Abstract
Breast cancer is one of the most frequently diagnosed cancer types in females worldwide. The aim of the present study was to investigate the expression levels, functional role and molecular mechanism of microRNA-616 (miR-616) in the progression of breast cancer cells. The relative expression levels of miR-616 in breast cancer cell lines and tumor tissues of 30 patients with breast cancer were analyzed using reverse transcription-quantitative PCR (RT-qPCR). Cell transfection was used to upregulate and downregulate the expression of miR-616 in MCF-7 and MDA-MB-231 cells, respectively. The regulatory effect of miR-616 on tissue inhibitor of metalloproteinases 2 (TIMP2) expression was also analyzed by dual-luciferase reporter assay, western blot analysis and RT-qPCR. The results of RT-qPCR analysis demonstrated significantly higher expression levels of miR-616 in tumor tissues and cancer cell lines compared with normal tissues and a normal epithelial cell line. In addition, overexpression of miR-616 significantly promoted MCF-7 cell proliferation, migration and invasion. By contrast, miR-616 silencing was associated with the opposite effects in MDA-MB-231 cells. Furthermore, the present study demonstrated that miR-616 could positively regulate the expression of matrix metalloproteinases (MMP)2 and MMP9, both at the mRNA and protein level. TIMP2 was further confirmed as a direct target of miR-616. Finally, the current study demonstrated that TIMP2 silencing rescued the proliferation and invasion capabilities and the expression levels of MMP2 and MMP9 in cells that were treated with the miR-616 inhibitor. In conclusion, the present data suggested that the miR-616/TIMP2/MMPs axis may serve an important role in the progression of breast cancer and may be a potential therapeutic target for this disease.
Keywords: microRNA-616, breast cancer, tissue inhibitor of metalloproteinases 2, proliferation, migration, invasion
Introduction
Breast cancer is one of the most prevalent types of malignant tumors in females (1). Significant advances in the treatment for breast cancer have been achieved (1,2). However, breast cancer remains one of the most common causes of cancer-associated mortality in females, accounting for ~14.3% of cancer-associated mortality worldwide in 2012 (2). The majority of mortalities due to breast cancer can be attributed to metastasis (2). Metastases occur when tumor cells disseminate from the primary tumor site to the surrounding extracellular matrix, travel through the vasculature, and finally extravasate at a distant site to form a secondary tumor (3,4). In total, ~10-15% of patients diagnosed with breast cancer present with distant metastasis within 3 years (4). Therefore, identification of new molecular targets for the effective treatment of breast cancer is important.
MicroRNAs (miRNAs) are a class of small non-coding RNAs of ~20 nucleotides in length that can regulate gene expression by directly targeting mRNAs for degradation (5,6). miRNAs regulate a wide range of physiological activities in the cell, including cell proliferation, metabolism, apoptosis, invasion and migration (7). Dysregulated miRNAs are also involved in the initiation and progression of certain types of cancer, including breast cancer (8,9). miR-616 may function as an oncogene in different types of cancer, including gastric cancer, glioma, non-small cell lung cancer, hepatocellular carcinoma and prostate cancer (10–15). However, the expression, function and molecular mechanism of miR-616 in breast cancer remain unclear.
Tissue inhibitor of metalloproteinases 2 (TIMP2) is a critical inhibitor of matrix metalloproteinases (MMPs) and has been largely studied in various types of human tumor. In addition, it was demonstrated that TIMP2 is associated with the invasive and metastatic abilities of various types of tumor cell including breast cancer cells (16). Previous studies reported that certain miRNAs, including miR-130a and miR-552, regulate TIMP2 expression (17,18); however, the association between TIMP2 and miRNAs in breast cancer remains unknown.
The results from the present study demonstrated that miR-616 was upregulated in breast cancer tissues and cell lines. In vitro functional assays indicated that miR-616 could promote breast cancer cell proliferation and metastasis. In addition, this study demonstrated that TIMP2 could be a direct target of miR-616. These results may help understanding the underlying mechanism of miR-616 in breast cancer.
Materials and methods
Tissue collection
The present study was approved by the Medical Ethics Committee of Dezhou No. 2 People's Hospital (Dezhou, China). All patients included in the current study provided written informed consent. In total, 30 paired breast tumor tissue and non-tumor breast tissue samples (>5 cm distant from tumor tissue) were obtained from 30 female patients (age range, 35–77 years; mean age, 62 years) who underwent surgical resection at Dezhou No. 2 People's Hospital (Dezhou, China) between January 2016 and July 2017. Patients who underwent chemotherapy or radiotherapy prior to surgery were excluded from the study.
Cell culture and transfection
The breast cancer cell lines MDA-MB-231, MCF-7, BT474 and MDA-MB-468, the immortal mammary epithelial cell line MCF-10A and the 293 cell line were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All cell lines were incubated in a 95% humidified chamber with 5% CO2 at 37°C. MCF-7, BT474, MDA-MB-231, MDA-MB-468 and 293 cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; GE Healthcare Life Sciences). MCF-10A cells were maintained in DMEM/F12 medium (GE Healthcare Life Sciences) containing 5% horse serum (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (GE Healthcare Life Sciences). The miR-616 mimic, negative control (miR-NC), inhibitor and inhibitor negative control (anti-NC) were purchased from Guangzhou RiboBio, Co., Ltd. The sequences were as follows: miR-616 mimics, 5′-AGUCAUUGGAGGGUUUGAGCAG-3′; miR-NC, 5′-ACUACUGAGUGACAGUAGA-3′; miR-616 inhibitor, 5′-GAGUAUCCCGUUGCCAACGAGA-3′; and anti-NC, 5′-UUCUCCGAACGUGUCACGUTT-3′. Small interfering RNA (siRNA) targeting human TIMP2 (siTIMP2) and si-control were obtained from Santa Cruz Biotechnology, Inc. The sequences were as follows: siTIMP2, 5′-CTCTGATTTGGTCGTATTGGG-3′ and si-control, 5′-CAGUACUUUUGUGUAGUACAA-3′. MCF-7 and MDA-MB-231 cells (5,000) were seeded into 6-well plates and incubated at 37°C until they reached 70–80% confluence. A total of 50 nM miR-616 mimics or miR-616 inhibitors or/and 50 nM siRNA were transfected into MCF-7 and MDA-MB-231 cells using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols. Following 24 h transfection, cells were collected for subsequent experiments.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was analyzed using a CCK-8 kit, according to the manufacturer's protocols (Beyotime Institute of Biotechnology). Briefly, MDA-MB-231 or MCF-7 cells were plated in 96-well plates at a density of 1,000 cells/well at 24 h post-transfection. Following incubation for 0, 24, 48 and 72 h at 37°C, 10 µl CCK-8 reagent was added to each well. The cells were incubated at 37°C in an atmosphere containing 5% CO2 for 2 h. Absorbance was determined at a wavelength of 450 nm using an ELx808 absorbance reader (BioTek Instruments, Inc.).
Colony formation assay
For the assessment of colony formation, transfected breast cancer cells were seeded in 6-well plates at a density of 500 cells/well in triplicate and incubated for 1 week at 37°C. Subsequently, the plates were washed with PBS and stained with 0.5% crystal violet at room temperature for 20 min. After washing three times, colonies with >50 cells were counted and analyzed under a light microscope (magnification, ×100).
Migration and invasion assays
The ability of migration and invasion was assessed using Transwell chambers (Corning Inc.). Cells were re-suspended in serum-free DMEM at a concentration of 1×105/ml and then 200 µl cell suspension was seeded onto the upper well of 8-µm pore Transwell inserts with or without Matrigel (Sigma Aldrich; Merck KGaA). Matrigel was only used for invasion assays. DMEM containing 10% FBS was added to the lower chamber. After 24 h incubation, cells in the upper chambers were removed with a cotton swab. The migrated and invaded cells were then stained with 0.1% crystal violet for 20 min at room temperature. Images from five different fields were captured and counted under a light microscope (magnification, ×100).
Western blot analysis
Cells were lysed in cold radioimmunoprecipitation assay buffer (Thermo Fisher Scientific, Inc.) and the protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Proteins (30 µg) were separated by 10% SDS-PAGE and then transferred to a polyvinylidene difluoride (PVDF) membrane (Thermo Fisher Scientific, Inc.) The PVDF membrane was blocked with 5% non-fat milk in PBS containing 0.1% Tween-20 (Sigma-Aldrich; Merck KGaA) at room temperature for 3 h. Subsequently, the PVDF membrane was incubated with TIMP2 antibody (cat. no. sc-21735; 1:200; Santa Cruz Biotechnology, Inc.), MMP2 antibody (cat. no. sc-13594; 1:200; Santa Cruz Biotechnology, Inc.), MMP9 antibody (cat. no. sc-21733; 1:200; Santa Cruz Biotechnology, Inc.) and GAPDH antibody (cat. no. AF0006; 1:500; Beyotime Institute of Biotechnology) at room temperature for 3 h. Following washing with PBS for 10 min, the PVDF membrane was incubated with a goat anti-mouse secondary antibody (cat. no. ab64255; 1:1,000; Abcam) at room temperature for 1 h. Following further washing with PBS for 10 min, the protein bands were detected using an Enhanced Chemiluminescence Western Blotting kit (Pierce; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocols.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA from the breast cancer cell lines was extracted using TRIzol® reagent (Thermo Fisher Scientific, Inc.) and 1 µg total RNA was reverse transcribed using the Reverse Transcription System kit (Promega Corporation). qPCR was performed with a Power SYBR-Green PCR Master mix (Thermo Fisher Scientific, Inc.) with human GAPDH used as an internal control. For miRNA analysis, qPCR was performed using an all-in-one miRNA RT-qPCR Detection kit (GeneCopoeia, Inc.) and U6 small nuclear RNA as an endogenous control. For the miRNA and mRNA amplifications, the PCR thermocycling conditions were as follows: One cycle at 95°C for 3 min, followed by 40 cycles at 95°C for 12 sec and 62°C for 35 sec, and a final extension step at 95°C for 15 sec. The relative expression levels were calculated using the 2−ΔΔCq method (19). The primer sequences used for amplification were as follows: miR-616, forward, 5′-ACACTCCAGCTGGGAGTCATTGGAGGGTTT-3′, reverse, 5′-TGGTGTCGTGGAGTCG′3′; TIMP2, forward, 5′-CTCTGATTTGGTCGTATTGGG-3′, reverse, 5′-TGGAAGATGGTGATGGGATT-3′; MMP2, forward, 5′-AAGTCTGAAGAGCGTGAAGTTTGGA-3′, reverse, 5′-TGAGGGTTGGTGGGATTGGAG-3′; MMP9, forward, 5′-AGTCCACCCTTGTGCTCTTCCC-3′, reverse, 5′-TCTGCCACCCGAGTGTAACCAT-3′; U6, forward, 5′-CTCGCTTCGGCAGCACA-3′, reverse, 5′-AACGCTTCACGAATTTGCGT-3′; and GAPDH, forward, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′, reverse, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′.
Dual-luciferase reporter assay
The TIMP2 3′-untranslated region (3′-UTR), containing target sequences complementary to the miR-616 seed sequence, was cloned downstream of the firefly luciferase gene in the psiCHECK-2™ luciferase vector (Promega Corporation). Mutated TIMP2 3′-UTR sequences were cloned into the same luciferase vector (TIMP2-mut). The indicated reporter constructs and miR-616 mimic or inhibitor were co-transfected with the phRGTK Renilla luciferase internal control plasmid (Promega Corporation) into 293 cell line using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). The luciferase activity was analyzed following 24 h transfection using a dual-luciferase reporter assay system (Promega Corporation), according to the manufacturer's protocols.
Bioinformatics analysis
The prediction of TIMP2 3′-UTR as a miR-616 binding target was determined using TargetScan 7.1 software (www.targetscan.org).
Statistical analysis
All experiments were performed three times and data were analyzed using GraphPad Prism 5 (GraphPad Software Inc.). Differences between two groups were assessed using a two-tailed Student's t-test. Data of >2 groups were analyzed using one-way analysis of variance with a post hoc Tukey's test. The correlation between miR-616 levels and TIMP2 mRNA expression levels in human breast cancer tissues was determined using Spearman's rank correlation coefficient. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-616 expression is upregulated in breast cancer tissues and cell lines
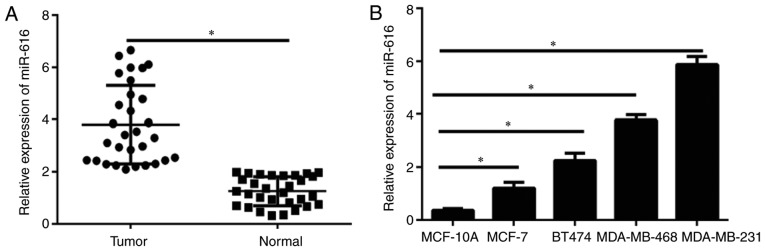
The expression levels of miR-616 in breast cancer tissues and cell lines were detected by RT-qPCR. The results demonstrated that miR-616 levels were significantly upregulated in breast cancer samples compared with normal tissues (P<0.05; Fig. 1A). Furthermore, the miR-616 levels were evaluated in the breast cancer cell lines MCF-7, BT474, MDA-MB-231 and MDA-MB-468, and the immortal mammary epithelial cell line MCF-10A. The miR-616 expression levels were significantly increased in all breast cancer cell lines compared with MCF-10A cells (P<0.05; Fig. 1B), although the difference varied across the cell lines. These data indicated that miR-616 may serve an important role in the progression of breast cancer. The expression levels of miR-616 was the highest in MDA-MB-231 cells and the lowest in MCF-7 cells; therefore, these two cell lines were selected for further experiments.
Figure 1.
Expression of miR-616 is upregulated in breast cancer tissues and cell lines. (A) miR-616 expression levels in the tumor and adjacentnn normal tissues were detected by RT-qPCR. (B) miR-616 expression levels in breast cancer cell lines and the immortal mammary epithelial cell line MCF-10A were detected by RT-qPCR. *P<0.05. RT-qPCR, reverse transcription-quantiative PCR; miR-616, microRNA-616.
miR-616 enhances cell growth in breast cancer cells
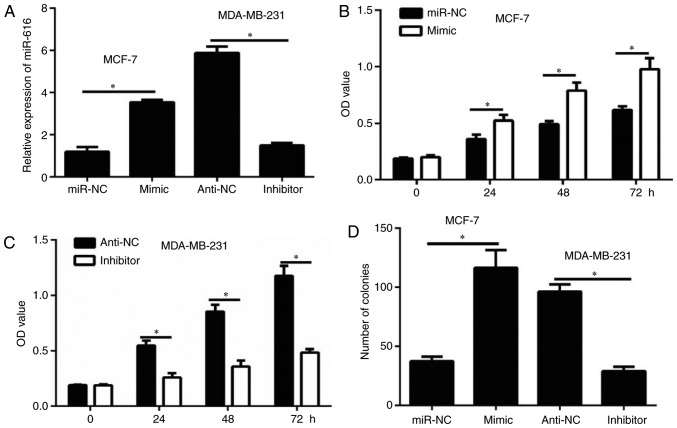
Overexpression of miR-616 was achieved by transfection of MCF-7 cells with miR-616 mimic. By contrast, miR-616 silencing was achieved by transfection of MDA-MB-231 cells with miR-616 inhibitor. Transfection efficiency for both the overexpression and the silencing experiments was confirmed by RT-qPCR (Fig. 2A). The results of CCK-8 assay demonstrated that miR-616 overexpression significantly enhanced the proliferation of MCF-7 cells (P<0.05; Fig. 2B). By contrast, miR-616 silencing significantly inhibited the proliferation of MDA-MB-231 cells (P<0.05; Fig. 2C). Similar results were observed with a colony formation assay (P<0.05; Fig. 2D). These data suggested that miR-616 enhanced breast cancer cell growth in vitro.
Figure 2.
miR-616 enhances cell growth in breast cancer cells. (A) miR-616 expression levels in transfected MCF-7 and MDA-MB-231 cells were detected by reverse transcription-quantitative PCR. (B) CCK-8 assay was performed to evaluate the cell viability following tranfection of MCF-7 cells with mimic or miR-NC. (C) CCK-8 assay was performed to evaluate the cell viability following tranfection of MDA-MB-231 cells with inhibitor or anti-NC. (D) Colony formation assay was performed to detect the colony formation ability of cells following transfection. *P<0.05. miR, microRNA; CCK-8, Cell Counting Kit-8; NC, negative control; OD, optical denisty.
miR-616 enhances breast cancer cell invasion in vitro
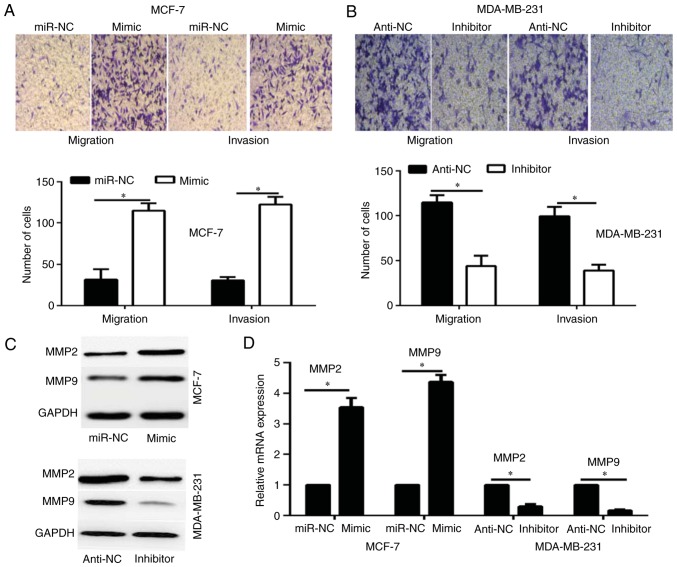
To investigate whether the overexpression of miR-616 affects the migration and invasion of breast cancer cells, Transwell assays were performed. The results revealed that overexpression of miR-616 significantly enhanced the migration and invasion potential of MCF-7 cells compared with the miR-NC group, whereas miR-616 silencing significantly inhibited the migration and invasion capacities of MDA-MB-231 cells compared with the anti-NC group (P<0.05; Fig. 3A and B).
Figure 3.
miR-616 enhances migration and invasion in breast cancer cells. (A) Cell migration and invasion capabilities were analyzed by Transwell assay in MCF-7 cells transfected with mimic or miR-NC, and in (B) MDA-MB-231 cells transfected with inhibitor or anti-NC. Magnification, ×100. (C) Western blot analysis for MMP2 and MMP9 protein expression levels. (D) MMP2 and MMP9 mRNA expression levels were detected by reverse transcription-quantitative PCR. *P<0.05. miR, microRNA; NC, negative control; MMP, matrix metalloproteinase.
A previous study has reported that MMP2 and MMP9 serve a critical role in the migration and invasion of breast cancer cells (20). Therefore, the current study investigated whether miR-616 affects the expression of MMP2 and MMP9. The results of RT-qPCR and western blot analysis demonstrated that overexpression of miR-616 enhanced the expression levels of MMP2 and MMP9, while miR-616 silencing significantly reduced their expression levels (P<0.05; Fig. 3C and D).
TIMP2 is a direct target of miR-616
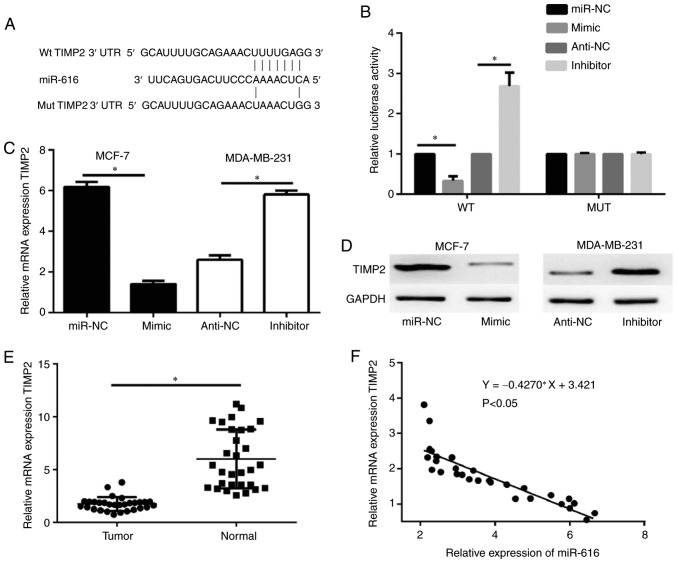
The potential targets of miR-616 were predicted by bioinformatics analysis. Previous studies have demonstrated that TIMP2 acts as a tumor suppressor and downregulates the expression of MMP2 and MMP9 in tumor cells (20,21); therefore, out of the predicted potential targets for miR-616, TIMP2 was selected for further analysis in the current study. A schematic of the target sequence in the 3′UTR of TIMP2 is shown in Fig. 4A. Dual-luciferase reporter assays were performed to evaluate whether miR-616 directly targets TIMP2. The results revealed that miR-616 mimic significantly decreased the luciferase activity of the wild-type TIMP2 and miR-616 silencing significantly increased the luciferase activity of the wild-type TIMP2; however, no significant difference was observed in the luciferase activity of the mutant TIMP2 (P<0.05; Fig. 4B). Furthermore, RT-qPCR and western blot analysis demonstrated that the mRNA and protein expression levels of TIMP2 were negatively regulated by miR-616 expression in MCF-7 and MDA-MB-231 cells (Fig. 4C and D). When examining the tissue samples from the patient cohort, RT-qPCR results revealed that the mRNA expression levels of TIMP2 were significantly downregulated in breast cancer tissue samples compared with normal tissue samples (P<0.05; Fig. 4E). Notably, the mRNA expression levels of TIMP2 were negatively correlated with miR-616 levels in the breast cancer tissues (P<0.05; Fig. 4F).
Figure 4.
TIMP2 is a direct target gene of miR-616. (A) Schematic presentation of the putative miR-616 target site in the 3′-UTR of the TIMP2 transcript. (B) A dual-luciferase reporter assay was performed with the 293 cell line co-transfected with TIMP2 3′-UTR-WT or 3′-UTR-MUT reporter plasmids and either miR-616 mimic or inhibitor. (C) Reverse transcription-quantitative PCR was used to detect the TIMP2 mRNA levels following changes in the expression of miR-616 in breast cancer cells. (D) Western blot assay was used to detect the TIMP2 protein levels following changes in the expression of miR-616 in breast cancer cells. GAPDH served as a loading control. (E) The mRNA expression levels of TIMP2 were analyzed in breast cancer tissues and adjacent normal breast tissues. (F) Spearman's correlation analysis was used to evaluate the correlation between the miR-616 levels and TIMP2 mRNA expression levels in breast cancer tissues. *P<0.05. TIMP2, tissue inhibitor of metalloproteinases 2; miR, microRNA; UTR, untranslated region; WT, wild-type; MUT, mutant; NC, negative control.
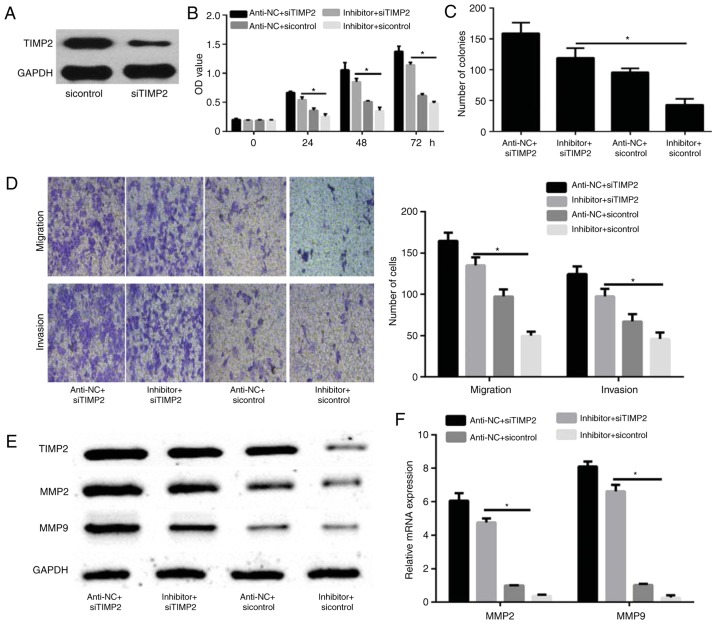
TIMP2 silencing reverses the effects of miR-616 in breast cancer cells
To confirm that TIMP2 is a functional target of miR-616, MDA-MB-231 cells were transfected with sicontrol or siTIMP2. Western blot analysis revealed that the expression of TIMP2 was decreased in siTIMP2-transfected MDA-MB-231 cells compared with sicontrol-transfected MDA-MB-231 cells (Fig. 5A). MDA-MB-231 cells were then transfected with miR-616 inhibitor and/or siTIMP2 and their corresponding controls. CCK-8 and colony formation assays demonstrated that TIMP2 knockdown significantly promoted cell proliferation and colony formation, which were initially reduced by miR-616 silencing (P<0.05; Fig. 5B and C). Furthermore, a Transwell assay demonstrated that TIMP2 silencing significantly increased the migration and invasion capabilities of MDA-MB-231 cells, which were initially reduced by miR-616 inhibitor (P<0.05; Fig. 5D). Finally, it was demonstrated that TIMP2 knockdown enhanced the protein and mRNA expression levels of MMP2 and MMP9, which were reduced by miR-616 inhibitor alone (P<0.05; Fig. 5E and F).
Figure 5.
TIMP2 silencing reverses the effect of miR-616 in breast cancer cells. (A) The protein expression of TIMP2 in MDA-MB-231 cells transfected with sicontrol or siTIMP2 was detected by western blot analysis. (B) Cell Counting Kit-8 and (C) colony formation assays were used to measure cell proliferation. (D) Cell migration and invasion were detected by Transwell assays. Magnification, ×100. (E) The protein expression levels of TIMP2, MMP2 and MMP9 in MDA-MB-231 cells transfected with siTIMP2 and/or miR-616 inhibitor were evaluated by western blot analysis. (F) The mRNA expression levels of TIMP2, MMP2 and MMP9 in MDA-MB-231 cells transfected with siTIMP2 and/or miR-616 inhibitor were analyzed by reverse transcription-quantitative PCR. *P<0.05. TIMP2, tissue inhibitor of metalloproteinases 2; miR, microRNA; si, small interfering; MMP, matrix metalloproteinase; OD, optical density.
Discussion
Investigation of the molecular mechanisms involved in tumor progression facilitates the identification of molecules that may act as new targets for the treatment of breast cancer (22,23). miRNAs serve important roles in tumorigenic processes, including cell viability, proliferation, apoptosis, migration and invasion (22,23). miRNAs may serve as oncogenes or tumor suppressors, depending on the type of tissue and the context of expression (5). However, the underlying molecular mechanisms of miRNAs in cancer progression remain unclear.
Certain types of miRNAs, including miR-155 and miR-21, serve critical roles in the progression of breast cancer (8,24). miR-616 is a novel miRNA that serves as an oncogene in glioma, gastric cancer, prostate cancer, non-small cell lung cancer and hepatocellular carcinoma (10–15). In breast cancer, to the best of our knowledge, the expression levels, biological function and molecular mechanism of miR-616 have not been fully elucidated. In the present study, the expression levels of miR-616 were significantly upregulated in breast cancer cell lines and tissues. Functional in vitro experiments demonstrated that miR-616 promoted the proliferation, migration and invasion of breast cancer cells. According to these data, miR-616 may act as an oncogene and may be a potential biomarker for breast cancer.
miRNAs exert their biological roles in cancer by regulating their target genes (5). Therefore, identifying the association of a miRNA and its target gene is important for elucidating the molecular mechanism underlying its action in cancer. miR-616 can regulate numerous genes, including phosphatase and tensin homolog, SRY-box 7, tissue factor pathway inhibitor 2 and glycogen synthase kinase 3β (10–14). In the present study, it was demonstrated that miR-616 could positively regulate the expression levels of MMP2 and MMP9. Using bioinformatics analysis, it was identified that the 3′-UTR of TIMP2 contained a miR-616 response element. TIMP2 inhibits cell proliferation and migration in vitro by inhibiting the function of MMPs (21,25). In addition, TIMP2 serves as a tumor suppressor in the progression of breast cancer (26). However, to the best of our knowledge, the mechanism of TIMP2 in the promotion of breast cancer remains unclear and the association of TIMP2 with miRNAs has not been fully investigated in breast cancer.
For the first time, the present study demonstrated the expression and function of miR-616 in breast cancer. A negative correlation was identified between TIMP2 and miR-616 in breast cancer, which was consistent with a previous study regarding ovarian cancer (15). Notably, the present study identified that miR-616 promoted the expression of MMP2 and MMP9 via TIMP2 in breast cancer. However, certain limitations were present in the current study. Firstly, the experiments were performed in vitro. Therefore, in vivo experiments are required to further support the conclusions of the current study. In addition, associations between the expression of miR-616 and clinical features were not investigated; therefore, this is required in future studies. Finally, other molecular mechanisms of miR-616 in the progression of breast cancer should be examined in the future.
In summary, the present data demonstrated that miR-616 enhanced the proliferation, migration and invasion of breast cancer cells by directly targeting TIMP2. Although clinical applications should be further investigated, the present study revealed that the miR-616/TIMP2/MMP axis may serve as role in the regulation of breast cancer progression and identified miR-616 as a potential novel therapeutic target for the treatment of breast cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data analyzed during this study are included in the published article.
Authors' contributions
CY conceived, designed and conducted all experiments, and wrote the manuscript.
Ethics approval and consent to participate
The present study was approved by the Medical Ethics Committee of Dezhou No. 2 People's Hospital (Dezhou, China). All patients included in the current study provided written informed consent.
Patients consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Valastyan S, Weinberg RA. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: Markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen J, Stass SA, Jiang F. MicroRNAs as potential biomarkers in human solid tumors. Cancer Lett. 2013;329:125–136. doi: 10.1016/j.canlet.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piasecka D, Braun M, Kordek R, Sadej R, Romanska H. MicroRNAs in regulation of triple-negative breast cancer progression. J Cancer Res Clin Oncol. 2018;144:1401–1411. doi: 10.1007/s00432-018-2689-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu W, Tan C, He Y, Zhang G, Xu Y, Tang J. Functional miRNAs in breast cancer drug resistance. Onco Targets Ther. 2018;11:1529–1541. doi: 10.2147/OTT.S152462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu ZH, Lin C, Liu CC, Jiang WW, Huang MZ, Liu X, Guo WJ. MiR-616-3p promotes angiogenesis and EMT in gastric cancer via the PTEN/AKT/mTOR pathway. Biochem Biophys Res Commun. 2018;501:1068–1073. doi: 10.1016/j.bbrc.2018.05.109. [DOI] [PubMed] [Google Scholar]
- 11.Bai QL, Hu CW, Wang XR, Shang JX, Yin GF. MiR-616 promotes proliferation and inhibits apoptosis in glioma cells by suppressing expression of SOX7 via the Wnt signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21:5630–5637. doi: 10.26355/eurrev_201712_14006. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Cao Q, Qu M, Xiao Z, Zhang M, Di S. MicroRNA-616 promotes the growth and metastasis of non-small cell lung cancer by targeting SOX7. Oncol Rep. 2017;38:2078–2086. doi: 10.3892/or.2017.5854. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Zhang D, Zhou P, Wang W, Wang X, Li J, Sun X, Zhang L. MicroRNA-616 promotes the migration, invasion and epithelial-mesenchymal transition of HCC by targeting PTEN. Oncol Rep. 2016;35:366–374. doi: 10.3892/or.2015.4334. [DOI] [PubMed] [Google Scholar]
- 14.Ma S, Chan YP, Kwan PS, Lee TK, Yan M, Tang KH, Ling MT, Vielkind JR, Guan XY, Chan KW. MicroRNA-616 induces androgen-independent growth of prostate cancer cells by suppressing expression of tissue factor pathway inhibitor TFPI-2. Cancer Res. 2011;71:583–592. doi: 10.1158/0008-5472.CAN-10-2587. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Zhu J, Zhu Y, Wang J. MicroRNA-616 promotes the progression of ovarian cancer by targeting TIMP2. Oncol Rep. 2018;39:2960–2968. doi: 10.3892/or.2018.6368. [DOI] [PubMed] [Google Scholar]
- 16.Chien YC, Liu LC, Ye HY, Wu JY, Yu YL. EZH2 promotes migration and invasion of triple-negative breast cancer cells via regulating TIMP2-MMP-2/-9 pathway. Am J Cancer Res. 2018;8:422–434. [PMC free article] [PubMed] [Google Scholar]
- 17.Chao Y, Hu K, Wang X, Wang L. MicroRNA-552 promotes migration and invasion of osteosarcoma through targeting TIMP2. Biochem Biophys Res Commun. 2019;511:63–68. doi: 10.1016/j.bbrc.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Yin S, Zhang Q, Wang Y, Li S, Hu R. MicroRNA-130a regulated by HPV18 E6 promotes proliferation and invasion of cervical cancer cells by targeting TIMP2. Exp Ther Med. 2019;17:2837–2846. doi: 10.3892/etm.2019.7226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Ren F, Tang R, Zhang X, Madushi WM, Luo D, Dang Y, Li Z, Wei K, Chen G. Overexpression of MMP family members functions as prognostic biomarker for breast cancer patients: A systematic review and meta-analysis. PLoS One. 2015;10:e0135544. doi: 10.1371/journal.pone.0135544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tjomsland V, Pomianowska E, Aasrum M, Sandnes D, Verbeke CS, Gladhaug IP. Profile of MMP and TIMP expression in human pancreatic stellate cells: Regulation by IL-1α and TGFβ and implications for migration of pancreatic cancer cells. Neoplasia. 2016;18:447–456. doi: 10.1016/j.neo.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iorio MV, Croce CM. MicroRNA dysregulation in cancer: Diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4:143–159. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasquinelli AE. MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 24.Lu L, Mao X, Shi P, He B, Xu K, Zhang S, Wang J. MicroRNAs in the prognosis of triple-negative breast cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7085. doi: 10.1097/MD.0000000000007085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurzawski M, Kaczmarek M, Kłysz M, Malinowski D, Kazienko A, Kurzawa R, Droździk M. MMP2, MMP9 and TIMP2 polymorphisms affect sperm parameters but not fertility in Polish males. Andrologia. 2017;49 doi: 10.1111/and.12654. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Park CI, Park BW, Lee HD, Jung WH. Expression of MT-1 MMP, MMP2, MMP9 and TIMP2 mRNAs in ductal carcinoma in situ and invasive ductal carcinoma of the breast. Yonsei Med J. 2006;47:333–342. doi: 10.3349/ymj.2006.47.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study are included in the published article.