Abstract
Long non-coding RNA GATA6 antisense RNA 1 (lncRNA GATA6-AS) is a recently identified lncRNA that is involved in endothelial-mesenchymal transition. The present study aimed to investigate the involvement of GATA6-AS in the progression of mantle cell lymphoma (MCL). It was found that plasma lncRNA GATA6-AS expression level was downregulated in patients with MCL, compared with that in healthy controls. Downregulation of lncRNA GATA6-AS has potential diagnostic value in early stage MCL. Overexpression of lncRNA GATA6-AS resulted in inhibited glucose uptake in the human cell lines JVM-2 and Z-138 MCL. Inhibited expression of glucose transporter 1 (GLUT1) was observed in MCL cells following lncRNA GATA6-AS overexpression, whilst GLUT1 overexpression did not alter the expression of lncRNA GATA6-AS. Additionally, lncRNA GATA6-AS overexpression inhibited, whilst GLUT1 overexpression promoted the proliferation of JVM-2 and Z-138 MCL cells; GLUT1 overexpression partially reversed the inhibitory effects of lncRNA GATA6-AS overexpression. It was therefore concluded that lncRNA GATA6-AS may inhibit cancer cell proliferation in MCL by downregulating GLUT1.
Keywords: mantle cell lymphoma, glucose transporter 1, long non-coding RNA GATA6 antisense RNA 1, glucose uptake, proliferation
Introduction
Tumor growth is the basis for all aspects of cancer development (1); therefore, the inhibition of tumor cell proliferation is considered to be a promising therapeutic strategy for cancer treatment (2). Mantle cell lymphoma (MCL), as a rare subtype of non-Hodgkin lymphoma is associated with poor patient prognosis even after active treatment (3). Following great efforts to improve the treatment of MCL, a series of novel drugs have been developed, the uses of which have resulted in markedly improved patient outcomes (4,5). However, the median survival time of patients with MCL remains at 10 years, thus further improvements are required (6).
The reprogramming of glucose metabolism is frequently observed in cancer cells (7), and accelerated glucose metabolism is able to distinguish cancer cells from normal cells (8). The initiation of glucose metabolism requires the uptake of glucose into cells, in which glucose transporter 1 (GLUT1) facilitates the transport of glucose across the mammalian plasma membrane (9). Overexpression of GLUT1 is believed to contribute to the proliferation of cancer cells (10,11). The recently identified long non-coding RNA GATA6 antisense RNA 1 (lncRNA GATA6-AS) plays a pivotal role in endothelial-mesenchymal transition (12). Preliminary microarray data, prior to the present study, identified downregulation of lncRNA GATA6-AS in patients with MCL. In the present study it was revealed that lncRNA GATA6-AS may inhibit cancer cell proliferation in MCL by downregulating GLUT1.
Materials and methods
Human samples and cell lines
Plasma samples were derived from the blood of 47 patients with MCL (patient group) and 42 healthy volunteers (control group), who were admitted to the Jilin Central Hospital (Jilin, China) between January 2015 and May 2018. All healthy volunteers possessed normal physiological conditions, as determined by systemic physiological examination. The inclusion criteria for patients were as follows: i) Patients with MCL at stage I or II, confirmed by histopathological testing; and ii) patients fully understood the experimental protocol and signed informed content. Exclusion criteria: i) Patients with MCL in addition to another disease/s; and ii) patients who had received treatment up to 3 months before admission. The patient group was composed of 25 males and 22 females, aged between 26 and 67 years (mean age, 46.8±4.8 years). The control group was composed of 22 females and 20 males, and aged between 25 and 66 years, (mean age, 45.9±4.4 years). Both groups possessed a similar age and gender distribution. The present study was approved by the Ethics committee of Jilin Central Hospital, and all participants gave written informed consent to participate.
JVM-2 and Z-138 human MCL cell lines were provided by the American Type Culture Collection (ATCC). ATCC-formulated RPMI-1640 medium (ATCC; cat. no. 30–2001) supplemented with 10% fetal bovine serum (ATCC; cat. no. 30-2020) was used to culture cells under normal conditions (37°C; 5% CO2).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The Monarch® Total RNA Miniprep kit (New England BioLabs, Inc.) was used to extract total RNA from cells and patient samples, and the High-Capacity cDNA RT kit (Thermo Fisher Scientific, Inc.) was used to synthesize cDNA (both according to the manufacturer's protocol) using following conditions: 25°C for 5 min, 55°C for 20 min and 80°C for 5 min. The SuperScript III Platinum One-Step RT-qPCR kit (Thermo Fisher Scientific, Inc.) was used to prepare all PCR reactions. The primer sequences were as follows: lncRNA GATA6-AS forward, 5′-ATGCGCTTTTTGCCCTGAAG-3′, and reverse, 5′-AGGTCAGCTGGGGAATGTTG-3′; β-actin forward, 5′-GACCTCTATGCCAACACAGT-3′, and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′. Thermocylcing conditions: 95°C for 2 min, followed by 40 cycles of 95°C for 15 sec and 56°C for 30 sec. RNA expression levels were quantified using the 2−ΔΔCq method (13), and normalized to β-actin.
Cell transfection
lncRNA GATA6-AS and GLUT1 expression vectors were designed and synthesized by Shanghai GenePharma Co., Ltd. Cells were cultured to 80–90% confluence and Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to transfect cancer cells with the appropriate vectors (15 nM). Cells transfected with empty vector were used as the negative control (NC), and untransfected cells were used as the control (C). lncRNA GATA6-AS and GLUT1 expression was detected 12 h post-transfection. The overexpression rates of lncRNA GATA6-AS and GLUT1 reached 180% (180–200%) prior to subsequent experimentation.
Glucose uptake assay
Following transfection, cells were harvested and counted. Subsequently, 3×105 cells were washed with PBS and resuspended in 2 ml Krebs-Ringer-HEPES (KRH) buffer, which was composed of 25 mM HEPES (pH 7.4), 120 mM NaCl, 1.3 mM CaCl2, 1.2 mM MgSO4, 1.3 mM KH2PO4 and 5 mM KCl. To initiate glucose uptake, 1 µCi of [3H]-2-deoxyglucose (PerkinElmer, Inc.) was added and cells were cultured at 37°C for 30 min. Cells were then washed in ice-cold KRH buffer to terminate glucose uptake. Liquid scintillation spectrometry was used to measure radioactivity, and the [3H]-2-deoxyglucose content of the cells was expressed as disintegrations per minute.
In vitro cell proliferation assay
Following transfection, the Cell Counting Kit-8 (CCK-8; Sigma-Aldrich; Merck KGaA) assay was used to assess cell proliferation ability. Briefly, cells were harvested to prepare a single cell suspension (3×104 cells/ml), which was transferred to a 96-well plate (100 µl/well). Cells were incubated at 37°C, 5% CO2, and CCK-8 solution was added at 24, 48, 72 and 96-h time points. Optical density values were determined using the Fisherbrand™ accuSkan™ GO UV/Vis Microplate Spectrophotometer (Thermo Fisher Scientific, Inc.) at 450 nm.
Western blot analysis
Western blotting was performed using conventional methods. RIPA solution (Sangon Biotech Co., Ltd.,) was used to extract the total protein from cells, and the protein concentrations were determined using a bicinchoninic acid assay kit (Sangon Biotech Co., Ltd.). Electrophoresis was performed using 10% SDS-PAGE gels with 35 µg protein per lane. Following gel transfer to PVDF membranes, blocking was performed using PBS containing 5% non-fat milk at room temperature for 2 h. Primary, rabbit anti-human antibodies for GLUT1 (1:1,200; cat. no. ab15309) and GAPDH (1:2,000; cat. no. ab8245) were purchased from Abcam, and incubated with the membranes at 4°C for 12 h. A secondary, goat anti-rabbit IgG-HRP antibody (1:1,000; cat. no. MBS435036) was purchased from MyBioSource, Inc., and incubation was performed at room temperature for 2 h. Signal development was performed using ECL™ Select Western Blotting Detection Reagent (Sigma-Aldrich; Merck KGaA) and ImageJ v1.46 software (National Institutes of Health) was used to capture gel images.
Statistical analysis
The data were processed using GraphPad prism 6 (GraphPad Software, Inc.). All experiments were performed in triplicate and the data are presented as the mean ± standard deviation. Student's t-test was used for comparisons between two groups, and one-way ANOVA followed by Tukey's test was used for comparisons among multiple groups. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic value of plasma lncRNA GATA6-AS in MCL, with MCL patients as true positive cases, and healthy volunteers as true negative cases. P<0.05 was considered to indicate a statistically significant difference.
Results
Downregulation of lncRNA GATA6-AS distinguishes MCL patients from healthy controls
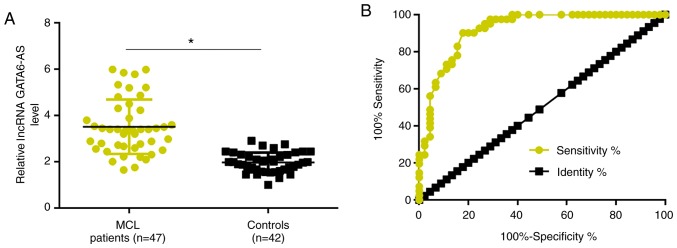
RT-qPCR was performed to detect the expression of lncRNA GATA6-AS in the plasma of patients with MCL and healthy controls. Compared with the healthy controls, plasma expression levels of lncRNA GATA6-AS were significantly reduced in patients with MCL (P<0.05; Fig. 1A). ROC curve analysis was performed to evaluate the diagnostic value of plasma lncRNA GATA6-AS in MCL. As revealed in Fig. 2B, the area under the curve was 0.9195, with a standard error of 0.02916 and 95% confidence interval of 0.8623–0.9767.
Figure 1.
Downregulation of lncRNA GATA6-AS distinguishes patients with MCL from healthy controls. (A) Compared with healthy controls, plasma expression levels of lncRNA GATA6-AS were significantly reduced in patients with MCL. (B) Downregulation of lncRNA GATA6-AS distinguished patients with MCL from healthy controls. *P<0.05. lncRNA GATA6-AS, long non-coding RNA GATA6 antisense RNA 1; MCL, mantle cell lymphoma.
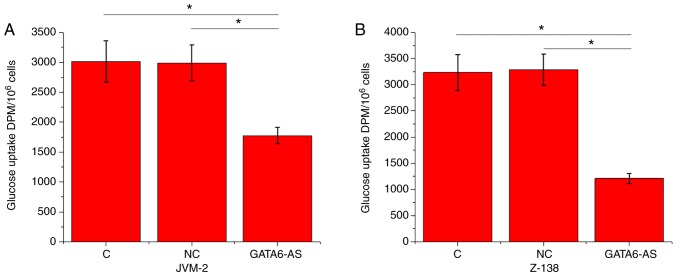
Figure 2.
lncRNA GATA6-AS overexpression inhibits glucose uptake in JVM-2 and Z-138 human MCL cells. lncRNA GATA6-AS overexpression resulted in significantly inhibited glucose uptake in (A) JVM-2 and (B) Z-138 human MCL cell lines. *P<0.05. lncRNA GATA6-AS, long non-coding RNA GATA6 antisense RNA 1; MCL, mantle cell lymphoma; C, control; NC, negative control; DPM, disintegrations per minute.
lncRNA GATA6-AS overexpression inhibits glucose uptake in JVM-2 and Z-138 human MCL cells
Glucose uptake in JVM-2 and Z-138 human MCL cells was detected using a glucose uptake assay. Compared with the control and negative control groups, glucose uptake was significantly reduced in JVM-2 (Fig. 2A) and Z-138 (Fig. 2B) human MCL cells following lncRNA GATA6-AS overexpression (P<0.05).
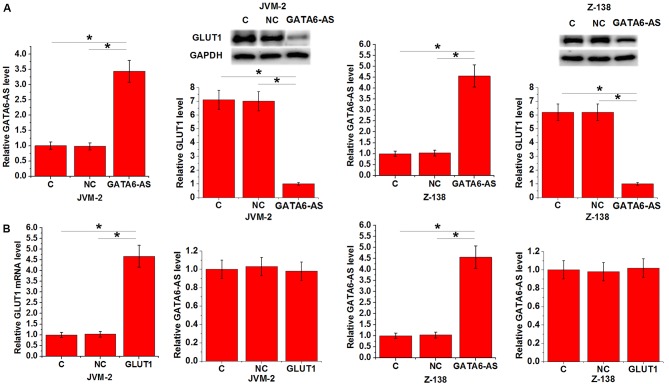
lncRNA GATA6-AS regulates GLUT1 expression in JVM-2 and Z-138 human MCL cells
GLUT1 is a one of the principal components of glucose uptake. Therefore, the expression of GLUT1 was detected in JVM-2 and Z-138 human MCL cell lines following lncRNA GATA6-AS overexpression. Compared with control and negative control groups, expression of GLUT1 was significantly reduced in both cell lines following lncRNA GATA6-AS overexpression (P<0.05; Fig. 3A). By contrast, no significant differences in the expression levels of lncRNA GATA6-AS were observed following GLUT1 overexpression (P<0.05; Fig. 3B).
Figure 3.
lncRNA GATA6-AS regulates GLUT1 expression in JVM-2 and Z-138 human MCL cells. (A) lncRNA GATA6-AS overexpression led to significantly inhibited expression of GLUT1 in JVM-2 and Z-138 human MCL cell lines, while (B) GLUT1 overexpression had no significant effects on lncRNA GATA6-AS expression in either cell line. *P<0.05. long non-coding RNA GATA6 antisense RNA 1; GLUT1, glucose transporter 1; MCL, mantle cell lymphoma; C, control; NC, negative control.
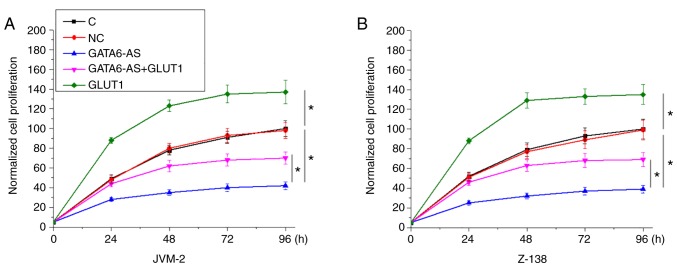
lncRNA GATA6-AS regulates GLUT1 involvement in the proliferation of JVM-2 and Z-138 human MCL cells
Following transfection with lncRNA GATA6-AS and GLUT1 expression vectors, the proliferation of JVM-2 and Z-138 human MCL cells was detected using a CCK-8 assay. Compared with control and negative control groups, GATA6-AS overexpression significantly inhibited, while GLUT1 overexpression significantly promoted the proliferation of JVM-2 (Fig. 4A) and Z-138 (Fig. 4B) human MCL cells. Compared with cells transfected with GATA6-AS expression vectors alone, cells transfected with both GATA6-AS and GLUT1 expression vectors showed significantly increased proliferation rates (P<0.05).
Figure 4.
lncRNA GATA6-AS regulates GLUT1 in the proliferation of JVM-2 and Z-138 human MCL cells. GATA6-AS overexpression significantly inhibited, whilst GLUT1 overexpression significantly promoted the proliferation of cells of (A) JVM-2 and (B) Z-138 human MCL cell lines. GLUT1 overexpression partially reversed the inhibitory effects of GATA6-AS overexpression on cell proliferation. *P<0.05. long non-coding RNA GATA6 antisense RNA 1; GLUT1, glucose transporter 1; MCL, mantle cell lymphoma; C, control; NC, negative control.
Discussion
lncRNA GATA6-AS has recently been identified as a key component in endothelial-mesenchymal transition, while its involvement in other physiological or pathological processes is unknown. In the present study, a novel function of lncRNA GATA6-AS was reported in MCL. This is the first report to indicate the involvement of lncRNA GATA6-AS in cancer cell proliferation, which is likely to be as a result of its interaction with GLUT1.
As a primary component of glucose uptake, GLUT1 is involved in the regulation of cancer cell proliferation in different types of human malignancies, including prostate cancer (10) and triple-negative breast cancer (11). The involvement of GLUT1 in MCL still hasn't been well studied. In the present study, GLUT1 overexpression resulted in significant promotion of MCL cell proliferation. Therefore, GLUT1 may also serve an oncogenic role in MCL by promoting cancer cell proliferation.
It has been reported that the development of MCL is accompanied by changes in the expression pattern of a large set of lncRNAs (14). However, the number of studies addressing the functions of these lncRNAs in MCL is limited (15). The findings of the present study indicate that the inhibition of lncRNA GATA6-AS expression may serve as a potential therapeutic target for the treatment of MCL. It was also observed that lncRNA GATA6-AS was downregulated in MCL, and that lncRNA GATA6-AS overexpression inhibited the proliferation of MCL cell lines. This suggests that in MCL, GATA6-AS is likely to be a tumor suppressor lncRNA, and that its overexpression may be used therapeutically.
Early diagnosis is the key to successful post-treatment survival in patients with cancer (16). The present study only included patients in the early cancer stages of MCL (stage I and II). ROC curve analysis revealed that downregulation of lncRNA GATA6-AS effectively distinguished between patients and healthy controls. Therefore, lncRNA GATA6-AS may have potential in the early diagnosis of MCL.
GLUT1 is involved in cancer development and progression through its interaction with different signaling molecules, including lncRNAs (17,18). The present study revealed that this involvement is likely to be downstream of lncRNA GATA6-AS in the regulation of MCL cell proliferation. However, as GLUT1 overexpression only attenuated, not reversed the inhibitory effects of GATA6-AS overexpression on cell proliferation, lncRNA GATA6-AS may interact with multiple signaling molecules. Future studies will focus on the identification of other downstream effectors of lncRNA GATA6-AS.
Notably, GLUT 2–4 (class 1 GLUT), GLUT5 (class 2 GLUT) and GLUT6 (class 3 GLUT) failed to respond to lncRNA GATA6-AS overexpression (preliminary data not shown), indicating a specific interaction between GLUT1 and lncRNA GATA6-AS. In addition, no potential targeting site of lncRNA GATA6 was identified on GLUT1; therefore, it was hypothesized that the interactions between lncRNA GATA6 and GLUT1 were mediated by other means, including pathological factors. Future studies aim to detect the levels of glycolytic intermediates to confirm the involvement of GLUT1 in MLC. Furthermore, the interaction between GATA6AS with GATA6S in MCL is also a topic for future investigation.
In conclusion, lncRNA GATA6-AS was downregulated in MCL. Its overexpression may serve as a potential therapeutic strategy for the treatment of MCL, by inhibiting cancer cell proliferation through the downregulation of GLUT1.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Jilin Provincial Health Department in China (grant no. 2017ZC029), and the Science and Technology Bureau project of Jilin City (grant no. 201830557).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
XW and PL designed the experiments. ZF performed the experiments with the assistance of CM, MZ and CZ, who also analyzed the data. ZF, XW and PL wrote the manuscript, which was revised by all of the authors.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Jilin Central Hospital, and all participants gave written informed consent to participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chang DW, Satterfield WC, Son D, Neto N, Madewell JE, Raymond AK, Patrick CW, Jr, Miller MJ, Costelloe CM, Weber KL. Use of vascularized periosteum or bone to improve healing of segmental allografts after tumor resection: An ovine rib model. Plast Reconstr Surg. 2009;123:71–78. doi: 10.1097/PRS.0b013e3181904baf. [DOI] [PubMed] [Google Scholar]
- 2.Sulciner ML, Serhan CN, Gilligan MM, Mudge DK, Chang J, Gartung A, Lehner KA, Bielenberg DR, Schmidt B, Dalli J, et al. Resolvins suppress tumor growth and enhance cancer therapy. J Exp Med. 2018;215:115–140. doi: 10.1084/jem.20170681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J Clin Oncol. 2016;34:1256–1269. doi: 10.1200/JCO.2015.63.5904. [DOI] [PubMed] [Google Scholar]
- 4.Robak T, Huang H, Jin J, Zhu J, Liu T, Samoilova O, Pylypenko H, Verhoef G, Siritanaratkul N, Osmanov E, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372:944–953. doi: 10.1056/NEJMoa1412096. [DOI] [PubMed] [Google Scholar]
- 5.Ruan J, Martin P, Shah B, Schuster SJ, Smith SM, Furman RR, Christos P, Rodriguez A, Svoboda J, Lewis J, et al. Lenalidomide plus rituximab as initial treatment for mantle-cell lymphoma. N Engl J Med. 2015;373:1835–1844. doi: 10.1056/NEJMoa1505237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eskelund CW, Kolstad A, Jerkeman M, Räty R, Laurell A, Eloranta S, Smedby KE, Husby S, Pedersen LB, Andersen NS, et al. 15-year follow-up of the second nordic mantle cell lymphoma trial (MCL2): Prolonged remissions without survival plateau. Br J Haematol. 2016;175:410–418. doi: 10.1111/bjh.14241. [DOI] [PubMed] [Google Scholar]
- 7.Hay N. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16:635–649. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberti MV, Locasale JW. The warburg effect: How does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr. 1996;16:235–256. doi: 10.1146/annurev.nu.16.070196.001315. [DOI] [PubMed] [Google Scholar]
- 10.Xiao H, Wang J, Yan W, Cui Y, Chen Z, Gao X, Wen X, Chen J. GLUT1 regulates cell glycolysis and proliferation in prostate cancer. Prostate. 2018;78:86–94. doi: 10.1002/pros.23448. [DOI] [PubMed] [Google Scholar]
- 11.Oh S, Kim H, Nam K, Shin I. Glut1 promotes cell proliferation, migration and invasion by regulating epidermal growth factor receptor and integrin signaling in triple-negative breast cancer cells. BMB Rep. 2017;50:132–137. doi: 10.5483/BMBRep.2017.50.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann P, Jaé N, Knau A, Glaser SF, Fouani Y, Rossbach O, Krüger M, John D, Bindereif A, Grote P, et al. The lncRNA GATA6-AS epigenetically regulates endothelial gene expression via interaction with LOXL2. Nat Commun. 2018;9:237. doi: 10.1038/s41467-017-02431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Hu G, Gupta SK, Troska TP, Nair A, Gupta M. Long non-coding RNA profile in mantle cell lymphoma identifies a functional lncRNA ROR1-AS1 associated with EZH2/PRC2 complex. Oncotarget. 2017;8:80223–80234. doi: 10.18632/oncotarget.17956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Sehgal L, Jain N, Khashab T, Mathur R, Samaniego F. LncRNA MALAT1 promotes development of mantle cell lymphoma by associating with EZH2. J Transl Med. 2016;14:346. doi: 10.1186/s12967-016-1100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vose JM. Mantle cell lymphoma: 2015 update on diagnosis, risk-stratification, and clinical management. Am J Hematol. 2015;90:739–745. doi: 10.1002/ajh.24094. [DOI] [PubMed] [Google Scholar]
- 17.Zou ZW, Ma C, Medoro L, Chen L, Wang B, Gupta R, Liu T, Yang XZ, Chen TT, Wang RZ, et al. LncRNA ANRIL is up-regulated in nasopharyngeal carcinoma and promotes the cancer progression via increasing proliferation, reprograming cell glucose metabolism and inducing side-population stem-like cancer cells. Oncotarget. 2016;7:61741–61754. doi: 10.18632/oncotarget.11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Gan B. lncRNA NBR2 modulates cancer cell sensitivity to phenformin through GLUT1. Cell Cycle. 2016;15:3471–3481. doi: 10.1080/15384101.2016.1249545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.