Abstract
Long intergenic non-coding RNA for kinase activation (LINK-A) has been characterized as an oncogenic long non-coding RNA (lncRNA) in triple-negative breast cancer. However, its involvement in non-small cell lung cancer (NSCLC) remains unknown. The aim of the present study was to investigate the involvement of LINK-A in NSCLC. Expression of LINK-A lncRNA in the plasma of patients with NSCLC collected on the day of admission and the day of discharge, and in the plasma of healthy controls, was detected by reverse transcription-quantitative PCR. Diagnostic values of plasma LINK-A for metastatic NSCLC were evaluated by receiver operating characteristic curve analysis. A LINK-A lncRNA expression vector was constructed and transfected into human NSCLC cell lines, and the effects on cell migration and invasion, and Akt activation were detected by Transwell and Matrigel assays, and western blotting, respectively. Plasma levels of LINK-A were found to be significantly higher in patients with different types of metastatic NSCLC than in patients with non-metastatic NSCLC and healthy controls. Plasma levels of LINK-A were lower in patients with metastatic NSCLC on the day of discharge than on the day of admission. Patients with high plasma LINK-A had a higher mortality rate and lower progression-free survival rate within 2 years of discharge. In conclusion, LINK-A is overexpressed in metastatic NSCLC, and may promote the migration and invasion of NSCLC by activating Akt signaling.
Keywords: non-small cell lung cancer, metastasis, long non-coding RNA, long intergenic non-coding RNA for kinase activation
Introduction
Non-small cell lung cancer (NSCLC) is a heterogeneous class of tumors that accounts for >85% of all newly diagnosed lung cancer cases (1). Despite the efforts that have been made on the development of treatments for NSCLC, outcomes for patients with this disease are generally poor due to the existence of distant tumor metastasis in more than two out of three cases (2). Cancer metastasis is a major challenge for the treatment of almost all types of human malignancies (3), and the prevention of metastasis remains a major task in clinical practice.
Epithelial-mesenchymal transition (EMT) serves a pivotal role in the metastasis of tumors (4). It has been well established that activation of Akt signaling is involved in EMT (5), and inhibition of Akt signaling is considered to be a promising target for the treatment of various types of malignancy including NSCLC (6). Akt signaling in some cases achieves its biological roles through interactions with long non-coding RNAs (lncRNAs) (7,8). lncRNAs are a group of noncoding RNAs of >200 nucleotides in length that have roles in cancer biology (9). Long intergenic non-coding RNA for kinase activation (LINK-A) is a recently identified lncRNA with oncogenic effects in triple-negative breast cancer (TNBC) (10). It has also been reported that LINK-A may induce oncogenic effects in TNBC through the activation of Akt signaling (11). However, the interaction between LINK-A and Akt signaling in other diseases remains unknown. In the present study, the role of LINK-A lncRNA in the metastasis of NSCLC through the activation of Akt signaling was investigated.
Materials and methods
Patients
The present study included 134 patients with NSCLC who were diagnosed and treated in the Navy General Hospital People's Liberation Army (PLA) (Beijing, China) between January 2014 and March 2016. These patients included 72 males and 62 females, with an age range of 28–71 years, and a mean age of 47±7.2 years. Inclusion criteria were as follows: i) Patients were pathologically diagnosed with NSCLC and undergoing first time treatment; ii) patients were educated to above primary high school level; iii) patients and their families were willing to participate; iv) the patients completed the whole treatment procedure in the Navy General Hospital PLA; v) the patients completed 2 years of follow-up; and vi) only a single type of metastasis was identified in cases of metastatic NSCLC. Exclusion criteria were as follows: i) NSCLS was combined with other types of malignancy; ii) the patients were treated prior to admission; iii) the patients were transferred to the Navy General Hospital PLA during treatment; or vi) the patients died of other causes during follow-up. A total of 45 healthy controls (age range, 28–70 years; mean age, 46±7.1 years) were enrolled at the physical health center of the same hospital. The control group included 31 males and 14 females. No significant differences in age and sex were found between two groups. The Ethics Committee of the Navy General Hospital PLA approved the present study and all subjects provided written informed consent.
Grouping and specimen collection
Among the 134 NSCLC patients, brain metastasis (BRM) was found in 32 cases, bone metastasis (BOM) in 44 patients, liver metastasis (LM) in 26 cases and no metastasis (NM) in the remaining 32 patients. Blood was extracted from the patients and the healthy controls on the day of admission. Blood was also extracted from patients on the day of discharge. Plasma was separated from blood by centrifuging blood samples in EDTA tubes at 1,200 × g for 14 min at room temperature. Plasma samples were stored in liquid nitrogen before use.
Cell lines, cell culture and transfection
The present study included 2 human NSCLC cell lines, NCI-H1993 (H1993) and NCI-H1581 (H1581), and a normal human lung cell line, HBEC3-KT (ATCC). RPMI-1640 medium containing 10% FBS (Sangon Biotech Co., Ltd.) was used to culture the cells at 37°C with 5% CO2. Full length LINK-A cDNA was obtained by PCR using Phusion® High-Fidelity DNA Polymerase (New England BioLabs, Inc.). The primer sequences used were: Forward, 5′-TGGAATTCAAGCTGTGGGTG-3′ and reverse, 5′-GCATTTTTATTTTAATTGAGGA-3′. Thermocycling conditions were as follows: 95°C for 5 min; followed by 30 cycles of 95°C for 30 sec, 57°C for 30 sec and 72°C for 30 sec; and 72°C for 10 min. The cDNA was inserted into a linearized pIRES2-EGFP vector (Clontech Laboratories, Inc.) to produce an LINK-A expression vector. Lipofectamine® 2000 reagent (cat. no. 11668-019; Invitrogen; Thermo Fisher Scientific, Inc.) and 10 nM vector was transfected into 5×105 cells. Untransfected cells were used as a control and cells transfected with empty vectors were used as the negative control. Expression of LINK-A, determined by RT-qPCR as aforementioned, at 200% of the control (200–220% in the three cell lines) was achieved at 24 h post-transfection prior to subsequent experiments. For Akt activator and inhibitor treatment, cells were treated with Akt activator SC79 (5 and 10 µg/ml; cat. no. SML0749; Sigma-Aldrich; Merck KGaA) or Akt inhibitor (SH-6; 50 µM; cat. no. sc-205974; Santa Cruz Biotechnology) at 37°C for 24 h prior to use in subsequent experiments.
Reverse transcription-quantitative PCR (RT-qPCR)
TRIzol® reagent (Thermo Fisher Scientific, Inc.) was used to extract total RNA from plasma and in vitro cultivated NCI-H1993 and NCI-H1581 cells. Reverse transcription was performed to synthesize cDNA using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) and oligo(dT) as the primer. Reaction conditions were as follows: 25°C for 5 min, 50°C for 20 min and 75°C for 10 min. The KAPA Taq PCR kit (Kapa Biosystems; Roche Diagnostics) was used to prepare samples for PCR and the thermocycling conditions were as follows: 95°C for 5 min, followed by 40 cycles of 95°C for 30 sec, 57°C for 30 sec and 72°C for 30 sec, and then 72°C for 10 min. The PCR product was checked by agraose gel electrophoresis with ethidium bromide staining at room temperature for 20 min. The gel was visulized using Gel Doc™ EZ Gel Documentation System (Bio-Rad Laboratories, Inc.). SYBR Green PCR Master mix (Thermo Fisher Scientific Inc.) was used in qPCR samples and the following primers were used: Human LINK-A forward, 5′-TTCCCCCATTTTTCCTTTTC-3′ and reverse, 5′-CTCTGGTTGGGTGACTGGTT-3′; and β-actin forward, 5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′. qPCR was performed using the CFX96 Touch™ Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.). The thermocycling parameters for qPCR were: 95°C for 45 sec, followed by 40 cycles of 95°C for 12 sec and 57°C for 28 sec. Data were processed using the 2−ΔΔCq method (12) and LINK-A expression was normalized to the lowest value of the endogenous control β-actin.
Transwell migration and invasion assays
The three cell lines were added to RPMI-1640 medium (Thermo Fisher Scientific, Inc.) containing 1% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA) and cell suspensions were prepared at a density of ~4×104 cells/ml. For the invasion assay, the upper Transwell chambers were precoated with Matrigel (cat. no. 356234; EMD Millipore), and for the migration assay, the Transwell chambers were uncoated. The upper Transwell chamber was filled with 0.1 ml cell suspension, and the lower chamber was filled with RPMI-1640 medium supplemented with 20% FBS to induce cell migration or invasion. The cells were incubated for 24 h at 37°C. The cells were removed from the membranes using cotton swabs and the invading and migrating cells were stained with 0.5% crystal violet (Sigma-Aldrich; Merck KGaA) for 15 min at room temperature. Cell migration and invasion were normalized to cell proliferation rate at 24 h, which was performed using a Cell Counting Kit-8 (Sigma-Aldrich; Merck KGaA) by measuring optical density at 450 nm, and data were expressed as a percentage of the control group.
Western blot analysis
Total protein was extracted using RIPA buffer (Thermo Fisher Scientific, Inc.), and bicinchoninic acid assay (BCA-1; Sigma-Aldrich; Merck KGaA) was performed to determine the protein concentration. SDS-PAGE using a 10% gel was performed with 20 µg denatured protein per well. Following transfer of protein to PVDF membranes, blocking was performed by incubation with skimmed milk (5%) in PBS at room temperature for 1 h. The membranes were then incubated with rabbit anti-human primary antibodies against Akt (1:1,200; cat. no. ab126811; Abcam), phosphorylated (p-)Akt (phospho T308; 1:1,400; cat. no. ab38449; Abcam), P13K (1:1,400; cat. no. ab227204; Abcam), p-PI3K (phospho Y607; 1:1,400; cat. no. ab182651; Abcam) and GAPDH (1:1,400; cat. no. ab9485; Abcam) at 4°C overnight. Membranes were washed with TBST (0.3% Tween-20) and incubated with horseradish peroxidase-conjugated immunoglobulin G secondary antibody (1:1,000; cat. no. MBS435036; MyBioSource, Inc.) for 3 h at room temperature. ECL™ Western Blotting Analysis System (Sigma-Aldrich; Merck KGaA) was used to develop signals. Grey values of Akt and p-Akt bands were normalized to GAPDH using ImageJ v 1.46 software (National Institutes of Health).
Statistical analysis
All statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software, Inc.). Data are presented as the mean ± standard deviation and were compared using Student's t-test for comparisons between 2 groups or one-way analysis of variance followed by Fisher's least significant difference test to compare >2 groups. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic values of plasma LINK-A for different types of NSCLC, using values from patients with NSCLC as true-positive cases and healthy controls as true-negative cases. Kaplan-Meier plotter (http://kmplot.com/analysis) was used to plot survival curves, which were compared by log-rank test. Comparisons of mortality rates were performed by χ2 test. P<0.05 was considered to indicate a statistically significant difference.
Results
Plasma levels of LINK-A in healthy controls and patients with different types of NSCLC
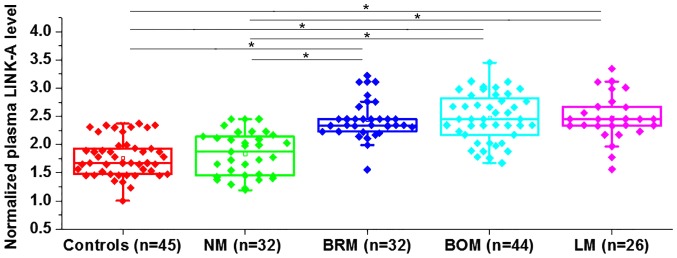
Differential expression indicates the involvement of a certain gene in a disease. The expression of LINK-A was detected in the plasma of healthy controls and in patients with non-metastatic NSCLC and different types of metastatic NSCLC. The expression level of LINK-A in the plasma was significantly higher in the patients with three types of metastatic NSCLC (BRM, BOM and LM) compared with that in patients with NM NSCLC and healthy controls (Fig. 1). However, no significant differences in plasma level of LINK-A were found between healthy controls and patients with NM NSCLC. Data were normalized to the sample with the lowest expression level. Therefore, upregulation of LINK-A may specifically participate in metastatic NSCLC.
Figure 1.
Plasma levels of LINK-A in healthy controls and patients with different types of non-small cell lung cancer. *P<0.05. LINK-A, long intergenic non-coding RNA for kinase activation; NM, non-metastasis; BRM, brain metastasis; BOM, bone metastasis; LM, liver metastasis.
Diagnostic values of plasma levels of LINK-A for different types of NSCLC
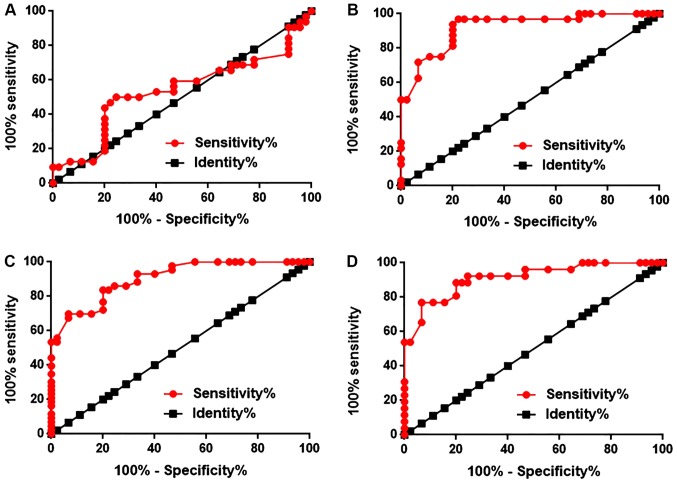
ROC curve analysis was performed to evaluate the diagnostic values of plasma LINK-A for different types of NSCLC. The area under the curve (AUC) of diagnosis of NM NSCLC was 0.5293, with a standard error of 0.07086 and a 95% confidence interval of 0.4007 to 0.6785 (P=0.5558; Fig. 2A). The AUC of BRM NSCLC was 0.9212, with a standard error of 0.03087 and a 95% confidence interval of 0.8607 to 0.9817 (P<0.0001; Fig. 2B). The AUC of BOM NSCLC was 0.9044, with a standard error of 0.02994 and a 95% confidence interval of 0.8457 to 0.9631 (P<0.0001; Fig. 2C). The AUC of LM NSCLC was 0.9120, with a standard error of 0.03587 and a 95% confidence interval of 0.8414 to 0.9825 (P<0.0001; Fig. 2D). Therefore, plasma LINK-A may serve as a diagnostic marker for metastatic NSCLC, but not NM NSCLC.
Figure 2.
Diagnostic values of plasma LINK-A for different types of NSCLC. Receiver operating characteristic curve analysis of the diagnostic values of plasma LINK-A for (A) non-metastatic NSCLC, (B) brain-metastatic NSCLC, (C) bone-metastatic NSCLC and (D) liver-metastatic NSCLC. *P<0.05. LINK-A, long intergenic non-coding RNA for kinase activation; NSCLC, non-small cell lung cancer.
Comparison of plasma levels of LINK-A prior to treatment and following discharge in patients with three types of metastatic NSCLC
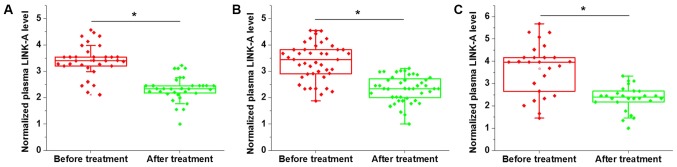
Comparison of plasma levels of LINK-A prior to treatment and following discharge in patients with three types of metastatic NSCLC indicated that, compared with the pre-treatment levels, the plasma level of LINK-A were significantly decreased in patients with BRM (Fig. 3A), BOM (Fig. 3B) and LM (Fig. 3C) NSCLC. Therefore, plasma LINK-A may serve as a marker for the treatment of metastatic NSCLC. Data were normalized to the sample with the lowest expression level.
Figure 3.
Comparison of plasma levels of LINK-A prior to and after treatment in patients with (A) brain-metastatic NSCLC (B) bone-metastatic NSCLC and (C) liver-metastatic NSCLC. *P<0.05. LINK-A, long intergenic non-coding RNA for kinase activation; NSCLC, non-small cell lung cancer.
Comparison of 2-year mortality and progression-free survival rates between patients with high and low plasma levels of LINK-A
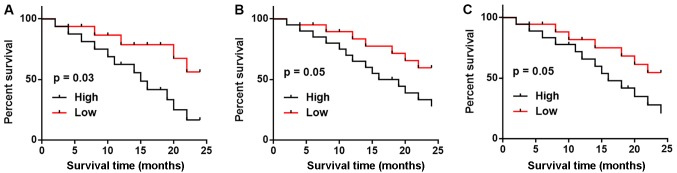
All patients with three types of metastatic NSCLC were followed-up for two years and the survival conditions were recorded. According to the median plasma level of LINK-A measured on the day of admission, patients with each type of metastatic NSCLC were divided into high and low groups. As shown in Table I, patients with high plasma levels of LINK-A had higher mortality and lower progression-free survival rates compared with patients with low plasma levels of LINK-A in all three subtypes of metastatic NSCLC. Kaplan-Meier curve was used to plot survival curves and these were compared by log-rank test. As shown in Fig. 4, patients with high LINK-A expression levels exhibited notably worse survival rates compared with patients with low LINK-A expression levels in BRM (Fig. 4A), BOM (Fig. 4B) and LM (Fig. 4C); however, the difference was only significant in patients with BRM.
Table I.
Comparison of 2-year MR and PFS rates between patients with high and low plasma levels of LINK-A.
| Type of NSCLC | LINK-A expression | Deceased, n | MR, % | Cases of PFS, n | PFS rate, % |
|---|---|---|---|---|---|
| Brain-metastatic | High (n=16) | 13 | 81.25 | 1 | 6.25 |
| Low (n=16) | 7 | 43.75 | 3 | 18.75a | |
| Bone-metastatic | High (n=22) | 14 | 63.64 | 2 | 9.09 |
| Low (n=22) | 8 | 36.36 | 4 | 18.18a | |
| Liver-metastatic | High (n=13) | 9 | 69.23 | 0 | 0.00 |
| Low (n=13) | 6 | 46.15 | 2 | 15.38a |
P<0.05, χ2 test. LINK-A, long intergenic non-coding RNA for kinase activation; MR, mortality rate; PFS, progression-free survival; NSCLC, non-small cell lung cancer.
Figure 4.
Comparison of survival curves of patients with high and low LINK-A expression levels in patients with (A) brain-metastatic NSCLC, (B) bone-metastatic NSCLC and (C) liver-metastatic NSCLC. LINK-A, long intergenic non-coding RNA for kinase activation; NSCLC, non-small cell lung cancer.
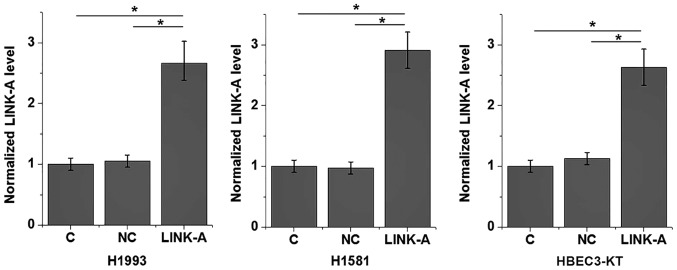
Effects of LINK-A overexpression on Akt and cell migration and invasion
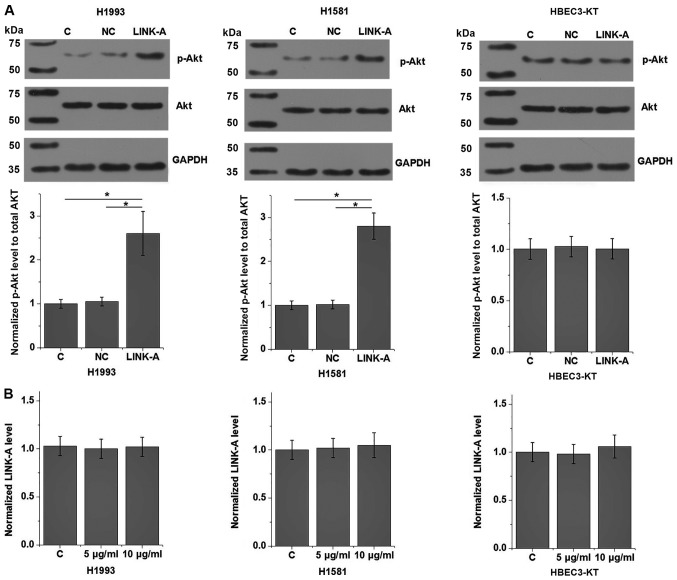
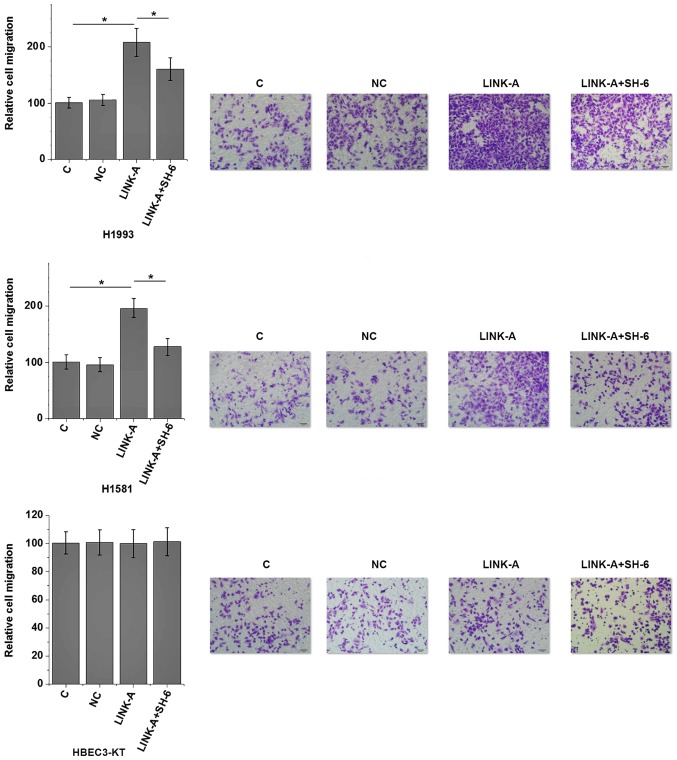
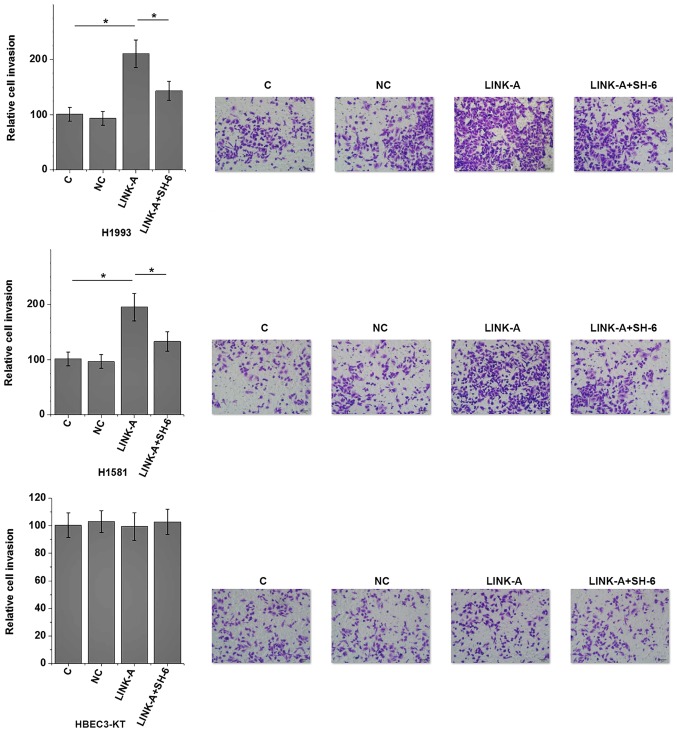
The present results indicate that LINK-A may be involved in the metastasis of NSCLC. Activation of Akt plays pivotal roles in tumor metastasis (13). Therefore, the potential interaction between LINK-A and Akt in NSCLC was explored by transfecting a LINK-A expression vector into cells of human NSCLC and normal lung tissue cell lines. LINK-A was overexpressed at 24 h post-transfection (Fig. 5). As shown in Fig. 6A, LINK-A overexpression significantly promoted the phosphorylation of Akt (P<0.05), but not the expression of Akt in the cells of the human NSCLC H1993 and H1581 cell lines compared with the control group (P<0.05). No effect on p-Akt was observed on cells of the normal human lung HBEC3-KT cell line compared with the control group. LINK-A overexpression exhibited no significant effects on PI3K and p-PI3K levels (data not shown), therefore PI3K was not further analyzed. By contrast, Akt activator SC79 treatment (5 and 10 µg/ml) had no significant effect on LINK-A expression (Fig. 6B). The involvement of LINK-A in NSCLC was further tested by in vitro cell migration and invasion assays. Results showed that LINK-A overexpression significantly promoted the migration (Fig. 7) and invasion (Fig. 8) of the NSCLC H1993 and H1581 cell lines (P<0.05), but not the normal human lung HBEC3-KT cell line (P>0.05). In addition, treatment with an Akt inhibitor (SH-6; 50 µM) significantly reduced the enhancing effect of LINK-A overexpression on cell migration (Fig. 7) and invasion (Fig. 8) in the NSCLC cell lines, but not the normal human lung cell line. Therefore, LINK-A may mediate NSCLC cell migration and invasion by functioning as an upstream activator of Akt signaling. In addition, SH-6 alone is unlikely to affect the migration and invasion of normal human lung HBEC3-KT cells, as both LINK-A and LINK-A + SH-6 failed to significantly affect the migration and invasion of these cells.
Figure 5.
LINK-A expression is increased at 24 h post-transfection with LINK-A-carrying plasmid. LINK-A, long intergenic non-coding RNA for kinase activation; C, control; NC, negative control.
Figure 6.
Effects of LINK-A overexpression on Akt signaling. (A) p-Akt and Akt protein expression, quantified by western blot analysis. (B) LINK-A expression in cells treated with Akt activator SC79 at 5 and 10 µg/ml. *P<0.05. LINK-A, long intergenic non-coding RNA for kinase activation; C, control; NC, negative control; p-, phosphorylated.
Figure 7.
Effects of LINK-A overexpression on cell migration. *P<0.05. LINK-A, long intergenic non-coding RNA for kinase activation; C, control; NC, negative control.
Figure 8.
Effects of LINK-A overexpression cell invasion. *P<0.05. LINK-A, long intergenic non-coding RNA for kinase activation; C, control; NC, negative control.
Discussion
In the present study, LINK-A was detected in the plasma of patients with NSCLC and healthy controls. Upregulation of LINK-A in metastatic NSCLC may promote the migration and invasion of NSCLC cells through the activation of Akt signaling. The interaction between LINK-A and Akt reported in the present study is consistent with the role of this lncRNA in TNBC (11), indicating that different malignancies may share similar pathological pathways.
Novel metastatic markers are required due to the clinical value in estimating patient prognosis, and as potential therapeutic targets (14). lncRNAs serve critical roles in the pathogenesis of numerous aspects of malignancies (9). Onset and development of NSCLC is usually accompanied by changes in expression of a large set of lncRNAs, and those lncRNAs show upregulated or downregulated expression patterns that are associated with the promotion or inhibition of disease progression (15). In previous studies, expression of lncRNAs SPRY4-IT1 and CCAT2 was increased in tumor tissues compared with expression in paired healthy tissues in patients with NSCLC (16,17), supporting their roles as oncogenic lncRNAs in this disease. By contrast, lncRNA LOC285194 is downregulated in NSCLC compared with that in healthy lung tissue, indicating its role as a tumor suppression lncRNA (18). However, altered expression of these lncRNAs has been demonstrated to correlate with tumor growth and metastasis (14). In the present study, upregulation of LINK-A was found in patients with metastatic NSCLC, but not in patients with NM NSCLC. In addition, ROC curve analysis also indicated that the plasma levels of LINK-A may be used to effectively distinguish patients with metastatic NSCLC from healthy controls, but not patients with NM NSCLC. Therefore, LINK-A may be associated with the metastasis of NSCLC.
The process of disease treatment is accompanied by changes in certain substances in the blood, and detecting those substances may provide guidance for treatment (19). In the present study, plasma levels of LINK-A were significantly lower on the day of discharge compared with pre-treatment levels measured on the day of admission. Therefore, plasma levels of LINK-A may reflect the treatment outcomes of metastatic NSCLC. It was also observed that high plasma LINK-A was associated with a higher mortality rate and lower progression-free survival rate compared with the rates for patients with low plasma LINK-A levels. Therefore, monitoring the changes in plasma levels of LINK-A may provide guidance for the prognosis of NSCLC.
It has been reported that LINK-A can directly interact with phosphatidylinositol (3,4,5)-trisphosphate (PIP3) and AKT pleckstrin homology domain at the single nucleotide level to promote the interactions between AKT and PIP3, and subsequent enzyme activation (11). In the present study, activation of Akt was observed in NSCLC cells with LINK-A overexpression, while an Akt activator failed to affect LINK-A expression. In addition, LINK-A overexpression promoted NSCLC cell migration and invasion, while an Akt inhibitor reduced these effects of LINK-A overexpression on migration and invasion. In the present study, LINK-A was not found to affect Akt expression, but impacted Akt phosphorylation. These data indicate that LINK-A and Akt signaling are associated in NSCLC. It is also worth noting that LINK-A overexpression had no significant effects on normal human lung cells. In this manner, LINK-A may serve as a potential therapeutic target for the treatment of NSCLCL by inhibiting tumor metastasis.
As the present study included 2 NSCLC cell lines and a normal control cell line only, future studies with more cell lines are required to further confirm the conclusions. The current study also did not include LINK-A siRNA silencing due to the low efficiency of knockdown, which will be resolved in future studies.
In conclusion, LINK-A is overexpressed in the plasma of patients with metastatic NSCLC. LINK-A may promote the migration and invasion of NSCLC by activating Akt signaling. However, future clinical studies are required to test the potential application of LINK-A as a diagnostic marker and treatment target for NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JQL and TG designed experiments. JQL, WS and JL performed experiments. XL and RZ collected and analyzed the data. TG drafted the manuscript and all authors approved the manuscript.
Ethics approval and consent to participate
The present study was approved by The Ethics Committee of the People's Liberation Army Navy General Hospital (Beijing, China) and all subjects provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, Petrella F, Spaggiari L, Rosell R. Non-small-cell lung cancer. Nat Rev Dis Primers. 2015;1:15009. doi: 10.1038/nrdp.2015.9. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta GP, Massagué J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–654. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- 5.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Yan Y, Cheng Z, Hu Y, Liu T. Sotetsuflavone suppresses invasion and metastasis in non-small-cell lung cancer A549 cells by reversing EMT via the TNF-α/NF-κB and PI3K/AKT signaling pathway. Cell Death Discov. 2018;4:26. doi: 10.1038/s41419-017-0186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu S, Sui S, Zhang J, Bai N, Shi Q, Zhang G, Gao S, You Z, Zhan C, Liu F, Pang D. Downregulation of long noncoding RNA MALAT1 induces epithelial-to-mesenchymal transition via the PI3K-AKT pathway in breast cancer. Int J Clin Exp Pathol. 2015;8:4881–4891. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y, Zhang X, Qi L, Cai Y, Yang P, Xuan G, Jiang Y. HULC long noncoding RNA silencing suppresses angiogenesis by regulating ESM-1 via the PI3K/Akt/mTOR signaling pathway in human gliomas. Oncotarget. 2016;7:14429–14440. doi: 10.18632/oncotarget.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108:2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin A, Li C, Xing Z, Hu Q, Liang K, Han L, Wang C, Hawke DH, Wang S, Zhang Y, et al. The LINK-A lncRNA activates normoxic HIF1α signalling in triple-negative breast cancer. Nat Cell Biol. 2016;18:213–224. doi: 10.1038/ncb3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin A, Hu Q, Li C, Xing Z, Ma G, Wang C, Li J, Ye Y, Yao J, Liang K, et al. The LINK-A lncRNA interacts with ptdIns (3, 4, 5) P 3 to hyperactivate AKT and confer resistance to AKT inhibitors. Nat Cell Biol. 2017;19:238–251. doi: 10.1038/ncb3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Qiao M, Sheng S, Pardee AB. Metastasis and AKT activation. Cell Cycle. 2008;7:2991–2996. doi: 10.4161/cc.7.19.6784. [DOI] [PubMed] [Google Scholar]
- 14.Ramankulov A, Lein M, Johannsen M, Schrader M, Miller K, Jung K. Plasma matrix metalloproteinase-7 as a metastatic marker and survival predictor in patients with renal cell carcinomas. Cancer Sci. 2008;99:1188–1194. doi: 10.1111/j.1349-7006.2008.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou D, Xie M, He B, Gao Y, Yu Q, He B, Chen Q. Microarray data re-annotation reveals specific lncRNAs and their potential functions in non-small cell lung cancer subtypes. Mol Med Rep. 2017;16:5129–5136. doi: 10.3892/mmr.2017.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun M, Liu XH, Lu KH, Nie FQ, Xia R, Kong R, Yang JS, Xu TP, Liu YW, Zou YF, et al. EZH2-mediated epigenetic suppression of long noncoding RNA SPRY4-IT1 promotes NSCLC cell proliferation and metastasis by affecting the epithelial-mesenchymal transition. Cell Death Dis. 2014;5:e1298. doi: 10.1038/cddis.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Z, Wang J, Wang S, Chang H, Zhang T, Qu J. LncRNA CCAT2 promotes tumorigenesis by over-expressed Pokemon in non-small cell lung cancer. Biomed Pharmacother. 2017;87:692–697. doi: 10.1016/j.biopha.2016.12.122. [DOI] [PubMed] [Google Scholar]
- 18.Shi X, Chen Y, Chen J, Feng F, Li J, He J. Antisense long noncoding RNA LOC285194 is downregulated in NSCLC and associated with poor prognosis. Chest. 2016;149:A256. doi: 10.1016/j.chest.2016.02.268. [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka Y, Nishi D, Tanima Y, Itakura M, Kojima M, Hamazaki K, Noguchi H, Hamazaki T. Serum pro-BDNF/BDNF as a treatment biomarker for response to docosahexaenoic acid in traumatized people vulnerable to developing psychological distress: A randomized controlled trial. Transl Psychiatry. 2015;5:e596. doi: 10.1038/tp.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.