletion of naïve T cells with cumulative chemotherapy cycles across multiple disease types as well as describe for the first time the variation in T-cell subsets at diagnosis. Although previous studies have assessed the effect of chemotherapy on T cells (11, 12, 14, 15), these studies were limited by patient number and grouped different regimens together. This study presents prospective data on multiple disease types including solid tumors and presents analysis of each type separately, representing the most comprehensive and largest analysis of this type to date. Furthermore, in the era of T-cell immune therapy, we assessed the potential for adoptive cell therapy manufacture from each disease type using a surrogate assay of successful CAR T-cell manufacture described previously. These data reveal intrinsic differences in T-cell distribution and proliferative capacity from patients with solid tumors, which may be important when considering CAR T-cell therapy protocols for these disease types. The difference in naïve T-cell content from patients with solid tumors was unexpected. These data do not explain if children with altered T-cell distributions are more at risk for solid tumors, or if solid tumors are influencing T-cell distribution in previously unidentified ways. The naïve T cells appear to have qualitative as well as quantitative defects, as using surface phenotype to define naïve T-cell content alone was not sufficient to explain the variance in response to CD3/CD28 bead stimulation. This variance is further suggested by the variable response to supplementary cytokines in the prechemotherapy samples, where some samples respond, others do not, and others overexpand the terminal effector subset. It would appear that normal naïve T cells are necessary for good proliferation potential, but that defining them by surface markers may miss qualitative defects that make some insufficient for good proliferation response. We did not find any significant differences in terminal effector subsets, but there may also be interactions between T-cell subsets that influence expansion quality that we lack the power to detect in this data set.
For most chemotherapy regimens, the potential of T cells to respond to ex vivo stimulation declined over time, though Wilms tumor and osteosarcoma held steady (see Supplementary Table S4 for chemotherapy regimens). Wilms tumor therapy is not considered clinically very intense (outpatient administration) but does include anthracyclines, and the patients tend to be very young. There may be unique biological features to this cancer compared with the others, which we plan to explore. Patients with osteosarcoma tend to be older, and the therapy is extremely intense, with cisplatinum, doxorubicin, and two consecutive doses of methotrexate. We are curious if the long time from cisplatinum and doxorubicin (typically about 6–8 weeks) prior to sample collection could be an influence. Ewing sarcoma therapy is also very intense and is compressed in time by design (called interval compression). Patients with Ewing sarcoma lost proliferative capacity quickly and did not recover. We would hypothesize longer times from a recent chemotherapy cycle might result in healthier T cells to collect, though this may depend on thymic function. Naïve T-cell content or recent thymic emigrants (TREC) may be a useful biomarker with which to explore this possibility. We do not have the power to detect the influence of any particular agent, though we are exploring in vitro mechanisms of toxicity against naïve T cells to gain insight into our observations here. Practically, collecting T cells for a potential cell therapy is a delicate balance between waiting for new healthy cells and administering appropriate disease-controlling therapy.
Studies have previously demonstrated that T-cell phenotype at time of cell infusion has a significant impact on engineered T-cell persistence and antitumor activity, observing that central memory CAR T cells persist longer and mediate more successful and sustained antileukemic responses (9, 20). Most, if not all, CAR T-cell manufacturing strategies incorporate T-cell stimulation, whether using CD3/CD28 beads or surrogate antigen presentation to the CAR as stimulation is often required to facilitate integration of retroviral vectors (1, 7). Antigen-driven antitumor activity of engineered T cells from patients could not be addressed in this study due to limitations on T-cell number, though this would be a better assay if this were possible. The expansion assay with CD3/CD28 beads used here and previously is a reasonable (though far from perfect) measure of T-cell potential for becoming effective adoptive immune therapy, as there is no established patient-validated in vitro “potency” assay for CAR T cells, though the field strongly desires one. We have previously established that a starting pool of naïve and SCM T cells results in a largely CM CART-cell product after manufacture with CD3/CD28 beads, and that this phenotype is highly potent in most patients. Whether this holds true in other manufacturing protocols is not known, as each center uses its own manufacturing protocol with several other variables and differing clinical results including efficacy and toxicity (2, 4, 5, 13, 21, 22). So although our results must be interpreted with caution and currently relate only to CD3/CD28 bead manufacture, the principles are likely relevant to other protocols that use T-cell stimulation.
Most patients with solid tumors and lymphoma demonstrated significantly lower naïve and SCM populations from diagnosis, a fact not explained by age in our population. The mechanism behind this is unclear, though the association of lower naïve and SCM cells does correlate with worse expansion potential. We would not be the first to hypothesize that the solid tumor microenvironment influences global immune function, or that children with subtle immune deficiencies maybe more predisposed to cancer (23). Solid tumors known to have bone marrow metastases, such as neuroblastoma, Ewing sarcoma, and rhabdomyosarcoma, may be able to more directly influence T-cell development than tumors more associated with liver and lung metastases, such as osteosarcoma or Wilms tumor. We are now gathering prospective data on tumor biopsies and negative checkpoint regulators to explore this possibility in the future.
Beyond the simple yet significant barrier of collecting sufficient numbers of T cells for ex vivo manufacturing is the quality of the cells collected after intense cycles of chemotherapy. We cannot extrapolate solid tumor potential from the leukemia CAR T-cell patients as so many of them were post-allogeneic transplant, which will not be the case with pediatric solid tumors. The clinical data very clearly suggest cumulative chemotherapy depletes T-cell potential, which is regimen- and time-specific. It is also unclear when to apply supplementary cytokines such as IL7 and IL15. Our data indicate that these cytokines may expand the terminal effector subset in some patients (Supplementary Fig. S2), which would be considered undesirable if it is at the expense of the efficacious central memory cells. In other patients, the cytokines increase cell number, and these are predominantly of the stem central memory phenotype, which is potentially desirable. In other cases, cytokines did not affect outcome at all, and it is unclear how to predict these effects in any given patient sample.
In summary, we provide here the largest cohort of pediatric patients with cancer with quantitative descriptions of T-cell number, subset, and ex vivo stimulation capacity at diagnosis and after each cycle of chemotherapy. We demonstrate intrinsic differences in T-cell subset distribution between tumor types, and further demonstrate a reproducible decline in naïve T cells with most chemotherapy regimens. These studies provide a foundation upon which to rationally design T cell-based therapies for pediatric solid tumors and potentially adapt T-cell collection or manufacturing strategies to ensure the highest possible activity for future cellular therapies.
METHODS
Patient Selection and Clinical Protocol
Patients were identified by the clinical practices at the Children’s Hospital of Philadelphia Division of Oncology in Philadelphia, PA. Patients were enrolled onto Children’s Hospital of Philadelphia IRB-approved clinical trial CHP-12–009915, with written informed consent obtained in accordance with the U.S. Common Rule. All samples were collected at the times indicated, with a cycle defined by the Children’s Oncology Group protocol on which the children were treated.
Ex Vivo T-cell Expansion and Culture
Lymphocytes were harvested from peripheral blood and expanded as previously described (8). Briefly, peripheral blood was collected in EDTA tubes at the time of clinical lab collection. Blood was processed using Ficoll-Paque the same day, washed in phosphate-buffered saline with 2% bovine serum albumin, counted, and plated in RPMI supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine and 1% penicillin/streptomycin at 6–8 × 105 cells/mL and 5–6 × 105 cells/cm2 in plastic culture vessels to allow for overnight adherence. Suspended cells were aspirated, recounted, and an aliquot taken for CD3/CD4/CD8 and surface phenotyping. CD3/CD28 beads were added to the remainder at a ratio of 3 beads to 1 T cell, followed by daily counts adjusting the media volume and surface area as needed. After 7 days, beads were removed by magnetic field, and cells were followed for an additional 7 days. Fold expansion was calculated by taking the maximum cell number and dividing by the starting cell number. T cells from normal adult donors were obtained under an IRB-approved protocol from the Human Immunology Core at the University of Pennsylvania and deidentified prior to receipt.
Flow Cytometry
T cells were stained for cell-surface markers to differentiate T-cell lineage. Previous work (24) has demonstrated that CCR7, CD62L, CD45RO, and CD95 can be used to differentiate the various T-cell phenotypes by using the following expression patterns: naïve (TN)—CCR7+, CD62L+, CD45RO−, CD95−; stem central memory (TSCM)—CCR7+, CD62L+, CD45RO−, CD95+; central memory (TCM)—CCR7+, CD62L+, CD45RO+, CD95+; effector memory (TEM)—CCR7−, CD62L−, CD45RO+, CD95+; terminal effector (TEff)—CCR7−, CD62L−, CD45RO−, CD95+. The antibodies used for this analysis as well as quantitation of T-cell bulk described above were CD8-FITC (BD Biosciences; #347313), CD3-PE (BD Biosciences; #555340), CD4-APC (BD Biosciences; #555349), CCR7-FITC (BD Biosciences; #561271), CD95-PE (BD Biosciences; #556641), CD45RO (BD Biosciences; #559865), and CD62L-PE/Cy7 (BioLegend; 304822). Samples were then washed twice, and flow cytometry acquisition was performed on a BD FACSVerse Flow Cytometer or BD Accuri C6 (BD Biosciences).
Statistical Analysis
All statistical analyses were performed using Prism 6 (Version 6.02, GraphPad Software) using analysis of variance testing for group comparisons. Regression analysis was used for analysis of trends over time. Fisher exact test was used for the comparison of percentages. All comparisons that were reported as significant reached a P value of <0.05 or as calculated after Bonferroni corrections for multiple comparisons.
Supplementary Material
SIGNIFICANCE:
Cumulative chemotherapy cycles deplete naïve T cells in many pediatric cancer regimens, reducing expansion potential associated with successful adoptive cellular therapies. Naïve T-cell deficits can be seen at diagnosis as well, implying immune deficits that exist prior to chemotherapy, which may also affect the development of immune-based therapies.
Acknowledgments
We thank Drs. Matthew Weitzman and Hamid Bassiri for review of this manuscript. Jessica Perazzelli and Julie Storm provided technical assistance, as did the Flow Cytometry Core at the Children’s Hospital of Philadelphia. Katelyn Oranges, Anne Grifo, and Sarah Getman provided invaluable assistance in patient recruitment and monitoring. This study was supported in part by a St. Baldrick’s Foundation Scholar Award, The Doris Duke Charitable Foundation Clinical Scientist Development Award, and the Jeffrey Pride Foundation (all to D.M. Barrett). This research was also supported by a Stand Up To Cancer Innovative Research Grant, grant number SU2C-AACR-IRG 12–17 (to D.M. Barrett). Stand Up To Cancer (SU2C) is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the Scientific Partner of SU2C.
Disclosure of Potential Conflicts of Interest
S.A. Grupp reports receiving commercial research grants from Novartis, Kite, and Servier and is a consultant/advisory board member for Novartis, Cellectis, Roche, CBMG, Adaptimmune, Eureka, TCR2, Juno, GSK, Vertex, Cure Genetics, and Humanigen. D.M. Barrett is a consultant/advisory board member for Eureka Therapeutics. No potential conflicts of interest were disclosed by the other authors.
Footnotes
Note: Supplementary data for this article are available at Cancer Discovery Online (http://cancerdiscovery.aacrjournals.org/).
REFERENCES
- 1.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Ann Rev Med 2014;65:333–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2018;380:45–56. [DOI] [PubMed] [Google Scholar]
- 4.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017;129:3322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Value in using CAR T cells for DLBCL. Cancer Discov 2018;8:131–2. [DOI] [PubMed] [Google Scholar]
- 7.Vormittag P, Gunn R, Ghorashian S, Veraitch FS. A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol 2018;53:164–81. [DOI] [PubMed] [Google Scholar]
- 8.Singh N, Perazzelli J, Grupp SA, Barrett DM. Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci Transi Med 2016;8:320ra3. [DOI] [PubMed] [Google Scholar]
- 9.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018;24:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett D, Fish JD, Grupp SA. Autologous and allogeneic cellular therapies for high-risk pediatric solid tumors. Pediatr Clin North Am 2010;57:47–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komada Y, Zhang SL, Zhou YW, Hanada M, Shibata T, Azuma E, et al. Cellular immunosuppression in children with acute lymphoblastic leukemia: effect of consolidation chemotherapy. Cancer Immunol Immunother 1992;35:271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackall CL, Fleisher TA, Brown MR, Magrath IT, Shad AT, Horowitz ME, et al. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood 1994;84:2221–8. [PubMed] [Google Scholar]
- 13.Fuks Z, Strober S, Bobrove AM, Sasazuki T, McMichael A, Kaplan HS. Long term effects of radiation of T and B lymphocytes in peripheral blood of patients with Hodgkin’s disease. J Clin Invest 1976;58:803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haining WN, Neuberg DS, Keczkemethy HL, Evans JW, Rivoli S, Gelman R, et al. Antigen-specific T-cell memory is preserved in children treated for acute lymphoblastic leukemia. Blood 2005;106:1749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood 1997;89:3700–7. [PubMed] [Google Scholar]
- 16.Hakim FT, Cepeda R, Kaimei S, Mackall CL, McAtee N, Zujewski J, et al. Constraints on CD4 recovery postchemotherapy in adults: thymic insufficiency and apoptotic decline of expanded peripheral CD4 cells. Blood 1997;90:3789–98. [PubMed] [Google Scholar]
- 17.Barrett DM, Singh N, Liu X, Jiang S, June CH, Grupp SA, et al. Relation of clinical culture method to T-cell memory status and efficacy in xenograft models of adoptive immunotherapy. Cytotherapy 2014;16:619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine BL, Bernstein WB, Connors M, Craighead N, Lindsten T, Thompson CB, et al. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol 1997;159:5921–30. [PubMed] [Google Scholar]
- 19.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol 2003;112:973–80. [DOI] [PubMed] [Google Scholar]
- 20.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells expressing chimeric receptors establish memory and potent antitumor effects in patients with advanced leukemia. Sei Translat Med 2011;3:95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill S, June CH. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev 2015;263:68–89. [DOI] [PubMed] [Google Scholar]
- 22.Ruella M, June CH. Chimeric antigen receptor T cells for B cell neoplasms: choose the right CAR for you. Curr Hematol Malign Rep 2016;11:368–84. [DOI] [PubMed] [Google Scholar]
- 23.Hegde M, Moll AJ, Byrd TT, Louis CU, Ahmed N. Cellular immunotherapy for pediatric solid tumors. Cytotherapy 2015;17:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med 2011;17:1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.