Abstract
Rationale:
Prior research with transgenic mice in which the kappa opioid receptor (KOR) has been suppressed or activated suggests that the aversive effects of THC are mediated by activity of this receptor subtype. If the activity of the KOR system is responsible for mediating the THC’s aversive effects, then selective antagonism of the KOR by norBNI should block such aversive effects. To test this hypothesis, rats were pretreated with norBNI 24 hours prior to place conditioning with THC to assess its effect on the acquisition of THC-induced place aversions.
Methods:
In Experiment 1, rats pretreated with norBNI (0 or 15 mg/kg) were exposed 24 hours later to one side of a place conditioning chamber and injected with THC (0, 0.56, 1 and 3.2 mg/kg). On the next day, they were injected with vehicle and placed on the opposite side of the chamber. This was repeated for a total of five cycles followed by a test of the animal’s aversion to the THC-paired side. In Experiment 2, rats were pretreated with norBNI (0 or 30 mg/kg) prior to place conditioning 24 hours later with THC (0 or 3.2 mg/kg).
Results:
In Experiment 1, THC produced dose-dependent place aversions that were unaffected by norBNI (15 mg/kg). In Experiment 2, THC induced significant place aversions that were fully attenuated by norBNI (30 mg/kg).
Conclusions:
Although 15 mg/kg norBNI was ineffective in antagonizing the aversive effects of THC, 30 mg/kg norBNI blocked the ability of THC to induce a place aversion. The results of the latter assessment are consistent with prior research with transgenic manipulations of the KOR and provide further evidence for the role of the KOR system in the aversive properties of THC.
Keywords: THC, norBNI, KOR, place aversion conditioning, rats, dose-response
1. Introduction
Drugs of abuse produce both rewarding and aversive effects with the balance of these affective properties impacting use and abuse vulnerability (Ettenberg et al., 2015; Simpson and Riley, 2005; Wise et al., 1976). Specifically, it has been argued that while the rewarding effects of a drug maintain use and abuse, its aversive effects limit it (for a review, see Riley, 2011). In this context, marijuana is of special interest given that despite its widespread use in human populations (World Health Organization, 2016) and the incidence of marijuana substance use disorder (SAMHSA, 2015), pre-clinical models demonstrating the rewarding effects of THC, the main psychoactive constituent of marijuana, are generally lacking (for a recent review, see Tanda, 2016; see also Wakeford et al., 2017). It has been suggested that the general failure of THC to induce a place preference or support steady rates of intravenous self-administration is the result of its aversive effects masking its potential rewarding effects (Cheng et al., 2004; Ghozland et al., 2002; Valjent and Maldonado, 2000; for recent discussions, see Hempel et al., 2016; Hempel et al., 2017; Wakeford et al., 2016; Wakeford et al., 2017). Accordingly, characterizing the aversive effects of THC and the host of factors that influence this property may provide some insight into the general failure to see THC self-administration in animals as well as the basis for marijuana use and abuse in humans.
In relation to the aversive effects of THC, it has been reported that the binding of THC to the cannabinoid 1 receptors (CB1) induces upregulated production and release of the endogenous kappa opioid receptor (KOR) peptide dynorphin (Houser et al., 2000; Mason et al., 1999; Welch and Eads, 1999). The subsequent binding of dynorphin to the KOR is thought in turn to produce stress and negative affect (Knoll and Carlezon, 2010). In support of this, Ghozland et al. (2002) reported that KOR knockout mice failed to display place aversions induced by 5 mg/kg of THC, while intact mice displayed robust place aversions at the same dose. Similarly, Cheng et al. (2004) found that mice lacking the gene coding for the downstream regulatory element antagonistic modulator (DREAM) for prodynorphin displayed potentiated place aversions when conditioned with both 1 and 5 mg/kg of THC compared to intact mice receiving the same doses. Mice lacking expression of DREAM displayed upregulated prodynorphin production and, thus, stronger responses to KOR activity because DREAM acts as a transcriptional repressor for prodynorphin.
If the upregulated binding of dynorphin to the KOR is responsible for the aversive effects of THC, then selective antagonism of the KOR should block these effects. In this context, the selective and long-term KOR antagonist nor-binaltorphimine (norBNI) has been reported to block a number of KOR mediated effects, e.g., antinociception. For example, in an assessment of selective opioid antinociception in a rat model of visceral pain Craft et al. (1995) found that norBNI selectively blocked analgesia induced by the KOR agonist BW373U86. In a similar report, Stein et al. (1988) found that norBNI selectively blocked KOR-mediated analgesia induced by the KOR agonist U50,488H (see also Horan et al., 1992 for a description of norBNI antagonism of analgesia induced by the selective KOR agonists U69,593 and bremazocine, but not analgesia mediated by selective mu or delta opioid receptor agonism.
Given that prior work with transgenic mice substantiates the role of the KOR system in the aversive effect of THC and that norBNI blocks a number of KOR-mediated effects, the present experiment evaluated whether long term and selective KOR antagonism by norBNI pretreatment would block the aversive properties of THC in rats as indexed by the conditioned place aversion design. To test this possibility, norBNI was administered to male Sprague-Dawley rats prior to place aversion conditioning with THC in two separate experiments. Specifically, in Experiment 1 rats pretreated with norBNI (0 or 15 mg/kg) were exposed 24 hours later to one side of a place conditioning chamber and injected with THC (0, 0.56, 1 and 3.2 mg/kg). In Experiment 2, rats were pretreated with norBNI (0 or 30 mg/kg) prior to place conditioning 24 hours later with THC (0 or 3.2 mg/kg).
2. Experiment 1
2.1. Materials and methods
2.1.1. Subjects and housing
Sixty-three experimentally naïve, male Sprague Dawley rats were obtained from Envigo (Indianapolis, IN) on postnatal day (PND) 21. They were group-housed (three rats per OptiRat Plus polycarbonate bins; 38.9 × 56.9 × 26.2 cm; 1,181 sq. cm) and maintained on ad libitum food and water for the entirety of the study. The animals remained experimentally and drug naïve until PND 87 when experimental procedures began. The study was approved by the Institutional Animal Care and Use Committee of American University and followed the National Research Council’s Guide for the Care and Use of Laboratory Subjects (2011) and the Guidelines for The Care and Use of Mammals in Neuroscience and Behavioral Research (2004).
2.1.2. Drugs and solutions
Norbinaltophimine (norBNI, synthesized at the Molecular Targets and Medications Discovery Branch of the National Institute on Drug Abuse) was dissolved in sterile H2O at concentrations of 15 (Experiment 1) and 30 mg/ml (Experiment 2) and administered once subcutaneously (SC) at doses of 15 (Experiment 1) and 30 mg/kg (Experiment 2) 24 hours prior to the initiation of place aversion conditioning with THC (see below). Sterile H2O was administered as vehicle to control animals at a volume equal to the 15 or 30 mg/kg dose of norBNI. The specific procedure utilized, i.e., a single injection of norBNI given 24 hour prior to the initiation of conditioning, was based on literature demonstrating KOR antagonism in various behavioral assessments. For example, 15 mg/kg norBNI has been shown to selectively antagonize the KOR-mediated antinociceptive effects of U50,588 in mice (Takemori et al., 1988) and rats (Craft et al., 1995) without influencing the antinociceptive effects mediated by mu and delta opioid receptors (for other examples of norBNI antagonism at this dose and below, see Knoll et al., 2007; Walker et al., 2010). The specific timing of the norBNI injections (24 hour prior to the initiation of conditioning) was based on research demonstrating that immediately after administration, and up to 2 hours, norBNI has a mild affinity for the μ-opioid receptor (MOR) that transitions to a highly selective antagonism at the KOR 24 hours after administration in both mice (Broadbear et al., 1994; Sperling et al., 2010) and rats (Grella et al., 2014; Walker et al., 2010; Wee et al., 2009). Only a single injection was administered given that systemic administration of norBNI produces KOR antagonism that persists for weeks or months (for reviews discussing the extended time course of norBNI antagonism, see Béguin and Cohen, 2009; Bruchas et al., 2007; Metcalf and Coop, 2005; for articles implementing such logic see Schlosburg et al., 2013).
THC (generously supplied by the National Institute on Drug Abuse, NIDA) was dissolved in a solution of 5% ethanol, 5% Cremophor (Sigma-Aldrich) and 90% saline at a concentration of 1 mg/ml and administered intraperitoneally (IP) at a dose of 0.56, 1 or 3.2 mg/kg. Such a range allowed an assessment of the effects of norBNI on specific doses that have been reported to have minimal to significant aversive effects (see Mallet and Beninger, 1998; McGregor et al., 1996; Parker and Gillies, 1995; Sanudo-Pena et al., 1997). The THC vehicle was also prepared as a 5% ethanol, 5% Cremophor (Sigma-Aldrich) and 90% saline solution and was administered to control animals at a volume equal to the highest dose of THC administered (3.2 mg/kg). All drug, vehicle and saline solutions were filtered through a 0.2 μl syringe filter to remove any possible contaminants before being administered.
2.1.3. Apparatus
Eight identical place conditioning apparatuses were used for this assessment. Each apparatus (San Diego Instruments, Place Preference System, San Diego, CA) consisted of two main conditioning chambers (28 cm × 21 cm × 34.5 cm) joined by a smaller middle chamber (14 cm × 21 cm × 34.5 cm). One of the conditioning chambers featured a white aluminum diamond plate floor with smooth white walls; the other conditioning chamber featured a haircell-textured black plastic floor with smooth black walls; the smaller middle chamber was outfitted with a steel rod floor and gray walls. While the chambers were outfitted with white LED houselights, these were left unused throughout the entire experiment in order to produce a completely unbiased conditioning apparatus (for a discussion on the impact of light in place conditioning; see Cunningham et al., 2003; Roma and Riley, 2005). Each apparatus featured a 16 × 4 photobeam array for recording time (in sec) spent in each chamber. The CPP room was illuminated by a 25-W red light mounted to the ceiling, and a white noise generator was used to mask background noise.
2.2. Method
2.2.1. Conditioned place aversion: Pretest and drug pretreatment
On PND 87, each animal was allowed 20 min to freely explore the entire place conditioning apparatus with time spent in each chamber recorded to obtain individual baseline preferences. Immediately following the Pretest, animals were returned to their home cages. On the following day (PND 88), the animals were randomly assigned to one of two groups [norBNI (n = 34) and Vehicle (n = 34)]. Subjects assigned to the norBNI group were injected subcutaneously (SC) with norBNI (15 mg/kg) 24 hours prior to place conditioning; subjects assigned to the Vehicle group were injected SC with the norBNI vehicle.
2.2.2. Conditioning and final test
Given that THC typically produces a conditioned place aversion in rats (Mallet and Beninger, 1998; McGregor et al., 1996; Parker and Gillies, 1995; Sanudo-Pena et al., 1997), each animal was given THC on its own initially preferred side (for similar procedures with drugs that typically induce place aversions, see Cunningham et al., 2003; Davis et al., 2007; Schenk et al., 1986). Specifically, 24 h after norBNI/vehicle administration (see above), animals in each pretreatment group were randomly assigned to receive an intraperitoneal (IP) injection of THC (0, 0.56, 1 or 3.2 mg/kg). This yielded eight groups [N0, N0.56, N1 N3.2, V0, V0.56, V1 and V3.2 (n = 8/group)], where N or V refers to the pretreatment group (norBNI or Vehicle) and the number refers to the dose of THC administered during conditioning. Following group assignment, each animal received its injection and was immediately confined to its preferred side (drug-paired side; DPS) of the CPP apparatus for 45 min. On the next day (PND 90), all animals were given an IP injection of the THC vehicle and then confined to the opposite chamber (non-drug paired side). This two-day cycle of the drug given prior to the animal’s placement on the preferred side followed by vehicle given prior to its placement on the non-preferred side was repeated four additional times (for a total of five cycles). Order of conditioning sessions (i.e., drug versus vehicle) was not counterbalanced (King and Riley, 2013; King et al., 2015; Lepore et al., 1995; Mayer and Parker, 1993). On the day following the final conditioning cycle (PND 99), all of the animals were given a final place conditioning test during which they were placed in the middle gray compartment and allowed to explore the apparatus freely for 20 min with time spent in each chamber recorded to obtain individual preferences. Following the Final Test, animals were returned to their home cages; no injections were given on this day.
2.3. Statistical analysis
Percent time spent on the drug-paired side on both the Pretest and the Final Test was compared with a 2 × 4 × 2 repeated measures ANOVA with between-subjects variables of Pretreatment Drug (0 and 30 mg/kg, norBNI) and Conditioning Dose (0, 0.56, 1 and 3.2 mg/kg, THC) and a within-subjects variable of Test (Pretest and Final Test). In the case of a three-way interaction, simple effects of Test at each Pretreatment Drug and Conditioning Dose (univariate analysis) and simple effects of Pretreatment Drug at each Conditioning Dose and Test (univariate analysis) were assessed with Bonferroni-corrected multiple comparisons as warranted. Significance level for all statistical tests was set to α = 0.05.
3. Results
3.1. norBNI (15 mg/kg)
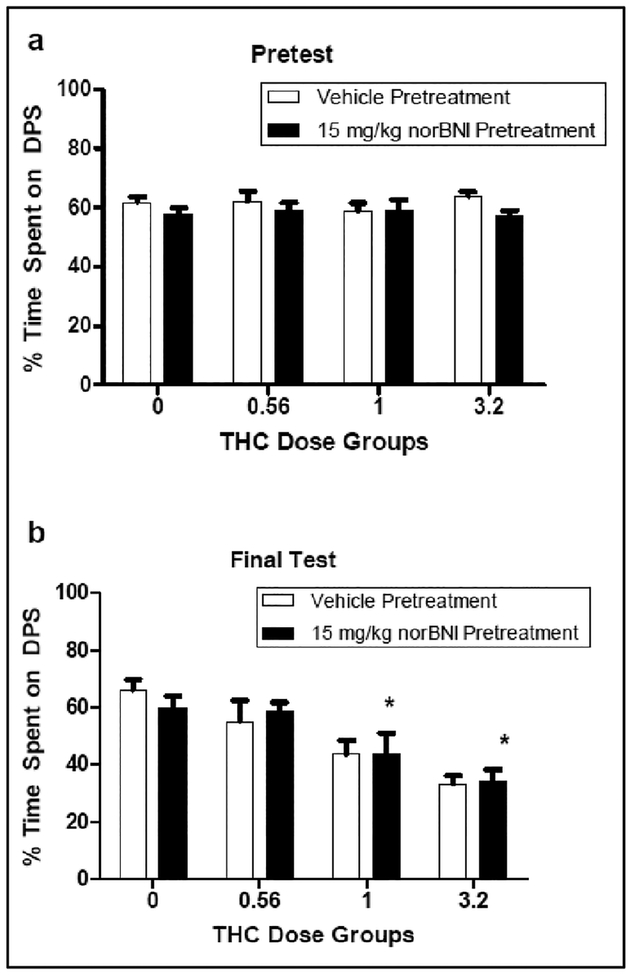
THC induced-dose dependent place aversions in both norBNI- and vehicle-pretreated rats. There was no effect of norBNI pretreatment. The 2 × 4 × 2 repeated measures ANOVA revealed significant effects of Test [F (1, 55) = 26.328] and Conditioning Dose [F (3, 55) = 11.773], as well as a significant Test × Conditioning Dose interaction [F (3, 55) = 10.898]. There was no effect of Pretreatment Drug [F (1, 55) = .945, p > 0.33], nor was there a significant Pretreatment Drug × Conditioning Dose [F (3, 55) = .442, p > 0.73], Pretreatment Drug × Test [F (1, 55) = .262, p > 0.61] or Pretreatment Drug × Conditioning Dose × Test [F (3, 55) = .265, p > 0.82] interaction. In relation to the significant Test × Conditioning Dose interaction (collapsed across Pretreatment Drug), all groups spent roughly 60% of their time on the drug-paired side during the Pretest (see Figure 1; Top Panel). On the Final Test, however, differences among groups emerged. Specifically, groups conditioned with 1 and 3.2 mg/kg of THC spent significantly less time on the drug-paired side than groups conditioned with vehicle and 0.56 mg/kg of THC (see Figure 1; Bottom Panel). There were no differences between groups conditioned with 0 and 0.56 mg/kg THC or between groups conditioned with 1 and 3.2 mg/kg THC in time spent on the drug-paired side. At no point did groups pretreated with norBNI differ from those pretreated with vehicle.
Figure 1.
Percentage time on the drug-paired side (DPS) at Pretest (Top Panel) and the Final Test (Bottom Panel) in animals (n = 8 per group) pretreated with either vehicle or nor-BNI (15 mg/kg) and injected with various doses of THC during conditioning in Experiment 1. *significantly different from Groups 0 and 0.56. There was no effect of norBNI pretreatment.
4. Experiment 2
In Experiment 1, THC induced dose-dependent place aversions that were unaffected by norBNI. It should be noted, however, that only a single dose of norBNI (15 mg/kg) was used in the pretreatment regimen of Experiment 1. Although the evidence of norBNI as a KOR antagonist with other behavioral endpoints and using injection procedures similar to those in Experiment 1 is well documented in rats (Anderson et al., 2013; Morales et al., 2014; Schlosburg et al., 2013, Wee et al., 2009), it is possible that the dose of norBNI in Experiment 1 was simply not sufficient to modulate THC-induced place aversions. Interestingly, multiple reports have demonstrated dose-dependent effects with norBNI in rats in which its effects were evident only at high doses. In one such example, Valdez and Harshberger (2012) reported that a 20 mg/kg dose of norBNI was able to attenuate anxiety-like behaviors during acute ethanol withdrawal, whereas a dose of 10 mg/kg was ineffective. Similarly, Wee et al. (2009) reported that while 15 mg/kg norBNI failed to influence short and long access intravenous self-administration (IVSA) of cocaine in male rats, 30 mg/kg norBNI acutely decreased short access IVSA of cocaine and significantly decreased long access cocaine IVSA. Consistent with this, Schlosburg et al. reported that 30 mg/kg norBNI prevented escalated heroin IVSA in 12-hour long sessions, although they did not evaluate whether lower doses of norBNI produced similar reductions in heroin IVSA. Given these reports, it is possible that a higher dose of norBNI may be required to impact THC-induced place aversions. Accordingly, Experiment 2 was conducted to broaden the dose-response assessment of norBNI in this design by evaluating whether THC-induced place aversion (3.2 mg/kg) is attenuated by a higher dose of norBNI (30 mg/kg).
4.1. Materials and methods
All experimental procedures for Experiment 2 were identical to those for Experiment 1 with the following exceptions. Thirty-one naïve Sprague-Dawley rats were obtained from Envigo (Indianapolis, IN). On PND 87, each animal was allowed 20 min to freely explore the entire place conditioning apparatus with time spent in each chamber recorded to obtain individual baseline preferences. Immediately following the Pretest, animals were returned to their home cages. On the following day (PND 88), the animals were randomly assigned to one of two groups [norBNI and Vehicle (n = 16 per group)] and injected SC with norBNI (30 mg/kg) and vehicle, respectively. Twenty-four hours later, animals in each pretreatment group were randomly assigned to receive an intraperitoneal (IP) injection of either 3.2 mg/kg THC or its vehicle. Only a single dose of THC (3.2 mg/kg) was used in this assessment given that in Experiment 1 aversions at the two higher doses (1.8 and 3.2 mg/kg) were significant and comparable. This yielded four groups [N0, N3.2, V0 and V3.2 (n = 7–8/group)], where N or V refers to the pretreatment group (norBNI or Vehicle) and the number refers to the dose of THC (0 or 3.2 mg/kg) administered during conditioning.
4.2. Statistical analysis
Percent time spent on the drug-paired side on both the Pretest and the Final Test was compared with a 2 × 2 × 2 repeated measures ANOVA with between-subjects variables of Pretreatment Drug (0 and 30 mgkg norBNI) and Conditioning Dose (0 and 3.2 mg/kg, THC) and a within-subjects variable of Test (Pretest and Final Test). In the case of a three-way interaction, simple effects of Test at each Pretreatment Drug and Conditioning Dose (univariate analysis) and simple effects of Pretreatment Drug at each Conditioning Dose and Test (univariate analysis) were assessed with Bonferroni-corrected multiple comparisons as warranted. Significance level for all statistical tests was set to α = 0.05.
5. Results
5.1. norBNI (30 mg/kg)
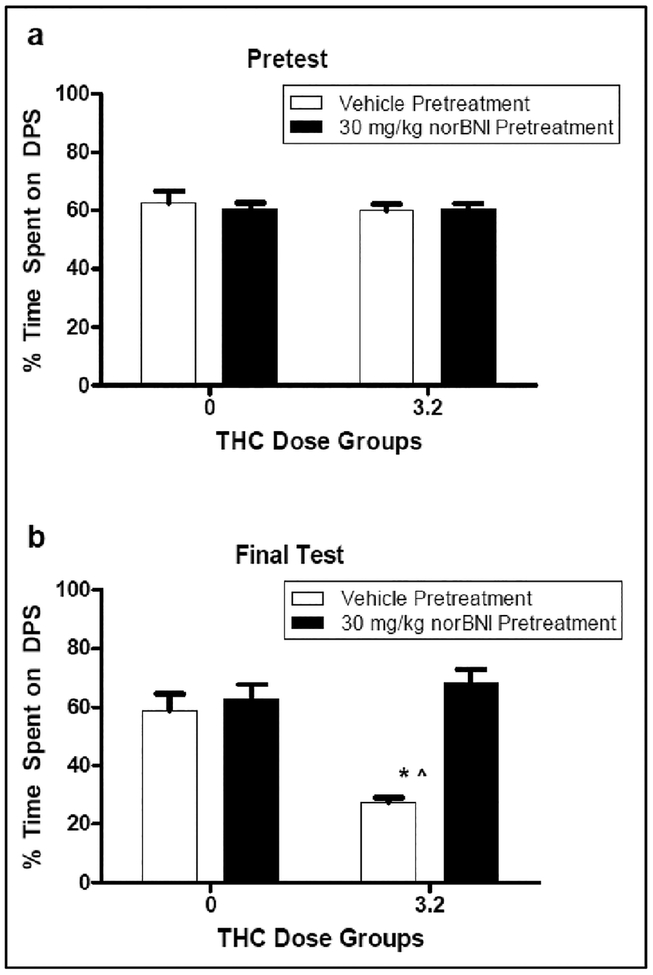
THC induced (3.2 mg/kg) a significant place aversion in vehicle-pretreated rats. Animals pretreated with norBNI (30 mg/kg) prior to place aversion conditioning failed to display such aversions. The 2 × 2 × 2 repeated measures ANOVA revealed significant effects of Pretreatment Drug [F (1, 27) = 19.880], Conditioning Dose [F (1, 27) = 5.822] and Test [F (1, 27) = 7.426], as well as a significant interactions of Conditioning Dose × Test [F (3, 55) = 10.898], Pretreatment Drug × Test [F (1, 27) = 22.280] and Pretreatment Drug × Conditioning Dose × Test [F (1, 27) = 7.088]. There was no significant interaction of Conditioning Dose × Test [F (1, 27) = 3.850, p = .060]. In relation to the significant Pretreatment Drug × Conditioning Dose × Test interaction, there were no group differences during the Pretest. All groups spent roughly 60% of their time on the drug-paired side prior to conditioning (see Figure 2; Top Panel). On the Final Test, however, differences among groups emerged. Specifically, Group V3.2 spent significantly less time on the drug-paired side than did Groups V0, indicative of a significant place aversion. On the other hand, Group N3.2 did not differ from its vehicle-injected control (Group N0), indicative of a blocking by norBNI of the THC-induced aversion. Further, Group V3.2 spent significantly less time on the drug-paired side than did Group N3.2 (see Figure 2, Bottom Panel).
Figure 2.
Percentage time on the drug-paired side (DPS) at Pretest (Top Panel) and the Final Test (Bottom Panel) in animals (n = 7–8 per group) pretreated with either vehicle or nor-BNI (30 mg/kg) and injected with THC or vehicle during conditioning in Experiment 2. *Significantly different from Groups V0 and N0. Ŝignificantly different from N3.2.
6. Discussion
Prior research with transgenic mice suggests that the aversive effects of THC are mediated by activity of the KOR system (Cheng et al., 2004; Ghozland et al., 2002). If the upregulated activity of the KOR system is responsible for mediating the aversive effects of THC, then selective antagonism of the KOR by norBNI should block such aversive effects (for reports of such antagonism in other behavioral and physiological preparations, see Craft et al., 1995; Horan et al., 1992; Stein et al., 1988). To test this hypothesis (Experiment 1), rats were pretreated with norBNI (0 or 15 mg/kg) 24 hours prior to place conditioning with THC (0, 0.56, 1 and 3.2 mg/kg). Under these conditions, subjects displayed dose-dependent THC-induced place aversions that were unaffected by norBNI (15 mg/kg) pretreatment. Given that the effects of norBNI are dose-dependent in a variety of preparations (Anderson et al., 2013; Knoll et al., 2007; Schlosberg et al., 2013; Valdez and Harshberger, 2012; Walker et al., 2010; Wee et al., 2009; Whitfield et al., 2015), Experiment 2 evaluated whether THC-induced place aversions could be attenuated (or blocked) by a higher dose of norBNI. As demonstrated, THC (3.2 mg/kg) induced a significant place aversion that was significantly attenuated by norBNI (30 mg/kg) pretreatment. The results of the latter assessment are consistent with the results of Cheng et al. (2004) and Ghozland et al. (2002) and provide further evidence for the role of the KOR system in the aversive properties of THC in both mice and rats.
Zimmer and his colleagues (2001) presented a similar argument following their demonstration that C57BL/6J mice pretreated with norBNI failed to acquire THC-induced place aversions at a dose of THC that was capable of inducing aversions in untreated mice. In this report, Zimmer et al. pretreated mice with 5 or 10 mg/kg norBNI 90 minutes prior to place conditioning with 5 mg/kg THC. As noted, THC induced significant avoidance of the THC-paired side that was blocked by pretreatment with 10 (but not 5) mg/kg norBNI. Although these results are certainly consistent with the data reported here (in terms of norBNI antagonism of THC-induced place aversion), the interpretation that the effect reported with mice is solely a function of kappa antagonism is less clear. For example, in an assessment of writhing induced by the administration of opioids selective for mu, delta or kappa subtypes of the opioid receptor, Broadbear et al. (1994) reported that pretreatment with norBNI 1 hour prior to morphine administration significantly attenuated morphine-induced writhing in male NIH mice, suggesting antagonism of the mu receptor subtype at this time period. Writhing induced by the selective KOR agonist CI-977 was also attenuated by 1-h pretreatment with norBNI. With longer pretreatment times, e.g., 24 hours up to 28 days, norBNI only affected kappa-mediated writhing, suggestive that mu antagonism was relatively short-lived compared to kappa. Similarly, Endoh et al. (1992) reported that in an assessment of opioid-induced analgesia in a tail-pinch preparation in male ddY mice norBNI antagonized the effects of the mu agonists morphine and fentanyl when given 30 minutes prior to their administration. norBNI at this same time period had no effect on the kappa agonist U-50,488H. The mu antagonist effects were still evident with 1 hour pretreatment at 5 mg/kg norBNI and at 2 hours pretreatment at 20 mg/kg norBNI. The effects of norBNI on U-50,488H began at 2 hours and persisted for 4 and 8 days (at 5 and 15 mg/kg norBNI, respectively).
Each of these behavioral studies indicates that in various pain and analgesia assays, norBNI does have mu and kappa antagonist effects for several hours post injection that become kappa selective with time. These behavioral assays parallel work done on receptor binding in male C57BL/6J mice in which mu, delta and kappa stimulated GTPyS coupling in mouse brain tissue was significantly blocked by norBNI at 0.05 uM. again suggestive of broad-based antagonism of the various opioid receptor subtypes by norBNI. No time course assessments were made in this analysis, so the onset and/or duration of the specific subtype antagonism is not known (Sirochi et al., 2016).
What these data indicate is that with short pretreatment intervals the effects of norBNI may not selectively antagonize the KOR and that the antagonism reported by Zimmer and his colleagues that was evident 90 minutes post norBNI injection may not necessarily be specific to antagonism to this receptor subtype (for a discussion, see Funk et al., 2014). In the present assessment, rats were treated with norBNI 24 hours prior to THC-induced place preference conditioning, using a time course consistent with other behavioral assays in rats assessing the effects of norBNI on various endpoints (for a discussion of norBNI’s specific KOR effects at the 24 hour time point, see Craft et al., 1995; see also Grella et al., 2014; Schlosburg et al., 2013; Walker et al., 2010; Wee et al., 2009; Zhou et al., 2013) and a time point at which norBNI’s antagonism in mice appears kappa specific (see Endoh et al., 1992; Broadbear et al., 1994). It is important to note in this context that similar time course analyses of norBNI antagonism have not been reported in rats, although mu-stimulated GTPyS coupling in rat brain immediately following norBNI is not affected, but kappa stimulated coupling is significantly attenuated (see Sirohi et al., 2016).
As stated previously, the general absence of preclinical models demonstrating the rewarding effects of THC, as indexed by CPP and IVSA, may be the result of THC’s aversive effects masking its potential rewarding effects. In this context, it should be noted that norBNI administration has been shown to affect responsivity to a number of abused drugs and not just THC. For example, Walker et al. (2010) found that norBNI pretreatment significantly attenuated ethanol self-administration in ethanol-dependent rats. In a related study, Schlosburg et al. (2013) observed a long term reduction in escalated heroin intravenous self-administration (IVSA) and heroin withdrawal-associated anxiety-like behavior in rats that were pretreated with norBNI. Pretreatment with norBNI also significantly attenuates escalated cocaine intake in dependent rats (Wee et al., 2009). In each of these studies, the authors argue that norBNI decreases the incidence of withdrawal symptoms mediated by kappa activity that negatively reinforce drug taking, thereby decreasing self-administration that typically occurs during such a period. Although such investigations did not assess the specific basis of norBNI’s action, i.e., if it was KOR specific, the fact that in each assessment norBNI was given 24 hours prior to the behavioral assessment is consistent with kappa mediation.
The possible role of the KOR in the present work with THC and the abovementioned assessments during withdrawal in dependent animals highlights the potential broad impact of the kappa receptor in drug use and abuse and illustrates the differences in various affective properties (reward and aversion) influencing the initial use and the negative affective state that arises during withdrawal. In this vein, work from our laboratory and others suggest that drug use is initiated and maintained by multiple affective states that vary across the addiction cycle (see Becker and Koob, 2016; Koob, 2013; Koob, 2015; Koob and Volkow, 2016). Understanding these affective states may provide some insight into the initiation and escalation of drug use and abuse. An understanding of how these effects are mediated and how they may vary among individuals may be important in predicting use and abuse liability.
Acknowledgements
This work was supported by a grant from the Mellon Foundation to Anthony L. Riley. Requests for reprints should be sent to Matthew M. Clasen, Psychopharmacology Laboratory, Center for Behavioral Neuroscience, American University, Washington, DC 20016 or mc0817a@student.american.edu.
This work was supported by a grant from the Mellon Foundation to ALR. A portion of this work was supported by the intramural research programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism. There are no conflicts of interest.
References
- Anderson RI, Agoglia AE, Morales M, Varlinskaya EI, Spear LP, 2013. Stress, kappa manipulations, and aversive effects of ethanol in adolescent and adult male rats. Neuroscience 249, 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex differences in animal models: focus on addiction. Pharmacol. Rev 68, 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin C, Cohen BM, 2009. Medicinal Chemistry of Kappa Opioid Receptor Antagonists In Opioid Receptors and Antagonists: from Bench to Clinic Edited by Dean RL, Bilsky EJ, Negus SS New York: Humana Press, pp 99–118. [Google Scholar]
- Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH, 1994. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on κ-opioid agonists in the mouse writhing assay. Psychopharmacology 115, 311–319. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Yang T, Schreiber S, DeFino M, Kwan SC, Li S, Chavkin C, 2007. Long-acting κ opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J. Bio. Chem 282, 29803–29811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HYM, Laviolette SR, Van Der Kooy D, Penninger JM, 2004. DREAM ablation selectively alters THC place aversion and analgesia but leaves intact the motivational and analgesic effects of morphine. Eur. J. Neurosci 19, 3033–3041. [DOI] [PubMed] [Google Scholar]
- Craft RM, Henley SR, Haaseth RC, Hruby VJ, Porreca F, 1995. Opioid antinociception in a rat model of visceral pain: systemic versus local drug administration. J. Pharmacol. Exp. Ther 275, 1535–1542. [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA, 2003. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology 170, 409–422. [DOI] [PubMed] [Google Scholar]
- Davis CM, Roma PG, Dominguez JM, Riley AL, 2007. Morphine-induced place conditioning in Fischer and Lewis rats: acquisition and dose-response in a fully biased procedure. Pharmacol. Biochem. Behav 86, 516–523. [DOI] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C and Nagase H, 1992. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch. Int. Pharmacod. T 316, 30–42. [PubMed] [Google Scholar]
- Ettenberg A, Fomenko V, Kaganovsky K, Shelton K, Wenzel JM, 2015. On the positive and negative affective responses to cocaine and their relation to drug self-administration in rats. Psychopharmacology 232, 2363–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Lê AD, 2014. The role of kappa opioid receptors in stress‐induced reinstatement of alcohol seeking in rats. Brain Behav 4, 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R, 2002. Motivational effects of cannabinoids are mediated by μ-opioid and κ-opioid receptors. J. Neurosci 22, 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella SL, Funk D, Coen K, Li Z, Lê AD, 2014. Role of the kappa-opioid receptor system in stress-induced reinstatement of nicotine seeking in rats. Behav. Brain Res 265,188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel B, Wakeford A, Clasen M, Friar M, Riley A, 2016. The effects of delta-9-tetrahydrocannabinol (THC) drug history on THC taste and place conditioning. Pharmacol. Biochem. Behav 144, 1–6. [DOI] [PubMed] [Google Scholar]
- Hempel BJ, Wakeford AG, Nelson KH, Clasen MM, Woloshchuk CJ, Riley AL, 2017. An assessment of sex differences in Δ9-tetrahydrocannabinol (THC) taste and place conditioning. Pharmacol. Biochem. Behav 153, 69–75. [DOI] [PubMed] [Google Scholar]
- Horan PE, Taylor JI, Yamamura HI, Porreca FR, 1992. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J. Pharmacol. Exp. Ther 260, 1237–1243. [PubMed] [Google Scholar]
- Houser SJ, Eads M, Embrey JP, Welch SP, 2000. Dynorphin B and spinal analgesia: induction of antinociception by the cannabinoids CP55, 940, Δ9-THC and anandamide. Brain Res 857, 337–342. [DOI] [PubMed] [Google Scholar]
- King HE, Riley AL, 2013. A history of morphine-induced taste aversion learning fails to affect morphine-induced place preference conditioning in rats. Learn. Behav 41, 433–442. [DOI] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL, 2015. An assessment of MDPV-induced place preference in adult Sprague-Dawley rats. Drug Alcohol Depen 146, 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA, 2007. Anxiolytic-like effects of κ-opioid receptor antagonists in models of unlearned and learned fear in rats. J. Pharmacol. Exp. Ther 323, 838–845. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA, 2010. Dynorphin, stress, and depression. Brain Res 1314, 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2013. Negative reinforcement in drug addiction: the darkness within. Curr. Opin. Neurobiol 23, 559–563. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2015. The dark side of emotion: the addiction perspective. Eur. J. Pharmacol 753, 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiat 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore M, Vorel SR, Lowinson J, Gardner EL, 1995. Conditioned place preference induced by Δ9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life Sci 56, 2073–2080. [DOI] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ, 1998. Δ9-tetrahydrocannabinol, but not the endogenous cannabinoid receptor ligand anandamide, produces conditioned place avoidance. Life Sci 62, 2431–2439. [DOI] [PubMed] [Google Scholar]
- Mason DJ, Lowe J, Welch SP, 1999. Cannabinoid modulation of dynorphin A: correlation to cannabinoid-induced antinociception. Eur. J. Pharmacol 378, 237–248. [DOI] [PubMed] [Google Scholar]
- Mayer LA, Parker LA, 1993. Rewarding and aversive properties of IP and SC cocaine: assessment by place and taste conditioning. Psychopharmacology 112, 189–194. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Issakidis CN, Prior G, 1996. Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacol. Biochem. Behav 53, 657–664. [DOI] [PubMed] [Google Scholar]
- Metcalf M, Coop A, 2005. Kappa opioid antagonists: Past successes and future prospects. AAPS J 7, 704–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Anderson RI, Spear LP, Varlinskaya EI, 2014. Effects of the kappa opioid receptor antagonist, nor‐binaltorphimine, on ethanol intake: impact of age and sex. Dev. Psychobiol 56, 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, 2004. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research National Academy Press, Washington, DC. [Google Scholar]
- National Research Council, 2011. Guide for the Care and Use of Laboratory Animals National Academy Press, Washington, DC. [Google Scholar]
- Parker LA, Gillies T, 1995. THC-induced place and taste aversions in Lewis and Sprague-Dawley rats. Behav. Neurosci 109, 71–78. [DOI] [PubMed] [Google Scholar]
- Riley AL, 2011. The paradox of drug taking: the role of the aversive effects of drugs. Physiol. Behav 103, 69–78. [DOI] [PubMed] [Google Scholar]
- Roma PG, Riley AL, 2005. Apparatus bias and the use of light and texture in place conditioning. Pharmacol. Biochem. Behav 82, 163–169. [DOI] [PubMed] [Google Scholar]
- Sañudo-Peña MC, Tsou K, Delay ER, Hohman AG, Force M, Walker JM, 1997. Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neurosci. Lett 223, 125–128. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Malovechko R, Robertson A, Klukowski G, Amit Z, 1986. Differential effects of isolation housing on the conditioned place preference produced by cocaine and amphetamine. Pharmacol. Biochem. Behav 24, 1793–1796. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Whitfield TW, Park PE, Crawford EF, George O, Vendruscolo LF, Koob GF, 2013. Long-term antagonism of κ opioid receptors prevents escalation of and increased motivation for heroin intake. J. Neurosci 33, 19384–19392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GR, Riley AL, 2005. Morphine preexposure facilitates morphine place preference and attenuates morphine taste aversion. Pharmacol. Biochem. Behav 80, 471–479. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Aldrich JV, Walker BM, 2016. Species differences in the effects of the κ-opioid receptor antagonist zyklophin. Alcohol 51, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP, 2010. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology 210, 199–209. [DOI] [PubMed] [Google Scholar]
- Stein C, Millan MJ, Shippenberg TS, Peter KL, Herz AL, 1989. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J. Pharmacol. Exp. Ther 248, 1269–1275. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2015. U.S. Department of Health and Human Services. Rockville, MD. [Google Scholar]
- Tanda G, 2016. Preclinical studies on the reinforcing effects of cannabinoids. A tribute to the scientific research of Dr. Steve Goldberg. Psychopharmacology 233, 1845–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori AE, Ho BY, Naeseth JS, Portoghese PS, 1988. Nor-binaltorphimine, a highly selective kappa-opioid antagonist in alalgesic and receptor binging assays. J. Pharmacol. Exp. Ther 246, 255–258. [PubMed] [Google Scholar]
- Valdez GR, Harshberger E, 2012. Kappa opioid regulation of anxiety-like behavior during acute ethanol withdrawal. Pharmacol. Biochem. Behav 102, 44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Maldonado R, 2000. A behavioural model to reveal place preference to Δ9-tetrahydrocannabinol in mice. Psychopharmacology 147, 436–438. [DOI] [PubMed] [Google Scholar]
- Wakeford AG, Flax SM, Pomfrey RL, Riley AL, 2016. Adolescent delta-9-tetrahydrocannabinol (THC) exposure fails to affect THC-induced place and taste conditioning in adult male rats. Pharmacol. Biochem. Behav 140, 75–81. [DOI] [PubMed] [Google Scholar]
- Wakeford AG, Wetzell BB, Pomfrey RL, Clasen MM, Taylor WW, Hempel BJ, Riley AL, 2017. The effects of cannabidiol (CBD) on Δ9-tetrahydrocannabinol (THC) self-administration in male and female Long-Evans rats. Exp. Clin. Psychopharm 25, 1–7. [DOI] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF, 2010. Systemic κ‐opioid receptor antagonism by nor‐binaltorphimine reduces dependence‐induced excessive alcohol self-administration in rats. Addict. Biol 16, 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF, 2009. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology 205, 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch SP, Eads M, 1999. Synergistic interactions of endogenous opioids and cannabinoid systems. Brain Res 848, 183–190. [DOI] [PubMed] [Google Scholar]
- Whitfield TW, Schlosburg JE, Wee S, Gould A, George O, Grant Y, Zamora-Martinez ER, Edwards S, Crawford E, Vendruscolo LF, Koob GF, 2015. κ Opioid receptors in the nucleus accumbens shell mediate escalation of methamphetamine intake. J.Neurosci 35, 4296–4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Yokel RA, DeWit H, 1976. Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science 191, 1273–1275. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2015. Management of substance abuse: cannabis. World Health Assembly, Geneva, Switzerland. [Google Scholar]
- Zimmer A, Valjent E, König M, Zimmer AM, Robledo P, Hahn H, Valverde O, Maldonado R, 2001. Absence of Δ−9-tetrahydrocannabinol dysphoric effects in dynorphin-deficient mice. J. Neurosci 21, 9499–9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Grella SL, Aldrich JV, Kreek MJ, 2013. Involvement of dynorphin and kappa opioid receptor in yohimbine‐induced reinstatement of heroin seeking in rats. Synapse 67, 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]