Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent cause of chronic liver disease worldwide. NAFLD is considerably more frequent in patients with type 2 diabetes mellitus (T2DM) than in the general population and is also more severe histologically in this group. Sodium-glucose co-transporter-2 (SGLT2) inhibitors, the newest class of antidiabetic agents, appear to represent a promising option for the management of NAFLD in patients with T2DM. In a number of studies, treatment with SGLT2 inhibitors resulted in a reduction in hepatic steatosis and in transaminase levels. However, existing studies are small, their follow-up period was short and none evaluated the effects of SGLT2 inhibitors on liver histology. Accordingly, larger studies are needed to verify these preliminary results and define the role of SGLT2 inhibitors in the treatment of NAFLD in patients with T2DM.
Keywords: Nonalcoholic fatty liver disease, Type 2 diabetes mellitus, Sodium-glucose co-transporter-2 inhibitors, Steatosis, Fibrosis, Transaminases
Core tip: Nonalcoholic fatty liver disease (NAFLD) is more frequent and more severe in patients with type 2 diabetes mellitus (T2DM) than in the general population. Sodium-glucose co-transporter-2 (SGLT2) inhibitors appear to represent a promising option for the management of NAFLD in patients with T2DM. However, existing studies are small, their follow-up period was short and none evaluated the effects of SGLT2 inhibitors on liver histology. Accordingly, larger studies are needed to verify these preliminary results and define the role of SGLT2 inhibitors in the treatment of NAFLD in patients with T2DM.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent cause of chronic liver disease worldwide and is defined as increased intrahepatic fat accumulation, in the absence of a history of alcohol abuse, intake of steatogenic medications and other causes of chronic liver disease[1]. NAFLD covers a wide range of histological and clinical disorders, from nonalcoholic fatty liver, which refers to isolated steatosis affecting hepatocytes, to nonalcoholic steatohepatitis (NASH), where inflammation and fibrosis coexist with steatosis and might progress to cirrhosis and hepatocellular carcinoma (HCC)[2-4]. The current prevalence of NAFLD is proportional to the increasing rates of obesity and is estimated to affect 24%-46% of the general population[4,5]. On the other hand, the prevalence of NAFLD is considerably higher in patients with type 2 diabetes mellitus (T2DM) than in the general population, ranging between 50%-75%[4,6,7]. Moreover, NAFLD appears to be more severe histologically in patients with T2DM[4,6,7]. Importantly, T2DM is a risk factor not only for NASH but also for the development of cirrhosis and HCC[8,9]. Indeed, NAFLD is considered as the hepatic phenotype of metabolic syndrome, a prediabetic disorder related to insulin resistance and abdominal obesity[10]. The pathogenesis of NAFLD also involves the increased efflux of free fatty acids to the liver as well as with oxidative stress, inflammation, mitochondrial dysfunction and hepatocellular apoptosis[11]. Notably, both T2DM and NAFLD are associated with increased risk for cardiovascular disease, which represents the leading cause of death in both diseases[12,13]. Currently, there are no approved pharmacological treatments for NAFLD and the mainstay of management is lifestyle changes, including diet and exercise[1]. Among antidiabetic agents, limited data suggest that glucagon-like peptide-1 receptor agonists might exert a beneficial effect on NAFLD whereas other classes do not appear to be effective[1]. Given the frequent coexistence of NAFLD and T2DM as well as the increased liver- and cardiovascular-related morbidity associated with their coexistence, there is a pressing need to develop effective therapeutic interventions for patients with T2DM-associated NAFLD.
ACTIONS OF SODIUM-GLUCOSE CO-TRANSPORTER 2 INHIBITORS
In this context, emerging evidence suggests that sodium-glucose co-transporter 2 (SGLT2) inhibitors might represent a useful tool for the management of these patients. SGLT2 inhibitors are the newest class of oral hypoglycemic agents and reduce blood glucose levels by inhibiting renal tubular glucose reabsorption[14]. This results in increased urinary glucose excretion without stimulating insulin release and hence without a risk of hypoglycemia. In addition to their hypoglycemic action, SGLT2 inhibitors induce weight loss by inducing urinary glucose excretion and osmotic diuresis[14]. They also reduce blood pressure by stimulating urinary sodium excretion[15]. Notably, recent large randomized controlled trials showed that SGLT-2 inhibitors reduce cardiovascular morbidity in patients with T2DM[16,17].
EFFECTS OF SGLT2 INHBITORS ON NAFLD
Regarding the effects of SGLT2 inhibitors on NAFLD in patients with T2DM, a number of small studies (n = 16-84) with a relatively short follow-up (12-24 wk) yielded encouraging results[18-23]. Indeed, a reduction in hepatic fat content was observed as evaluated with magnetic resonance imaging or computed tomo-graphy[18-21,23]. A decrease in transaminase levels was also recorded in most studies[18-22]. Moreover, a reduction in markers of hepatocellular apoptosis (cytokeratin 18-M30 and 18-M65) was observed[18]. A small study (n = 16) reported a decrease in type IV collagen 7S levels, a marker of hepatic fibrosis, after treatment with dapagliflozin for 24 weeks[22] but another study (n = 40) reported no change in type IV collagen 7S levels or in other markers of fibrosis (Fibrosis-4 index and NAFLD fibrosis score) after treatment with luseogliflozin for 24 wk[21]. Weight loss, a reduction in blood pressure, a decrease inHbA1c and fasting glucose levels as well as an improvement of the lipid profile were also recorded[18-23]. Treatment with SGLT2 inhibitors was generally well-tolerated, apart from an increased incidence of genitourinary tract infections[18-23]. Interestingly, in a comparative study, ipragliflozin was as effective as pioglitazone in the reduction of hepatic steatosis[19]. Moreover, in another comparative study, luseogliflozin was more effective than metformin in reducing hepatic steatosis[23].
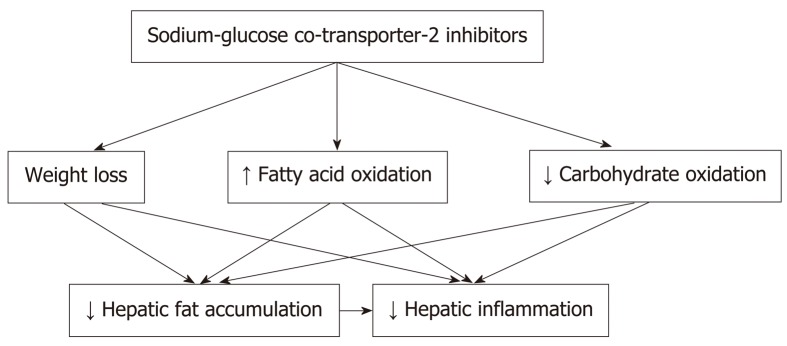
Several mechanisms appear to be implicated in the beneficial effects of SGLT-2 inhibitors on T2DM-associated NAFLD (Figure 1). Weight loss is an important mediator of the improvement in hepatic steatosis[18-21,23]. Furthermore, a relative increase in fatty acid oxidation instead of carbohydrate oxidation could also play a role in the reduction of hepatic fat accumulation and might also suppress hepatic inflammation[14]. Moreover, data from animal models support a direct positive effect of SGLT-2 inhibitors on insulin resistance and an inhibitory effect on liver injury and lipotoxicity[24,25]. Importantly, a recent preclinical study also showed that canagliflozin reduces the risk for hepatocellular cancer in an animal model of NASH[26].
Figure 1.
Mechanisms implicated in the beneficial effects of sodium-glucose co-transporter-2 inhibitors on type 2 diabetes mellitus-associated nonalcoholic fatty liver disease.
CONCLUSION
SGLT2 inhibitors appear to represent a promising option for the management of NAFLD in patients with T2DM. However, existing studies are small, their follow-up period was short and none evaluated the effects of SGLT2 inhibitors on liver histology. Moreover, these agents induce a notable increase in non-serious adverse events, particularly urinary and genital tract infections, and their glucose-lowering benefit might have been overestimated[27]. In addition, even though the phar-macokinetics of SGLT2 inhibitors are unlikely to be affected by the presence of hepatic impairment, there are limited data regarding the safety of these agents in patients with severe liver dysfunction (e.g., Child-Pugh grade C)[28-30]. Therefore, close monitoring is required during the administration of SGLT2 inhibitors in patients with advanced cirrhosis, particularly in patients with ascites who are receiving diuretics. Overall, larger studies are needed to verify the preliminary findings suggesting a benefit of SGLT2 inhibitors in NAFLD and to define their role in the treatment of this common comorbidity in patients with T2DM.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement: All authors declare no conflict of interest related to this publication.
Peer-review started: March 18, 2019
First decision: May 9, 2019
Article in press: June 26, 2019
P-Reviewer: Enomoto H, Miyoshi E, Tarantino G, Trovato GM, Xu CF S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
Contributor Information
Anastasia Kontana, First Propedeutic Department of Internal Medicine, Medical School, Aristotle University of Thessaloniki, AHEPA Hospital, Thessaloniki 54636, Greece.
Konstantinos Tziomalos, First Propedeutic Department of Internal Medicine, Medical School, Aristotle University of Thessaloniki, AHEPA Hospital, Thessaloniki 54636, Greece. ktziomalos@yahoo.com.
References
- 1.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci. 2011;48:97–113. doi: 10.3109/10408363.2011.596521. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 6.Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig Liver Dis. 2015;47:181–190. doi: 10.1016/j.dld.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Lee YH, Cho Y, Lee BW, Park CY, Lee DH, Cha BS, Rhee EJ. Nonalcoholic Fatty Liver Disease in Diabetes. Part I: Epidemiology and Diagnosis. Diabetes Metab J. 2019;43:31–45. doi: 10.4093/dmj.2019.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, Kobayashi M, Saitoh S, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Ohmoto Y, Amakawa K, Tsuji H, Kumada H. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–261. doi: 10.1038/ajg.2011.327. [DOI] [PubMed] [Google Scholar]
- 9.Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, Chim AM, Yu J, Sung JJ, Chan HL. Disease progression of non-alcoholic fatty liver disease: A prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–974. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Baker RD, Bhatia T, Zhu L, Baker SS. Pathogenesis of nonalcoholic steatohepatitis. Cell Mol Life Sci. 2016;73:1969–1987. doi: 10.1007/s00018-016-2161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day CP, James OF. Steatohepatitis: A tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 12.Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Mudaliar S, Polidori D, Zambrowicz B, Henry RR. Sodium-Glucose Cotransporter Inhibitors: Effects on Renal and Intestinal Glucose Transport: From Bench to Bedside. Diabetes Care. 2015;38:2344–2353. doi: 10.2337/dc15-0642. [DOI] [PubMed] [Google Scholar]
- 15.Desouza CV, Gupta N, Patel A. Cardiometabolic Effects of a New Class of Antidiabetic Agents. Clin Ther. 2015;37:1178–1194. doi: 10.1016/j.clinthera.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 17.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson JW, Lundkvist P, Jansson PA, Johansson L, Kvarnström M, Moris L, Miliotis T, Forsberg GB, Risérus U, Lind L, Oscarsson J. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: A double-blind randomised placebo-controlled study. Diabetologia. 2018;61:1923–1934. doi: 10.1007/s00125-018-4675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, Akiyama Y, Morimoto Y, Noda M, Shimada A. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Active-Controlled Trial. Diabetes Care. 2017;40:1364–1372. doi: 10.2337/dc17-0518. [DOI] [PubMed] [Google Scholar]
- 20.Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, Bansal B, Kaur P, Jevalikar G, Gill HK, Choudhary NS, Mithal A. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial) Diabetes Care. 2018;41:1801–1808. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 21.Sumida Y, Murotani K, Saito M, Tamasawa A, Osonoi Y, Yoneda M, Osonoi T. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective, single-arm trial (LEAD trial) Hepatol Res. 2019;49:64–71. doi: 10.1111/hepr.13236. [DOI] [PubMed] [Google Scholar]
- 22.Tobita H, Sato S, Miyake T, Ishihara S, Kinoshita Y. Effects of Dapagliflozin on Body Composition and Liver Tests in Patients with Nonalcoholic Steatohepatitis Associated with Type 2 Diabetes Mellitus: A Prospective, Open-label, Uncontrolled Study. Curr Ther Res Clin Exp. 2017;87:13–19. doi: 10.1016/j.curtheres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibuya T, Fushimi N, Kawai M, Yoshida Y, Hachiya H, Ito S, Kawai H, Ohashi N, Mori A. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective randomized controlled pilot study. Diabetes Obes Metab. 2018;20:438–442. doi: 10.1111/dom.13061. [DOI] [PubMed] [Google Scholar]
- 24.Honda Y, Imajo K, Kato T, Kessoku T, Ogawa Y, Tomeno W, Kato S, Mawatari H, Fujita K, Yoneda M, Saito S, Nakajima A. The Selective SGLT2 Inhibitor Ipragliflozin Has a Therapeutic Effect on Nonalcoholic Steatohepatitis in Mice. PLoS One. 2016;11:e0146337. doi: 10.1371/journal.pone.0146337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komiya C, Tsuchiya K, Shiba K, Miyachi Y, Furuke S, Shimazu N, Yamaguchi S, Kanno K, Ogawa Y. Ipragliflozin Improves Hepatic Steatosis in Obese Mice and Liver Dysfunction in Type 2 Diabetic Patients Irrespective of Body Weight Reduction. PLoS One. 2016;11:e0151511. doi: 10.1371/journal.pone.0151511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiba K, Tsuchiya K, Komiya C, Miyachi Y, Mori K, Shimazu N, Yamaguchi S, Ogasawara N, Katoh M, Itoh M, Suganami T, Ogawa Y. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep. 2018;8:2362. doi: 10.1038/s41598-018-19658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storgaard H, Gluud LL, Bennett C, Grøndahl MF, Christensen MB, Knop FK, Vilsbøll T. Benefits and Harms of Sodium-Glucose Co-Transporter 2 Inhibitors in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0166125. doi: 10.1371/journal.pone.0166125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahasrabudhe V, Terra SG, Hickman A, Saur D, Raje S, Shi H, Matschke K, Zhou S, Cutler DL. Pharmacokinetics of Single-dose Ertugliflozin in Patients With Hepatic Impairment. Clin Ther. 2018;40:1701–1710. doi: 10.1016/j.clinthera.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Devineni D, Curtin CR, Marbury TC, Smith W, Vaccaro N, Wexler D, Vandebosch A, Rusch S, Stieltjes H, Wajs E. Effect of hepatic or renal impairment on the pharmacokinetics of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Ther. 2015;37:610–628.e4. doi: 10.1016/j.clinthera.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Macha S, Rose P, Mattheus M, Cinca R, Pinnetti S, Broedl UC, Woerle HJ. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes Obes Metab. 2014;16:118–123. doi: 10.1111/dom.12183. [DOI] [PubMed] [Google Scholar]