Abstract
The process by which naïve CD8 T cells become activated, accumulate, and terminally differentiate as well as develop into memory cytotoxic T lymphocytes (CTLs) is central to the development of potent and durable immunity to intracellular infections and tumors. In this review, we discuss recent studies that have elucidated ancestries of short-lived and memory CTLs during infection, others that have shed light on gene expression programs manifest in individual responding cells and chromatin remodeling events, remodeling factors, and conventional DNA-binding transcription factors that stabilize the differentiated states after activation of naïve CD8 T cells. Several models have been proposed to conceptualize how naïve cells become memory CD8 T cells. A parsimonious solution is that initial naïve cell activation induces metastable gene expression in nascent CTLs, which act as progenitor cells that stochastically diverge along pathways that are self-reinforcing and result in shorter- versus longer-lived CTL progeny. Deciphering how regulatory factors establish and reinforce these pathways in CD8 T cells could potentially guide their use in immunotherapeutic contexts.
Keywords: Memory CD8 T cells, chromatin structure, transcriptional control
Introduction
During a prototypical acute intracellular infection that will be cleared, naïve antigen-specific CD8 T cells become activated and their progeny accumulates dramatically, a period generally referred to as the “effector” phase. Near the point of maximal accumulation, cells in the responding population manifest substantial phenotypic and functional heterogeneity. As the infection clears, most effector cells die and the population “contracts”. Cells that survive this period ultimately give rise to an array of memory CD8 T-cell subsets 1.
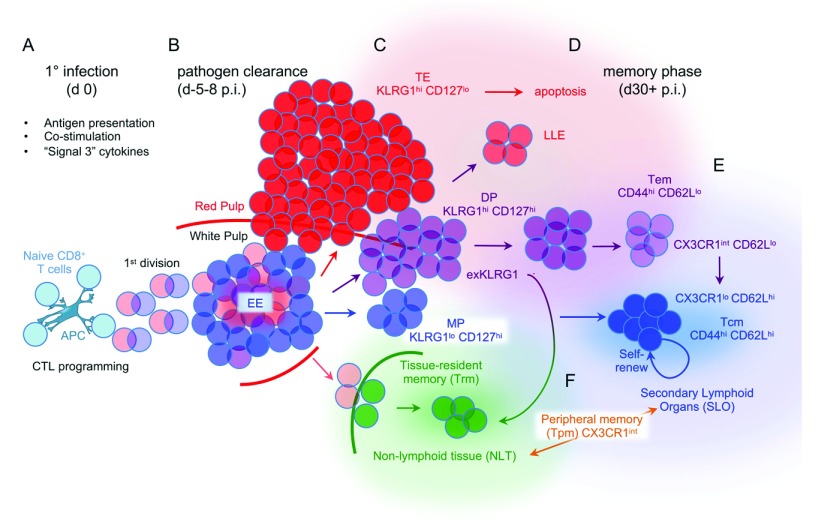
Many excellent recent reviews have comprehensively outlined the tapestry and importance of distinct memory CD8 T-cell subsets that arise after infection 2– 4. An illustration of the main effector and memory CD8 T-cell subsets in mice depicts their general inter-relationships ( Figure 1) ( Table 1). Memory T cells are classically categorized into central memory T (Tcm) cells, which localize in secondary lymphoid organs (SLOs), and effector memory T (Tem) cells, which recirculate between peripheral tissues and SLOs 4. However, at early memory time points, a substantial fraction of the classically defined Tem cells are more effector-like and have been termed effector-like memory cells or long-lived effector (LLE) cells 5, 6. Moreover, another subset of classic Tem cells, called peripheral memory T cells, has been delineated as those that recirculate through peripheral tissues via SLOs and has been distinguished from Tem cells that do not recirculate 7. In addition, memory T cells that enter and stably reside within tissues have been defined as tissue resident memory (Trm) cells 8. Further emphasizing the diversity of memory T-cell subsets is that analysis of human CD8 T cells using cytometry by time of flight has demonstrated that substantial heterogeneity exists between individual cells defined classically as Tcm and Tem cells 9. The extent to which all of these phenotypically distinguishable populations of effector and memory T cells comprise stable cell “lineages” is an open set of questions 3.
Figure 1. Patterns and inter-relationships of effector and memory CD8 T-cell subsets induced by acute intracellular infection.
( A) Antigen presentation, co-stimulation, and additional inflammatory signals induce multiple individual naïve CD8 T cells to undergo a prototypical pattern of geometric expansion. ( B) Individual cells within the nascent CTL population of early effector (EE) cells differentiate along any one of multiple trajectories. ( C) Multiple phenotypic subsets with distinct memory CD8 T-cell potentials are detectable at the peak response, near the time when most pathogen has been eliminated. Cells that are KLRG1 hi CD127 lo have the shortest half-lives after the infection resolves and are referred to as short-lived effector cells (SLECs) or simply terminal effector (TE) CD8 T cells (red). Conversely, KLRG1 lo CD127 hi cells are termed memory precursor (MP) effector CD8 T cells (light blue) because they most efficiently generate memory CD8 T cells. However, some double-positive (DP) effector cells that are KLRG1 hi CD127 hi 9 (purple) downregulate KLRG1 and give rise to memory CD8 T cells. Trm precursors (light green) derived from KLRG1 lo intermediates in the spleen begin populating non-lymphoid tissues (NLTs) near the peak response. ( D) Most TE cells persist poorly into the memory phase. At early memory time points, some KLRG1 hi cells persist and have been termed effector-like memory cells or long-lived effector (LLE) cells but they wane over time. Tem cells preferentially localize in the vasculature (light red background), some of which convert into Tcm cells (dark blue) that reside in secondary lymphoid organs (light blue background) later during the memory phase. Arrows indicate the general ancestry of the different cell populations and are colored according to the main classes of effector and memory CTL subsets.
Table 1. Key definitions.
|
Effector phase: Time period between the initial infection and when the accumulation of effector cells has peaked.
Contraction phase: Time period between the peak accumulation of effector cells and when the decreasing effector population numbers have stabilized. Memory phase: Time period after pathogen clearance and when the effector cell population has contracted and the antigen-specific cell numbers have stabilized. Effector cells: The antigen-activated cells that expand during infection and then die during contraction of the response as pathogen is cleared. Memory cells: The stable populations of antigen-specific cells that persist after the effector cell population undergoes contraction. Early effector (EE) cells: KLRG1 lo CD127 lo cells defined around the time of peak cellular accumulation in response to infection. EE cells retain potential to give rise to terminal effector (TE), double-positive (DP), and memory precursor (MP) cells and ultimately memory T cells. Terminal effector (TE) or short-lived effector cells: KLRG1 hi CD127 lo cells identified around the time of peak cellular accumulation in response to infection. TE cells are prone to apoptosis during contraction and manifest very weak persistence into the memory phase and weak secondary proliferative capacity upon re-stimulation. Memory precursor (MP) effector cells: KLRG1 lo CD127 hi cells identified around the time of peak cellular accumulation in response to infection. MP cells efficiently give rise to effector and central memory T cells (Tcm) and manifest strong capacity for persistence and secondary proliferation upon re-stimulation. Double-positive (DP) effector cells: KLRG1 hi CD127 hi cells defined around the time of peak cellular accumulation in response to infection. Intermediate capacity to contribute to effector memory and Tcm. Tcm cells: CD62L hi CCR7 hi CD44 hi (also CD127 hi and KLRG1 lo and CD27 hi and CX3CR1 lo) cells defined after expanded T-cell numbers following infection have contracted and stabilized. Mainly reside in secondary lymphoid organs, exhibit lower constitutive expression of effector molecules, and manifest strongest proliferation upon re-stimulation. Effector memory T (Tem) cells: CD62L lo CCR7 lo CD44 hi (also CD127 hi and KLRG1 lo/hi and CD27 lo and CX3CR1 hi) cells defined after expanded T-cell numbers following infection have contracted and stabilized. Mainly reside in vasculature and intravascular spaces, exhibit higher constitutive expression of effector molecules, and manifest less strong proliferation upon re-stimulation compared with Tcm cells. Peripheral memory T (Tpm) cells: CX3CR1 int cells defined after expanded T-cell numbers following infection have contracted and stabilized. Tpm cells are located in both intravascular spaces and recirculating through secondary lymphoid organs and exhibit strong homeostatic renewal. Tissue resident memory (Trm) cells: Operationally defined antigen-specific cells that enter non-lymphoid tissues during the effector phase, that are non-vascular-associated, and that do not recirculate. Trm cells have variable phenotypes depending on their host tissues but are frequently CD69 + and CD103 +. Long-lived effector (LLE) or effector-like memory (ELM) cells: LLE cells are KLRG1 hi CD127 hi/lo (and CD62L lo) and are mainly CD27 lo and CD43 lo (defined as ELM with these markers), probably correspond to most CD27 lo CX3CR1 hi cells, and are most frequent at early times of the memory phase. LLE/ELM cells have strong protective capacity and expression of effector molecules but weak capacity for proliferation upon secondary antigen stimulation. |
Although a generally agreed upon concept is that memory CD8 T cells derive from effector cells, this general explanation is somewhat unsatisfying because of the semantics in defining what an “effector” cell is 11– 13. Phenotypically distinct populations of cells that arise in the effector phase differ in their propensity to form specific types of memory cytotoxic T lymphocytes (CTLs). The phenotypes of cells representing some of these populations are relatively stable and do not readily interconvert whereas others do so more easily 7, 9, 14, 15, which likely reflects a spectrum of differentiated states that, on the one end, are terminally differentiated and have relatively short-term roles and, on the other, are stem cell–like and participate in populating and re-populating multiple memory cell niches during iterative infections over time. It is still unclear exactly how all of these differentiated states are initially established and how they are maintained.
Here, we discuss recent studies that have helped to define how activated CD8 T cells terminally differentiate or become memory CD8 T cells, and we focus specifically on the regulation of gene expression and chromatin structure in distinct effector CD8 T-cell populations. Our conclusion is a model that incorporates many of these observations and that might help to clarify how memory CD8 T cells develop from activated cells in the effector phase.
The descent of memory T cells: individual naïve CD8 T cells initiate memory CD8 T-cell programming rapidly and stochastically undergo terminal differentiation
A brief encounter of T-cell receptors (TCRs) on naïve CD8 T cells with their cognate peptide–major histocompatibility complex together with co-stimulation is sufficient to induce a complete program of memory cell differentiation 16, 17. Individual naïve T cells have the potential to differentiate into all phenotypic effector cell subsets and ultimately memory CD8 T cells 9, 18, 19. Aspects of this decision could be programmed during the first naïve cell division, as antigen-presenting cell contact establishes molecular asymmetry in nascent daughter T cells which is associated with their ultimate fate 20, 21, and cells that have undergone their first cell division exhibit distinct single-cell mRNA expression profiles that can be correlated with either gene expression signatures from mature KLRG1 hi IL-7Rα (CD127) lo terminal effector (TE) CD8 T cells at the peak response, or from memory CD8 T cells 22, 23. However, the gene expression profiles in single cells 4 days later are neither strongly distinct between each other nor analogous to the profiles observed after the initial cell division. The expression profiles in single cells on day 4 are also distinct from those in mature TE and memory CD8 T cells 23. However, the day 4 cells could be classified as putative pre-terminal and pre-memory cells on the basis of their expression of “fate-classifier” genes associated with mature memory or TE CD8 T cells 23. Therefore, distinctly fated cells could be present at early times. However, it is unclear whether the distinct gene expression patterns in cells after the initial division derived from the same or different naïve parents and whether the fate-associated gene expression regimes in the single cells are reinforced in their descendants or whether they convert.
The ancestry of CD8 T cells at the single-cell level indicates that the overall pattern of TE and memory precursor (MP) CD8 T-cell differentiation is an average resulting from stochastic behavior of cells recruited into the response ( Figure 2). Studies applying DNA barcodes to follow CD8 T-cell families from individual naïve cells using next-generation DNA sequencing 24, or the transfer of individual congenically marked cells 9, 18, concur that the differentiated fates of single cells are highly variable 19. The overall response comprises relatively few clones that grow into very large CD8 T-cell families whose individual members manifest a phenotype that is indicative of shorter-lived TE CD8 T cells ( Figure 2A–E), together with many smaller CD8 T-cell families derived from a larger number of initial clones that manifest an MP CD8 T-cell phenotype that develop into most long-lived memory cells ( Figure 2B–D). These data are best fit into a model in which naïve cells differentiate linearly into MP cells that proliferate slowly and serve as precursors of more rapidly dividing Tem cells that finally give rise to shorter-lived TE CD8 T cells 18, 19.
Figure 2. The descent of individual naïve CD8 T cells into effector and memory CD8 T-cell progeny on the basis of lineage tracing and single-cell transfer studies.
( A) Individual naïve CD8 T cells are recruited into the response and undergo geometric accumulation resulting in distinct CD8 T-cell families (numbers) derived from individual naïve cells. ( B) Each naïve cell has the potential to differentiate into progeny that exhibit central memory T (Tcm) (blue), effector memory T (Tem) (purple), or terminal effector (TE) (red) CD8 T-cell phenotypes. ( C) Central memory precursors (light blue) are composed of diverse families that divide slowly, ( D) some of which give rise to faster-dividing Tem precursors (purple). ( E) TE CD8 T cells comprise relatively few CD8 T-cell families that have accumulated dramatically and most die.
Activated naïve CD8 T cells acquire effector cell attributes before diverging into subsets with distinct potential to form memory cells
Very soon after naïve CD8 T cells become activated, they differentiate into a population of nascent CTLs that express genes which are indicative of multiple effector cell functions 11, 25, even though only some of these cells terminally differentiate while others give rise to memory CTLs 14. Moreover, although cells from early times after infection that express higher amounts of KLRG1 produce fewer memory cells, both KLRG1 hi and KLRG1 int subsets generate substantial memory cell numbers 25. In addition, gene expression in KLRG1 hi cells at day 5 after lymphocytic choriomeningitis virus (LCMV) infection is substantially different than in canonical TE CD8 T cells on day 8 after infection 26, 27, and gene expression profiles in single activated CD8 T cells 4 days after Listeria infection are distinct from those in single cells on day 1 after infection as well as those in single cells at the peak response on day 7 and in the memory phase 23. These results imply that, at early times, gene expression in the nascent CTL population is not fixed, despite having established the capacity for multiple effector functions, and that this gene program diverges as cells become TE and MP subsets as defined by KLRG1 and CD127 expression near the peak response.
The flexibility in gene expression of nascent CTLs is consistent with the stochastic nature of whether activated CD8 T cells will terminally differentiate or become memory T cells and is also born out of recent genetic experiments. An engineered reporter mouse in which Cre-recombinase is expressed from the endogenous Klrg1 locus to activate constitutive expression of fluorescent proteins and indelibly mark cells which have expressed Klrg1 in their history demonstrates that a substantial fraction of KLRG1 lo cells are marked with the reporter prior to the absolute peak effector response, indicating that they had previously expressed Klrg1 and subsequently downregulated it 28. These “exKLRG1” cells also frequently derived from KLRG1 hi CD127 hi double-positive (DP) effector cells at the peak response and are found in all memory CD8 T-cell populations at later times ( Figure 1).
The strong memory potential of exKLRG1 cells is an indication that many, if not all, memory cells are the progeny of nascent CTLs that manifest promiscuous gene expression regimes before acquiring a more stably differentiated phenotype. This suggests that unstable gene expression in nascent CTLs facilitates differentiation along both memory and terminal differentiation paths, which are reinforced in only some progeny stochastically, a process that might be similar to multi-lineage gene expression in hematopoietic precursors which precedes and primes lineage commitment of myeloid and monocyte subsets 29.
TCR stimulation rapidly induces chromatin remodeling in naïve cells which persists in differentiated effector and memory T cells
Initial TCR stimulation induces widespread alterations in chromatin accessibility of cis-regulatory regions prior to the initial cell division of naïve CD4 and CD8 T cells 27, 30. Analysis of enriched DNA motifs encoded within differentially accessible regions has provided insight into the potential transcription factors (TFs) that control the early programming of effector and memory T cells. Sequences in regions that become accessible during initial TCR stimulation in naïve cells, and that are also accessible in mature memory CD8 T cells, most frequently encode enriched motifs recognized by TFs in the RUNX, ETS, bZIP, T-BOX, IRF, RHD, PRDM1, and ZF-KLF families, which implies that these TFs might induce transcriptional reprogramming of naïve CD8 T cells, and also stabilize the differentiation of memory CD8 T cells 27, 31– 33. Many TFs that can bind these motifs have established functions for driving the differentiation of both effector and memory CD8 T-cell subsets and have been reviewed in detail fairly recently, but still many others have yet to be explored 34– 36.
The mechanism that reprograms the chromatin structure of cis-regulatory regions and promotes effector and memory CD4 and CD8 T-cell differentiation involves transient activation of TFs that are activated by TCR signals (that is, NFAT and AP-1), which facilitates binding of constitutively expressed or lineage-specific TFs, such as the ETS and RUNX family TFs, and presumably others 30, 37, 38. TCR stimulation drives transient chromatin accessibility at “inducible” regions of accessibility in conjunction with adjacent “primed” regions that remain accessible persistently after cessation of TCR signals in the differentiated progeny 30. Sequences within inducible regions are strongly enriched with binding sites for NFAT (RHD family) and AP-1 (bZIP family) TFs, whereas sequences within primed regions are enriched with ETS and RUNX binding sites 30. This process results in ETS and RUNX family TFs and presumably many others, gaining stable access to cis-acting regions in immune activation–relevant genes 27, 38.
Chromatin remodeling of distal cis-regulatory regions correlates with the stability of gene expression in naïve and distinct effector and memory CD8 T-cell subsets
Analysis of chromatin accessibility in purified naïve, effector, exhausted, reinvigorated, and memory CD8 T-cell subsets indicates that an extensive accessible cis-regulatory landscape develops during the differentiation of both TE and MP cells, most of which is preserved in memory CD8 T cells 31, 32, 39– 42. Even though TE and MP CD8 T-cell populations have distinct proclivities to form memory CD8 T cells, there is considerable similarity in the chromatin accessibility profiles between both cell subsets. Consistent with the notion of a common early path of differentiation, accessibility to many of the regions from both effector cell subsets is established within the first 24 hours of TCR stimulation of naïve cells 27, 32. Moreover, some regions that are accessible in memory CD8 T cells but not TE cells are established by initial TCR stimulation, which indicates that specific aspects of memory CTLs are induced prior to extensive effector cell differentiation.
TE and MP CD8 T cells both manifest more accessible regions than memory CD8 T cells, when one considers regions that are also different than in naïve T cells, and most are located distal to gene transcription start sites (TSSs) 31, 32, 42. However, consistent with MP cells being more efficient precursors of memory CD8 T cells than TE cells, their accessibility profile is biased toward that found in memory CD8 T cells 32. Nevertheless, the differences in the numbers of accessible regions between MP cells and memory CTLs indicate that both chromatin condensation and chromatin opening likely occur as effector cells convert into mature memory CD8 T cells. Consistent with this, other changes to chromatin structure, such as DNA methylation, are acquired during the effector phase but are erased as MP CD8 T cells convert into memory CTLs 15.
The most well-defined alterations to chromatin structure that differ between effector and memory CD8 T-cell subsets appear to occur in distal intragenic regions. Distinct histone modification profiles occur at TSSs compared with transcriptional enhancers and have been used to define cis-regulatory function and transcriptional activity in ex vivo CD8 T-cell subsets. Chromatin immunoprecipitation and sequencing (ChIP-seq) analyses of multiple histone modifications (H3K4me3, H3K4me1, H3K27me3, and H3K27Ac) combined with algorithms trained to predict enhancer regions based on these modifications have identified many distal intergenic regions that potentially comprise enhancers in specific CD8 T-cell subsets 42– 50. The apparent differential activity of these putative enhancers based on histone modifications 42, 44– 46 and three-dimensional interactions with their target gene promoters 44 positively correlates with gene expression signatures of naïve, TE, and memory CD8 T cells. Thus, cis-regulatory regions, mainly in distal intergenic regions, undergo dynamic alterations as naïve CD8 T cells become activated and differentiate into distinct populations of effector and ultimately memory CD8 T cells.
Promoter proximal regulation is also likely to be important for the gene activity that defines the distinct differentiated states of CD8 T-cell subsets. Although neither differential histone modifications near TSSs 44 nor the accessibility of promoter-proximal regions in TE and memory CD8 T cells correlates with the differential gene expression patterns between these subsets 32, 44, a complete assessment of chromatin modifications that influence promoter activities has not been performed in CD8 T cells 51, and additional analyses could reveal important differences. In line with this idea, the occupancy of RNA polymerase II (Pol II) at the promoters of multiple effector genes differs in naïve, effector, and memory CD8 T cells 52, which suggests that recruitment and activity of Pol II at target gene promoters are associated with effector and memory CD8 T-cell differentiation. In addition, both subunits of P-TEFb (positive transcription elongation factor b) are essential for TE cell differentiation 53. P-TEFb is recruited to paused Pol II molecules at TSSs and is necessary for inducing transcriptional elongation 54. Therefore, overcoming Pol II pausing might be a key step that drives terminal differentiation, whereas ensuring Pol II pausing could be a mechanism that ensures that MP CD8 T cells form and perhaps the transcriptional “capacitance” of effector genes in memory CD8 T cells. Such promoter-proximal regulation is likely conferred by the differential activity and long-range interactions observed at distal cis-regulatory regions in distinct CD8 T-cell subsets.
Chromatin structure and transcriptional regulation that initializes effector CTL differentiation and preserves memory CTL potential
Memory CTL differentiation involves de-activating gene expression programs of naïve cells and concurrently establishing gene expression that accounts for effector functions, tissue relocalization, persistence, and re-expansion after a secondary antigen encounter. The enrichment of Runx motifs in accessible regions that are induced during TCR stimulation and that persist in memory CTLs suggests that they might contribute to this process. Indeed, insufficiency in either Runx3 or Cbfb (the partner of all three Runx TFs that is obligatory for DNA binding) impairs the acquisition of key effector functions of CTLs 27, 55, 56, and the activated cells do not differentiate into genuine MP CTLs or circulating memory CTLs and instead preferentially develop a TE-like phenotype 27. Moreover, Runx3-deficient cells do not repress Tcf7 and Bcl6, which results in aberrant acquisition of a follicular T helper cell phenotype and trafficking into B-cell follicles 56. In addition, Runx3 deficiency impairs the differentiation of Trm cells and their homeostasis in non-lymphoid tissue (NLT) 39; the transcriptional control of Trm cell differentiation was recently reviewed in detail 36. Runx2-deficient T cells also exhibit defects in memory CTL generation and long-term persistence 57, which confirms an earlier computational prediction that Runx2 is critical for memory CD8 T-cell development 58. Thus, Runx family TFs drive programming of effector attributes of nascent effector CTLs and also ensure that these cells develop into memory CTLs.
Runx3 is required during TCR stimulation to establish chromatin accessibility of cis-regulatory regions that form stably in effector and memory CD8 T cells 27 and most likely depends on cooperativity with many additional TFs. The cis-regulatory regions that do become accessible in CD8 T cells lacking Runx3 encode many fewer motifs for RUNX, IRF, bZIP, RHD, PRDM1, and T-BOX motifs, suggesting that TFs which normally bind these sites in Runx3-sufficient cells could be collaborating factors. Runx and T-box motifs frequently co-occur within stably remodeled cis-regulatory regions of memory CD8 T cells, and binding regions for the two T-box TFs—T-bet and Eomesodermin—each extensively overlap those of the obligate Runx TF partner Cbfb 33. Together, these observations indicate that cooperativity between Runx and T-box proteins is a core regulatory mechanism that establishes the identity of effector and memory CD8 T cells 27, 33, 55, 59, perhaps by outcompeting nucleosomes that otherwise would form at these sites 60. Furthermore, the overlapping binding of Runx3, IRF4, and multiple bZIP family TFs suggests that potential cooperativity with these TFs is also important 27. Thus, complex cooperative interactions between multiple TFs are likely to establish a chromatin accessibility landscape during initial naïve CD8 T-cell stimulation that induces effector CD8 T-cell subsets and is stabilized in memory cells.
In addition, the cis-regulatory regions that are operational in Tcm cells relative to TE cells encode multiple TF motifs that predict potential TFs that promote memory CTL differentiation 42, 44. Binding motifs for Tcf1, Lef1, Foxo1, Foxp1, Eomes, Stat5, Gabpa, Gfi1, and Nr3c1 (as well as others) are enriched in these regions, suggesting that these TFs promote cis-regulatory activity that establishes and maintains memory CTL differentiation. Most of these TFs have established roles for activating gene expression that promotes T-cell quiescence, lymphoid homing, homeostasis, and the potential for self-renewal 61– 65. At the same time, memory CTL differentiation appears to involve actively repressing the activity of some genes to prevent terminal differentiation. RNA interference (RNAi)-mediated suppression of the glucocorticoid receptor ( Nr3c1) and its canonical co-repressor encoded by the chromatin regulator Ncor1 both impaired the differentiation of MP cells and memory CTLs and increased terminal differentiation 42.
A somewhat paradoxical feature of memory CTL cell differentiation is that genes that promote memory CTL formation and homeostasis are initially downregulated during activation of naïve CD8 T cells, only to be re-expressed in some effector cells that become memory CTLs. The entire population of effector cells near the peak response to infection exhibits increased CpG DNA methylation genome-wide, including at representative genes such as Il7r, Sell (CD62L), and Tcf7, which correlates with their reduced expression in most effector CD8 T cells at the peak response 15. A large fraction of CD62L lo MP CD8 T cells upregulate Sell and undergo demethylation of its locus prior to their initial homeostatic cell division, indicating that CpG methylation is actively removed as MP cells from the effector phase convert into memory CTLs. This process does not occur at an appreciable rate in TE CD8 T cells, which remain CD62L lo. CD8 T cells from mice in which the de novo DNA methyltransferase (Dnmt3a) was deleted early during the effector response undergo more rapid re-expression of Il7r, Sell, and Tcf7 genes near the peak response and during the contraction phase, which indicates that initiation of DNA methylation at early times correlates with gene silencing that enforces terminal differentiation of some cells but that, in others, it can be erased at later times 15, 66. CD8 T cells lacking the maintenance DNA methyltransferase Dnmt1 also appear to have reduced differentiation of effector cells, and although memory CTLs appear to form, they exhibit defective recall function 67. Thus, DNA methylation appears to be important for proper memory CTL differentiation, although the mechanisms that account for why some cells are able to undergo demethylation of key loci that promote memory CTL development whereas others do not and progress toward terminal differentiation are not yet clear. However, multiple chromatin reader proteins that bind methylated DNA and recruit additional chromatin-modifying factors or enzymes that chemically convert methylated cytosine residues appear to be involved. CD8 T cells from mice lacking Mbd2, one of four genes encoding methyl CpG-binding DNA proteins, exhibit skewing toward terminal differentiation and defective differentiation of memory CTLs that are not protective 68. In addition, CD8 T cells deficient in methylcytosine dioxygenase ten-eleven translocation 2 ( Tet2) exhibit DNA hypermethylation in multiple transcriptional regulators and enhanced memory CD8 T-cell differentiation 69.
Molecular regulation that imposes terminal differentiation on effector CD8 T cells
Terminal differentiation of activated CD8 T cells positively correlates with extensive proliferative history 19. Even though the outcome is probabilistic at the single-cell level, the pattern of terminal differentiation of the population of cells seems to be programmed by signals received very early during activation 18, 24. Stimulation of T cells via their antigen receptors and co-stimulatory molecules, together with inflammatory cytokines (for example, interleukin-12 [IL-12] and IL-2), integrates to form a calculus that determines the amount of cell division in the resulting progeny 70. Cells accumulating larger sums of the integrated signals during priming extends their proliferative capacity and likely predisposes them to terminal differentiation 14, 71– 74. The same signals that induce extensive proliferation in the responding cell population also prolong their responsiveness to these stimuli, which sustains or increases the expression of TFs (such as T-bet, Zeb2, and Blimp-1) that jointly promote terminal differentiation 14, 72, 74– 76.
A critical feedforward transcriptional circuit involving the TFs T-bet and Zeb2 positively regulates terminal differentiation 77, 78. T-bet binds to the Zeb2 locus and induces Zeb2 expression, and both TFs appear to be necessary for optimal T-bet binding to cis-regulatory regions it controls; although (owing to the lack of a reliable antibody) Zeb2 occupancy was not analyzed, its putative binding motif was highly enriched within T-bet occupied regions, and T-bet binding was compromised in Zeb2-deficient CD8 T cells 77. In addition, both factors are expressed in LLE cells from the memory phase but are more highly expressed in TE cells from the peak response, which suggests that each TF has roles in both terminally differentiated and memory CTLs 5.
Consistent with this, memory CD8 T cells remain differentiated from TE CD8 T cells in part by preventing high expression of T-bet and Zeb2 14, 77. An antagonistic relationship between the TFs Zeb1 and Zeb2 and the action of mir-200 family microRNAs 79 form an important regulatory circuit that determines the memory potential of effector T cells. Zeb1 is necessary for memory CD8 T-cell differentiation and is induced in response to transforming growth factor-beta (TGF-β) signals. Together with mir-200 family microRNAs, it represses Zeb2 expression. Runx3 also prevents high expression of T-bet and Zeb2 that normally occurs in TE cells 27.
In addition, the bZIP family TF, Bach2, deactivates terminal CTL differentiation by preventing TCR-induced AP-1–driven signals by competing with Jun proteins for DNA occupancy within cis-regulatory regions 58, 80 and is necessary for exKLRG1 cells to develop into memory CTLs 28. Runx3 appears to contribute to this process because Runx3-deficient CD8 T cells fail to induce chromatin accessibility of cis-regulatory regions encoding Bach2-binding motifs 27. Therefore, negative feedback is provided by TFs that initially drive acquisition of effector cell attributes during CD8 T-cell activation which prevents terminal differentiation.
Terminal CTL differentiation involves stable repression of genes encoding stem cell–like qualities that normally promote the long-lived nature of Tcm cells 64, 81, 82. Both T-bet and Zeb2 repress features of memory CD8 T cells (for example, by binding directly to the Il2 and Il7r genes and repressing their expression). In addition, high Blimp-1 expression causes repression of Id3, which retards the ability of effector cells to contribute to the memory CTL compartment 83, 84. Also, chromatin-level repression of genes that promote lymphoid homing and quiescence and other features of “stemness” that can be considered “pro-memory” promotes commitment to terminal differentiation 15, 26, 48, 85. Methylation of histone H3K9 and H3K27 is a well-studied mechanism that promotes chromatin condensation and gene silencing during cell development 86. Upon activation, naïve CD8 T cells rapidly accumulate islands of histone H3K9 trimethylation (H3K9me3), especially at genes such as Il7r and Sell 85. H3K9me3 is deposited by multiple methyltransferases, including the suppressor of variegation 3-9 homolog 1 ( Suv39h1), and is a histone modification that recruits multiple proteins in the chromobox (Cbx) family to bind adjacent nucleosomes together, a process that reinforces recruitment of additional Suv39h1 and promotes spreading of H3K9me3 deposition 87, 88. In addition, Suv39h1 interacts with Mbd family proteins, which suggests that DNA methylation could instigate or enhance Suv39h1 recruitment and H3K9me3 deposition 89. Suv39h1-deficient CD8 T cells fail to repress naïve and stem cell–associated genes and exhibit a loss in the inverse correlation between H3K9me3 density and stem cell gene expression 85. These cells accumulate poorly and develop a normal TE CD8 T-cell phenotype inefficiently, and the resulting memory cells are not protective 85.
Similarly, repression of MP cell signature genes by enhanced deposition of H3K27me3 in cis-regulatory regions of TE CD8 T cells also promotes terminal differentiation 23, 48. H3K27me3 deposition is catalyzed by the methyltransferase Ezh2, which is upregulated upon stimulation of naïve CD8 T cells 48, and its mRNA is more highly expressed in a subset of responding CD8 T cells classified as pre-terminal effector cells 23. Disruption of Ezh2 impairs CD8 T-cell accumulation and effector cell differentiation 23, 48. This phenotype correlates with reduced H3K27me3 and enhanced expression of Eomes, Tcf7, and Klf2 genes, which encode TFs that promote competitive fitness of Tcm cells, their maintenance, and lymphoid retention 23, 61, 65, 90. Thus, coordinated targeting of histone methyltransferase activity in effector cells leads to methylation of H3K9 and H3K27 residues in nucleosomes of genes that are essential for memory cell homeostasis, which represses their expression and may ensure terminal differentiation.
Finally, the stability of phenotypes in CTL subsets, as in many other developmental systems, is enforced by TFs that drive particular cell states by continuously directing the activity of chromatin regulators to their appropriate gene targets 91. In the earliest part of the memory phase, LLEs that are KLRG1 hi retain properties that endow them with additional effector capacities and persistence at early times during the memory phase 5 ( Figure 1). The phenotype of these cells depends on continued expression of the proteins Id2 and Zeb2 6, 78. Conditional disruption of Id2 in KLRG1 hi cells after differentiation of LLE results in loss of KLRG1 expression and in conversion of their transcriptional profile into one reminiscent of that found in Tcm cells 6. These results demonstrate that the persistent activity of certain TFs is essential for maintaining the differentiated state of memory CTLs after they have been generated. Thus, while these differentiation programs depend on chromatin remodeling, they are maintained by the continuous activity of specific TFs.
Toward a unified model of memory CD8 T-cell differentiation
Several models have been proposed to conceptualize how naïve CD8 T cells differentiate into memory CD8 T cells 19, 35, 92. An amenable solution that bears similarity to the decreasing potential and progressive differentiation models but that includes insight from single-cell tracing studies and population analyses of chromatin structure suggests that naïve CD8 T cells rapidly acquire critical features of both effector and memory CD8 T cells upon TCR activation and thus comprise effector and memory precursor progenitor (EMPpro) cells ( Figure 3). Cells in the EMPpro population manifest metastable transcriptional states characterized by promiscuous gene expression among individual cells and stochastic proclivity for acceleration or diversion into effector memory–like cells and further commitment to extensive proliferation and terminal differentiation, or reversion to a slowly dividing EMPpro state, relaxation into MP cells, and ultimately differentiation into memory CD8 T cells. Between these extremes, some cells depart the spleen and seed peripheral NLTs to form precursors of Trm CD8 T cells 39. The probability that cells opt to proliferate extensively and differentiate into TE CTLs is influenced by the integration of signals that individual naïve cells experience during initial activation. In addition, signals in the local microenvironment as the nascent EMPpro families accumulate may sustain or antagonize these signals in some cells 93, and influence the binding activity of specific TFs and alterations to chromatin structure that drive the gene expression programs specific to TE and MP cells, thus progressively reinforcing (or reversing) the fates of individual cells that tend to diverge along these pathways to memory. Therefore, even though individual T cells arrive at their fates randomly, the patterns of memory CTL differentiation are influenced deterministically.
Figure 3. An integrated model of memory CD8 T-cell differentiation.
( A) Individual naïve CD8 T cells undergo memory CD8 T-cell programming wherein they acquire fundamental traits of fully developed memory CD8 T cells prior to the first cell division. ( B) The growing nascent CD8 T-cell population comprises a transitional population of effector and memory precursor progenitor (EMPpro) cells that are metastable at the chromatin and transcriptional level and can give rise to all subsets of differentiated CD8 T-cell progeny. ( C) A small number of EMPpro cells randomly undergo massive proliferation coupled to higher expression of multiple transcription factors (TFs) in response to inflammatory signals that drive transcription underlying the phenotypic and functional profiles of terminally differentiated CD8 T cells. ( D) Multiple chromatin regulatory factors that methylate DNA and histones persistently repress genes that otherwise favor quiescence, lymphoid retention, and overall “stemness” and thus enforce terminal differentiation. Factors generally associated with terminal CD8 T-cell differentiation (red) and memory (blue) are highlighted by color but are not intended to imply exclusive correlations.
Abbreviations
CTL, cytotoxic T lymphocyte; EMPpro, progenitor effector and memory precursor; H3K9me3, histone H3 lysine 9 tri-methylation; H3K27me3, histone H3 lysine 27 tri-methylation; IL, interleukin; LLE, long-lived effector; MP, memory precursor; NLT, non-lymphoid tissue; Pol II, polymerase II; P-TEFb, positive transcription elongation factor b; SLO, secondary lymphoid organ; Tcm, central memory; TCR, T-cell receptor; TE, terminal effector; Tem, effector memory; TF, transcription factor; Trm, tissue-resident memory; TSS, transcription start site.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Peter N Cockerill, Institute of Immunology and Immunotherapy, Institute of Biomedical Research, University of Birmingham, Birmingham, UK
Axel Kallies, Department of Microbiology and Immunology, The University of Melbourne, Parkville, Australia
Funding Statement
This work was supported by National Institutes of Health grants R01 AI095634 and U19 AI109976 and US Department of Defense grant W81XWH-16-1-0006 (to MEP) and the Frenchman’s Creek Women for Cancer Research.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Kaech SM, Wherry EJ, Ahmed R: Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–62. 10.1038/nri778 [DOI] [PubMed] [Google Scholar]
- 2. Masopust D, Soerens AG: Tissue-Resident T Cells and Other Resident Leukocytes. Annu Rev Immunol. 2019;37:521–46. 10.1146/annurev-immunol-042617-053214 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Jameson SC, Masopust D: Understanding Subset Diversity in T Cell Memory. Immunity. 2018;48(2):214–26. 10.1016/j.immuni.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Mueller SN, Gebhardt T, Carbone FR, et al. : Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–61. 10.1146/annurev-immunol-032712-095954 [DOI] [PubMed] [Google Scholar]
- 5. Olson JA, McDonald-Hyman C, Jameson SC, et al. : Effector-like CD8 + T cells in the memory population mediate potent protective immunity. Immunity. 2013;38(6):1250–60. 10.1016/j.immuni.2013.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Omilusik KD, Nadjsombati MS, Shaw LA, et al. : Sustained Id2 regulation of E proteins is required for terminal differentiation of effector CD8 + T cells. J Exp Med. 2018;215(3):773–83. 10.1084/jem.20171584 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Gerlach C, Moseman EA, Loughhead SM, et al. : The Chemokine Receptor CX3CR1 Defines Three Antigen-Experienced CD8 T Cell Subsets with Distinct Roles in Immune Surveillance and Homeostasis. Immunity. 2016;45(6):1270–84. 10.1016/j.immuni.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Schenkel JM, Masopust D: Tissue-resident memory T cells. Immunity. 2014;41(6):886–97. 10.1016/j.immuni.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plumlee CR, Sheridan BS, Cicek BB, et al. : Environmental cues dictate the fate of individual CD8 + T cells responding to infection. Immunity. 2013;39(2):347–56. 10.1016/j.immuni.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Newell EW, Sigal N, Bendall SC, et al. : Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8 + T cell phenotypes. Immunity. 2012;36(1):142–52. 10.1016/j.immuni.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bannard O, Kraman M, Fearon DT: Secondary replicative function of CD8 + T cells that had developed an effector phenotype. Science. 2009;323(5913):505–9. 10.1126/science.1166831 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Harrington LE, Janowski KM, Oliver JR, et al. : Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452(7185):356–60. 10.1038/nature06672 [DOI] [PubMed] [Google Scholar]
- 13. Opferman JT, Ober BT, Ashton-Rickardt PG: Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 1999;283(5408):1745–8. 10.1126/science.283.5408.1745 [DOI] [PubMed] [Google Scholar]
- 14. Joshi NS, Cui W, Chandele A, et al. : Inflammation directs memory precursor and short-lived effector CD8 + T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–95. 10.1016/j.immuni.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Youngblood B, Hale JS, Kissick HT, et al. : Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature. 2017;552(7685):404–9. 10.1038/nature25144 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Kaech SM, Ahmed R: Memory CD8 + T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2(5):415–22. 10.1038/87720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Stipdonk MJ, Lemmens EE, Schoenberger SP: Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2(5):423–9. 10.1038/87730 [DOI] [PubMed] [Google Scholar]
- 18. Buchholz VR, Flossdorf M, Hensel I, et al. : Disparate individual fates compose robust CD8 + T cell immunity. Science. 2013;340(6132):630–5. 10.1126/science.1235454 [DOI] [PubMed] [Google Scholar]
- 19. Buchholz VR, Schumacher TN, Busch DH: T Cell Fate at the Single-Cell Level. Annu Rev Immunol. 2016;34:65–92. 10.1146/annurev-immunol-032414-112014 [DOI] [PubMed] [Google Scholar]
- 20. Chang JT, Ciocca ML, Kinjyo I, et al. : Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity. 2011;34(4):492–504. 10.1016/j.immuni.2011.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Chang JT, Palanivel VR, Kinjyo I, et al. : Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315(5819):1687–91. 10.1126/science.1139393 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Arsenio J, Kakaradov B, Metz PJ, et al. : Early specification of CD8 + T lymphocyte fates during adaptive immunity revealed by single-cell gene-expression analyses. Nat Immunol. 2014;15(4):365–372. 10.1038/ni.2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kakaradov B, Arsenio J, Widjaja CE, et al. : Early transcriptional and epigenetic regulation of CD8 + T cell differentiation revealed by single-cell RNA sequencing. Nat Immunol. 2017;18(4):422–432. 10.1038/ni.3688 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Gerlach C, Rohr JC, Perié L, et al. : Heterogeneous differentiation patterns of individual CD8 + T cells. Science. 2013;340(6132):635–9. 10.1126/science.1235487 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Sarkar S, Kalia V, Haining WN, et al. : Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205(3):625–40. 10.1084/jem.20071641 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Best JA, Blair DA, Knell J, et al. : Transcriptional insights into the CD8 + T cell response to infection and memory T cell formation. Nat Immunol. 2013;14(4):404–12. 10.1038/ni.2536 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Wang D, Diao H, Getzler AJ, et al. : The Transcription Factor Runx3 Establishes Chromatin Accessibility of cis-Regulatory Landscapes that Drive Memory Cytotoxic T Lymphocyte Formation. Immunity. 2018;48(4):659–74.e6. 10.1016/j.immuni.2018.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herndler-Brandstetter D, Ishigame H, Shinnakasu R, et al. : KLRG1 + Effector CD8 + T Cells Lose KLRG1, Differentiate into All Memory T Cell Lineages, and Convey Enhanced Protective Immunity. Immunity. 2018;48(4):716–29.e8. 10.1016/j.immuni.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Laslo P, Spooner CJ, Warmflash A, et al. : Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126(4):755–66. 10.1016/j.cell.2006.06.052 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Bevington SL, Cauchy P, Piper J, et al. : Inducible chromatin priming is associated with the establishment of immunological memory in T cells. EMBO J. 2016;35(5):515–35. 10.15252/embj.201592534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scharer CD, Bally AP, Gandham B, et al. : Cutting Edge: Chromatin Accessibility Programs CD8 T Cell Memory. J Immunol. 2017;198(6):2238–2243. 10.4049/jimmunol.1602086 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Scott-Browne JP, Lopez-Moyadó IF, Trifari S, et al. : Dynamic Changes in Chromatin Accessibility Occur in CD8 + T Cells Responding to Viral Infection. Immunity. 2016;45(6):1327–40. 10.1016/j.immuni.2016.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. van der Veeken J, Zhong Y, Sharma R, et al. : Natural Genetic Variation Reveals Key Features of Epigenetic and Transcriptional Memory in Virus-Specific CD8 T Cells. Immunity. 2019;50(5):1202–1217.e7. 10.1016/j.immuni.2019.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Chang JT, Wherry EJ, Goldrath AW: Molecular regulation of effector and memory T cell differentiation. Nat Immunol. 2014;15(12):1104–15. 10.1038/ni.3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaech SM, Cui W: Transcriptional control of effector and memory CD8 + T cell differentiation. Nat Rev Immunol. 2012;12(11):749–61. 10.1038/nri3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Milner JJ, Goldrath AW: Transcriptional programming of tissue-resident memory CD8 + T cells. Curr Opin Immunol. 2018;51:162–169. 10.1016/j.coi.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Bevington SL, Cauchy P, Cockerill PN: Chromatin priming elements establish immunological memory in T cells without activating transcription: T cell memory is maintained by DNA elements which stably prime inducible genes without activating steady state transcription. BioEssays. 2017;39(2). 10.1002/bies.201600184 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Bevington SL, Cauchy P, Withers DR, et al. : T Cell Receptor and Cytokine Signaling Can Function at Different Stages to Establish and Maintain Transcriptional Memory and Enable T Helper Cell Differentiation. Front Immunol. 2017;8:204. 10.3389/fimmu.2017.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Milner JJ, Toma C, Yu B, et al. : Runx3 programs CD8 + T cell residency in non-lymphoid tissues and tumours. Nature. 2017;552(7684):253–257. 10.1038/nature24993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pauken KE, Sammons MA, Odorizzi PM, et al. : Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354(6316):1160–5. 10.1126/science.aaf2807 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Sen DR, Kaminski J, Barnitz RA, et al. : The epigenetic landscape of T cell exhaustion. Science. 2016;354(6316):1165–9. 10.1126/science.aae0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu B, Zhang K, Milner JJ, et al. : Epigenetic landscapes reveal transcription factors that regulate CD8 + T cell differentiation. Nat Immunol. 2017;18(5):573–82. 10.1038/ni.3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calo E, Wysocka J: Modification of enhancer chromatin: what, how, and why? Mol Cell. 2013;49(5):825–37. 10.1016/j.molcel.2013.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He B, Xing S, Chen C, et al. : CD8 + T Cells Utilize Highly Dynamic Enhancer Repertoires and Regulatory Circuitry in Response to Infections. Immunity. 2016;45(6):1341–54. 10.1016/j.immuni.2016.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Russ BE, Olshanksy M, Smallwood HS, et al. : Distinct Epigenetic Signatures Delineate Transcriptional Programs during Virus-Specific CD8 + T Cell Differentiation. Immunity. 2014;41(5):853–65. 10.1016/j.immuni.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Russ BE, Olshansky M, Li J, et al. : Regulation of H3K4me3 at Transcriptional Enhancers Characterizes Acquisition of Virus-Specific CD8 + T Cell-Lineage-Specific Function. Cell Rep. 2017;21(12):3624–36. 10.1016/j.celrep.2017.11.097 [DOI] [PubMed] [Google Scholar]
- 47. Shen Y, Yue F, McCleary DF, et al. : A map of the cis-regulatory sequences in the mouse genome. Nature. 2012;488(7409):116–20. 10.1038/nature11243 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Gray SM, Amezquita RA, Guan T, et al. : Polycomb Repressive Complex 2-Mediated Chromatin Repression Guides Effector CD8 + T Cell Terminal Differentiation and Loss of Multipotency. Immunity. 2017;46(4):596–608. 10.1016/j.immuni.2017.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Firpi HA, Ucar D, Tan K: Discover regulatory DNA elements using chromatin signatures and artificial neural network. Bioinformatics. 2010;26(13):1579–86. 10.1093/bioinformatics/btq248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rajagopal N, Xie W, Li Y, et al. : RFECS: a random-forest based algorithm for enhancer identification from chromatin state. PLoS Comput Biol. 2013;9(3):e1002968. 10.1371/journal.pcbi.1002968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kouzarides T: Chromatin modifications and their function. Cell. 2007;128(4):693–705. 10.1016/j.cell.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 52. Zediak VP, Johnnidis JB, Wherry EJ, et al. : Cutting edge: persistently open chromatin at effector gene loci in resting memory CD8 + T cells independent of transcriptional status. J Immunol. 2011;186(5):2705–9. 10.4049/jimmunol.1003741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen R, Bélanger S, Frederick MA, et al. : In vivo RNA interference screens identify regulators of antiviral CD4 + and CD8 + T cell differentiation. Immunity. 2014;41(2):325–38. 10.1016/j.immuni.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Adelman K, Lis JT: Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat Rev Genet. 2012;13(10):720–31. 10.1038/nrg3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cruz-Guilloty F, Pipkin ME, Djuretic IM, et al. : Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J Exp Med. 2009;206(1):51–9. 10.1084/jem.20081242 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Shan Q, Zeng Z, Xing S, et al. : The transcription factor Runx3 guards cytotoxic CD8 + effector T cells against deviation towards follicular helper T cell lineage. Nat Immunol. 2017;18(8):931–939. 10.1038/ni.3773 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Olesin E, Nayar R, Saikumar-Lakshmi P, et al. : The Transcription Factor Runx2 Is Required for Long-Term Persistence of Antiviral CD8 + Memory T Cells. Immunohorizons. 2018;2(7):251–261. 10.4049/immunohorizons.1800046 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Hu G, Chen J: A genome-wide regulatory network identifies key transcription factors for memory CD8 + T-cell development. Nat Commun. 2013;4:2830. 10.1038/ncomms3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Intlekofer AM, Takemoto N, Wherry EJ, et al. : Effector and memory CD8 + T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6(12):1236–44. 10.1038/ni1268 [DOI] [PubMed] [Google Scholar]
- 60. Li G, Levitus M, Bustamante C, et al. : Rapid spontaneous accessibility of nucleosomal DNA. Nat Struct Mol Biol. 2005;12(1):46–53. 10.1038/nsmb869 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Banerjee A, Gordon SM, Intlekofer AM, et al. : Cutting edge: The transcription factor eomesodermin enables CD8 + T cells to compete for the memory cell niche. J Immunol. 2010;185(9):4988–92. 10.4049/jimmunol.1002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chandele A, Joshi NS, Zhu J, et al. : Formation of IL-7Rα high and IL-7Rα low CD8 T Cells during Infection Is Regulated by the Opposing Functions of GABPα and Gfi-1. J Immunol. 2008;180(8):5309–19. 10.4049/jimmunol.180.8.5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kerdiles YM, Beisner DR, Tinoco R, et al. : Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10(2):176–84. 10.1038/ni.1689 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Lin WW, Nish SA, Yen B, et al. : CD8 + T Lymphocyte Self-Renewal during Effector Cell Determination. Cell Rep. 2016;17(7):1773–82. 10.1016/j.celrep.2016.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou X, Yu S, Zhao DM, et al. : Differentiation and Persistence of Memory CD8 + T Cells Depend on T Cell Factor 1. Immunity. 2010;33(2):229–40. 10.1016/j.immuni.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Ladle BH, Li KP, Phillips MJ, et al. : De novo DNA methylation by DNA methyltransferase 3a controls early effector CD8 + T-cell fate decisions following activation. Proc Natl Acad Sci U S A. 2016;113(38):10631–6. 10.1073/pnas.1524490113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chappell C, Beard C, Altman J, et al. : DNA Methylation by DNA Methyltransferase 1 Is Critical for Effector CD8 T Cell Expansion. J Immunol. 2006;176(8):4562–72. 10.4049/jimmunol.176.8.4562 [DOI] [PubMed] [Google Scholar]
- 68. Kersh EN: Impaired memory CD8 T cell development in the absence of methyl-CpG-binding domain protein 2. J Immunol. 2006;177(6):3821–6. 10.4049/jimmunol.177.6.3821 [DOI] [PubMed] [Google Scholar]
- 69. Carty SA, Gohil M, Banks LB, et al. : The Loss of TET2 Promotes CD8 + T Cell Memory Differentiation J Immunol. 2018;200(1):82–91. 10.4049/jimmunol.1700559 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Marchingo JM, Kan A, Sutherland RM, et al. : T cell signaling. Antigen affinity, costimulation, and cytokine inputs sum linearly to amplify T cell expansion. Science. 2014;346(6213):1123–7. 10.1126/science.1260044 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Curtsinger JM, Valenzuela JO, Agarwal P, et al. : Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174(8):4465–9. 10.4049/jimmunol.174.8.4465 [DOI] [PubMed] [Google Scholar]
- 72. Pipkin ME, Sacks JA, Cruz-Guilloty F, et al. : Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32(1):79–90. 10.1016/j.immuni.2009.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Starbeck-Miller GR, Xue HH, Harty JT: IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. J Exp Med. 2014;211(1):105–20. 10.1084/jem.20130901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kalia V, Sarkar S, Subramaniam S, et al. : Prolonged interleukin-2Ralpha expression on virus-specific CD8 + T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32(1):91–103. 10.1016/j.immuni.2009.11.010 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Rutishauser RL, Martins GA, Kalachikov S, et al. : Transcriptional repressor Blimp-1 promotes CD8 + T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31(2):296–308. 10.1016/j.immuni.2009.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Xin A, Masson F, Liao Y, et al. : A molecular threshold for effector CD8 + T cell differentiation controlled by transcription factors Blimp-1 and T-bet. Nat Immunol. 2016;17(4):422–32. 10.1038/ni.3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dominguez CX, Amezquita RA, Guan T, et al. : The transcription factors ZEB2 and T-bet cooperate to program cytotoxic T cell terminal differentiation in response to LCMV viral infection. J Exp Med. 2015;212(12):2041–56. 10.1084/jem.20150186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Omilusik KD, Best JA, Yu B, et al. : Transcriptional repressor ZEB2 promotes terminal differentiation of CD8 + effector and memory T cell populations during infection. J Exp Med. 2015;212(12):2027–39. 10.1084/jem.20150194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Guan T, Dominguez CX, Amezquita RA, et al. : ZEB1, ZEB2, and the miR-200 family form a counterregulatory network to regulate CD8 + T cell fates. J Exp Med. 2018;215(4):1153–1168. 10.1084/jem.20171352 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Roychoudhuri R, Clever D, Li P, et al. : BACH2 regulates CD8 + T cell differentiation by controlling access of AP-1 factors to enhancers. Nat Immunol. 2016;17(7):851–860. 10.1038/ni.3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gattinoni L, Zhong XS, Palmer DC, et al. : Wnt signaling arrests effector T cell differentiation and generates CD8 + memory stem cells. Nat Med. 2009;15(7):808–13. 10.1038/nm.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Graef P, Buchholz VR, Stemberger C, et al. : Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8 + central memory T cells. Immunity. 2014;41(1):116–26. 10.1016/j.immuni.2014.05.018 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Ji Y, Pos Z, Rao M, et al. : Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8 + T cells. Nat Immunol. 2011;12(12):1230–7. 10.1038/ni.2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yang CY, Best JA, Knell J, et al. : The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8 + T cell subsets. Nat Immunol. 2011;12(12):1221–9. 10.1038/ni.2158 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Pace L, Goudot C, Zueva E, et al. : The epigenetic control of stemness in CD8 + T cell fate commitment. Science. 2018;359(6372):177–86. 10.1126/science.aah6499 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Blackledge NP, Rose NR, Klose RJ: Targeting Polycomb systems to regulate gene expression: modifications to a complex story. Nat Rev Mol Cell Biol. 2015;16(11):643–9. 10.1038/nrm4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bannister AJ, Zegerman P, Partridge JF, et al. : Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410(6824):120–4. 10.1038/35065138 [DOI] [PubMed] [Google Scholar]
- 88. Lachner M, O'Carroll D, Rea S, et al. : Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410(6824):116–20. 10.1038/35065132 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Rose NR, Klose RJ: Understanding the relationship between DNA methylation and histone lysine methylation. Biochim Biophys Acta. 2014;1839(12):1362–72. 10.1016/j.bbagrm.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schober SL, Kuo CT, Schluns KS, et al. : Expression of the transcription factor lung Krüppel-like factor is regulated by cytokines and correlates with survival of memory T cells in vitro and in vivo. J Immunol. 1999;163(7):3662–7. [PubMed] [Google Scholar]
- 91. Ptashne M: Epigenetics: core misconcept. Proc Natl Acad Sci U S A. 2013;110(18):7101–3. 10.1073/pnas.1305399110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kaech SM, Wherry EJ: Heterogeneity and cell-fate decisions in effector and memory CD8 + T cell differentiation during viral infection. Immunity. 2007;27(3):393–405. 10.1016/j.immuni.2007.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Seo YJ, Jothikumar P, Suthar MS, et al. : Local Cellular and Cytokine Cues in the Spleen Regulate In Situ T Cell Receptor Affinity, Function, and Fate of CD8 + T Cells. Immunity. 2016;45(5):988–98. 10.1016/j.immuni.2016.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]