Abstract
Rovalpituzumab tesirine is a promising delta-like protein 3 (DLL3)-targeted antibody-drug conjugate for the treatment of small-cell lung cancer (SCLC). Thyroid transcription factor-1 (TTF-1) and DLL3 protein are associated with SCLC, and may be used to identify patients, who respond to the DLL3-targeted therapy. However, little is known about the expression pattern of the DLL3 protein, and the prognostic value of DLL3 and TTF-1 for SCLC. A total of 335 patients with SCLC were identified, including 11 patients with paired biopsy of primary site and lobectomy specimens, and 37 patients with paired specimens of primary and metastatic site. The DLL3 expression levels of individuals were evaluated using the anti-DLL3 antibody. No differences in DLL3 expression levels were observed in paired biopsy and lobectomy specimens (P=0.774), and paired primary and metastatic sites (P=0.472). SCLC cases with high DLL3 expression levels were more frequent in male patients (P=0.041), smokers (P=0.023) and patients with positive TTF-1 expression (P=0.006) compared with DLL3-low SCLC. DLL3-high SCLC exhibited worse overall survival compared with DLL3-low SCLC (log-rank test, P=0.007). Patients with TTF-1+ SCLC experienced a significantly worse overall survival compared with patients with TTF-1- SCLC (P<0.001). DLL3-low/TTF-1- was defined as a distinct molecular subgroup of SCLC with optimal prognosis (P<0.001). DLL3-low/TTF-1- was an independent prognostic marker for SCLC (P=0.001). In conclusion, the present study, to the best of our knowledge, provided novel evidence for SCLC intratumoral and intertumoral homogeneity with the identification of DLL3 protein levels. Therefore, it is reliable to use biopsy specimens to evaluate DLL3 expression levels for identification of patients who may benefit from DLL3-targeted therapy. In addition, DLL3 and TTF-1 are two protein markers with potential clinical value in risk stratification for patients with SCLC.
Keywords: delta-like protein 3, thyroid transcription factor-1, small-cell lung cancer, prognosis
Introduction
Small-cell lung cancer (SCLC) is a highly aggressive malignancy with early development of widespread metastases, in addition to a limited number of effective treatments and particularly poor prognoses (1–3). SCLC accounted for ~15% of all diagnosed novel lung cancer cases worldwide between 1995 and 2011 (4,5).
Recently, SCLC-targeted therapy research has progressed. Delta-like protein 3 (DLL3), an inhibitory Notch ligand, is particularly upregulated in SCLC (6–8). Rovalpituzumab tesirine (Rova-T), a novel DLL3-targeted antibody-drug conjugate, has demonstrated in vivo efficacy in eradicating DLL3-expressing tumor-initiating cells in SCLC patient-derived xenograft tumors (7). A phase I clinical trial demonstrated single-agent antitumor activity of Rova-T in patients with recurrent SCLC (6,9). The objective response rate and disease-control rate were 38 (10/26) and 88% (23/26) in the DLL3-high patient subgroup compared with 0 (0/8) and 50% (4/8) in the DLL3-low subgroup (6,9). This early clinical trial provides evidence that DLL3 is a potential predictive molecular marker for DLL3-targeted treatment. Although a recent study reported that DLL3-high expression was not a prognostic factor for Japanese patients with SCLC (10), the prognostic value of DLL3 for patients from China remains unknown.
Biopsies are used often in clinical practice to determine SCLC diagnoses prior to treatment. According to our previous studies, in Guangdong Provincial People's Hospital between January 2006 and June 2015, 93% of SCLC cases were diagnosed based on biopsy specimens (unpublished data). Accurate diagnosis is the premise of accurate treatment. However, sampling bias caused by intratumoral and intertumoral heterogeneity may reduce its value as a biomarker for targeted therapy (11). Therefore, it is crucial for any ongoing and future clinical trials to examine the DLL3 expression pattern. At present, the DLL3 expression pattern in SCLC remains unknown.
SCLC is positive for thyroid transcription factor-1 (TTF-1) in ≤90-95% of cases due to its neuroendocrine differentiation (12–14). TTF-1 protein is routinely used for differential diagnosis in clinical pathology labs (15). A previous study revealed a positive correlation between the expression of DLL3 and TTF-1 in SCLC, suggesting the potential application of TTF-1 to predict the DLL3 expression level and response to targeted therapy (16). However, a limited number of studies have been published on the prognostic significance of TTF-1 in SCLC.
Taking this into consideration, the aim of the present study was to examine the expression pattern of DLL3 in SCLC by immunohistochemical staining of formalin-fixed, paraffin-embedded (FFPE) tumor tissues. Furthermore, the association between DLL3 and TTF-1 expression in SCLC was analyzed. Finally, the prognostic value of protein markers DLL3 and TTF-1 was examined.
Materials and methods
Human tissues
A retrospective study of patients with de novo SCLC was performed. Hematoxylin and Eosin (H&E) staining of the tissue sections included nuclear staining with 1% hematoxylin for 5–15 min at room temperature and counterstaining with 1% eosin for 2–3 min at room temperature. Expert lung cancer pathologists Dr Yan-Hui Liu, Dr Li-Xu Yan and Dr Yu-Fa Li from The Guangdong Provincial People's Hospital of Guangdong Academy of Medical Sciences (Guangzhou, China) independently reviewed the diagnoses of the histological samples according to the 2015 World Health Organization classification (17). Inconsistent diagnoses were submitted to the expert panel to reach a consensus diagnosis. A total of 335 cases were identified at Guangdong Provincial People's Hospital (Guangzhou, China) between January 2006 and June 2015. All cases had adequate tumor tissues and complete clinical and prognostic data. Patients with only cytology specimens were excluded. Out of the 335 patients, 11 had paired biopsy of primary site and lobectomy specimens. A total of 37 patients had paired specimens of primary and metastatic site, including mediastinal lymph node, supraclavicular lymph node and distant metastatic site.
Tumor staging was classified according to the 8th Edition of The Tumor-Node-Metastasis Classification for Lung Cancer (18). A total of 324 of the 335 patients received cytotoxic therapy (75 or 80 mg/m2 cisplatin on day 1 and 100 or 80 mg/m2 etoposide on days 1, 2 and 3), and 11 patients received surgery with paired biopsy of primary site and lobectomy specimens. The Research Ethics Committee of Guangdong Provincial People's Hospital (Guangzhou, China) approved the present study (approval no. GDREC2016373H).
Immunohistochemistry (IHC)
The specimens were fixed in 10% neutral buffered formalin for 24 to 48 h at room temperature. Pretreated FFPE tumor specimens were used for testing. Each tumor tissue block was sectioned at 4 µm. Slides were stained with a DLL3-specific antibody (dilution, 1:100; cat. no. ab103102; Abcam, Cambridge, UK) at 37°C for 32 min. The OptiView DAB IHC Detection Kit (cat. no. 760–700; Ventana Medical Systems, Inc.), including blocking reagent and secondary antibody, was used according to the manufacturer's protocol. IHC staining was performed using an automated immunostaining instrument (Ventana BenchMark XT; Ventana Medical Systems, Inc., Tucson, AZ, USA) according to the manufacturer's protocol. For the detection of TTF-1, IHC was performed with anti-TTF-1 antibody (undiluted; cat. no. 790-4398; Ventana Medical Systems, Inc.) combined with the same detection kit and procedures as DLL3. DLL3-positive SCLC tissue was used as a positive control, and DLL3-negative lung adenocarcinoma tissue was used as a negative control; positive and negative control tumor slides were included in each assay. An Olympus BX51 light microscope (magnification, ×20-400), equipped with a DP72 camera and DP2-BSW software (Olympus, Tokyo, Japan), was used. Positive TTF-1 staining was defined as >5% of tumor cells stained for the marker for TTF-1- targeted treatment (13).
Scoring for DLL3
The results of immunohistochemical staining for DLL3 were semi-quantitatively evaluated using an immunohistochemical H-score (HS) method (7,11,19). Staining intensity of DLL3 was categorized into the following four groups: i) 0, no membrane or cytoplasmic staining; ii) 1+, weak membranous with or without cytoplasmic staining; iii) 2+, moderate membranous with or without cytoplasmic staining; and iv) 3+, strong membranous (observable with ×10 objective) with or without cytoplasmic staining. HS (range, 0–300) was calculated using the formula: HS=Σ(i+1) × Pi, in which i=staining intensity and Pi=percentage of stained cells (7,11,19).
Statistical analysis
Statistical analyses were conducted using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). Wilcoxon matched-pairs test was used to analyze DLL3 expression in paired samples. The receiver operating characteristic (ROC) curve was used to define the optimal cut-off value for DLL3 in predicting 5-year overall survival rate (36.1%) of SCLCs. Pearson's χ2 test was used to analyze the potential association between DLL3 level and TTF-1 and the clinicopathological features. The concordance analyses between paired specimens were estimated using Kappa test. Kaplan-Meier curve with log-rank test was used to analyze the impact of DLL3 expression levels on survival of patients. Univariate and multivariate analysis was performed using a Cox proportional hazard regression model. Multivariable models were constructed using a forward selection (likelihood ratio, LR) test, starting with variables with P<0.05 in univariable analyses. Two-sided P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
The clinical characteristics of the 335 patients with SCLC are summarized in Table I. The median age was 63 years (range, 34–87), and the majority of the patients, 91.3%, were male (306/335), while 72.2% were smokers (242/335). Distant metastasis at diagnosis was observed in approximately one-half (50.7%) of the patients (170/335).
Table I.
Association analysis between DLL3 and TTF-1 expression and clinicopathological characteristics of patients with small-cell lung cancer.
| Variables | Number of patients, N=335 (%) | DLL3-Low, N=126 (%) | DLL3-High, N=209 (%) | P-value | TTF-1 negative, N=65 (%) | TTF-1 positive, N=270 (%) | P-value |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| ≤60 | 143 (42.7) | 51 (40.5) | 92 (44.0) | 0.525 | 25 (38.5) | 118 (43.7) | 0.443 |
| >60 | 192 (57.3) | 75 (59.5) | 117 (56.0) | 40 (61.5) | 152 (56.3) | ||
| Sex | |||||||
| Female | 29 (8.7) | 16 (12.7) | 13 (6.2) | 0.041a | 8 (12.3) | 21 (7.8) | 0.244 |
| Male | 306 (91.3) | 110 (87.3) | 196 (93.8) | 57 (87.7) | 249 (92.2) | ||
| Smoking history | |||||||
| Non-smokersc | 93 (27.8) | 44 (34.9) | 49 (23.4) | 0.023a | 17 (26.2) | 76 (28.1) | 0.747 |
| Smokers | 242 (72.2) | 82 (65.1) | 160 (76.6) | 48 (73.8) | 194 (71.9) | ||
| Distant metastasis | |||||||
| Negative | 165 (49.3) | 69 (54.8) | 96 (45.9) | 0.117 | 38 (58.5) | 127 (47.0) | 0.098 |
| Positive | 170 (50.7) | 57 (45.2) | 113 (54.1) | 27 (41.5) | 143 (53.0) | ||
| TNM stage (18) | |||||||
| I | 12 (3.6) | 5 (4.0) | 7 (3.3) | 0.661b | 4 (6.2) | 8 (3.0) | 0.244 |
| II | 17 (5.1) | 7 (5.6) | 10 (4.8) | 4 (6.2) | 13 (4.8) | ||
| III | 136 (40.6) | 57 (45.2) | 79 (37.8) | 30 (46.2) | 106 (39.3) | ||
| IV | 170 (50.7) | 57 (45.2) | 113 (54.1) | 27 (41.5) | 143 (53.0) | ||
| TTF-1 | |||||||
| Negative | 65 (19.4) | 34 (27.0) | 31 (14.8) | 0.006a | – | – | – |
| Positive | 270 (80.6) | 92 (73.0) | 178 (85.2) | – | – | ||
P<0.05. All P-values were derived from Pearson's χ2 test.
P-values were compared between stage I/II and III/ IV.
<100 cigarettes in patient lifetime. DLL3, delta-like protein 3; TTF-1, thyroid transcription factor-1; TNM, Tumor-Node-Metastasis.
Cut-off value for DLL3
The optimal cut-off value for DLL3 in predicting overall survival of patients with SCLC was determined by ROC curves (data not shown). DLL3-low was defined as an H-score ≤150 and DLL3-high was defined as an H-score >150.
Expression pattern of DLL3 protein in SCLCs
Tumor cells labeled by DLL3 exhibited a membranous staining with or without a cytoplasmic staining pattern as previously reported (6). Representative images of DLL3 protein are indicated in Fig. 1. Intratumoral distribution of DLL3 indicated homogeneity in 11 lobectomy specimens (Fig. 2). To compare intratumoral expression of DLL3, paired biopsy of primary site and lobectomy specimens were used (Fig. 3A). No difference in DLL3 H-scores was observed in paired biopsy [median HS, 140; interquartile range (IQR), 100–280] and lobectomy specimens (median HS, 150; IQR 100–270; Wilcoxon test; P=0.774; H-score). Concordant staining, either high or low for DLL3 in paired biopsy and lobectomy specimens, was observed in 11 out of the 11 (100%) SCLCs. Kappa test indicated significant concordance between the paired specimens (P<0.001; Fig. 3B).
Figure 1.
Representative images of DLL3 protein in small-cell lung cancer (×400 magnification). (A) DLL3 H-score=0. (B) DLL3 H-score=150. (C) DLL3 H-score=300. DLL3, Delta-like protein 3.
Figure 2.
Representative images of two lobectomy specimens with immunohistochemical staining for DLL3, indicating the intratumoral homogeneity of DLL3 protein in small-cell lung cancer. (A) DLL3 H-score=100. (B) DLL3 H-score=300. Black boxes indicate the corresponding high magnification fields. H&E, hematoxylin and eosin; DLL3, delta-like protein 3.
Figure 3.
Comparison of DLL3 expression in paired specimens of patients with small-cell lung cancer. (A) DLL3 H-scores in paired biopsy and lobectomy specimens (N=11). (B) Comparison of DLL3 expression levels in paired biopsy and lobectomy specimens (N=11). DLL3-low was defined as an H-score ≤150 and DLL3-high was defined as an H-score >150. (C) DLL3 H-scores in paired primary and metastatic site specimens (N=37). (D) Comparison of DLL3 expression levels in paired primary and metastatic site specimens (N=37). DLL3, delta-like protein 3.
To compare intertumoral expression of DLL3, paired biopsies of primary and metastatic sites were used; no difference of DLL3 H-scores was observed in paired primary sites (median HS, 150; IQR, 100–260) and metastatic sites (median HS, 150; IQR, 100–285; P=0.472; Fig. 3C). Concordant staining, either high or low for DLL3 in paired specimens of primary and metastatic sites, was also observed in 37 of the 37 (100%) SCLCs (P<0.001; Fig. 3D).
Association of DLL3 and TTF-1 with clinicopathological characteristics
SCLC with high DLL3 expression levels were more frequent in males (P=0.041), smokers (P=0.023) and TTF-1 expression (P=0.006) compared with SCLCs with low DLL3 expression levels (Table I). There was no significant association of DLL3 expression level with age, distant metastasis status or TNM stage. No significant association of TTF-1 expression with clinical characteristics was observed.
DLL3 combined with TTF-1 predicts survival of SCLC
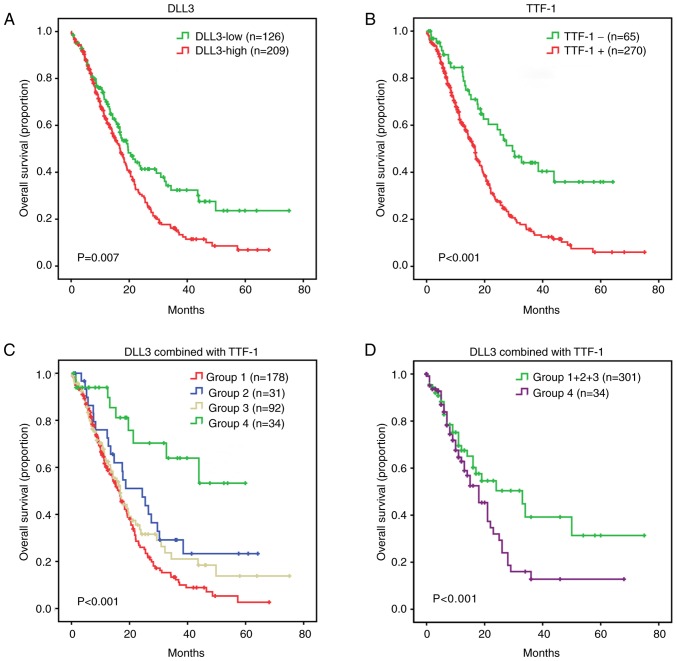
The median follow-up time for the patients with SCLCs was 11.3 months (range, 0.1–75.1). The extent to which analysis of DLL3 and TTF-1 levels delineate prognosis in SCLC was investigated. A Kaplan-Meier survival curve indicated that patients with SCLC with a high DLL3 expression level exhibited a lower overall survival compared with patients with DLL3-low expression (log-rank test; P=0.007; Fig. 4A). TTF-1+ SCLCs experienced a decrease in overall survival compared with TTF-1- SCLCs (P<0.001; Fig. 4B). Based on the DLL3 and TTF-1 features, the SCLC cohort was divided into the following four subgroups: Group 1 consisting of DLL3-high/TTF-1+; Group 2 consisting of DLL3-high/TTF-1-; Group 3 consisting of DLL3-low/TTF-1+; and Group 4 consisting of DLL3-low/TTF-1-. A Kaplan-Meier survival curve of the four different subgroups indicated significant differences (P<0.001; Fig. 4C). Group 4, representing DLL3-low/TTF-1- had improved overall survival compared with Group 1+2+3 (P<0.001; Fig. 4D). Group 1 had inferior survival compared with Group 2 (P=0.024) and Group 4 (P<0.001; data not shown). Group 1 and 3 (P=0.192), and Group 2 and 3 (P=0.298) exhibited a similar survival pattern (data not shown). A univariate model was fitted for each patient characteristic, including age, sex, smoking history, distant metastasis, clinical stage, DLL3, TTF-1, and combination of DLL3 and TTF-1. Univariate analysis indicated that non-distant metastasis (P<0.001), early clinical stage (P<0.001), DLL3-low (P=0.008), TTF-1- (P<0.001) and DLL3-low/TTF-1- (P<0.001) were prognostic factors for improved overall survival of patients with SCLC. All eight characteristics were included in the multivariate analysis using a forward selection (LR) test in SPSS software, with P<0.05 as the entry criterion. Therefore, only significant characteristics were included in the final multivariate cox model. Multivariate analysis indicated that early clinical stage (P<0.001) and DLL3-low/TTF-1- (P=0.001) were independent prognostic factors for improved overall survival of patients with SCLC (Table II).
Figure 4.
Kaplan-Meier survival analyses for patients with SCLC, according to DLL3 and TTF-1. Kaplan-Meier survival analyses for patients with SCLC according to (A) DLL3 and (B) TTF-1. (C) Combination of DLL3 and TTF-1 separated patients into distinct prognostic groups. (D) Combination of DLL3 and TTF-1 segregated patients into two main prognostic groups, Group 1+2+3 and Group 4. Group 1 consisted of DLL3-high/TTF-1+; Group 2 consisted of DLL3-high/TTF-1-; Group 3 consisted of DLL3-low/TTF-1+; and Group 4 consisted of DLL3-low/TTF-1-. SCLC, small-cell lung cancer; DLL3, delta-like protein 3; TTF-1, thyroid transcription factor-1.
Table II.
Cox regression analyses of overall survival of patients with small-cell lung cancer.
| Univariate Cox model | Multivariate Cox modelb | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age (≤60 years vs. >60 years) | 1.05 | 0.80–1.37 | 0.753 | NS | ||
| Sex (male vs. female) | 1.08 | 0.68–1.72 | 0.743 | NS | ||
| Smoking history (no vs. yes) | 1.01 | 0.74–1.36 | 0.968 | NS | ||
| Distant metastasis (negative vs. positive) | 2.01 | 1.52–2.64 | <0.001a | NS | ||
| TNM stage (18) (I vs. II vs. III vs. IV) | 1.82 | 1.49–2.22 | <0.001a | 1.73 | 1.43–2.10 | <0.001a |
| DLL3 (low vs. high) | 1.49 | 1.11–1.99 | 0.008a | NS | ||
| TTF-1 (negative vs. positive) | 2.27 | 1.53–3.34 | <0.001a | NS | ||
| Combination of DLL3 and TTF-1 | ||||||
| (Group 4 vs. Group 1–3) | 3.71 | 1.90–7.25 | <0.001a | 3.26 | 1.67–6.39 | 0.001a |
P<0.05.
All eight variables were included using forward selection likelihood ratio test. HR, hazard ratio; CI, confidence interval; DLL3, delta-like protein 3; TTF-1, thyroid transcription factor-1; NS, not significant; Group 1, DLL3-high/TTF-1+; Group 2, DLL3-high/TTF-1-; Group 3, DLL3-low/TTF-1+; Group 4, DLL3-low/TTF-1-.
Discussion
The aim of the present study was to examine the expression pattern of DLL3 protein in pretreated tumor tissues of patients with SCLC. The main finding was that the intratumoral and intertumoral distribution of DLL3 protein in SCLC is homogeneous, supporting the conclusion that biopsy specimens are a reliable source for DLL3 evaluation for targeted therapy. In addition, the clinical and prognostic significance of DLL3 and TTF-1 for SCLC were examined, given the fact that high DLL3 in SCLCs was associated with the smoking history of the patient, TTF-1 expression and poor survival. One of the most notable findings of the present study was that DLL3-low/TTF-1- was an independent prognostic marker and defined a distinct subpopulation of patients with SCLC with improved overall survival.
Rova-T, a novel DLL3-targeted conjugate, has been reported to exhibit single-agent antitumor activity in preclinical and early clinical studies with a strong correlation between DLL3 expression level and antitumor activity (6,7). These results indicate that DLL3 is a potential predictive biomarker for therapy with Rova-T. Multiple clinical trials of Rova-T are ongoing (20). Therefore, DLL3 expression pattern is essential from a clinical point of view. The present study indicated high consistency of DLL3 expression levels in paired specimens, adding novel information on DLL3 expression and further demonstrating its predictive value for DLL3-targeted agents.
However, these clinical trials support the role of DLL3 as a predictive marker for the therapeutic utility of Rova-T therapy. However, the prognostic value of DLL3 in SCLC remains unclear. One of the main purposes of the present study was to investigate the prognostic value of DLL3. The present study indicated that DLL3-high was an inferior survival marker for SCLC.
Previous preclinical studies suggested that DLL3 may lead to high-grade neuroendocrine tumorigenesis, by inhibiting the Notch receptor activation (7,21). SCLC is positive for TTF-1 in ≤90-95% of cases, due to its neuroendocrine differentiation (12–14). Therefore, the association between DLL3 and TTF-1 protein expression was analyzed. It was indicated that high expression of DLL3 in SCLCs are associated with the expression of TTF-1, suggesting that DLL3 may be associated with the neuroendocrine phenotype. The findings of the present study are in agreement with a recent study with a small sample size, reporting that TTF-1 and DLL3 were highly associated in SCLC (16).
The prognostic value of TTF-1 for patients with SCLC is supported by a limited number of previous studies (13,22,23). Patients with SCLC with TTF-1 expression had worse disease-free survival and overall survival (22). The results of the present study demonstrated that TTF-1 predicts inferior survival, which reinforces the prognostic value of TTF-1 in SCLC. In addition, the prognostic value of the combination of DLL3 and TTF-1 was examined. It was indicated that the combination of DLL3 and TTF-1 was an independent prognostic marker, and had a higher prognostic value compared with a single marker. The cohort was further divided into four subgroups based on TTF-1 and DLL3 protein expression levels. It was indicated that DLL3-low/TTF-1- was an independent marker and defined a distinct molecular subgroup of patients with SCLC exhibiting the optimal prognosis. The combination of the two markers has potential clinical value to stratify patients with SCLC into subgroups of different prognosis.
It should be noted that the optimal cut-off value for DLL3 in the present study was used for predicting overall survival. Further clinical trials to determine the optimal cut-off value for DLL3 as a predictive biomarker for DLL3-targeted agents are required. The main limitation of the present study is the small sample size of paired specimens, due to a limited number of patients with SCLC receiving lobectomy (patients with T1-2N0), dissection or sampling of metastatic site. A total of 1,145 consecutive cases of high-grade pulmonary neuroendocrine carcinomas were reviewed in Guangdong Provincial People's Hospital between January 2006 and June 2015, of which 335 SCLC cases had adequate tissues for IHC detection. In order to reduce selection bias, all eligible cases were recruited. A second limitation is the relatively high proportion of early-censored patients, the majority of whom received treatments at other hospitals following diagnosis or first-line treatment. The original follow-up data was updated. The updated one-year and five-year censored rate of the total cohort were 16.1 (54/335) and 34.6% (116/335), respectively. There was no bias of early-censored cases between Group 1–3 (46/301, censored/total) and Group 4 (8/34; P=0.215; data not shown). Therefore, it is suggested that the survival analyses of the present study are reliable.
In conclusion, this is the first study, to the best of our knowledge, to examine DLL3 expression in Chinese patients with SCLC. Novel information on the homogeneous expression pattern of DLL3 was indicated in the present study, and evidence supporting the reliability of biopsy specimens for evaluating DLL3 expression level in SCLC for targeted therapy has been provided. Additionally, it was indicated that high DLL3 was associated with smoking history, TTF-1 expression and poor survival of patients with SCLC. A total of two subgroups of SCLC with distinct prognoses were further identified, defined by the combination of TTF-1 and DLL3. The combination of the two protein markers has potential clinical value in risk stratification for patients with SCLC.
Acknowledgements
The authors would like to thank Mr. Xin-Chuang Cai from Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences (Guangzhou, China) for support with the archive collection.
Funding
The present study was supported by The National Natural Science Foundation of China (grant nos. 81202111 and 81673031); The Guangdong Medical Science and Technology Research Foundation (grant no. A2017566); and The National Clinical Key Subject Construction Project Fund of China.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
LXY, YHL and ZL participated in the conception and design of the study. YHL, LXY and YFL reviewed all the slides and collected pathologic data. JTZ, DLL, JHY, JH and CL contributed to the acquisition and interpretation of the clinical data. LXY and JH performed immunohistochemical staining. LXY, DLL, JHY, JTZ, XHL and CL performed the statistical analysis. All authors interpreted the data. LXY drafted the manuscript, and YHL, ZL and JH edited it. All authors gave final approval of the version to be published.
Ethics approval and consent to participate
The Research Ethics Committee of Guangdong Provincial People's Hospital (Guangzhou, China) approved the present study (approval no. GDREC2016373H). Due to the retrospective design of the current study and patient anonymization, the review board determined that informed consent was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Baize N, Monnet I, Greillier L, Quere G, Kerjouan M, Janicot H, Vergnenegre A, Auliac JB, Chouaid C. Second-line treatments of small-cell lung cancers. Expert Rev Anticancer Ther. 2017;17:1033–1043. doi: 10.1080/14737140.2017.1372198. [DOI] [PubMed] [Google Scholar]
- 2.Asamura H, Kameya T, Matsuno Y, Noguchi M, Tada H, Ishikawa Y, Yokose T, Jiang SX, Inoue T, Nakagawa K, et al. Neuroendocrine neoplasms of the lung: A prognostic spectrum. J Clin Oncol. 2006;24:70–76. doi: 10.1200/JCO.2005.04.1202. [DOI] [PubMed] [Google Scholar]
- 3.Metro G, Ricciuti B, Chiari R, Baretti M, Falcinelli L, Giannarelli D, Sidoni A, Mountzios G, Crinò L, Bellezza G, et al. Survival outcomes and incidence of brain recurrence in high-grade neuroendocrine carcinomas of the lung: Implications for clinical practice. Lung Cancer. 2016;95:82–87. doi: 10.1016/j.lungcan.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD. Advances in neuroendocrine lung tumors. Ann Oncol. 2010;21(Suppl 7):vii65–71. doi: 10.1093/annonc/mdq380. [DOI] [PubMed] [Google Scholar]
- 5.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378:1741–1755. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 6.Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, Byers LA, Johnson ML, Burris HA, III, Robert F, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: A first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18:42–51. doi: 10.1016/S1470-2045(16)30565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, Desai R, Escarpe PA, Hampl J, Laysang A, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015;7:302ra136. doi: 10.1126/scitranslmed.aac9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: What we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17:725–737. doi: 10.1038/nrc.2017.106. [DOI] [PubMed] [Google Scholar]
- 9.Bauer TM, Spigel D, Ready N, Morgensztern D, Glisson BS, Byers LA, Burris H, Robert F, Strickland DK, Pietanza MC, Govindan R Dylla SJ, Peng S, Rudin C. ORAL02.01: Safety and efficacy of single-agent rovalpituzumab tesirine, a DLL3-targeted ADC, in recurrent or refractory SCLC: Topic: Medical oncology. J Thorac Oncol. 2016;11:S252–S253. doi: 10.1016/j.jtho.2016.09.010. [DOI] [Google Scholar]
- 10.Tanaka K, Isse K, Fujihira T, Takenoyama M, Saunders L, Bheddah S, Nakanishi Y, Okamoto I. Prevalence of delta-like protein 3 expression in patients with small cell lung cancer. Lung Cancer. 2018;115:116–120. doi: 10.1016/j.lungcan.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Sharma SK, Pourat J, Abdel-Atti D, Carlin SD, Piersigilli A, Bankovich AJ, Gardner EE, Hamdy O, Isse K, Bheddah S, et al. Noninvasive interrogation of DLL3 expression in metastatic small cell lung cancer. Cancer Res. 2017;77:3931–3941. doi: 10.1158/0008-5472.CAN-17-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaeda M, Sato H, Ishii J, Miyata C, Kamma H, Shishido-Hara Y, Shimoyamada H, Fujiwara M, Endo T, Tanaka R, et al. Neural lineage-specific homeoprotein BRN2 is directly involved in TTF1 expression in small-cell lung cancer. Lab Invest. 2013;93:408–421. doi: 10.1038/labinvest.2013.2. [DOI] [PubMed] [Google Scholar]
- 13.Misch D, Blum T, Boch C, Weiss T, Crolow C, Griff S, Mairinger T, Bauer TT, Kollmeier J. Value of thyroid transcription factor (TTF)-1 for diagnosis and prognosis of patients with locally advanced or metastatic small cell lung cancer. Diagn Pathol. 2015;10:21. doi: 10.1186/s13000-015-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitamura H, Yazawa T, Sato H, Okudela K, Shimoyamada H. Small cell lung cancer: Significance of RB alterations and TTF-1 expression in its carcinogenesis, phenotype, and biology. Endocr Pathol. 2009;20:101–107. doi: 10.1007/s12022-009-9072-4. [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay S, Katzenstein AL. Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: Utility of an immunohistochemical panel containing TTF-1, napsin a, p63, and CK5/6. Am J Surg Pathol. 2011;35:15–25. doi: 10.1097/PAS.0b013e3182036d05. [DOI] [PubMed] [Google Scholar]
- 16.Cardnell RJ, Li L, Sen T, Bara R, Tong P, Fujimoto J, Ireland AS, Guthrie MR, Bheddah S, Banerjee U, et al. Protein expression of TTF1 and cMYC define distinct molecular subgroups of small cell lung cancer with unique vulnerabilities to aurora kinase inhibition, DLL3 targeting, and other targeted therapies. Oncotarget. 2017;8:73419–73432. doi: 10.18632/oncotarget.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brambilla E, Beasley MB, Austin JHM, Capelozzi VL, Chirieac LR, Devesa SS, Frank GA, Gazdar A, Ishikawa Y, Jen J, et al. Small cell carcinoma. In: Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, editors. WHO classification of tumors of the lung, pleura, thymus and heart. IARC; Lyon: 2015. pp. 62–68. [Google Scholar]
- 18.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151:193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Welti J, Rodrigues DN, Sharp A, Sun S, Lorente D, Riisnaes R, Figueiredo I, Zafeiriou Z, Rescigno P, de Bono JS, Plymate SR. Analytical validation and clinical qualification of a new immunohistochemical assay for androgen receptor splice variant-7 protein expression in metastatic castration-resistant prostate cancer. Eur Urol. 2016;70:599–608. doi: 10.1016/j.eururo.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabari JK, Lok BH, Laird JH, Poirier JT, Rudin CM. Unravelling the biology of SCLC: Implications for therapy. Nat Rev Clin Oncol. 2017;14:549–561. doi: 10.1038/nrclinonc.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman G, Sparrow DB, Kremmer E, Dunwoodie SL. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Hum Mol Genet. 2011;20:905–916. doi: 10.1093/hmg/ddq529. [DOI] [PubMed] [Google Scholar]
- 22.Hiroshima K, Iyoda A, Shida T, Shibuya K, Iizasa T, Kishi H, Tanizawa T, Fujisawa T, Nakatani Y. Distinction of pulmonary large cell neuroendocrine carcinoma from small cell lung carcinoma: A morphological, immunohistochemical, and molecular analysis. Mod Pathol. 2006;19:1358–1368. doi: 10.1038/modpathol.3800659. [DOI] [PubMed] [Google Scholar]
- 23.Myong NH. Thyroid transcription factor-1 (TTF-1) expression in human lung carcinomas: Its prognostic implication and relationship with expressions of p53 and Ki-67 proteins. J Korean Med Sci. 2003;18:494–500. doi: 10.3346/jkms.2003.18.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.