Abstract
BACKGROUND
Malnutrition is prevalent in inflammatory bowel disease (IBD). Multiple nutrition screening (NST) and assessment tools (NAT) have been developed for general populations, but the evidence in patients with IBD remains unclear.
AIM
To systematically review the prevalence of abnormalities on NSTs and NATs, whether NSTs are associated with NATs, and whether they predict clinical outcomes in patients with IBD.
METHODS
Comprehensive searches performed in Medline, CINAHL Plus and PubMed. Included: English language studies correlating NSTs with NATs or NSTs/NATs with clinical outcomes in IBD. Excluded: Review articles/case studies; use of body mass index/laboratory values as sole NST/NAT; age < 16.
RESULTS
Of 16 studies and 1618 patients were included, 72% Crohn’s disease and 28% ulcerative colitis. Four NSTs (the Malnutrition Universal Screening Tool, Malnutrition Inflammation Risk Tool (MIRT), Saskatchewan Inflammatory Bowel Disease Nutrition Risk Tool (SaskIBD-NRT) and Nutrition Risk Screening 2002 (NRS-2002) were significantly associated with nutritional assessment measures of sarcopenia and the Subjective Global Assessment (SGA). Three NSTs (MIRT, NRS-2002 and Nutritional Risk Index) were associated with clinical outcomes including hospitalizations, need for surgery, disease flares, and length of stay (LOS). Sarcopenia was the most commonly evaluated NAT associated with outcomes including the need for surgery and post-operative complications. The SGA was not associated with clinical outcomes aside from LOS.
CONCLUSION
There is limited evidence correlating NSTs, NATs and clinical outcomes in IBD. Although studies support the association of NSTs/NATs with relevant outcomes, the heterogeneity calls for further studies before an optimal tool can be recommended. The NRS-2002, measures of sarcopenia and developments of novel NSTs/NATs, such as the MIRT, represent key, clinically-relevant areas for future exploration.
Keywords: Nutrition, Inflammatory bowel disease, Ulcerative colitis, Crohn’s disease, Screening, Outcomes research
Core tip: Malnutrition is highly prevalent amongst patients with inflammatory bowel disease (IBD) and negatively impacts various clinical outcomes. This review highlights the Malnutrition Universal Screening Tool, Malnutrition Inflammation Risk Tool, Saskatchewan Inflammatory Bowel Disease Nutrition Risk Tool, Nutrition Risk Screening 2002 and cross-sectional imaging assessments of sarcopenia as promising nutrition screening and assessment tools in IBD. By becoming familiar with and consistently applying these tools we can move towards early recognition, diagnosis and management of malnutrition in clinical practice. Further research will elucidate the optimal tools and the impact of their integration into routine practice on clinical outcomes in IBD.
INTRODUCTION
Malnutrition is highly prevalent in inflammatory bowel disease (IBD); present in up to 70% of patients with active disease and up to 38% of patients in remission[1-3]. Closely related to malnutrition, sarcopenia is a syndrome defined by the presence of low muscle mass and either decreased muscle strength or physical performance[4]. Sarcopenia and malnutrition represent separate entities but often overlap; notably, the Global Leadership Initiative on Malnutrition, American Society for Parenteral and Enteral Nutrition and European Society for Clinical Nutrition and Metabolism (ESPEN) include components of reduced muscle mass and impaired muscle function in their respective consensus definitions of malnutrition[5-7].
In patients with IBD, sarcopenia and malnutrition have been associated with increased hospitalizations, disease flares, need for surgery, and post-operative complications[8-13]. Early identification of malnourished patients using a two-step approach of nutritional screening and subsequent assessment[6] may allow for earlier intervention and impact on clinical outcomes[14-17]. Recent data from Zhang et al[10] showed fewer major complications in patients who received peri-operative enteral nutrition than those who did not (6.5% vs 29%, P = 0.045). In line with these findings, ESPEN recommends implementing nutrition support therapy in malnourished peri-operative patients with IBD[18].
Nutritional risk screening (NRS) is a process to predict those at risk of malnutrition so that they can be referred to a registered dietitian (RD) for detailed nutritional assessment and intervention. Nutrition screening tools (NSTs) (i.e., the malnutrition universal screening tool, MUST) are rapid evaluations that can be completed by any member of the medical team whereas nutrition assessment tools (NATs) (i.e., the subjective global assessment, SGA) are usually more detailed and require greater specialized resources[5]. As recent studies have demonstrated a close relationship between malnutrition and sarcopenia, many expert groups now incorporate measures of lean muscle mass within the definition of malnutrition[19,20]. For the purposes of our review, measurements of lean muscle mass and sarcopenia will be classified as a NAT.
To date, there are no published recommendations that exist for use of a specific NST or NAT in IBD[14,18,21]. Although there have been isolated reviews of sarcopenia in IBD[22], a practical approach to nutrition screening and treatment is more extensive than sarcopenia assessment alone. Given the current lack of consensus, high prevalence and the significant health and economic burden of malnutrition in IBD, we performed a systematic review of the available literature surrounding NSTs and NATs for IBD patients, including sarcopenia. In patients with IBD, our aims were to provide a descriptive overview of: (1) The prevalence of abnormalities on NSTs and NATs; (2) Whether the findings on NSTs are associated with abnormalities on NATs; and (3) Whether NSTs or NATs are associated with clinical outcomes. Evidence of clear associations between NSTs and NATs may simplify the nutrition care process, allow for much needed risk stratification and targeted use of limited dietitian resources.
MATERIALS AND METHODS
Data sources/search strategy (Appendix S1)
The initial literature review was completed on December 20, 2017 using the following databases: National Institutes of Health PubMed (1946-present), Ovid MEDLINE (1946-present) and CINAHL Plus (1937-present). Medical library search heading terms were used to combine “nutrition screening”, “nutrition assessment”, “malnutrition”, or “sarcopenia” with either terms of “inflammatory bowel disease”, Crohn’s/Crohn disease” or “ulcerative colitis”. Filters applied included human subjects, English language and adult population (age 16 years and above). An updated search was conducted to identify articles published between December 20, 2017 and January 14, 2019 on PubMed. Further eligible studies were extracted from a review of reference lists of full texts retrieved after initial screening of search results.
Study selection
Initial search results were screened against inclusion and exclusion criteria through review of article titles and abstracts. Inclusion criteria encompassed studies whose population was > 16 years old, had a confirmed diagnosis of IBD [either Crohn’s disease (CD) or ulcerative colitis (UC)], and (1) Associated NSTs with a diagnosis of malnutrition using NATs; or (2) Associated either NST or NATs with prospective clinical outcomes. Study designs eligible for inclusion included randomized controlled trials, cross-sectional studies, cohort studies and case control studies.
Records were excluded if a formal NST/NAT was not utilized, if there were no prospective clinical outcomes evaluated and/or the study lacked comparisons between NSTs and NATs. Additionally, studies that utilized body mass index (BMI) as the sole NAT were excluded as previous studies have shown that BMI does not accurately predict body composition in IBD patients[23]. Studies that used NST/NATs based only on laboratory parameters (i.e., CONUT, OPNI) were also excluded. Significant laboratory abnormalities can be seen in IBD patients at baseline due to the inflammatory nature of their illness that do not necessarily accurately reflect nutrition status[24]. Articles that did not have an English translation available were excluded. Articles of interest or that were unclear as to meeting inclusion/exclusion criteria had their full text retrieved and reviewed by two independent reviewers (SL and MN) for eligibility. Disagreements between reviewers were settled through discussions with a third reviewer (PT).
Data extraction
The following data was extracted from each study where possible by an independent reviewer: First author’s surname, journal, year of publication, study design (patient selection) and duration, number of participants, underlying disease (CD or UC), patient demographics (age, duration of disease, severity of disease, concomitant treatments), type of NST or NAT used, reported correlations between NST and NAT or NST/NAT, and clinical outcomes.
Quality assessment
As most studies were observational non-randomized non-interventional studies without control groups, quality was assessed with a modified Newcastle-Ottawa assessment scale (NOS)[25]. Of available instruments, the NOS is highlighted as one of the most useful tools for assessing methodological quality and risk of bias in non-randomized studies in the Cochrane Handbook for Systematic Review of Interventions[26]. Study quality was assessed by two independent reviewers utilizing the modified NOS (SL and MN). Disagreements between reviewers were settled through discussions with a third reviewer (PT).
Data synthesis
Statistical results relating to outcomes of interest were retrieved from each study and categorized as per objectives. Authors and a third-party statistician (BV) reviewed all data. Given the heterogeneity of results, they were not suitable for a formal meta-analysis.
RESULTS
Literature search results
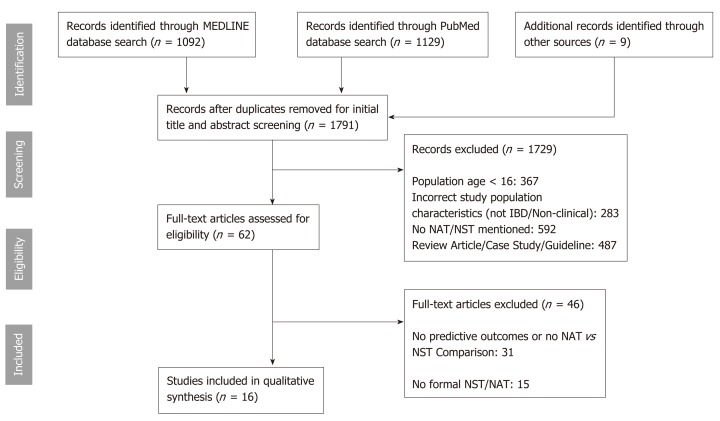
The summary of the literature search and selection process is shown in Figure 1. In total, 1782 studies were identified from the initial search after removal of duplicates. An additional 9 studies were identified through review of the full-text of articles of interest. 62 studies were identified for full-text review of which 16 studies met inclusion/exclusion criteria. 31 studies were excluded because they lacked predictive outcomes or comparisons between NST/NATs. 15 studies were excluded as there was no formal NST or NAT utilized in the study or the NST/NAT utilized included only laboratory parameters or was based solely on BMI.
Figure 1.
PRISMA flow diagram. IBD: Inflammatory bowel disease; NST: Nutrition screening tools; NAT: Nutrition assessment tools.
Study populations
Included studies were published between 2015-2018. Seven studies were conducted in Asia[9-11,13,27-29], four in Europe[8,30-32], four in North America[12,33-35], and one in Oceania[36]. In total, 1618 patients with IBD were included from all studies, 1158 (72%) had the diagnosis of CD, 454 (28%) were UC patients and 4 (0.2%) had indeterminate colitis. The age of participants ranged from 16 to 86 years (Table 1).
Table 1.
Demographics of patients with inflammatory bowel disease included in the studies
| Study ID | Total (n) (M:F) | CD:UC:ID (n) | Age (yr) | BMI (kg/m2) | Steroid n (%) | Immunomo-dulator n (%) | Biologics n (%) | Previous resection n (%) |
| Adams et al[33] | 90 (38:52) | 76:14 | Median: 35 (26-50) | Median: 22.5 | 30 (33) | 40 (44) | 15 (17) | 40 (44) |
| Bamba et al[9] | 72 (52:19) | 43:29 | UC Median: 39 (28-55) | Median: 19.5 | - | - | - | 25 (35) |
| CD Median: 29 (25-37) | ||||||||
| Csontos et al[30] | 173 (92:81) | 126:47 | Mean: 34.8 ± 12.3 | Mean: 23.6 | - | - | - | - |
| Cushing et al[34] | 89 (53:29) | 0:89 | Mean: 43 (9 – 86) | Non-sarcopenic: 26 ± 8 | - | 33 (37) | 26 (29) | - |
| Sarcopenic: 23 ± 6 | ||||||||
| Fujikawa et al[29] | 69 (45:24) | 0:69:0 | Mean: 39.8 ± 14.4 | Mean: 20.40 ± 3.65 | - | - | - | - |
| Haskey et al[35] | 110 (47:63) | 75:35 | Mean: 39 ± 15 | Mean BMI: 26.4 ± 5.8 | 5 (4.5) | 17 (15.5) | 17 (15.5) | - |
| Holt et al[36] | 44 (20:24) | 44:0 | Mean: 37.8 ± 14.2 | Mean: 23.5 | 20 (45) | 26 (59) | 10 (24) | 44 (100) |
| Jansen et al[8] | 55 (19:36) | 55:0 | Mean: 40 ± 11 | Mean: 24.9 | 10 (18) | 31 (56) | 21 (38) | - |
| O’Brien et al[31] | 77 (46:31) | 52:21:4 | Median: 42 (20-80) | Median: 24 (16-37) | 42 (55) | - | - | - |
| Pedersen et al[12] | 178 (86:92) | 127:51 | Mean: 42.71 (18-86) | - | 86 (48) | 63 (35) | 42 (24) | 178 (100) |
| Sumi et al[27] | 16 (12:4) | 16:0 | Responders median: 34 (18-68) | Responders median: 21.7 | 5 (31) | 8 (50) | - | 9 (56) |
| Non-responders median: 31 (23-46) | Non-responders Median: 16.8 | |||||||
| Takaoka et al[13] | 40 (30:10) | 40:0 | Median: 32.4 (25.3-37.8) | Median:19.2 | 12 (30) | 15 (38) | 30 (75) | 13 (33) |
| Thiberge et al[32] | 149 (68:81) | 149:0 | Mean: 41.0 ± 17.5 | Mean: 22.7 ± 6.1 | 108 | 85 | 86 | 85 |
| Zhang T et al[10] | 114 (75:39) | 114:0 | Mean: 32 ± 11.47 | Median: 13.66 | - | - | - | 114 (100) |
| Zhang T et al[11] | 204 (NR) | 105:99 | NR (min 18; max 65) | Median: 18.41 | 99 (49) | 53 (26) | 25 (12) | 14 (7) |
| Zhang W et al[28] | 138 (86:52) | 138:0 | Median: 29 (16-60) | Median: 17.9 | 13 (9) | 50 (36) | - | 37 (27) |
NR: Not reported; CD: Crohn’s disease; UC: Ulcerative colitis.
Nutrition screening or assessment tools
NSTs that were examined in the included studies were the NRS-2002, MUST, Nutritional Risk Index (NRI), Malnutrition Inflammation Risk Tool (MIRT), and the Saskatchewan Inflammatory Bowel Disease Nutrition Risk Tool (SaskIBD-NRT)[8,9,13,27,30,35]. Table 2 illustrates the basic components, categories and interpretations of included NSTs.
Table 2.
Components and interpretation of nutrition screening tools
| NST | NRS-2002[9] | MUST[9] | NRI[27] | MIRT[8] | SaskIBD-NR[35] |
| NST components | |||||
| Initial screening | BMI | Serum albumin | BMI | Symptoms (nausea/vomiting/diarrhea/poor appetite > 2 wk) | |
| BMI | Weight loss (last 3-6 mo) | Present weight/usual weight | Weight loss (last 3 mo) | Weight loss (last month) | |
| Weight loss (last 6 mo) | Acute disease effect3 | CRP | Anorexia | ||
| Dietary intake (last week) | Food restriction | ||||
| ICU patient | |||||
| Final Screening1 | |||||
| Weight loss | |||||
| Food intake | |||||
| Disease severity2 | |||||
| NST score indicating risk of malnutrition | |||||
| 0 = Low | 0 = Low | > 97.5 = No Risk | Score range = 0-8 | 0-2 = Low risk | |
| 1 = Mild | 1 = Medium | 83.5-97.5 = Moderate | 0 = Lowest | 3-4 = Medium risk | |
| 2 = Moderate | ≥ 2 = High | < 83.5 = High | 8 = Highest | ≥ 5 = High risk | |
| ≥ 3 = High | |||||
To be conducted if there is a “YES” to any one of initial screening questions;
Categorized into mild/moderate/severe based on descriptors in Nutrition Risk Screening 2002 Tool;
Patient is acutely ill AND there has been/likely to be no nutrition intake > 5 d. BMI: Body mass index; CRP: C-reactive protein; ICU: Intensive care unit. NST: Nutrition screening tools; NRS-2002: Nutrition Risk Screening 2002; MUST: Malnutrition universal screening tool; NRI: Nutritional Risk Index; MIRT: Malnutrition Inflammation Risk Tool; SaskIBD-NR: Saskatchewan Inflammatory Bowel Disease Nutrition Risk.
The NATs evaluated in the studies in this review included the SGA, comprehensive RD and gastroenterologist (GI) assessment, Skeletal Muscle Percentage (SMP), Fat Free Mass Index (FFMI), L3 Skeletal Muscle Index (L3 SMI), Appendicular Skeletal Muscle Indices, Skeletal Muscle Area (SMA), Total Psoas Muscle Area, and the mean Hounsfield unit average calculation (mHUAC) at L3[8-13,28-30,33,35,36]. Table 3 illustrates the basic components, categories and interpretations of included NATs.
Table 3.
Components and interpretation of nutrition assessment tools
| Nutrition Assessment Tools | |||
| SGA[8,9,13] | Comprehensive RD/GI Assessment[35] | BIA[28,30] | CT Scan[9-12,29,31-34,36] |
| NAT Components | |||
| Nutrient Intake | BMI | SMP | mHUAC |
| Weight loss | GI symptoms, oral intake | FFMI | L3 SMI |
| Symptoms affecting oral intake | IBD location, severity, concurrent conditions | L4 TPA | |
| Functional capacity | Surgical history, medications | ASMI | |
| Metabolic requirement | Laboratory parameters (Albumin/Vit D/Iron/Vit B12) | SMA | |
| Physical examination | SCAI, HBS | ||
| NAT interpretation | |||
| A = Well nourished | At risk | Sarcopenia: | Sarcopenia: |
| B = Mild/moderately malnourished | Not at risk | FFMI: | mHUAC: Lowest sex quartile at level of L3 vertebrae |
| C = Severely malnourished | Men: ≤ 17 kg/m2 | L3 SMI: Lowest sex quartile, variable between studies (Male: < 42-55 cm2/m2; Female: < 35.6-41 cm2/m2) | |
| Women: ≤ 15 kg/m2 | L4 TPA: Lowest sex quartile (Male < 56.7 cm2/m2, Female: < 35.6 cm2/m2) | ||
| SMP: Continuous variable | ASMI/SMA: Continuous variable | ||
BMI: Body mass index; SCAI: Simple Colitis Activity Index; HBS: Harvey Bradshaw Score; SGA: Subjective Global Assessment; SMP: Skeletal Muscle Percentage; FFMI: Fat Free Mass Index; mHUAC: Mean Hounsfield Unit Area Calculation; ASMI: Appendicular Skeletal Muscle Index; L3 SMI: L3 Vertebrae Skeletal Muscle Index; SMA: Skeletal Muscle Area; IBD: Inflammatory bowel disease; CT: Computed tomography; RD: Registered dietitian; GI: Gastroenterologist.
Quality assessment results
Given the non-randomized observational design of all studies, with the majority lacking well-defined cohorts, all studies carry a high relative inherent risk of bias. Utilizing the modified NOS scale, half of the studies (8/16) were assessed to be of acceptable quality, scoring four or more stars out of five, with the other half assessed to be of relatively poor-quality scoring three or less stars (Table S2).
What is the prevalence of abnormalities on nutrition screening and assessment?
Five studies utilized various NSTs (MUST, NRI, NRS-2002, and SaskIBD-NRT) to categorize patients ordinally into low, moderate and high nutrition risk categories[9,13,27,30,35]. The most commonly utilized NST was the MUST (4/5 studies) showing 28.0% (n = 115) to be at high nutrition risk (MUST ≥ 2) across a composite of inpatient/outpatient studies[9,13,30,35]. Of the two inpatient studies, both utilizing the NRS-2002, 67.0% (n = 75) of patients were found to be at high nutrition risk (NRS-2002 ≥ 3)[9,13]. Of the three outpatient studies, 29.1% (n = 87) of patients were found to have at least a mild/moderate degree of nutrition risk via MUST (score ≥ 1), NRI (score ≥ 97.5) and SaskIBD-NRT (score ≥ 3)[27,30,35] (Table 4).
Table 4.
Proportion of nutrition abnormalities via nutrition screening tools
| NST | Proportion of low risk patient’s n (%) | Proportion of mild-moderate risk patient’s n (%) | Proportion of high-risk patient’s n (%) | Study ID |
| MUST | 12 (16.7) | 27 (37.5) | 49 (68.1) | Bamba et al[9] |
| 118 (68.2) | 18 (10.4) | 37 (21.4) | Csontos et al[30] | |
| 93 (84.5) | 12 (10.9) | 5 (4.5) | Haskey et al[35] | |
| 10 (25.0) | 6 (15) | 24 (60) | Takaoka et al[13] | |
| NRI | 5 (31.3) | 11 (68.8) | Sumi et al[27] | |
| NRS-2002 | 0 (0) | 24 (33.3) | 48 (66.7) | Bamba et al[9] |
| 13 (32.5) | 27 (67.5) | Takaoka et al[13] | ||
| SaskIBD-NRT | 89 (80.9) | 12 (10.9) | 9 (8.2) | Haskey et al[35] |
NST: Nutrition screening tools; NRS-2002: Nutrition Risk Screening 2002; MUST: Malnutrition universal screening tool; NRI: Nutritional Risk Index; SaskIBD-NRT: Saskatchewan Inflammatory Bowel Disease Nutrition Risk Tool.
Ten studies evaluated the presence of sarcopenia in their respective populations, with a total prevalence of 39.5% (n = 477) across all studies[9-12,29-34]. Three studies utilized the SGA in categorizing patients into well-nourished (SGA-A), mild/ moderately malnourished (SGA-B) and severely malnourished (SGA-C) with two of the studies based on an inpatient IBD population. The total proportion of patients diagnosed with some degree of malnutrition based on SGA (SGA B/C) was 61.7% (n = 103)[8,9,13] (Table 5).
Table 5.
Proportion of nutrition abnormalities via nutrition assessment tools
| NAT measure | ||||
| Proportion of non-sarcopenic patients n (%) | Proportion of sarcopenic patients n (%) | Study ID | ||
| Sarcopenia | 49 (54.4) | 41 (45.6) | Adams et al[33] | |
| 42 (58.3) | 30 (41.7) | Bamba et al[9] | ||
| 125 (72.3) | 48 (27.7) | Csontos et al[30] | ||
| 25 (30.5) | 57 (69.5) | Cushing et al[34] | ||
| 51 (73.9) | 18 (26.1) | Fujikawa et al[29] | ||
| 47 (67.1) | 30 (38.9) | O’Brien et al[31] | ||
| 134 (75.3) | 44 (24.7) | Pedersen et al[12] | ||
| 99 (66.4) | 50 (33.6) | Thiberge et al[32] | ||
| 115 (56.4) | 89 (43.6) | Zhang et al[11] | ||
| 44 (35.1) | 70 (61.4) | Zhang et al[10] | ||
| Comprehensive RD/GI Assessment | Proportion of patients not at risk n (%) | Proportion of patients at risk of malnutrition n (%) | Study ID | |
| 87 (79.1) | 23 (20.9) | Haskey et al[35] | ||
| SGA | Proportion of SGA A | Proportion of SGA B | Proportion of SGA C | Study ID |
| 8 (11.1) | 37 (51.4) | 27 (37.5%) | Bamba et al[9] | |
| 8 (20.0) | 17 (42.5) | 15 (37.5%) | Takaoka et al[13] | |
| 48 (87.3) | 7 (12.7) | Jansen et al[8] | ||
NAT: Nutrition assessment tools; RD/GI: Registered dietitian/Gastroenterologist.
How did findings on nutrition screening compare to those on nutrition asses-sment?
Four studies (25%) included comparisons between an abnormal score on an NST and how that compared to a diagnosis of malnutrition using a NAT[8,9,30,35]. All four NSTs (MUST, NRS-2002, MIRT, and SaskIBD-NRT) showed significant association with NAT measures[8,9,30,35]. In both inpatients and outpatients from two separate studies[9,30], the MUST showed a significant association via logistic regression [odds ratio (OR) = 0.934, P = 0.014] and fair inter-rater agreement (Cohen’s kappa=0.53) to SMI and FFMI. One study demonstrated poor inter-rater agreement (Cohen’s kappa = 0.15) of MUST with com-prehensive RD/GI nutritional assessment among outpatients[35]. NRS-2002 was significantly associated with SMI (OR = 0.928, P = 0.008) in one inpatient study[9]. MIRT also demonstrated significance with a moderate correlation to SGA in one outpatient study (Spearman Rank Correlation = 0.394, P = 0.005)[8]. The SaskIBD-NRT showed strong inter-rater agreement (Cohen’s kappa = 0.73) with comprehensive RD/GI assessment in outpatients[35] (Table 6).
Table 6.
Nutrition screening tools correlating with nutrition assessment tools
| NST | Comparative NAT measure | Statistical Variable | Value | Study ID |
| MUST | FFMI | Cohen’s Kappa (low/normal FFMI vs low MUST) | κ = 0.53 (95%CI: 0.39-0.67) | Csontos et al[30] |
| SMI | Logistic Regression (MUST 0,1 vs ≥ 2) | OR: 0.934, P = 0.014a | Bamba et al[9] | |
| RD/GI Assessment | Cohen’s Kappa | κ = 0.15 | Haskey et al[35] | |
| MIRT | SGA | Spearman’s Rank Correlation | ρ = 0.394, P = 0.005a | Jansen et al[8] |
| NRS-2002 | SMI | Logistic Regression (NRS-2002 1, 2 vs ≥ 3) | OR: 0.928, P = 0.008a | Bamba et al[9] |
| SaskIBD-NR | RD/GI Assessment | Cohen’s Kappa | κ = 0.73 | Haskey et al[35] |
Indicates significant P value < 0.05. FFMI: Fat Free Mas Index; SMI: Skeletal Muscle Index; SGA: Subjective Global Assessment; OR: Odds ratio; NST: Nutrition screening tools; NRS-2002: Nutrition Risk Screening 2002; MUST: Malnutrition universal screening tool; SaskIBD-NR: Saskatchewan Inflammatory Bowel Disease Nutrition Risk Tool; MIRT: Malnutrition Inflammation Risk Tool; RD/GI: Registered dietitian/Gastroenterologist.
Were nutrition screening tools associated with clinical outcomes?
Three studies (18.8%) associated NSTs with clinical outcomes. The NSTs utilized in these studies included the MUST, MIRT, NRS-2002, and NRI[8,13,27], the latter three showing significance[8,13,27]. Baseline MIRT was significantly correlated via Spearman rank correlation at 6 mo with hospitalizations (ρ = 0.398, Ρ = 0.003), disease flares (ρ = 0.299, Ρ = 0.030), disease complications (ρ = 0.333, Ρ = 0.015), and need for surgery (ρ = 0.371, Ρ = 0.006)[8]. Interestingly, the study did not find a significant association between MIRT and CDAI or Harvey-Bradshaw index (HBI) scores at 6 mo (Ρ = 0.077 and 0.195 respectively)[8] (Table 7).
Table 7.
Significant nutrition screening tool correlations with clinical outcomes
| NST | Comparative outcome measure | Statistical variable | Value | Study ID |
| MIRT | Hospitalization | Spearman’s rank correlation | ρ = 0.398, P = 0.003a | Jansen et al[8] |
| Disease flare | ρ = 0.299, P = 0.030a | |||
| Disease complications1 | ρ = 0.333, P = 0.015a | |||
| Need for surgery | ρ = 0.371, P = 0.006a | |||
| NRI | Response to infliximab | Fischer’s exact test | P = 0.037a | Sumi et al[27] |
| NRS-2002 | Length of stay (< 28 vs ≥ 28 d) | Chi-square test | P = 0.032a | Takaoka et al[13] |
Indicates significant P value < 0.05);
Newly occurred stenosis, fistula or abscess. NST: Nutrition screening tools; NRS-2002: Nutrition risk screening 2002; NRI: Nutritional risk index; MIRT: Malnutrition inflammation risk tool.
NRS-2002 (scores ≥ 3 vs ≤ 2) significantly predicted hospital length of stay (Ρ = 0.032), however did not significantly predict the need for surgery (Ρ = 0.109)[13]. A high NRI score (> 97.5) significantly predicted response to infliximab among CD patients (Ρ = 0.037)[27]. MUST was examined in only one study and showed a trend towards significance in predicting length of stay (Ρ = 0.058) and had no significance in predicting need for intestinal resection (Ρ = 0.314)[13] (Table S3).
Were nutrition assessment tools associated with clinical outcomes?
Thirteen studies (81.3%) examined NATs for the prediction of clinical out-comes[8-13,28,29,31-34,36]. The majority of studies (11/13) that evaluated NATs utilized measures of sarcopenia via computed tomography of the L3/4 vertebrae or BIA[8-13,28,29,31-34,36]. Five studies evaluated the correlation of sarcopenia with the need for intestinal resection[9,11,31,33,34], with only two demonstrating a significant correlation with need for intestinal resection (P = 0.003 on operation free survival curves)[9,11] (Table 8).
Table 8.
Significant nutrition assessment tool correlations with clinical outcomes
| NAT | Comparative outcome measure | Statistical analysis | Result | Study ID |
| SGA | Length of stay in hospital | Chi-square test | P = 0.008 | Takaoka et al[13] |
| Sarcopenia | Change in IBD disease activity at 6 mo (HBI) | Paired t-test (baseline vs 6 mo) | Sarcopenic: 0.4 (P = 0.80) | Adams et al[33] |
| Non-sarcopenic: -2.3 (P = 0.004) | ||||
| Need for operation (operation free survival curve) | Kaplan-Meier Analysis | P = 0.003 | Bamba et al[9] | |
| P = 0.003 | Zhang et al[11] | |||
| Need for operation | Cox-regression (multivariate) | HR 0.318 (0.126-0.802), P = 0.015 | Bamba et al[9] | |
| Need for any rescue therapy (medical/surgical) | Fischers exact test | P = 0.02 | Cushing et al[34] | |
| Multivariate logistic regression | OR 3.98 (95%CI 1.12-14.1), P = 0.033 | |||
| Post-operative complications (Major)1 | OR 9.24 (95%CI 1.10-77.50). P = 0.04 | Zhang et al[10] | ||
| UC disease activity (Mayo Score ≥ 6) | OR 8.49 (95%CI 1.80-40.10), P = 0.007 | Zhang et al[11] | ||
| Post-operative surgical site infection | OR 4.91 (95%CI 1.09-23.50), P = 0.03 | Fujikawa et al[29] | ||
| Need for red blood cell transfusion | OR 1.31, P = 0.014 | Pedersen et al[12] | ||
| ICU admission | OR 1.32, P = 0.016 | |||
| Post-operative sepsis | OR 1.325, P = 0.009 | |||
| Deep vein thrombosis | OR 1.265, P = 0.0173 | |||
| Clavien-Dindo grade 4 complication | OR 1.329, P = 0.0052 | |||
| ASMI | Fecal calprotectin | Spearman’s Rank Correlation | ρ = -0.564, P = 0.005 | Holt et al[36] |
| L3 SMI | UC disease activity (Mayo Score) | ρ = -0.523, P ≤ 0.01 | Zhang et al[11] | |
| SMA | ρ = -0.445, P ≤ 0.01 | |||
| SMP | Post-operative complications (Overall)2 | Multivariate logistic regression analysis | OR: 0.487 (95%CI 0.307-0.772) P = 0.002a | Zhang et al[28] |
| Post-op complications (Major)1 | OR: 0.588 (95%CI 0.422-0.820) P = 0.002a |
Indicates significant P value < 0.05);
Clavien-Dindo Score ≥ 3;
Clavien-Dindo Score 1-5. HR: Hazard ratio; OR: Odds ratio; CI: Confidence interval; HBI: Harvey-bradshaw index; mHUAC: Mean hounsfield unit area calculation; ASMI: Appendicular skeletal muscle index; SMI: Skeletal muscle index; SMA: Skeletal muscle area; SMP: Skeletal muscle percentage; SGA: Subjective global assessment.
The presence of sarcopenia (via L3 SMI or mHUAC) was significantly associated with major post-operative complications with Clavien-Dindo grade (CDG) ≥ 3 in one study (OR = 9.24, P = 0.04) and life-threatening complications (CDG = 4) in another[10,12]. SMP was protective against major (OR 0.588, P = 0.002) and overall (OR = 0.487, P = 0.002) post-operative complications in one study[28] but not another[31]. Additionally the need for post-operative blood transfusions (OR = 1.31, P = 0.014), ICU admissions (OR = 1.32, P = 0.016), post-operative sepsis (OR = 1.325, P = 0.009), post-operative surgical site infections (OR = 4.91, P = 0.03) and deep vein thrombosis (OR = 1.265, P = 0.017) was found to be significantly associated with sarcopenia[12,29]. The need for either surgical or medical rescue therapy (P = 0.02) in patients with acute severe UC was significantly associated with the presence of sarcopenia[34] (Table S4).
One study demonstrated that various measures of sarcopenia (SMI, SMA) correlated significantly with Mayo disease activity scores[11]. A separate study showed that SMA did not significantly predict endoscopic recurrence (P = 0.096)[36]. Two studies associated SGA with clinical outcomes based on SGA score[8,13] with discordant results. One inpatient study found that SGA did not predict the need for surgery (P = 0.071)[13] but it did predict length of stay (P = 0.008)[13]. A second outpatient study did not find any correlation between SGA and hospitalizations, disease flares, disease complications, or need for surgery[8].
DISCUSSION
This review of the literature is the first to systematically evaluate the use of NSTs and NATs in IBD-their performance in relation to each other and to clinical outcomes. Our review highlights both the adverse clinical implications of malnutrition in IBD as well as the paucity of NST and NAT data available in this population in comparison to other chronic disease populations[37-40]. Although the reviewed studies were not amenable to meta-analysis due to heterogeneity and observational non-randomized, non-controlled study designs, multiple conclusions can still be drawn to summarize the current state and guide future work in the area.
First, our review reinforces the high prevalence of malnutrition in patients with IBD. One in four outpatients and approximately two in three inpatients were found to be at nutritional risk. These results are consistent with prior studies confirming the substantial prevalence of malnutrition in IBD[1,3,22]. Secondly, we evaluated how the findings on Nutrition Screening compared to the findings on Nutrition Assessment. This demonstration of an association between NSTs and NATs is required, to demonstrate face and content validity of the NST for use in screening[41].
There is a limited amount of data available to compare NSTs to NATs. Four NSTs (the MUST, NRS-2002, MIRT and SaskIBD-NRT) showed promise. The MUST includes BMI, unplanned weight loss in the past 3-6 mo and an acute disease effect score. The MIRT measures similar criteria, including BMI, unintentional weight loss and CRP. Therefore, these two tools vary only in the method that acute disease is assessed. The NRS-2002 differs from the two previous tools, as it captures reduced dietary intake in addition to BMI, weight loss and ICU admission status, and has been validated only in the inpatient population. The SaskIBD-NRT is a novel tool based on patient history evaluating gastrointestinal symptoms and food restriction behaviors commonly seen in the IBD population in addition to the more common screening questions of weight loss and poor oral intake[35]. The SaskIBD-NRT does not capture disease severity and is reliant only on nutrition specific data points to assess risk.
The MIRT and SaskIBD-NRT although not yet compared to SMI in IBD, have shown significant associations with more comprehensive nutritional assessment methods. The MIRT for example demonstrating an association with an abnormal SGA[8]. Similarly, the SaskIBD-NRT showed strong agreement to subsequent comprehensive assessment by RD/GI[35]. This association has not been consistent with one study noting poor inter-rater agreement between MUST and a comprehensive RD/GI assessment[35]. Recognizing sarcopenia as an integral, objective component of malnutrition, both the MUST and NRS-2002 demonstrated a significant association with sarcopenia as measured by the SMI[9]. To summarize, although limited, the data on NSTs is encouraging for a strong association with a diagnosis of malnutrition by NATs (both sarcopenia and more comprehensive NATs).
Thirdly, we evaluated whether NSTs were associated with clinical outcomes. Although traditionally used to determine which patients require further nutritional assessment and therapy, the summary of findings from the current review would suggest that NSTs also hold promise in the prediction of clinical outcomes. Notably, all studies were performed in patients with CD and therefore the results are at this time only generalizable to this population. The outcomes associated with the three NSTs (NRS-2002, NRI and MIRT) were all of clinical were of clinical relevance. For inpatients, the NRS-2002 predicted hospital length of stay[13]. For outpatients, the MIRT correlated well with hospitalizations, disease flares and need for surgery[8,27]. All three NSTs included a component to reflect disease severity. Although this parameter itself can correlate with adverse clinical outcomes, its inclusion in IBD nutrition screening and assessment is appropriate, as disease severity may exacerbate poor oral intake, malabsorption and catabolism. The SaskIBD-NRT (did not include measure of disease severity) has not yet been studied with reference to clinical outcomes.
Notably, the MUST was not associated with clinical outcomes among inpatients. This is perhaps not surprising as the European Society for Clinical Nutrition and Metabolism has recommended against the use of MUST in inpatients, citing concern regarding confounders from the lack of grading the severity of the acute illness[14]. In other studies, the MUST has been associated with CD severity as measured by the HBI (P = 0.005) on cross-sectional analysis[42]. Further studies utilizing this tool are required to evaluate its use in outpatient IBD populations.
From the available NST data therefore, the NRS-2002 in inpatients, and the MIRT and MUST in outpatients, are promising candidates for further evaluation. This is consistent with previous reviews suggesting NSTs such as the NRS-2002 which use combined simple measures of malnutrition are most appropriate to assess malnutrition in IBD[24]. Further evaluation is needed as it remains unclear whether the associations noted in CD patients will be generalizable to the UC population and, furthermore, if these findings will apply across inpatient and outpatient populations. It is also important to recognize that there are other NSTs that have not yet been explored in the IBD setting, including the patient-generated SGA, and Canadian Nutrition Risk Screening Tool. These screening tools have performed well in other chronic disease populations[43,44]. Further research into the use of patient-led versions of malnutrition screens would also be of interest. Although the studies evaluating patient-led NSTs did not meet eligibility criteria for this review, the patient-led MUST has correlated with a practitioner-led MUST in IBD[45,46] and is in keeping with the utility of these screens in other chronic disease populations[47,48]. As a direct translation to clinical practice, the signal that NSTs predict clinical outcomes supports their importance. In future studies it will be of interest to evaluate the impact of nutrition therapies on NST results and on clinical outcomes.
Lastly, we explored the association between NATs and clinical outcome measures. Notably, most studies correlating NATs and clinical outcomes used measures of sarcopenia as the primary assessment method, in particular the L3 SMI[9,11,28,29]. By adding an additional 6 studies (Zhang 2015, Holt 2017, Cushing 2018, Fujikawa 2017, O’Brien 2018, and Thiberge 2018)[28,29,31,32,34,36] the current review extends the recent sarcopenia focused systematic review carried out by Ryan et al[22]. Ryan’s group reported a sarcopenia prevalence rate over 40%, similar to the 39.5% seen in our current study. They also concluded that sarcopenia was a significant independent predictor for the need for surgery and it correlated with an increased rate of major post-operative complications, as was seen our study[22].
It must be noted that although measures of sarcopenia are among some of the most objective assessment tools for malnutrition, given the inherent cost, risk of radiation and contrast exposure with computed tomography, research into more practical alternatives such as bed-side ultrasound, is required[49,50]. Moreover, the underlying pathogenesis of sarcopenia remains multifactorial, and may include additional physiological factors independent of malnutrition[51,52]. In the IBD population, active inflammation may be reflective of disease severity and contribute to malnutrition through anorexia, hypermetabolism and malabsorption. Additionally, anorexia, malabsorption and active inflammation underpin some pathophysiological mechanisms of sarcopenia[53]. Nutrition risk screening and assessment is made even more complex with the increasing prevalence of overweight patients with IBD. Over-nourishment and obesity affects up to 55% of patients with IBD in the Western hemisphere[23,54]. In spite of this, decreased muscle mass and micronutrient deficiencies remain prevalent even among the obese population with IBD (i.e., sarcopenic obesity), and are not accurately assessed by traditional nutrition assessment methods[33,55-57]. Although there is conflicting data on the association of obesity itself with IBD related clinical outcomes[58-61], the syndrome of “sarcopenic-obesity” likely does have implications in predicting relevant clinical outcomes, and warrants further in-vestigation[33].
Additionally, our review of NATs highlights the discordant data regarding the ability of the SGA, a familiar nutritional assessment tool, to predict clinical outcomes in IBD. Notably, a large percentage of IBD patients with decreased body cell mass as determined by BIA and sarcopenia can be missed by SGA alone[50]. In this review, SGA was not significantly associated with clinical outcomes in IBD populations other than length of hospital stay[8,13].
In conclusion, our study has summarized the currently available evidence for NSTs/NATs in the IBD population. Although some studies support the association of NSTs/NATs with specific clinical outcomes, the heterogeneity in study design, lack of data from large cohorts, and lack of comprehensive validation of existing NSTs, does not translate into the recommendation of a single optimal NST or NAT at this time. The high prevalence of malnutrition seen across these recent studies reaffirms the ongoing significance of malnutrition in the IBD population and the need to utilize appropriate NST/NATs. Consistent with guideline recommendations, nutrition screening should be conducted on every patient with IBD both at diagnosis and at least annually, with more frequent measures as needed[18]. Referral should be made to a RD to patients at moderate or high risk of malnutrition for more definitive assessment. The strengths and limitations of the tools have been highlighted in this review. Going forward, clinically relevant research areas include larger scale studies evaluating the assessment of alternate measures of sarcopenia, the development and validation of novel NSTs/NATs, such as the MIRT/SaskIBD-NRT and an assessment of the responsiveness of the tools to measure change with a nutrition intervention. Based on the promising data from these tools, the optimal NST/NAT for the IBD population is likely to be one that takes into account the unique dietary habits and chronic inflammatory nature of this population. It is encouraging to note that the majority of articles included within this review have been published within the last 2 years. We anticipate that continued activity and interest will lead to the development and validation of tools in concert with clinical care pathways, embedding the important processes of nutrition screening and assessment within routine IBD clinic visits.
ARTICLE HIGHLIGHTS
Research background
Malnutrition is highly prevalent in patients with inflammatory bowel disease (IBD), however the optimal nutrition screening tools (NST) and nutrition assessment tools (NAT) to detect and diagnosis malnutrition respectively are unclear.
Research motivation
Given the negative clinical and economic impacts of malnutrition in IBD, identification of a simple, accurate and efficient process for identifying malnutrition may allow for increased recognition and earlier nutritional intervention.
Research objectives
To systematically review the prevalence of malnutrition in patients with IBD, whether available NSTs correlate with NATs, and whether NSTs and NATs are predictive of clinical outcomes.
Research methods
PubMed and MEDLINE databases were systematically searched utilizing a comprehensive search strategy. Articles were reviewed and extracted by two independent reviewers against inclusion/exclusion criteria. Included articles underwent quality assessment review utilizing the modified Newcastle Ottawa Scale as well as data extraction, synthesis and review by the authors and a biostatistician.
Research results
A total of 1791 studies were identified from the initial search, 16 of which met all inclusion criteria and were included for qualitative synthesis. Prevalence of patients at high risk of malnutrition amongst inpatient and outpatient IBD patients as assessed by NSTs ranged from 28%-67%. Sarcopenia was identified in 39.5% of IBD patients. The malnutrition universal screening tool (MUST), Nutrition Risk Screening 2002 (NRS-2002), Malnutrition Inflammation Risk Tool (MIRT) and Saskatchewan Inflammatory Bowel Disease Nutrition Risk Tool (SaskIBD-NRT) all showed significant associations with various NAT measures. Of NSTs, the MIRT, NRS-2002 and NRI demonstrated significance in predicting clinical outcomes of relevant clinical outcomes. Presence of sarcopenia was significantly associated with various clinical and post-operative outcomes. The Subjective Global Assessment was not consistent in its association with clinical outcomes.
Research conclusions
Malnutrition and sarcopenia remain highly prevalent in the IBD population as assessed by currently available NSTs and NATs. No single optimal NST or NAT can be recommended based on our review at this time. Based on current evidence, previously available NSTs including the NRS-2002 and MUST, as well as novel IBD-specific NSTs (MIRT, SaskIBD-NRT) are the most useful to screen for malnutrition in this population. Sarcopenia evaluation (via cross-sectional imaging) has promise as a robust nutrition assessment method given its significant associations with clinical outcomes. However, more accurate, practical and cost-effective methods of evaluating sarcopenia in the IBD population outside of conventional methods of body composition analysis should be explored.
Research perspectives
The utility as well as strengths and weaknesses of available NSTs and NATs have been reviewed. Future research is needed to test and validate available tools in the IBD population. The development of novel tools will aid clinicians in identifying, diagnosing and intervening on malnourishment in the IBD patient population.
ACKNOWLEDGEMENTS
The authors are grateful to the Digestive Health Strategic Clinical Network and the Nutrition Center of Excellence in Alberta and for supporting the knowledge translation of this work.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement: All authors have no conflict of interest related to the manuscript.
PRISMA 2009 Checklist statement: Authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Peer-review started: March 18, 2019
First decision: May 16, 2019
Article in press: July 3, 2019
P-Reviewer: Lv XP, Rowland M, Tang ZP S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
Contributor Information
Suqing Li, Division of Internal Medicine, Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta T6G 2G3, Canada.
Michael Ney, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, Alberta T2N 2T9, Canada.
Tannaz Eslamparast, Cirrhosis Care Clinic, Division of Gastroenterology, Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta T6G 2X8, Canada.
Ben Vandermeer, Alberta Research Centre for Health Evidence, Biostatistician, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta T6G 1C9, Canada.
Kathleen P Ismond, Cirrhosis Care Clinic, Division of Gastroenterology, Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta T6G 2X8, Canada.
Karen Kroeker, Cirrhosis Care Clinic, Division of Gastroenterology, Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta T6G 2X8, Canada.
Brendan Halloran, Cirrhosis Care Clinic, Division of Gastroenterology, Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta T6G 2X8, Canada.
Maitreyi Raman, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, Alberta T2N 2T9, Canada. mkothand@ucalgary.ca.
Puneeta Tandon, Cirrhosis Care Clinic, Division of Gastroenterology, Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta T6G 2X8, Canada.
References
- 1.Mijac DD, Janković GL, Jorga J, Krstić MN. Nutritional status in patients with active inflammatory bowel disease: Prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010;21:315–319. doi: 10.1016/j.ejim.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Vadan R, Gheorghe LS, Constantinescu A, Gheorghe C. The prevalence of malnutrition and the evolution of nutritional status in patients with moderate to severe forms of Crohn's disease treated with Infliximab. Clin Nutr. 2011;30:86–91. doi: 10.1016/j.clnu.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin J, Makharia GK, Kalaivani M, Joshi YK. Nutritional status of patients with Crohn's disease. Indian J Gastroenterol. 2008;27:195–200. [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White JV, Guenter P, Jensen G, Malone A, Schofield M Academy of Nutrition and Dietetics Malnutrition Work Group; A. S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) J Acad Nutr Diet. 2012;112:730–738. doi: 10.1016/j.jand.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, Baptista G, Barazzoni R, Blaauw R, Coats A, Crivelli A, Evans DC, Gramlich L, Fuchs-Tarlovsky V, Keller H, Llido L, Malone A, Mogensen KM, Morley JE, Muscaritoli M, Nyulasi I, Pirlich M, Pisprasert V, de van der Schueren MAE, Siltharm S, Singer P, Tappenden K, Velasco N, Waitzberg D, Yamwong P, Yu J, Van Gossum A, Compher C GLIM Core Leadership Committee; GLIM Working Group. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1–9. doi: 10.1016/j.clnu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, Muscaritoli M, Nyulasi I, Ockenga J, Schneider SM, de van der Schueren MA, Singer P. Diagnostic criteria for malnutrition - An ESPEN Consensus Statement. Clin Nutr. 2015;34:335–340. doi: 10.1016/j.clnu.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Jansen I, Prager M, Valentini L, Büning C. Inflammation-driven malnutrition: A new screening tool predicts outcome in Crohn's disease. Br J Nutr. 2016;116:1061–1067. doi: 10.1017/S0007114516003044. [DOI] [PubMed] [Google Scholar]
- 9.Bamba S, Sasaki M, Takaoka A, Takahashi K, Imaeda H, Nishida A, Inatomi O, Sugimoto M, Andoh A. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn's disease. PLoS One. 2017;12:e0180036. doi: 10.1371/journal.pone.0180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang T, Cao L, Cao T, Yang J, Gong J, Zhu W, Li N, Li J. Prevalence of Sarcopenia and Its Impact on Postoperative Outcome in Patients With Crohn's Disease Undergoing Bowel Resection. JPEN J Parenter Enteral Nutr. 2017;41:592–600. doi: 10.1177/0148607115612054. [DOI] [PubMed] [Google Scholar]
- 11.Zhang T, Ding C, Xie T, Yang J, Dai X, Lv T, Li Y, Gu L, Wei Y, Gong J, Zhu W, Li N, Li J. Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clin Nutr. 2017;36:1586–1592. doi: 10.1016/j.clnu.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen M, Cromwell J, Nau P. Sarcopenia is a Predictor of Surgical Morbidity in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23:1867–1872. doi: 10.1097/MIB.0000000000001166. [DOI] [PubMed] [Google Scholar]
- 13.Takaoka A, Sasaki M, Nakanishi N, Kurihara M, Ohi A, Bamba S, Andoh A. Nutritional Screening and Clinical Outcome in Hospitalized Patients with Crohn's Disease. Ann Nutr Metab. 2017;71:266–272. doi: 10.1159/000485637. [DOI] [PubMed] [Google Scholar]
- 14.Kondrup J, Allison SP, Elia M, Vellas B, Plauth M Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN) ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415–421. doi: 10.1016/s0261-5614(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 15.Stoner PL, Kamel A, Ayoub F, Tan S, Iqbal A, Glover SC, Zimmermann EM. Perioperative Care of Patients with Inflammatory Bowel Disease: Focus on Nutritional Support. Gastroenterol Res Pract. 2018;2018:7890161. doi: 10.1155/2018/7890161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan GT, Ha I, Hogan C, Nguyen E, Jamal MM, Bechtold ML, Nguyen DL. Does preoperative enteral or parenteral nutrition reduce postoperative complications in Crohn's disease patients: A meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:997–1002. doi: 10.1097/MEG.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 17.Wędrychowicz A, Zając A, Tomasik P. Advances in nutritional therapy in inflammatory bowel diseases: Review. World J Gastroenterol. 2016;22:1045–1066. doi: 10.3748/wjg.v22.i3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes A, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, Shamir R, Stardelova K, Wierdsma N, Wiskin AE, Bischoff SC. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36:321–347. doi: 10.1016/j.clnu.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Landi F, Camprubi-Robles M, Bear DE, Cederholm T, Malafarina V, Welch AA, Cruz-Jentoft AJ. Muscle loss: The new malnutrition challenge in clinical practice. Clin Nutr. 2018:30553578. doi: 10.1016/j.clnu.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Vandewoude MF, Alish CJ, Sauer AC, Hegazi RA. Malnutrition-sarcopenia syndrome: Is this the future of nutrition screening and assessment for older adults? J Aging Res. 2012;2012:651570. doi: 10.1155/2012/651570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller C, Compher C, Ellen DM American Society for Parenteral and Enteral Nutrition (A. S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: Nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35:16–24. doi: 10.1177/0148607110389335. [DOI] [PubMed] [Google Scholar]
- 22.Ryan E, McNicholas D, Creavin B, Kelly ME, Walsh T, Beddy D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm Bowel Dis. 2019;25:67–73. doi: 10.1093/ibd/izy212. [DOI] [PubMed] [Google Scholar]
- 23.Bryant RV, Trott MJ, Bartholomeusz FD, Andrews JM. Systematic review: Body composition in adults with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:213–225. doi: 10.1111/apt.12372. [DOI] [PubMed] [Google Scholar]
- 24.Valentini L, Schulzke JD. Mundane, yet challenging: The assessment of malnutrition in inflammatory bowel disease. Eur J Intern Med. 2011;22:13–15. doi: 10.1016/j.ejim.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. Available from: https://www.researchgate.net/publication/261773681_The_Newcastle-Ottawa_Scale_NOS_for_Assessing_the_Quality_of_Non-Randomized_Studies_in_Meta-Analysis. [Google Scholar]
- 26.Higgins J, Green S (editors) 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available from: http://handbook-5-1.cochrane.org/ [Google Scholar]
- 27.Sumi R, Nakajima K, Iijima H, Wasa M, Shinzaki S, Nezu R, Inoue Y, Ito T. Influence of nutritional status on the therapeutic effect of infliximab in patients with Crohn's disease. Surg Today. 2016;46:922–929. doi: 10.1007/s00595-015-1257-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Zhu W, Ren J, Zuo L, Wu X, Li J. Skeletal muscle percentage: A protective factor for postoperative morbidity in Crohn's disease patients with severe malnutrition. J Gastrointest Surg. 2015;19:715–721. doi: 10.1007/s11605-015-2763-x. [DOI] [PubMed] [Google Scholar]
- 29.Fujikawa H, Araki T, Okita Y, Kondo S, Kawamura M, Hiro J, Toiyama Y, Kobayashi M, Tanaka K, Inoue Y, Mohri Y, Uchida K, Kusunoki M. Impact of sarcopenia on surgical site infection after restorative proctocolectomy for ulcerative colitis. Surg Today. 2017;47:92–98. doi: 10.1007/s00595-016-1357-x. [DOI] [PubMed] [Google Scholar]
- 30.Csontos ÁA, Molnár A, Piri Z, Pálfi E, Miheller P. Malnutrition risk questionnaire combined with body composition measurement in malnutrition screening in inflammatory bowel disease. Rev Esp Enferm Dig. 2017;109:26–32. doi: 10.17235/reed.2016.4557/2016. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien S, Kavanagh RG, Carey BW, Maher MM, O'Connor OJ, Andrews EJ. The impact of sarcopenia and myosteatosis on postoperative outcomes in patients with inflammatory bowel disease. Eur Radiol Exp. 2018;2:37. doi: 10.1186/s41747-018-0072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiberge C, Charpentier C, Gillibert A, Modzelewski R, Dacher JN, Savoye G, Savoye-Collet C. Lower Subcutaneous or Visceral Adiposity Assessed by Abdominal Computed Tomography Could Predict Adverse Outcome in Patients With Crohn's Disease. J Crohns Colitis. 2018;12:1429–1437. doi: 10.1093/ecco-jcc/jjy124. [DOI] [PubMed] [Google Scholar]
- 33.Adams DW, Gurwara S, Silver HJ, Horst SN, Beaulieu DB, Schwartz DA, Seidner DL. Sarcopenia Is Common in Overweight Patients with Inflammatory Bowel Disease and May Predict Need for Surgery. Inflamm Bowel Dis. 2017;23:1182–1186. doi: 10.1097/MIB.0000000000001128. [DOI] [PubMed] [Google Scholar]
- 34.Cushing KC, Kordbacheh H, Gee MS, Kambadakone A, Ananthakrishnan AN. Sarcopenia is a Novel Predictor of the Need for Rescue Therapy in Hospitalized Ulcerative Colitis Patients. J Crohns Colitis. 2018 doi: 10.1093/ecco-jcc/jjy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haskey N, Peña-Sánchez JN, Jones JL, Fowler SA. Development of a screening tool to detect nutrition risk in patients with inflammatory bowel disease. Asia Pac J Clin Nutr. 2018;27:756–762. doi: 10.6133/apjcn.112017.01. [DOI] [PubMed] [Google Scholar]
- 36.Holt DQ, Moore GT, Strauss BJ, Hamilton AL, De Cruz P, Kamm MA. Visceral adiposity predicts post-operative Crohn's disease recurrence. Aliment Pharmacol Ther. 2017;45:1255–1264. doi: 10.1111/apt.14018. [DOI] [PubMed] [Google Scholar]
- 37.Stenvinkel P, Barany P, Chung SH, Lindholm B, Heimbürger O. A comparative analysis of nutritional parameters as predictors of outcome in male and female ESRD patients. Nephrol Dial Transplant. 2002;17:1266–1274. doi: 10.1093/ndt/17.7.1266. [DOI] [PubMed] [Google Scholar]
- 38.Lin H, Zhang H, Lin Z, Li X, Kong X, Sun G. Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail Rev. 2016;21:549–565. doi: 10.1007/s10741-016-9540-0. [DOI] [PubMed] [Google Scholar]
- 39.Lew CCH, Yandell R, Fraser RJL, Chua AP, Chong MFF, Miller M. Association Between Malnutrition and Clinical Outcomes in the Intensive Care Unit: A Systematic Review [Formula: See text] JPEN J Parenter Enteral Nutr. 2017;41:744–758. doi: 10.1177/0148607115625638. [DOI] [PubMed] [Google Scholar]
- 40.Choi WJ, Kim J. Nutritional Care of Gastric Cancer Patients with Clinical Outcomes and Complications: A Review. Clin Nutr Res. 2016;5:65–78. doi: 10.7762/cnr.2016.5.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Correia MITD. Nutrition Screening vs Nutrition Assessment: What's the Difference? Nutr Clin Pract. 2018;33:62–72. doi: 10.1177/0884533617719669. [DOI] [PubMed] [Google Scholar]
- 42.Rahman A, Williams P, Sandhu A, Mosli M. Malnutrition Universal Screening Tool (MUST) predicts disease activity in patients with Crohn’s disease. Can J Nutr. 2016;1:1–5. [Google Scholar]
- 43.Faramarzi E, Mahdavi R, Mohammad-Zadeh M, Nasirimotlagh B. Validation of nutritional risk index method against patient-generated subjective global assessment in screening malnutrition in colorectal cancer patients. Chin J Cancer Res. 2013;25:544–548. doi: 10.3978/j.issn.1000-9604.2013.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy M, Hunter P, Perry JA, Cross KM. Development of a Universal Nutritional Screening Platform for Plastic Surgery Patients. Plast Reconstr Surg Glob Open. 2017;5:e1342. doi: 10.1097/GOX.0000000000001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keetarut K, Zacharopoulou-Otapasidou S, Bloom S, Majumdar A, Patel PS. An evaluation of the feasibility and validity of a patient-administered malnutrition universal screening tool ('MUST') compared to healthcare professional screening in an inflammatory bowel disease (IBD) outpatient clinic. J Hum Nutr Diet. 2017;30:737–745. doi: 10.1111/jhn.12481. [DOI] [PubMed] [Google Scholar]
- 46.Sandhu A, Mosli M, Yan B, Wu T, Gregor J, Chande N, Ponich T, Beaton M, Rahman A. Self-Screening for Malnutrition Risk in Outpatient Inflammatory Bowel Disease Patients Using the Malnutrition Universal Screening Tool (MUST) JPEN J Parenter Enteral Nutr. 2016;40:507–510. doi: 10.1177/0148607114566656. [DOI] [PubMed] [Google Scholar]
- 47.Gabrielson DK, Scaffidi D, Leung E, Stoyanoff L, Robinson J, Nisenbaum R, Brezden-Masley C, Darling PB. Use of an abridged scored Patient-Generated Subjective Global Assessment (abPG-SGA) as a nutritional screening tool for cancer patients in an outpatient setting. Nutr Cancer. 2013;65:234–239. doi: 10.1080/01635581.2013.755554. [DOI] [PubMed] [Google Scholar]
- 48.Vigano AL, di Tomasso J, Kilgour RD, Trutschnigg B, Lucar E, Morais JA, Borod M. The abridged patient-generated subjective global assessment is a useful tool for early detection and characterization of cancer cachexia. J Acad Nutr Diet. 2014;114:1088–1098. doi: 10.1016/j.jand.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 49.Tandon P, Low G, Mourtzakis M, Zenith L, Myers RP, Abraldes JG, Shaheen AA, Qamar H, Mansoor N, Carbonneau M, Ismond K, Mann S, Alaboudy A, Ma M. A Model to Identify Sarcopenia in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1473–1480.e3. doi: 10.1016/j.cgh.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 50.Valentini L, Schaper L, Buning C, Hengstermann S, Koernicke T, Tillinger W, Guglielmi FW, Norman K, Buhner S, Ockenga J, Pirlich M, Lochs H. Malnutrition and impaired muscle strength in patients with Crohn's disease and ulcerative colitis in remission. Nutrition. 2008;24:694–702. doi: 10.1016/j.nut.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 51.Liguori I, Russo G, Aran L, Bulli G, Curcio F, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Sarcopenia: Assessment of disease burden and strategies to improve outcomes. Clin Interv Aging. 2018;13:913–927. doi: 10.2147/CIA.S149232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu X, Zhang L, Wang H, Hao Q, Dong B, Yang M. Malnutrition-sarcopenia syndrome predicts mortality in hospitalized older patients. Sci Rep. 2017;7:3171. doi: 10.1038/s41598-017-03388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scaldaferri F, Pizzoferrato M, Lopetuso LR, Musca T, Ingravalle F, Sicignano LL, Mentella M, Miggiano G, Mele MC, Gaetani E, Graziani C, Petito V, Cammarota G, Marzetti E, Martone A, Landi F, Gasbarrini A. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol Res Pract. 2017;2017:8646495. doi: 10.1155/2017/8646495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nic Suibhne T, Raftery TC, McMahon O, Walsh C, O'Morain C, O'Sullivan M. High prevalence of overweight and obesity in adults with Crohn's disease: Associations with disease and lifestyle factors. J Crohns Colitis. 2013;7:e241–e248. doi: 10.1016/j.crohns.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 55.Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: A new form of malnutrition? Part A: vitamins. Obes Surg. 2008;18:870–876. doi: 10.1007/s11695-007-9349-y. [DOI] [PubMed] [Google Scholar]
- 56.Bryant RV, Schultz CG, Ooi S, Goess C, Costello SP, Vincent AD, Schoeman SN, Lim A, Bartholomeusz FD, Travis SPL, Andrews JM. Obesity in Inflammatory Bowel Disease: Gains in Adiposity despite High Prevalence of Myopenia and Osteopenia. Nutrients. 2018;10:pii: E1192. doi: 10.3390/nu10091192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrade MI, Maio R, Dourado KF, Macêdo PF, Barreto Neto AC. Excessive weight--muscle depletion paradox and cardiovascular risk factors in outpatients with inflammatory bowel disease. Arq Gastroenterol. 2015;52:37–45. doi: 10.1590/S0004-28032015000100009. [DOI] [PubMed] [Google Scholar]
- 58.Seminerio JL, Koutroubakis IE, Ramos-Rivers C, Hashash JG, Dudekula A, Regueiro M, Baidoo L, Barrie A, Swoger J, Schwartz M, Weyant K, Dunn MA, Binion DG. Impact of Obesity on the Management and Clinical Course of Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:2857–2863. doi: 10.1097/MIB.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 59.Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2118–2124. doi: 10.1097/MIB.0b013e31829cf401. [DOI] [PubMed] [Google Scholar]
- 60.Flores A, Burstein E, Cipher DJ, Feagins LA. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig Dis Sci. 2015;60:2436–2445. doi: 10.1007/s10620-015-3629-5. [DOI] [PubMed] [Google Scholar]
- 61.Blain A, Cattan S, Beaugerie L, Carbonnel F, Gendre JP, Cosnes J. Crohn's disease clinical course and severity in obese patients. Clin Nutr. 2002;21:51–57. doi: 10.1054/clnu.2001.0503. [DOI] [PubMed] [Google Scholar]