Abstract
Decades of research have shown that women’s and men’s immune systems function differently. During the reproductive years, women have a stronger immune response than men. This gender difference is believed to be controlled by differences in the blood levels of gonadal steroid hormones—including the female hormone, estrogen, which stimulates immune responses, and the male hormone, testosterone, which is immunosuppressive. In both males and females, alcohol exposure suppresses immune responses; however, it is unclear whether there are significant gender differences in this suppression. Chronic exposure to alcohol alters the production of this same set of hormones (i.e., estrogen and testosterone), and hence alcohol’s effects on immunity could involve an indirect mechanism in which alcohol alters hormone levels and, in turn, the hormones regulate immune responses. This article discusses evidence that these hormonal changes play a role in the regulation of the immune response following alcohol exposure in males and females. In addition, the article considers the possible reasons why it takes less time and lower doses of alcohol exposure to cause liver damage in females than in males.
Keywords: immune response, gender differences, chronic AODE (alcohol and other drug effects), alcoholic beverage, hormones, estrogens, testosterone, cytokines, alcoholic liver disorder, literature review
Clinical and experimental research has demonstrated naturally occurring gender differences in immune response, but the reasons for these differences have yet to be determined. This article examines alcohol’s effects on the immune systems of both genders and the differential effects of alcohol on males’ and females’ immune responses. It then discusses whether alcohol-induced changes in stress hormones and in gonadal steroid hormones such as estrogen and testosterone are sufficient to trigger the observed defects in immune response and to explain gender differences in alcohol-induced immune suppression. Finally, the article considers the reasons why women are at higher risk than men of developing liver disease at any given level of alcohol intake.
Alcohol and Immune Responses
An overwhelming amount of evidence reveals that both acute and chronic alcohol exposure suppresses all branches of the immune system, including early responses to infection and the tumor surveillance system (for reviews, see Cook 1998; Diaz et al. 2002; Nelson and Kolls 2002; Messingham et al. 2002). For example, there is a decrease in the ability to recruit and activate germ-killing white blood cells (Deaciuc 1997; Szabo et al. 1999) and an increase in the incidence of breast cancer in people who consume alcohol (Warner-Smith et al. 1998; Zhang et al. 1999).
Some experts suspect that alcohol exerts an “all-or-none” effect on immune response—that is, the presence or absence of alcohol, rather than its amount, dictates the immune response (McGill et al. 1995; Messingham et al. 2002). Other researchers believe that low doses of alcohol—the amount equivalent to a glass of wine—can confer health benefits, including protection against damage to the cardiovascular (Holman et al. 1996) and immune systems (Mendenhall et al. 1997). Such benefits, if they are present, may be attributable to antioxidants in alcoholic beverages such as red wine. In any case, health experts agree that the beneficial effects of antioxidants in some alcoholic beverages are lost if the level of alcohol consumption is elevated (Hanna et al. 1992).
There are several mechanisms by which alcohol impedes immune function. First, alcohol impairs the ability of white blood cells known as neutrophils to migrate to sites of injury and infection, a process called chemotaxis (Bautista 2001). (See the sidebar for a general description of how the immune system works, pp. 261–262) In addition, removing germ-fighting white blood cells (macrophages) and proteins that act as messengers between immune cells (cytokines) from an animal that has not been given alcohol and culturing them in the presence of alcohol, or isolating these cells from humans or animals after administering alcohol, has been shown to alter production of these macrophages and cytokines (Deaciuc 1997; Szabo 1998; Szabo 1999).
How the Immune System Works.
The immune system is designed to provide protection from invading organisms, including bacteria and viruses, tumor cells, dirt, pollen, and other foreign material. Normally, barriers—including the skin and the lining of the lungs and gastrointestinal and reproductive tracts— protect the underlying delicate tissues from the outside environment. However, when there is a breakdown in that protective lining, germs and other irritants can enter the body. The immune system’s function is to conquer these foreign molecules by engulfing them or by destroying them with enzymes or other detoxifying means. In addition to fighting off these foreign invaders, the immune system has evolved to destroy abnormal cells (such as tumor cells) but occasionally reacts against the body’s own normal tissues (autoimmunity).
Innate and Acquired Immunity
There are two principal types of immune response, innate and adaptive (or acquired) immunity, which are distinguished from one another by both their speed and specificity. The innate immune system, so called because it is present from birth, involves nonspecific responses that are the first line of defense against common infectious agents, including bacteria and viruses. This system is generally able to recognize foreign organisms but is unable to distinguish between particular invaders. Thus, an innate response does not require stimulation by sophisticated cell-to-cell interactions to remove bacteria or other foreign material and degrade it.
In contrast to the innate immune system, the more specific adaptive (acquired) immune system must be triggered by a specific virus, bacterium, or other foreign material, which stimulates lymphocytes (see below) to produce antibodies that can combat the foreign substance. At the next exposure, the preformed antibodies will allow the person to respond with an even stronger, more specific response. This is called immunological memory.
Cells of the Immune System
The immune system consists of white blood cells (leukocytes), which are produced in the bone marrow and mature there or in the thymus and other lymphoid organs. Leukocytes circulate in the blood along with oxygen-carrying red blood cells. Under normal conditions, leukocytes leave the circulation and migrate into organs, including the skin, lungs, intestine, and reproductive tract, as these are places where germs can appear. There, they can wait for infectious agents, or they can migrate back through the circulation to other organs. There are three major types of leukocytes.
Neutrophils are the most plentiful of the white blood cells in humans. They are the immune system’s first line of defense, as they contain an arsenal of preformed chemicals known as enzymes, which are capable of destroying bacteria. In addition, they are phagocytic, meaning that they can engulf viruses, bacteria, or other foreign material, protecting the host from further damage. Neutrophils are very short-lived and are often destroyed during the process of fighting infection.
Monocytes are leukocytes that, after migrating to tissues, mature into macrophages. Like neutrophils, macrophages are phagocytic and can remove foreign material and parts of dead cells from the tissues. They too contain enzymes that can destroy infectious material but live longer than neutrophils and do not tend to self-destruct as easily. The tissue macrophage in the liver is called the Kupffer cell.
Lymphocytes, the most selective cells of the immune system, are specialized white blood cells that can combat specific infectious agents. There are two types of lymphocytes: B cells and T cells. B cells, which are responsible for humoral immunity (so-called because it takes place in the body fluids, classically known as the humors), release specialized, soluble proteins known as antibodies into the blood and other body fluids. The antibodies recognize and bind to the surface of foreign substances (i.e., pathogens), immobilizing them and further labeling them as foreign so that they can be more readily taken up by phagocytic cells.
T cells, in contrast, act directly on other cells rather than manufacturing antibodies to combat infectious agents. Because of this direct interaction with other cells, T cells are responsible for cellular immunity. They can be further divided into helper T cells, which recognize foreign invaders and stimulate immune responses from other cells; and cytotoxic T cells, which destroy infected cells. Whereas some of these cells survive only briefly, others are extremely long-lived, including “memory cells,” which are capable of remembering certain features on the foreign molecules so that, if the organism encounters that foreign molecule in the future, it can quickly stimulate its response team.
Communication Between Immune Cells
One form of communication between immune cells is direct cell-to-cell contact, which can occur either as a loose, transient association or as a tighter, more long-lasting encounter. Either way, cells must make physical contact with one another.
In the second form of contact, cells release small proteins called cytokines, which bind to specific receptors on the surface of target cells. This enables cytokines to interact only with the appropriate target cell with no effect on surrounding cells. Although many of the effects of cytokines are local, they have been called the hormones of the immune system, because like hormones, they are transported by the circulating blood.
Cytokines can affect the same cell that produced them, a neighboring cell, or a cell far away. They stimulate or dampen cell proliferation (replication), production of other cytokines, killing of damaged cells or tumor cells (cytotoxicity), and cell migration (chemotaxis). The latter response is controlled by a subset of cytokines called chemokines. Just as there are cells that can stimulate or inhibit immune response, cytokines produced by those cells can regulate a variety of cell functions either positively or negatively.
—Elizabeth J. Kovacs and Kelly A.N. Messingham
Rodent studies also show that animals are more vulnerable to infection after chronic or acute exposure to alcohol (Deaciuc 1997; Cook 1998; Messingham et al. 2002). This increase in susceptibility is equally dramatic in human patients who sustain traumatic injury (Smith and Kraus 1988; Brezel et al. 1988). Those who have consumed alcohol prior to their injury are six times more likely to die than are alcohol-free patients with comparable injuries (McGill et al. 1995). The mechanisms responsible for this increased mortality are unknown, but it is thought that alcohol compromises the immune system’s ability to quickly fight infection by unidentified invaders—a function of the innate immune system (Faunce et al. 1997; Cook 1998; Messingham et al. 2001).
Gender Differences in Immune Response Following Alcohol Exposure
To date, only a handful of studies have directly examined gender differences in the effects of alcohol on inflammatory and immune responses (Grossman et al. 1993; Spitzer and Zhang 1996a, b; Lee and Rivier 1996; Li et al. 1998; Spitzer 1999; Spitzer and Spitzer 2000). These studies were conducted in rodents and employed different methods, including varying the quantity and duration of alcohol exposure. These reports show that in the absence of alcohol exposure, inflammatory and immune responses are stronger in females than in males (Grossman et al. 1993; Spitzer and Zhang 1996a, b; Spitzer 1999). However, the increased immunity in females is nullified by alcohol exposure. For example, in one study, proliferation of white blood cells was suppressed in alcohol-exposed female rats (Grossman et al. 1993); however, investigation also showed that alcohol induced an increase in antibody production. In two other studies, female rats were less able to fight infection when intoxicated (Spitzer and Zhang 1996b; Li et al. 1998). The mechanisms driving these effects remain uncertain. One possibility is that gender differences in inflammatory and immune responses following alcohol exposure stem from alcohol-induced changes in the production of gonadal steroid hormones, such as estrogen and testosterone.
In general, estrogen stimulates immune responses and testosterone is immunosuppressive (Grossman 1989; Morell 1995; Cannon and St. Pierre 1997; Verthelyi 2001; Burger and Dayer 2002). During their reproductive years, females have more vigorous cellular and humoral immune responses than do males (see the sidebar for a description of these two types of immune response). This heightened immunity in females is evidenced by a more developed thymus,1 higher antibody concentrations, and a greater ability to reject tumors and transplanted tissues. Ironically, the enhanced immune function in women of reproductive age is associated with a higher prevalence of autoimmune disorders than is found in postmenopausal women or in men.2
The effects of alcohol on production of the gonadal steroid hormones are well documented (Van Thiel et al. 1987; Gavaler and Van Thiel 1992; Gavaler et al. 1993; Gill 2000; Emanuele and Emanuele 2001). In women, chronic alcohol exposure causes an initial increase in estrogen levels, followed by a marked decrease (Gavaler and Van Thiel 1992; Gavaler et al. 1993). In men, chronic alcohol consumption causes a decrease in testosterone (Emanuele and Emanuele 2001). The alcohol-induced decrease in testosterone levels is significant enough to cause shrinkage (atrophy) of the testes, impotence, and loss of secondary sex characteristics (Van Thiel et al. 1987).
Estrogen and Cytokines
From the limited information available, it is thought that fluctuations in estrogen may alter immune cell function, in part, by increasing or decreasing the production of cytokines (Olsen and Kovacs 1996; Cannon and St. Pierre 1997; Verthelyi 2001; Burger and Dayer 2002). There are several pieces of evidence for this idea. First, researchers found that removing the ovaries of adult rodents (eliminating the primary source of estrogen) lowered the level of cytokine production by certain types of white blood cells (Frazier-Jessen and Kovacs 1995; Chao et al. 1995; D’Agostino et al. 1999; Deshpande et al. 1997). This lower level of cytokine production was comparable to that of males and could be restored by administering estrogen (Gregory et al. 2000a).
In other studies, drugs known as estrogen receptor antagonists inhibited the effect of estrogen on immune cells in animals (Gregory et al. 2000b; Wu et al. 1999). While receptor antagonists are bound to the same receptors that normally interact with estrogen, they block the binding of the hormone. Thus, it is possible to alter immune responses by blocking estrogen at one of its sites of action in white blood cells.
Further evidence that estrogen affects immune cell function, in part, by altering production of cytokines comes from cell-culture studies in which estrogen was added to a culture of white blood cells (Burger and Dayer 2002). The effects of estrogen on cytokine production by immune target cells may involve direct interaction (binding) of the hormone and hormone receptors within those cells (Hall et al. 2001). The idea of direct effects of estrogen on target cells is supported by the existence of estrogen receptors not only in reproductive tissues, including the uterus, ovaries, and testes, where one would expect the hormone’s actions to occur, but also in white blood cells (Weusten et al. 1986; Gulshan et al. 1990; Benten et al. 2001).
Alcohol, Stress Responses, and Immunity
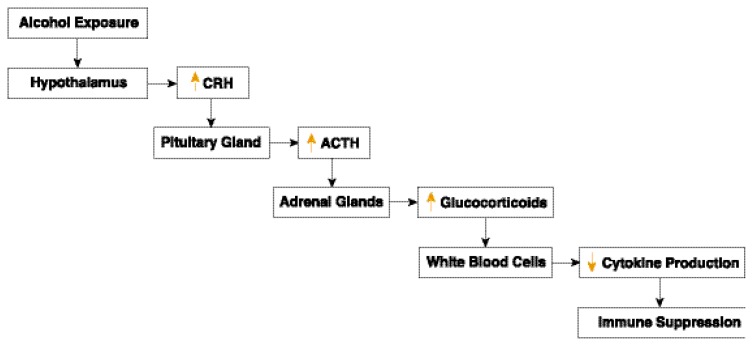
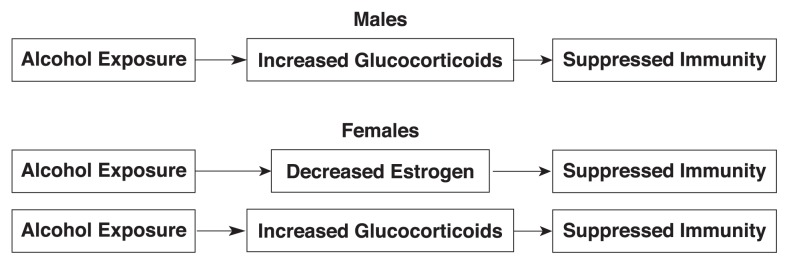
Like other stressors, alcohol stimulates a neuroendocrine network known as the hypothalamic–pituitary–adrenal (HPA) axis, resulting in a dampening of the immune response (Eskandari and Sternberg 2002) (see figure 1). This process begins with activation of the hypothalamus (near the base of the brain), which produces a molecule called corticotropin-releasing hormone (CRH). This triggers the pituitary gland (below the hypothalamus) to secrete adrenal corticotropic hormone (ACTH). Finally, ACTH stimulates the adrenal glands (above the kidneys) to release glucocorticoids (cortisol in humans and corticosterone in rodents). These steroid hormones, which direct the activity of many cell types, are transmitted throughout the body in the blood. At high levels, they suppress inflammatory and immune responses (Guyre and Goulding 1993; Da Silva 2002). Several studies have documented that under resting (baseline) conditions and in response to stress, females have higher levels of glucocorticoids than do men (Kant et al. 1983; Chasari et al. 1995). Furthermore, estrogen stimulates glucocorticoid production in females (Burgess and Handa 1992; Carey et al. 1995), whereas testosterone suppresses its production in both male and female subjects (Carlstrom and Stege 1990; De Weerdt and Gooren 1992; Handa et al. 1994). Alcohol exposure stimulates glucocorticoid production in both males and females (Ogilvie et al. 1998; Eskandari and Sternberg 2002). Thus, there are two possible pathways by which alcohol-induced changes in steroid hormones could suppress immune responses in females, whereas there is only one such potential pathway in males (as shown in figure 2). Further study will be required to determine if and how the two pathways interact to mediate alcohol-induced effects on immune function in females.
Figure 1.
Involvement of the hypothalamic–pituitary–adrenal axis in alcohol-induced immune suppression. Alcohol exposure stimulates the hypothalamus to produce corticotropin-releasing hormone (CRH). This triggers the pituitary gland to secrete adrenal corticotropic hormone (ACTH), which in turn stimulates the adrenal glands to release glucocorticoids. At high levels, glucocorticoids signal white blood cells to alter cytokine production, suppressing inflammatory and immune responses. (Yellow arrows indicate increased or decreased production or activity.)
Figure 2.
Hormone-dependent pathways by which alcohol could suppress immune responses. As the figure illustrates, there is only one potential pathway in males, but there are two in females, which could result in an additive effect.
Gender, Alcohol, and Liver Damage
Epidemiologic evidence clearly indicates that the adverse consequences of alcohol consumption, including severe liver disease, such as alcoholic cirrhosis, develop more quickly and require lower levels of alcohol exposure for females than for males (Ashley et al. 1977; Loft et al. 1987; Schenker 1997; for reviews, see Thurman 2000; Diehl 2002). At any given level of alcohol intake, women are at higher risk than men of developing liver disease (Ashley et al. 1977; Loft et al. 1987; Dawson 1994; Schenker 1997). It has been shown that a daily alcohol ingestion of as low as two drinks per day increases the risk of developing cirrhosis in women, although at least four drinks per day are required to increase this risk in men (Ashley et al. 1977; Loft et al. 1987; Thomasson 1995; Frezza et al. 1990; Taylor et al. 1996). These observations were made taking into account differences in body weight, fat distribution, body water, and other potentially confounding variables.
The mechanisms responsible for the gender difference in alcohol-related liver injury are currently under intense investigation and have been better described in animal studies (Kono et al. 2000; Nanji et al. 2001). Performing studies in animals allows the investigator to include experiments involving hormone manipulations that would not be feasible in human experimentation. These experiments could include removing ovaries (the primary site of estrogen production) or giving a hormone receptor antagonist (i.e., a molecule that blocks the hormone from binding to its receptor).
It is possible that gender differences in alcohol-related liver disease could be explained by gender differences in:
The breakdown and elimination of alcohol and its byproducts, including the resulting differences in acetaldehyde levels within the liver (Thomasson 1995; Li et al. 2000).
The level of activation of inflammatory and immune cells within the liver in response to alcohol ingestion, including Kupffer cells (Adachi et al. 1994; Kono et al. 2000; Nanji et al. 2001; McClain et al. 2002).3 Upon stimulation, these cells produce free oxygen radicals and cytokines, which damage and destroy liver cells (Adachi et al. 1994).
The amount of alcohol that is metabolized in the stomach (first-pass metabolism). Some research has indicated that women break down less alcohol in the stomach than men do, leading to higher blood alcohol levels—and hence greater risk to the liver—for a given dose of alcohol (Pozzato et al. 1995; Baraona et al. 2001).
Summary
Taken together, these studies show clearly that there are dramatic suppressive effects of both acute and chronic alcohol exposure on inflammation and immunity, regardless of gender. This results in decreased ability of the immune system to fight infections and tumors. The decrease in immunity after consumption of larger quantities of alcohol is in marked contrast to the effects of very low levels of some alcoholic beverages (such as a single glass of red wine), which contain immunoprotective antioxidants. By depressing estrogen levels, chronic or acute alcohol exposure may cause females to lose the important boost to the immune system that estrogen normally provides. This could act additively or synergistically with an elevation in immunosuppressive glucocorticoids (through activation of the HPA axis) to attenuate immune response, thus leading to a weakened ability to fight infections and tumors. Finally, although chronic alcohol exposure causes liver damage in both males and females, it takes less alcohol and shorter periods of consumption to raise the risk of liver damage for females than for males. Like the observed gender differences in alcohol-induced immune suppression, this effect may involve the combined effect of stimulating glucocorticoid production and inhibiting estrogen production (see figure 2).
Further studies will be required to determine whether the alcohol-induced changes in gonadal steroid hormone production are sufficient to explain the observed gender differences in immune function. These will require using a similar model system in which both males and females are given alcohol at doses designed to raise blood alcohol levels to the same extent, after which immune responses can be examined. Because of the complexity of studying these parameters in humans, it may be necessary to conduct these studies in animal models of alcohol exposure. By using animal models, it will also be possible to manipulate the levels of estrogen, testosterone, and glucocorticoids by removing organs (ovaries, testes, and adrenal glands, respectively) and administering hormones and hormone antagonists to determine the role of those hormones in regulating inflammatory and immune responses after alcohol exposure.
Footnotes
The thymus is a gland located in the upper chest that is involved in the maturation of immune cells.
Although estrogen is present in males, its concentration is too low to affect immune response.
Kupffer cells are star-shaped immune cells that reside in the microscopic blood vessels of the liver. They are phagocytic, meaning that they are capable of ingesting other cells and foreign particles. Alcohol exposure can cause leakiness of the digestive tract, which can result in the release of endotoxins (bacterial cell wall products) from the gut into the blood. These endotoxins travel through the blood to the liver, where they can activate Kupffer cells.
This work was supported in part by the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism grants AA–12034 and AA–12034–S1, and the Illinois Excellence in Academic Medicine Grant.
References
- Adachi Y, Bradford BU, Guo W, et al. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–460. [PubMed] [Google Scholar]
- Ashley MJ, Olin JS, le Riche WH, et al. Morbidity in alcoholics: Evidence for accelerated development of disease in women. Archives of Internal Medicine. 1977;137:883–887. doi: 10.1001/archinte.137.7.883. [DOI] [PubMed] [Google Scholar]
- Baraona E, Abittan CS, Dohmen K, et al. Gender differences in pharmacokinetics of alcohol. Alcoholism: Clinical and Experimental Research. 2001;25:502–507. [PubMed] [Google Scholar]
- Bautista AP. Free radicals, chemokines, and cell injury in HIV–1 and SIV infections and alcoholic hepatitis. Free Radical Biology Medicine. 2001;31:1527–1532. doi: 10.1016/s0891-5849(01)00745-6. [DOI] [PubMed] [Google Scholar]
- Benten WP, Stephan C, Lieberherr M, et al. Estradiol signaling via sequestrable surface receptors. Endocrinology. 2001;142:1669–1677. doi: 10.1210/endo.142.4.8094. [DOI] [PubMed] [Google Scholar]
- Brezel BS, Kassenbrock JM, Stein JM. Burns in substance abusers and in neurologically and mentally impaired patients. Journal of Burn Care & Rehabilitation. 1988;9:169–171. doi: 10.1097/00004630-198803000-00009. [DOI] [PubMed] [Google Scholar]
- Burger D, Dayer JM. Cytokines, acute-phase proteins, and hormones: IL–1 and TNF-alpha production in contact-mediated activation of monocytes by T lymphocytes. Annals of the New York Academy of Sciences. 2002;966:464–473. doi: 10.1111/j.1749-6632.2002.tb04248.x. [DOI] [PubMed] [Google Scholar]
- Burgess L, Handa RJ. Chronic estrogen induced alterations in adrenocorticotropin and cortricosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Cannon JG, StPierre BA. Gender differences in host defense mechanisms. Journal of Psychiatric Research. 1997;31:99–113. doi: 10.1016/s0022-3956(96)00055-6. [DOI] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koenig J, et al. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. Journal of Endocrinology. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Carlstrom K, Stege R. Adrenocortical function in prostatic cancer patients: Effects of orchidectomy or different modes of estrogen treatment on basal steroid levels and on the response to exogenous adrenocorticotropic hormone. Urology International. 1990;45:160–163. doi: 10.1159/000281699. [DOI] [PubMed] [Google Scholar]
- Chao TC, Van Alten PJ, Greager JA, et al. Steroid sex hormones regulate the release of tumor necrosis factor by macrophages. Cellular Immunology. 1995;160:43–49. doi: 10.1016/0008-8749(95)80007-6. [DOI] [PubMed] [Google Scholar]
- Chasari A, Carino M, Perone M, et al. Sex and strain variability in the rat hypothalamic-pituitary-adrenal axis function. Journal of Endocrinological Investigation. 1995;18:25–33. doi: 10.1007/BF03349692. [DOI] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system—A review. Alcoholism: Clinical and Experimental Research. 1998;22:1927–1942. [PubMed] [Google Scholar]
- D’Agostino P, Milano S, Barbera C, et al. Sex hormones modulate inflammatory mediators produced by macrophages. Annals of the New York Academy of Sciences. 1999;876:426–429. doi: 10.1111/j.1749-6632.1999.tb07667.x. [DOI] [PubMed] [Google Scholar]
- Da Silva JAP. Sex hormones and glucocorticoids: Interactions with the immune system. Annals of the New York Academy of Sciences. 2002;699:158–165. doi: 10.1111/j.1749-6632.1999.tb07628.x. [DOI] [PubMed] [Google Scholar]
- Dawson DA. Consumption indicators of alcohol dependence. Addiction. 1994;89:345–350. doi: 10.1111/j.1360-0443.1994.tb00901.x. [DOI] [PubMed] [Google Scholar]
- De Weerdt O, Gooren LJ. Patterns of serum cortisol in ovariectomized females with and without androgen administration. Hormone Metabolism Research. 1992;28:82–84. doi: 10.1055/s-2007-1003261. [DOI] [PubMed] [Google Scholar]
- Deaciuc IV. Alcohol and cytokine networks. Alcohol. 1997;14:421–430. doi: 10.1016/s0741-8329(97)00039-6. [DOI] [PubMed] [Google Scholar]
- Deshpande R, Khalili H, Pergolizzi RG, et al. Estradiol down-regulates LPS-induced cytokine production and NF-κB activation in murine macrophages. American Journal of Reproductive Immunology. 1997;38:46–54. doi: 10.1111/j.1600-0897.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Diaz LE, Montero A, Gonzalez-Gross M, et al. Influence of alcohol consumption on immunological status: A review. European Journal of Clinical Nutrition. 2002;56(Suppl 3):S50–S52. doi: 10.1038/sj.ejcn.1601486. [DOI] [PubMed] [Google Scholar]
- Diehl AM. Liver disease in alcohol abusers: Clinical perspective. Alcohol. 2002;27:7–11. doi: 10.1016/s0741-8329(02)00204-5. [DOI] [PubMed] [Google Scholar]
- Emanuele MA, Emanuele N. Alcohol and the male reproductive system. Alcohol Research & Health. 2001;25:282–287. [PMC free article] [PubMed] [Google Scholar]
- Eskandari F, Sternberg EM. Neural-immune interactions in health and disease. Annals of the New York Academy of Sciences. 2002;966:20–27. doi: 10.1111/j.1749-6632.2002.tb04198.x. [DOI] [PubMed] [Google Scholar]
- Faunce DE, Gregory MS, Kovacs EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. Journal of Leukocyte Biology. 1997;62:733–740. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- Frazier-Jessen MR, Kovacs EJ. Estrogen modulation of JE/monocyte chemoattractant protein-1 mRNA expression in murine macrophages. Journal of Immunology. 1995;154:1838–1845. [PubMed] [Google Scholar]
- Frezza M, di Padova C, Pozzato G, et al. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. New England Journal of Medicine. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- Gavaler JS, Van Thiel DH. The association between moderate alcoholic beverage consumption and serum estradiol and testosterone levels in normal postmenopausal women: Relationship to the literature. Alcoholism: Clinical and Experimental Research. 1992;16:87–92. doi: 10.1111/j.1530-0277.1992.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Gavaler JS, Deal SR, Van Thiel DH, et al. Alcohol and estrogen levels in postmenopausal women: The spectrum of effect. Alcoholism: Clinical and Experimental Research. 1993;17:786–90. doi: 10.1111/j.1530-0277.1993.tb00842.x. [DOI] [PubMed] [Google Scholar]
- Gill J. The effects of moderate alcohol consumption on female hormone levels and reproductive function. Alcohol and Alcoholism. 2000;35:417–423. doi: 10.1093/alcalc/35.5.417. [DOI] [PubMed] [Google Scholar]
- Gregory MS, Faunce DE, Duffner LA, et al. Estrogen mediates the gender difference in post-burn immunosuppression. Journal of Endocrinology. 2000a;146:129–138. doi: 10.1677/joe.0.1640129. [DOI] [PubMed] [Google Scholar]
- Gregory MS, Faunce DE, Kovacs EJ. The gender difference in cell-mediated immunity following thermal injury is mediated in part by elevated levels of interleukin-6. Journal of Leukocyte Biology. 2000b;67:319–326. [PubMed] [Google Scholar]
- Grossman CJ. Possible underlying mechanisms of sexual dimorphism in the immune response, fact and hypothesis. Journal of Steroid Biochemistry. 1989;34:241–251. doi: 10.1016/0022-4731(89)90088-5. [DOI] [PubMed] [Google Scholar]
- Grossman CJ, Neinabar M, Mendenhall CL, et al. Sex differences in the effects of alcohol on immune response in male and female rats. Alcoholism: Clinical and Experimental Research. 1993;17:832–840. doi: 10.1111/j.1530-0277.1993.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Gulshan S, McCruden AB, Stimson WH. Oestrogen receptors in macrophages. Scandinavian Journal of Immunology. 1990;31:691–697. doi: 10.1111/j.1365-3083.1990.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Guyre PM, Goulding N. Glucocorticoids, lipocortins and the immune response. Current Opinion in Immunology. 1993;5:108–113. doi: 10.1016/0952-7915(93)90089-b. [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. Journal of Biological Chemistry. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nutley KM, Lorens SA, et al. Androgen regulation of adrenocorticotrophin and corticosterone secretion in the male following novelty and foot shock stress. Physiology and Behavior. 1994;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Hanna E, Dufour MC, Elliott S, et al. Dying to be equal: Women, alcohol and cardiovascular disease. British Journal of Addiction. 1992;87:1593–1597. doi: 10.1111/j.1360-0443.1992.tb02667.x. [DOI] [PubMed] [Google Scholar]
- Holman CDJ, English DR, Milne E, et al. Meta-analysis of alcohol and all cause mortality: A validation of NHMRC recommendations. Medical Journal of Australia. 1996;164:141–145. doi: 10.5694/j.1326-5377.1996.tb122011.x. [DOI] [PubMed] [Google Scholar]
- Kant GJ, Lenox RH, Bunnell BN, et al. Comparison of stress responses in male and female rats: Pituitary cyclic AMP and plasma prolactin, growth hormone and corticosterone. Psychoneuroendocrinology. 1983;8:421–428. doi: 10.1016/0306-4530(83)90021-5. [DOI] [PubMed] [Google Scholar]
- Kono H, Wheeler MD, Rusyn I, et al. Gender differences in early alcohol-induced liver injury: Role of CD14, NF-κB, and TNF-κ. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2000;278:G652–G661. doi: 10.1152/ajpgi.2000.278.4.G652. [DOI] [PubMed] [Google Scholar]
- Lee S, Rivier C. Gender differences in the effect of prenatal alcohol exposure on the hypothalamic-pituitary-adrenal axis response to immune signals. Psychoneuroendocrinology. 1996;21:145–155. doi: 10.1016/0306-4530(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Li X, Grossman CJ, Mendenhall CL, et al. Host response to mycobacterial infection in the alcoholic rat: Male and female dimorphism. Alcohol. 1998;16:207–212. doi: 10.1016/s0741-8329(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Li T-K, Beard JD, Orr WE, et al. Variation in ethanol pharmacokinetics and perceived gender and ethnic differences in alcohol elimination. Alcoholism: Clinical and Experimental Research. 2000;24:415–416. [PubMed] [Google Scholar]
- Loft S, Olesen K, Dossing M. Increased susceptibility to liver disease in relation to alcohol consumption in women. Scandinavian Journal of Gastroenterology. 1987;22:1251–1256. doi: 10.3109/00365528708996472. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Hill DB, Song A, et al. Monocyte activation in alcoholic liver disease. Alcohol. 2002;27:53–61. doi: 10.1016/s0741-8329(02)00212-4. [DOI] [PubMed] [Google Scholar]
- McGill V, Kowal-Vern A, Fisher SG, et al. The impact of substance use on mortality and morbidity from thermal injury. Journal of Trauma. 1995;38:931–934. doi: 10.1097/00005373-199506000-00019. [DOI] [PubMed] [Google Scholar]
- Mendenhall CL, Theus SA, Roselle GA, et al. Biphasic in vivo immune function after low-versus high-dose alcohol consumption. Alcohol. 1997;14:255–260. doi: 10.1016/s0741-8329(96)00150-4. [DOI] [PubMed] [Google Scholar]
- Messingham KAN, Heinrich SA, Kovacs EJ. Estrogen restores cellular immunity in injured male mice via suppression of inter-leukin-6 production. Journal of Leukocyte Biology. 2001;70:887–895. [PubMed] [Google Scholar]
- Messingham KAN, Faunce DE, Kovacs EJ. Alcohol, injury and cellular immunity. Alcohol. 2002;28:137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- Morell V. Zeroing in on how hormones affect the immune system. Science. 1995;269:773–775. doi: 10.1126/science.7638587. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Jokelainen K, Fotouhinia M, et al. Increased severity of alcoholic liver injury in female rats: Role of oxidative stress, endotoxin, and chemokines. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2001;281:G1348–G1356. doi: 10.1152/ajpgi.2001.281.6.G1348. [DOI] [PubMed] [Google Scholar]
- Nelson S, Kolls JK. Alcohol, host defence and society. Nature Reviews Immunology. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Weiss B, et al. Mechanisms mediating the influence of alcohol on the hypothalamic-pituitary-adrenal axis responses to immune and nonimmune signals. Alcoholism: Clinical and Experimental Research. 1998;22(Suppl 5):243S–247S. doi: 10.1097/00000374-199805001-00005. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocrinology Reviews. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- Pozzato G, Moretti M, Franzin F, et al. Ethanol metabolism and aging: The role of “first pass metabolism” and gastric alcohol dehydrogenase activity. Journal of Gerontology. 1995;50:B135–B141. doi: 10.1093/gerona/50a.3.b135. [DOI] [PubMed] [Google Scholar]
- Schenker S. Medical consequences of alcohol abuse: Is gender a factor? Alcoholism: Clinical and Experimental Research. 1997;21:179–181. doi: 10.1111/j.1530-0277.1997.tb03746.x. [DOI] [PubMed] [Google Scholar]
- Smith GS, Kraus JF. Alcohol and residential, recreational, and occupational injuries: A review of the epidemiologic evidence. Annual Review of Public Health. 1988;9:99–121. doi: 10.1146/annurev.pu.09.050188.000531. [DOI] [PubMed] [Google Scholar]
- Spitzer JA. Gender differences in some host defense mechanisms. Lupus. 1999;8:380–383. doi: 10.1177/096120339900800510. [DOI] [PubMed] [Google Scholar]
- Spitzer JA, Spitzer JJ. Lipopolysaccharide tolerance and ethanol modulate hepatic nitric oxide production in a gender-dependent manner. Alcohol. 2000;21:27–35. doi: 10.1016/s0741-8329(99)00098-1. [DOI] [PubMed] [Google Scholar]
- Spitzer JA, Zhang P. Gender differences in neutrophil function and cytokine-induced neutrophil chemoattractant generation in endotoxic rats. Inflammation. 1996a;20:485–498. doi: 10.1007/BF01487041. [DOI] [PubMed] [Google Scholar]
- Spitzer JA, Zhang P. Gender differences in phagocytic responses in the blood and liver, and the generation of cytokine-induced neutrophil chemoattractant in the liver of acutely ethanol-intoxicated rats. Alcoholism: Clinical and Experimental Research. 1996b;20:914–920. doi: 10.1111/j.1530-0277.1996.tb05271.x. [DOI] [PubMed] [Google Scholar]
- Szabo G. Monocytes, alcohol use, and altered immunity. Alcoholism: Clinical and Experimental Research. 1998;22(Suppl 5):216S–219S. doi: 10.1097/00000374-199805001-00002. [DOI] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defense. Alcohol and Alcoholism. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Szabo G, Chavan S, Mandrekar P, et al. Acute alcohol consumption attenuates interleukin-8 (IL–8) and monocyte chemoattractant peptide-1 (MCP–1) induction in response to ex vivo stimulation. Journal of Clinical Immunology. 1999;19:67–76. doi: 10.1023/a:1020518703050. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Dolhert N, Friedman L, et al. Alcohol elimination and simulator performance of male and female aviators: A preliminary report. Aviation Space and Environmental Medicine. 1996;67:407–413. [PubMed] [Google Scholar]
- Thomasson HR. Gender differences in alcohol metabolism. In: Galanter M, editor. Recent Developments in Alcoholism: Women and Alcoholism. New York: Plenum Press; 1995. pp. 163–179. [DOI] [PubMed] [Google Scholar]
- Thurman RG. Sex-related liver injury due to alcohol involves activation of Kupffer cells by endotoxin. Canadian Journal of Gastroenterology. 2000;14(Suppl D):129D–135D. doi: 10.1155/2000/735262. [DOI] [PubMed] [Google Scholar]
- Van Thiel DH, Gavaler JS, Rosenblum ER, et al. Effects of ethanol on endocrine cells: Testicular effects. Annals of the New York Academy of Sciences. 1987;492:287–302. doi: 10.1111/j.1749-6632.1987.tb48682.x. [DOI] [PubMed] [Google Scholar]
- Verthelyi D. Sex hormone as immunomodulators in health and disease. International Immunopharmacology. 2001;1:983–993. doi: 10.1016/s1567-5769(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Warner-Smith SA, Speigelman D, Yaun SS, et al. Alcohol and breast cancer in women: A pooled analysis of cohort studies. JAMA: Journal of the American Medical Association. 1998;279:535–540. doi: 10.1001/jama.279.7.535. [DOI] [PubMed] [Google Scholar]
- Weusten JJ, Blankenstein MA, Gmelig-Meyling FH, et al. Presence of oestrogen receptors in human blood mononuclear cells and thymocytes. Acta Endocrinologica. 1986;112:409–414. doi: 10.1530/acta.0.1120409. [DOI] [PubMed] [Google Scholar]
- Wu AH, Pike MC, Stram DO. Meta-analysis: Dietary fat intake, serum estrogen levels, and the risk of breast cancer. Journal of the National Cancer Institute. 1999;91:529–534. doi: 10.1093/jnci/91.6.529. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kreger BE, Dorgan JF, et al. Alcohol consumption and risk of breast cancer: The Framingham study revisited. American Journal of Epidemiology. 1999;149:93–101. doi: 10.1093/oxfordjournals.aje.a009791. [DOI] [PubMed] [Google Scholar]