Abstract
Background
The efficacy of sodium citrate supplementation (SC) in exercise performance is unclear. Therefore, the aim of this study was to investigate the effect of SC on skilled tennis performance.
Methods
Ten Brazilian nationally-ranked young male tennis players (age: 17 ± 1 yrs.; stature: 176.7 ± 5.2 cm; body mass: 68.4 ± 7.9 kg) participated in this crossover, placebo-controlled, double-blind study. Upon arrival, at baseline, in both experimental sessions blood was collected, then subjects ingested either sodium citrate (SC - 0.5 g.kg−1BM in capsules of 500 mg) or a placebo (PLA). Two hours later, pre-match blood was collected then skills tests (skill tennis performance test - STPT, repeated-sprint ability shuttle test - RSA) were performed followed by a 1-h simulated match. Immediately following the match, blood was again collected, and STPT, and RSA were administered.
Results
All metabolic parameters (i.e. base excess, pH, bicarbonate, and blood lactate) increased (p < 0.001) from baseline to pre-match and post-match in SC condition. Each metabolic parameter was greater (p < 0.001) in SC compared to PLA condition at both pre- and post-match. The SC condition elicited a greater (p < 0.01) shot consistency at post-match in the STPT vs. PLA condition (SC: 58.5 ± 14.8% vs. PLA: 40.4 ± 10.4%). A greater (p < 0.001) amount of games won was observed in the simulated match for SC condition vs. PLA condition (SC: 8.0 ± 1.6 vs. PLA: 6.0 ± 1.7). Additionally, the games won during the simulated match in SC condition was positively correlated with percentage shot consistency (r = 0.67, p < 0.001).
Conclusions
The current findings suggest that SC supplementation is an effective ergogenic aid to enhance skilled tennis performance.
Keywords: Buffering agent, Fatigue, Tennis skills, Blood lactate, Supplementation
Introduction
Tennis match play consists of intense intermittent activity and potentially long duration (i.e. up to 5 h); thus, the magnitude of physiological demands during match play can be considerable. Although, blood lactate concentration ([La]) usually remains lower than 5 mmol.L− 1 [1–4] data have reported [La] concentration to reach 7-8 mmol.L− 1 during match play, which has led to both technical and tactical performance declines [5]. The rise in [La] is a consequence of the ≈1:2 work-to-rest ratio during a match [2, 5–7]. Correspondingly, HR consistently reaches 60–80% of maximum with absolute values up to 200 bpm [2] along with VO2 rising to 60–70% of maximum [8]. Additionally, the elevation of cortisol [9] and creatine kinase [9, 10] following match play signifies both acute stress and muscle damage.
Although various forms of fatigue exist during tennis match play [11], peripheral fatigue due to diminished ATPase activity compromises muscle contraction capabilities [12]. Further, debate exists regarding if an increase in H+ causing metabolic acidosis (i.e. decreased muscle pH) harms [13, 14], helps [15], or has a neutral performance effect [16]. Controversy is also present regarding the influence of the allocation of other ions (Ca2+, K+, and CL−) [17] in addition to decreased pH on exercise performance. However, in tennis, the muscle damage associated with the intermittent nature of tennis may lead to decreased Ca2+ release from the sarcoplasmic reticulum negatively affecting force production capabilities, and possibly lead to mistimed strokes [18]. Therefore, strategies, such as nutritional supplementation, which may delay these mechanism of fatigue during match play warrant investigation.
Specifically, extracellular buffers, sodium bicarbonate (NaHCO3) and sodium citrate (SC), serve as alkalizing agents and can elicit acute performance benefits in intermittent-type exercise [8, 17, 19–22]. Specifically, data have demonstrated NaHCO3 to significantly improve exercise performance, which lasts between 1 and 7 min. [19, 20] and meta-analytics have determined a moderate effect size between NaHCO3 and acute exercise performance [21]. Indeed, Wu et al. [23] demonstrated NaHCO3 to improve skilled tennis performance following a simulated match, which was associated with increased pH. However, an important limitation of NaHCO3 supplementation is that ingestion has consistently resulted in gastrointestinal (GI) distress and even vomiting [24], which may preclude some individuals from experiencing performance benefits [25]. On the other hand, SC supplementation may provide similar benefits to NaHCO3 with less GI distress [26].
Similar to NaHCO3, SC directly improves extracellular buffering capacity along with indirectly enhancing intramuscular pH conditions via facilitation of H+ efflux [22]. Specifically, SC supplementation causes the negatively charged citrate anion to be ejected from the plasma leading to decreased plasma H+ along with a concomitant increase in bicarbonate (HCO3−) [8], subsequently improving buffering capabilities. Conflicting results exist with SC as 0.5 g.kg−1BM (body mass) has been shown to improve anaerobic cycling power [27] performance [28], and has enhanced 200 m swim performance [29]. However, SC failed to improve 5.000 m treadmill running and repeated 60s sprint performance [30]. A meta-analysis from Carr et al. [31] revealed an unclear effect for SC to improve exercise performance, however, limited data was available for analysis and confidence limits were wide [31]. Moreover, Carr et al. [31] did report that SC provided similar benefits to pre-exercise alkalosis as NaHCO3 and did note that GI disturbance should be taken into account when considering an alkalizing agent. Despite the potential benefits for SC and tennis performance, to the authors’ knowledge, SC has not yet been examined in this regard.
Therefore, the primary aim of this study was to investigate the effects of SC supplementation (vs. a placebo condition) on specific tennis skill tests in nationally ranked young male tennis players. It was hypothesized that SC would create a condition of metabolic alkalosis; thus, preventing decline in tennis skill performance compared to a condition.
Methods
Experimental approach to the problem
This study used a randomized crossover, placebo-controlled, double blind design. Each subject reported for data collection on 3 occasions. The first and second sessions were separated by 3 days and the second and third were separated by 10 days. The first visit served to familiarize subjects with the skill tennis performance test (STPT) and repeated-sprint ability shuttle test (RSA). The second and third visits served as experimental sessions to examine the effects of SC (vs. a placebo-PLA condition) on 4 metabolic parameters (1. base excess - BE, 2. pH, 3. HCO3−, and 4. [La]), 2 performance tests (i.e. STPT and RSA) and session rating of perceived exertion (RPE) following 1-h of simulated match play. The 10-day period between sessions served as a washout period. All sessions were performed on the same outdoor hard-surface tennis court at 9:30 AM. Environmental conditions, temperature and humidity, were similar between sessions (Second visit: Temperature: 27 ± 2 °C; Humidity: 68 ± 4%; Third visit: Temperature: 26 ± 3 °C; Humidity: 72 ± 3%). Additionally, players were asked to maintain their regular dietary intake (24 h prior to each visit) and their current training program between laboratory visits. Athletes were also instructed to keep their regular diet throughout the duration of the study.
Subjects
Ten young male tennis players (age: 17 ± 1 years; stature: 176.7 ± 5.2 cm; body mass: 68.4 ± 7.9 kg; body fat: 11.7 ± 1.4%) who were competitive at the Brazilian national level participated in this study. Body fat was estimated by skinfold thickness measurements using a skinfold caliper (Lange, USA). The measurements were taken from the subjects’ right side at the following 7 sites: subscapular, triceps, pectoral, mid-axillary, supra-iliac, abdominal, and anterior mid-thigh. Body density was estimated using the formula proposed by Jackson and Pollock (1978) [32], and body fat was calculated with the equation from Siri [33]. These players were all ranked between 10 and 55 nationally and volunteered for the study. Additionally, all subjects had a training experience of at least 5 years and were currently engaged in training volume of 25–30 h per week. To fully inform the athletes of the protocol the investigators explained the experimental procedures and any potential risks to all subjects and their parents prior to participation. Written informed consent was obtained from the players and their parents. The protocol was approved by the University’s Ethics Committee (protocol #217.695, UNICAMP).
Experimental protocol
In the experimental sessions, subjects received a standardized meal (shake containing carbohydrates 2 g.kg−1BM and whey protein 1 g.kg−1BM) (07:00 AM). At the tennis academy (7:30 AM), 1 mL of blood was collected from subjects’ antecubital vein using a syringe without anticoagulant. Next, either SC in the amount of 0.5 g.kg−1BM in capsules of 500 mg [8, 29, 31] or a placebo consisting of NaCl 0.1 g.kg−1BM, microcrystalline cellulose powder 0.3 g.kg−1BM, and monohydrate lactose 0.1 g.kg−1BM in capsules of 500 mg were ingested with 1 L of water. Players were instructed to finish the entire 1 L of water within 2 h, which was just before the next blood collection. All capsules were prepared and distributed to subjects by a pharmacist. Following SC or placebo ingestion, players rested for 2 h before a pre-match blood collection in the amount of 1 mL. Immediately following this blood collection, the players then underwent the STPT and RSA tests and then participated in 1-h of simulated match play as outlined by Gomes et al. [34]. Kilit et al. [35] reported similar HR [5] along with higher RPE in service vs. return games [36, 37] in a 1-h simulated match, which is consistent with data observed in actual match play. The simulated match was carried out according to official international tennis federation rules, qualified umpires kept the match score, and matches took place on a clay court. At the conclusion of match play (post-match) a final 1 mL blood collection was conducted and players then performed a post-match STPT and RSA tests. Thirty minutes following the simulated match, players recorded a session RPE value and completed a GI distress questionnaire [38]. Finally, in the simulated match, the coaches paired players against each other according to skill level. All players faced the same opponent in both conditions.
Physical performance tests
Skill tennis performance test (STPT)
This test, previously used [39] assessed fatigue via measurements of stroke accuracy (%), stroke consistency (%), and number of strokes. To perform this test, tennis players received a random ball-feed of 30 balls per minute (1 ball every 2 s) from a tennis ball-serving machine (Lobmaster Poplob™). Balls were fed at a 10° projection with a constant initial velocity of 26.8 m∙s− 1 or 96.6 km∙h− 1) and landed 2 m in front of the baseline. Determination of fatigue was when the hitting frequency could not be maintained for 2 consecutive ball feeds or when a player voluntarily chose to cease the test. Further, tennis players were instructed to return the ball using the same hitting style, spin, and pace for all shots, and hit each shot at maximal effort. Players were also instructed to aim the shot to land within the singles court but beyond the service line and toward 1 of 2 targets placed in the opposite court. All shots were recorded with video so that analysis could determine stroke accuracy (%), stroke consistency (%), and total number of strokes during the test.
Repeated-Sprint ability shuttle test (RSA)
The RSA has been previously utilized to in part determine profile of a tennis player [52]. To measure RSA, a total of 10 shuttle sprints each totaling 22 m were performed as previously outlined by Fernandez-Fernandez et al. [40]. To begin, tennis players stood with his racket in the front position and in the middle of the baseline, while looking at the net. In response to a signal from the investigator players turned and ran to the prescribed backhand (left) or forehand (right) corner. Next, players ran toward the prescribed corner forward and in a straight line and when their feet were in line with a turning point (marked with a cone) players touched the cone with their racket and turned 180°. Then, the players ran back to the opposite side of the court while running forward to touch a second cone, and finally turned at that cone to run back to the initial starting position. Following the completion of the RSA trial, players had 15 s of passive recovery before completing the subsequent trial and this continued until all 10 trials were complete. Additionally, the tennis players assumed the ready position 5 s before each trial and waited for the investigator’s signal. The mean time of 10 trials was used for analysis, and the change in time from the first to 10th sprint was calculated in each condition.
Rating of perceived exertion (RPE)
Session RPE was recorded to gauge intensity and fatigue of the total session (simulated match, STPT, and RSA) using the CR-10 RPE scale as previously explained by Foster [41]. To assess exertion of the entire session RPE was collected 30 min following completion of the post-match STPT and RSA and the players were individually asked, “How was your workout?” and shown the RPE scale. The same investigator collected all RPE data; and players were familiarized with the RPE scale during the initial visit.
Metabolic parameters and blood analysis
The 1 mL sample of the metabolic parameters (BE, pH, HCO3−, and [La]) was analyzed via the iSTAT Blood Gas Analyzer using the disposable cartridge (CG8+) (iSTAT© Abbott, USA).
Statistical analyses
Means and standard deviations were calculated for all dependent variables at all time points and a Shapiro-wilk test was used to test normality of the data. Metabolic parameters as well as percentage of stroke accuracy and consistency during the STPT were analyzed using a 2-way repeated measures analysis of variance (ANOVA). In the event of a significant F-ratio, a Tukey post hoc test was performed for pairwise comparisons. Number of total strokes during the STPT, mean RSA test time, and games won during the simulated match were analyzed by a paired t-test. Additionally, a linear regression was utilized to determine if any correlation existed between changes in any of the metabolic parameters or STPT with games won during the simulated match. In all cases the level of significance was set at p ≤ 0.05. Correlations were interpreted and reported as “weak” if they were less than or equal to 0.35, “moderate” if they fell between 0.36 to 0.67, “strong” if they fell between 0.68 to 0.89, and “very strong” if they were equal or greater than 0.90 [42]. Finally, effect size (ES) was determined using Cohen’s d and interpreted in accordance with Cohen (1988) [43].
Results
Physical performance tests
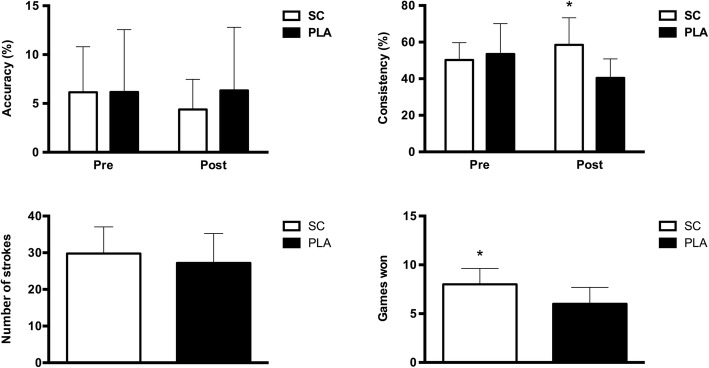
A bar graph depiction of results for the STPT and games won during the simulated match can be seen in Fig. 1. Regarding the STPT, there was no difference (p > 0.05) in percentage shot accuracy or percentage shot consistency at the pre-match testing time point between PLA and SC. Further, no difference existed (p > 0.05) between conditions in shot accuracy or total strokes between conditions as post-match. However, SC did show a significantly greater (p < 0.01) percentage shot consistency at post-match compared to PLA (SC: 58.5 ± 14.8% vs. PLA: 40.4 ± 10.4%).
Fig. 1.
Data are mean ± standard deviation. Accuracy (%), consistency (%), number of strokes and games won in both experimental conditions. * different from Placebo (PLA)
In SC, players won a significantly greater (p < 0.001) amount of games compared to PLA. Additionally, in SC there was a significant correlation between number of games won during the simulated match with both post-match percentage shot consistency (r = 0.67, p < 0.001) and post-match pH (r = 0.70, p < 0.0005) (Fig. 2). There was no difference in RSA performance from pre- to post-match for PLA (5.368 ± 0.413 to 5.205 ± 0.484 s; p > 0.05) or SC (5.342 ± 0.400 to 5.280 ± 0.382 s; p > 0.05), nor was there any difference between conditions (p > 0.05). Between conditions, ES for RSA at pre-match was 0.06, while ES at post-match was 0.17. For RSA there was also an average decrement from the first to the 10th sprint of 4.76% in performance in PLA (1st: 5.441 ± 0.470 to 10th: 5.713 ± 0.284). However, a decrement of only 0.1% occurred in SC from the first to the 10th sprint (1st: 5.371 ± 0.460 to 10th: 5.424 ± 0.322).
Fig. 2.
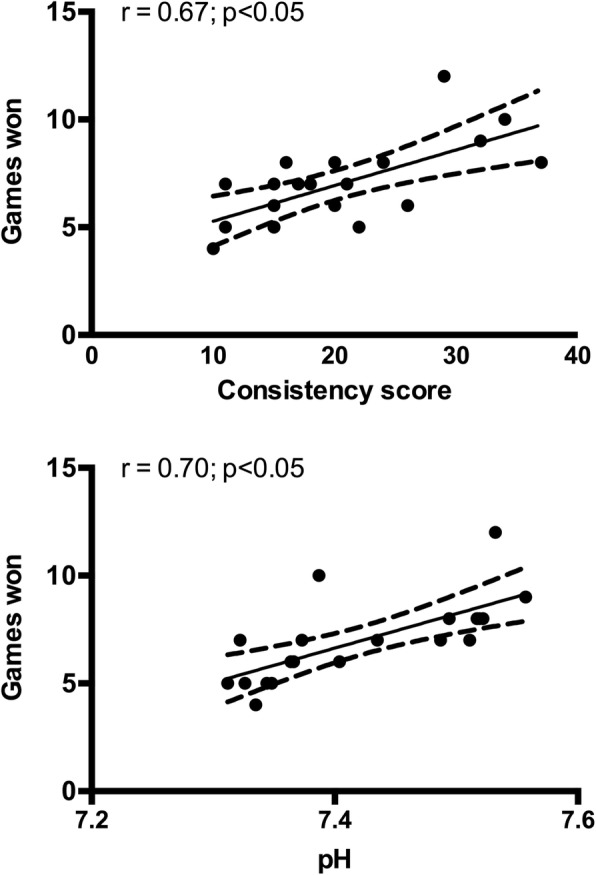
Correlation analysis between games won and post-match consistency score and games won and post-match pH
RPE and GI questionnaire
No difference (p > 0.05) was observed in total session RPE score between SC (6.4 ± 1.2) and PLA (6.7 ± 1.8) conditions. In terms of the GI questionnaire only 3 subjects in SC reported mild symptoms of discomfort (i.e. abdominal pain, epigastric pain, abdominal noises, bloating, urge to burp, loss of appetite and flatulence), and only 2 subjects in SC noted a mild headache, while no subjects reported sever GI distress symptoms. No subjects reported GI discomfort following PLA.
Metabolic parameters
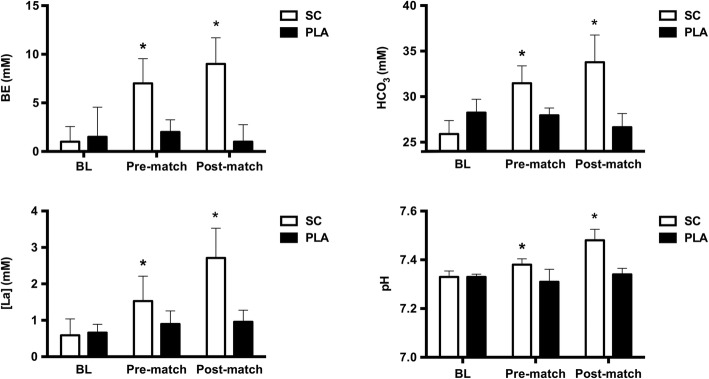
There was no difference (p > 0.05) in the level of any metabolic parameter at BL between conditions (Fig. 3) and BE, pH, and HCO3− significantly increased (p < 0.05) from BL to pre-match in SC. All 4 metabolic markers showed an increase from BL to post-match in SC (p < 0.05), while there was no significant change (p > 0.05) from BL to pre- or post-match in any metabolic parameter in PLA. Between conditions, there was a statistically greater level at both pre- and post-match for BE (Pre, p < 0.001; Post, p < 0.001), pH (Pre, p < 0.001; Post, p < 0.001), HCO3−, and [La] (Pre, p < 0.05; Post, p < 0.001) in SC vs. PLA. Effect sizes demonstrated a large effect (> 0.80) in favor of SC for every metabolic parameter at both pre- and post-match. Percentage changes from BL to pre- and post-match of the metabolic parameters in SC were as follows: BE: 1.30 ± 1.57 to 6.30 ± 2.69 mM at pre-match (+ 384.62%) and 9.10 ± 2.69 mM at post-match (+ 600%), pH: 7.33 ± 0.02 to 7.38 ± 0.020 at pre-match (+ 0.68%) and 7.48 ± 0.05 at post-match (+ 2.05%), HCO3−: 25.90 ± 1.49 to 31.48 ± 2.00 mM at pre-match (+ 21.54%) and 33.78 ± 2.99 mM at post-match (+ 0.42%), [La]: 1.62 ± 1.06 to 2.54 ± 0.31 at pre-match (+ 56.79%) and 5.46 ± 1.11 mM at post-match (+ 237.04%).
Fig. 3.
Metabolic parameters Data are mean ± standard deviation. Base excess (BE), bicarbonate (HCO3), lactate concentration ([La]) and pH level at baseline (BL), pre-match and post-match in both conditions. a - Greater than Placebo (PLA). b - Greater than Baseline (BL)
Discussion
The primary aim of the present study was to examine changes in metabolic parameters, tennis skill performance, and the perceptual response following SC supplementation or PLA in nationally ranked young Brazilian tennis players. The main findings of this investigation supported the hypothesis the SC would induce alkalosis and benefit performance. These findings were: 1) All metabolic parameters (i.e. BE, pH, HCO3−, and [La]) increased from BL to both pre- and post-match in SC, 2) The level of all metabolic parameters in SC compared to PLA at both pre- and post-match, 3) The SC condition resulted in a greater performance vs. PLA in terms of percentage shot consistency during the STPT and more games won during the simulated match, and 4) In SC, games won during the simulated match was positively correlated with both percentage shot consistency and pH.
It has been suggested that when [La] reaches up to 7-8 mM, tennis performance declines [5]. However, [La] more commonly increases to only 2-4 mM during match play [2, 18, 23]. Girard and Millet have suggested that elevated blood lactate may lead to mistimed strokes [18]. Presently, it was observed [La] concentration in SC to reach 5.46 ± 1.11 mM at post-match, which is lower than the upper end previously observed, however, the only 1-h of match time in the present study likely accounts for this lower level. Although a relationship between acidity and mistimed strokes was not observed presently, a positive relationship between pH and games won (r = 0.70) was observed suggesting that inducing alkalosis may enhance tennis performance.
This study showed a significantly greater level of BE, pH, HCO3−, and [La] in the players at post-match in SC compared to the PLA condition. Interestingly, the increase in [La] in SC occurred despite an increase in pH. However, a concomitant increase [La] and extracellular pH is in agreement with previous findings in which alkalosis has been induced [44–46]. Specifically, Wu et al. [23] induced alkalosis via NaHCO3 in male collegiate tennis players and still observed increased [La] just as the present study. Further, Stephens et al. [46] induced alkalosis via NaHCO3 during endurance cycling and concomitantly observed increased [La]. Mechanistically, the increased alkalosis observed in these studies is due to an increased H+ gradient leading to higher H+ and lactate expulsion from the working skeletal muscles due to monocarboxylate co-transporter (a carrier of H+ and lactate), which explains increased blood [La] in the presence of alkalosis [46]. Additionally, all metabolic parameters experienced a non-significant increase from pre- to post-match, which seems incongruent with fatigue that may have been caused by the simulated match. However, post-match blood collection occurred 3 h following supplementation, which is the duration (120 min) that Potteiger et al. [47] have previously demonstrated HCO3− level to peak.
Fatigue has been explained as a reduction in maximal force capabilities of a muscle over time during exercise [48], thus delaying fatigue can maintain neuromuscular capabilites and potentially performance. Indeed, the current study not only noted superior shot consistency (%) in STPT and a greater amount of games won in the simulated match in SC compared to PLA, but also a positive correlation (r = 0.70) between pH level and games won. Therefore, it seems that SC was able to prolong muscle contractile capabilities. Theoretically, prolonging muscle force production would enhance an athlete’s ability to perform training volume, which is the training variable most closely and positively associated with muscle performance adaptations [49].
Interestingly, the present investigation did not report a significant difference (p > 0.05) between session RPE in SC (6.45 ± 1.21) vs. PLA (6.7 ± 1.84) despite the improved metabolic profile and performance in SC. However, this finding is in agreement with previous data, which has induced alkalosis via NaHCO3, and noted specific skill performance increase in tennis [23] and boxing [50], but no difference between experimental and control conditions in terms of session RPE. Thus, the perceptual response may not be related to specific skill performance.
The dosage of SC in this study was based off of McNaughton and Cedaro [8], which demonstrated 0.5 g.kg−1BM of SC significantly increased buffering capacity, total work, and peak power during anaerobic cycling performance of durations of 120 s and 240 s in healthy males. Additionally, McNaughton [28] reported a performance enhancement after SC supplementation in a 1 min maximal cycle ergometer test; however, Cox and Jenkins [30] did not find SC supplementation to improve performance in repeated 60 s cycling sprint performance despite inducement of alkalosis. Furthermore, McNaughton and Cedaro [8] did not observe a performance benefit in cycling of a 10 s duration, which may explain the lack of difference in RSA times between SC and PLA in the present study; as each RSA test lasted approximately 5 s. It has been suggested that metabolic factors may not be of great consequence in such a short trials [26], since reliance on glycolysis is decreased and buffering H+ is not of great consequence in this short duration. However, Carr et al. [31] concluding in a meta-analysis that NaHCO3 improves 1 min sprint performance by 1.7% and has compared favorably to SC for running sprint performance lasting approximately 80s. To explain the differential findings between SC and NaHCO3 for sprint performance, Van Montfoort et al. [51] noted that despite SC inducing extracellular alkalosis the intracellular increase in citrate may blunt ATP resynthesis, whereas NaHCO3 would enhance ATP resynthesis. This explanation may account for the varying results between the 2 alkalizing agents and the lack of benefit of SC for RSA performance in the present study.
Even though NaHCO3 has been an effective buffer a possible drawback is the possible GI distress. Importantly, the GI distress with both NaHCO3 and SC is equivocal [26]. The current results for GI distress revealed that only 3 subjects reported even mild symptoms of GI discomfort (i.e. abdominal pain epigastric pain, abdominal noises, bloating, urge to burp, loss of appetite and flatulence), and only 2 subjects acknowledged a mild headache with no reports of severe GI discomfort following the SC condition. Thus, although NaHCO3 and SC may both be effective as extracellular buffers, SC may be the more attractive option in an effort to avoid GI discomfort. However, more research should be conducted with NaHCO3 in tennis players, in which GI discomfort is specifically monitored.
A possible limitation to this study was the small sample size. However, a previous investigation [23], also in male tennis players, used only 9 subjects (one fewer than the present study) to examine NaHCO3 supplementation in a double blind fashion. Further, this study is unique as it is the first study to measure specific tennis skills as an outcome measure in response to SC supplementation, adding novelty to the investigation.
Conclusion
In conclusion, SC supplementation (0.5 g.kg−1BM in capsules of 500 mg) significantly altered metabolic parameters (i.e. BE, pH, HCO3−, [La]) and improved skilled tennis performance (i.e. percent shot consistency and games won during simulated match play) compared to PLA. Importantly, no players reported any significant GI discomfort following SC supplementation. Practically, it appears that SC supplementation can be safely and effectively utilized in tennis players to enhanced skilled performance. Although, it cannot be known from these results if SC supplementation would be beneficial in long lasting matches, previous data has shown SC to be effective for up to 30 km of cycling, thus it is possible that SC could be beneficial in matches lasting for multiple hours. Importantly, if SC is utilized in training sessions, training volume could be increased to allow for greater duration of quality skill practice. Additionally, it should be noted that individual athletes may have varying GI responses to SC, even though no athletes reported discomfort in the present study, thus future studies should continue to investigate the efficacy/safety of SC supplementation in various athletic populations.
Acknowledgements
The authors would like to acknowledge all players and coaches involved in this study for their committed participation. RVG would like to thank the CAPES (Brazil) for his scholarship. VCRC would like to thank the FAEPEX (UNICAMP, São Paulo, Brazil) for her scholarship (Grant: 519292 #130/13). This research was supported by FAPESP (Fundação de Amparo à Pesquisa no Estado de São Paulo, São Paulo, Brazil – Grant: 2012/19529-9).
Authors’ contributions
VCRC carried out data collection, analyzed the data, and helped to draft the manuscript. RVG, MCZ and AM participated in the design and coordination of the study and helped to write the manuscript. MSA and CDC conceived the study, and participated in the design and coordination and helped to write the manuscript. All of the authors have been involved in the writing of this manuscript and have read and approved the final text.
Funding
Not applicable.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The protocol was approved by the University’s Ethics Committee (Protocol #217.695, UNICAMP).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davey PR, Thorpe RD, Williams C. Fatigue decreases skilled tennis performance. J Sports Sci. 2002;20:311–318. doi: 10.1080/026404102753576080. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez J, Mendez-Villanueva A, Pluim B. Intensity of tennis match play. Br J Sports Med. 2006;40:387–391. doi: 10.1136/bjsm.2005.023168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrauti A, Bergeron MF, Pluim BM, Weber K. Physiological responses in tennis and running with similar oxygen uptake. Eur J Appl Physiol. 2001;85:27–33. doi: 10.1007/s004210100425. [DOI] [PubMed] [Google Scholar]
- 4.Ferrauti A, Pluim BM, Weber K. The effect of recovery duration on running speed and stroke quality during intermittent training drills in elite tennis players. J Sports Sci. 2001;19:235–242. doi: 10.1080/026404101750158277. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs MS. Tennis physiology Sports Med. 2007;37:189–198. doi: 10.2165/00007256-200737030-00001. [DOI] [PubMed] [Google Scholar]
- 6.Bergeron MF, Maresh C, Kraemer W, Abraham A, Conroy B, Gabaree C. Tennis: a physiological profile during match play. Int J Sports Med. 1991;12:474–479. doi: 10.1055/s-2007-1024716. [DOI] [PubMed] [Google Scholar]
- 7.Christmass M, Richmond S, Cable N, Hartmann P. A metabolic characterisation of single tennis. Science and Racket Sports. 1994:3–9.
- 8.McNaughton L, Cedaro R. Sodium citrate ingestion and its effects on maximal anaerobic exercise of different durations. Eur J Appl Physiol. 1992;64:36–41. doi: 10.1007/BF00376437. [DOI] [PubMed] [Google Scholar]
- 9.Ojala T, Häkkinen K. Effects of the tennis tournament on players’ physical performance, hormonal responses, muscle damage and recovery. Journal of Sports Science & Medicine. 2013;12:240. [PMC free article] [PubMed] [Google Scholar]
- 10.Hornery DJ, Farrow D, Mujika I, Young W. An integrated physiological and performance profile of professional tennis. Br J Sports Med. 2007;41:531–536. doi: 10.1136/bjsm.2006.031351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid M, Duffield R. The development of fatigue during match-play tennis. Br J Sports Med. 2014;48:i7–i11. doi: 10.1136/bjsports-2013-093196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacIntosh BR, Holash RJ, Renaud J-M. Skeletal muscle fatigue-regulation of excitation-contraction coupling to avoid metabolic catastrophe. J Cell Sci. 2012;125:2105–2114. doi: 10.1242/jcs.093674. [DOI] [PubMed] [Google Scholar]
- 13.Debold EP, Beck SE, Warshaw DM. Effect of low Ph on single skeletal muscle myosin mechanics and kinetics. Am J Phys Cell Phys. 2008;295:C173–C179. doi: 10.1152/ajpcell.00172.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spriet L, Lindinger M, McKelvie R, Heigenhauser G, Jones N. Muscle glycogenolysis and H+ concentration during maximal intermittent cycling. J Appl Physiol. 1989;66:8–13. doi: 10.1152/jappl.1989.66.1.8. [DOI] [PubMed] [Google Scholar]
- 15.Overgaard K, Højfeldt GW, Nielsen OB. Effects of acidification and increased extracellular potassium on dynamic muscle contractions in isolated rat muscles. J Physiol. 2010;588:5065–5076. doi: 10.1113/jphysiol.2010.195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westerblad H, Allen D. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol. 1992;453:413–434. doi: 10.1113/jphysiol.1992.sp019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegler JC, Marshall PW, Bishop D, Shaw G, Green S. Mechanistic insights into the efficacy of sodium bicarbonate supplementation to improve athletic performance. Sports Med. 2016;2:41. doi: 10.1186/s40798-016-0065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girard O, Millet GP. Neuromuscular fatigue in racquet sports. Phys Med Rehabil Clin N Am. 2009;20:161–173. doi: 10.1016/j.pmr.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Lindh A, Peyrebrune M, Ingham S, Bailey D, Folland J. Sodium bicarbonate improves swimming performance. Int J Sports Med. 2008;29:519–523. doi: 10.1055/s-2007-989228. [DOI] [PubMed] [Google Scholar]
- 20.McNaughton LR, Siegler J, Midgley A. Ergogenic effects of sodium bicarbonate. Current Sports Medicine Reports. 2008;7:230–236. doi: 10.1249/JSR.0b013e31817ef530. [DOI] [PubMed] [Google Scholar]
- 21.Peart DJ, Siegler JC, Vince RV. Practical recommendations for coaches and athletes: a meta-analysis of sodium bicarbonate use for athletic performance. J Strength Cond Res. 2012;26:1975–1983. doi: 10.1519/JSC.0b013e3182576f3d. [DOI] [PubMed] [Google Scholar]
- 22.Requena B, Zabala M, Padial P, Feriche B. Sodium bicarbonate and sodium citrate: ergogenic aids? J Strength Cond Res. 2005;19:213. doi: 10.1519/13733.1. [DOI] [PubMed] [Google Scholar]
- 23.Wu C-L, Shih M-C, Yang C-C, Huang M-H, Chang C-K. Sodium bicarbonate supplementation prevents skilled tennis performance decline after a simulated match. Journal of the International Society of Sports Nutrition. 2010;7:33. doi: 10.1186/1550-2783-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breitkreutz J, Gan TG, Schneider B, Kalisch P. Enteric-coated solid dosage forms containing sodium bicarbonate as a drug substance: an exception from the rule? J Pharm Pharmacol. 2007;59:59–65. doi: 10.1211/jpp.59.1.0008. [DOI] [PubMed] [Google Scholar]
- 25.Saunders B, Sale C, Harris RC, Sunderland C. Sodium bicarbonate and high-intensity-cycling capacity: variability in responses. International Journal of Sports Physiology and Performance. 2014;9:627–632. doi: 10.1123/ijspp.2013-0295. [DOI] [PubMed] [Google Scholar]
- 26.Junior AHL, de Salles Painelli V, Saunders B, Artioli GG. Nutritional strategies to modulate intracellular and extracellular buffering capacity during high-intensity exercise. Sports Med. 2015;45:71–81. doi: 10.1007/s40279-015-0397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parry-Billings M, MacLaren D. The effect of sodium bicarbonate and sodium citrate ingestion on anaerobic power during intermittent exercise. Eur J Appl Physiol. 1986;55:524–529. doi: 10.1007/BF00421648. [DOI] [PubMed] [Google Scholar]
- 28.McNaughton LR. Sodium citrate and anaerobic performance: implications of dosage. Eur J Appl Physiol. 1990;61:392–397. doi: 10.1007/BF00236058. [DOI] [PubMed] [Google Scholar]
- 29.Russell C, Papadopoulos E, Mezil Y, Wells GD, Plyley MJ, Greenway M, Klentrou P. Acute versus chronic supplementation of sodium citrate on 200 M performance in adolescent swimmers. J Int Soc Sports Nutr. 2014;11:26. doi: 10.1186/1550-2783-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox G, Jenkins D. The physiological and ventilatory responses to repeated 60 S sprints following sodium citrate ingestion. J Sports Sci. 1994;12:469–475. doi: 10.1080/02640419408732197. [DOI] [PubMed] [Google Scholar]
- 31.Carr AJ, Hopkins WG, Gore CJ. Effects of acute alkalosis and acidosis on performance. Sports Med. 2011;41:801–814. doi: 10.2165/11591440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497–504. doi: 10.1079/BJN19780152. [DOI] [PubMed] [Google Scholar]
- 33.Siri W. Body composition from fluid spaces and density: analysis of methods. 1961 Nutrition. 1993;9:480. [PubMed] [Google Scholar]
- 34.Gomes RV, Santos R, Nosaka K, Moreira A, Miyabara E, Aoki MS. Muscle damage after a tennis match in young players. Biol Sport. 2014;31:27. doi: 10.5604/20831862.1083276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilit B, Şenel Ö, Arslan E, Can S. Physiological responses and match characteristics in professional tennis players during a one-hour simulated tennis match. J Hum Kinet. 2016;51:83–92. doi: 10.1515/hukin-2015-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendez-Villanueva A, Fernandez-Fernández J, Bishop D, Fernandez-Garcia B. Ratings of perceived exertion-lactate association during actual singles tennis match play. J Strength Cond Res. 2010;24:165–170. doi: 10.1519/JSC.0b013e3181a5bc6d. [DOI] [PubMed] [Google Scholar]
- 37.Mendez-Villanueva A, Fernandez-Fernandez J, Bishop D, Fernandez-Garcia B, Terrados N. Activity patterns, blood lactate concentrations and ratings of perceived exertion during a professional singles tennis tournament. Br J Sports Med. 2007;41:296–300. doi: 10.1136/bjsm.2006.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bovenschen HJ, Janssen M, van Oijen M, Laheij R, van Rossum L, Jansen J. Evaluation of a gastrointestinal symptoms questionnaire. Dig Dis Sci. 2006;51:1509–1515. doi: 10.1007/s10620-006-9120-6. [DOI] [PubMed] [Google Scholar]
- 39.Davey P, Thorpe R, Williams C. Simulated tennis matchplay in a controlled environment. J Sports Sci. 2003;21:459–467. doi: 10.1080/0264041031000101926. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez-Fernandez J, Zimek R, Wiewelhove T, Ferrauti A. High-intensity interval training vs. repeated-sprint training in tennis. J Strength Cond Res. 2012;26:53–62. doi: 10.1519/JSC.0b013e318220b4ff. [DOI] [PubMed] [Google Scholar]
- 41.Foster C. Monitoring training in athletes with reference to overtraining syndrome. Med Sci Sports Exerc. 1998;30:1164–1168. doi: 10.1097/00005768-199807000-00023. [DOI] [PubMed] [Google Scholar]
- 42.Taylor R. Interpretation of the correlation coefficient: a basic review. J Diagn Med Sonogr. 1990;6:35–39. doi: 10.1177/875647939000600106. [DOI] [Google Scholar]
- 43.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Earlbaum Associates; 1988. pp. 20–26. [Google Scholar]
- 44.Bishop D, Edge J, Davis C, Goodman C. Induced metabolic alkalosis affects muscle metabolism and repeated-sprint ability. Med Sci Sports Exerc. 2004;36:807–813. doi: 10.1249/01.MSS.0000126392.20025.17. [DOI] [PubMed] [Google Scholar]
- 45.Price M, Moss P, Rance S. Effects of sodium bicarbonate ingestion on prolonged intermittent exercise. Med Sci Sports Exerc. 2003;35:1303–1308. doi: 10.1249/01.MSS.0000079067.46555.3C. [DOI] [PubMed] [Google Scholar]
- 46.Stephens TJ, McKENNA MJ, Canny BJ, Snow RJ, McConell GK. Effect of sodium bicarbonate on muscle metabolism during intense endurance cycling. Med Sci Sports Exerc. 2002;34:614–621. doi: 10.1097/00005768-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Potteiger J, Nickei G, Webster M, Haub M, Palmer R. Sodium citrate ingestion enhances 30 km cycling performance. Int J Sports Med. 1996;17:7–11. doi: 10.1055/s-2007-972800. [DOI] [PubMed] [Google Scholar]
- 48.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 49.Flann KL, LaStayo PC, McClain DA, Hazel M, Lindstedt SL. Muscle damage and muscle remodeling: no pain, no gain? J Exp Biol. 2011;214:674–679. doi: 10.1242/jeb.050112. [DOI] [PubMed] [Google Scholar]
- 50.Siegler JC, Hirscher K. Sodium bicarbonate ingestion and boxing performance. J Strength Cond Res. 2010;24:103–108. doi: 10.1519/JSC.0b013e3181a392b2. [DOI] [PubMed] [Google Scholar]
- 51.Van Montfoort M, Van Dieren L, Hopkins WG, Shearman JP. Effects of ingestion of bicarbonate, citrate, lactate, and chloride on sprint running. Med Sci Sports Exerc. 2004;36:1239–1243. doi: 10.1249/01.MSS.0000132378.73975.25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.