Abstract
Historically, a single research project involving numerous practice-based research networks (PBRNs) required multiple institutional review boards (IRBs) to be involved in approval of the project. However, to avoid redundancies, federal IRB regulations now allow cooperative research projects that involve more than one institution to use reasonable methods of cooperative IRB review and to cede authority for review and oversight of the project to a single lead IRB. Through ceding, a lead IRB has the authority for review and oversight of the project delegated by all participating sites’ IRBs and becomes the IRB of record for the ceded sites. In the conduct of cooperative research projects, each institution or primary care office site is still responsible for safeguarding the rights and welfare of human subjects and for complying with applicable regulations. The purpose of this report is to delineate the process, including cooperation and effort of personnel, for accomplishing IRB approval for the Implementing Networks’ Self-management Tools Through Engaging Patients and Practices (INSTTEPP) clinical trial. This process involved 4 PBRNs, 16 family physician offices, 4 academic institution’s IRBs, and 4 family practice office external IRBs ceding to the lead IRB. Once ceding was accomplished, subsequent IRB modifications and continuing reviews were the responsibility of the lead IRB, ultimately saving time for all participants and keeping the project on schedule.
Keywords: institutional review board, cooperative research, practice-based research networks, regulation, ceding
Project Initiation
The Meta-Network Learning and Research Center (Meta-LARC) is a collaboration of 6 well-established practice-based research networks (PBRNs) whose mission is to sustain a consortium of PBRNs dedicated to increasing the quality, effectiveness, and safety of primary care through accelerated research and collaborative learning. Meta-LARC provides a robust infrastructure capable of managing large clinical trials and practice transformation initiatives. In September 2013, Meta-LARC received funding from the Agency for Healthcare Research and Quality to conduct a study titled “Implementing Networks’ Self-management Tools Through Engaging Patients and Practices (INSTTEPP).”1 The purpose of INSTTEPP was to provide an enhanced practice-patient partnership model, through boot camp translation,2 to increase patient self-management skills and engagement for improving their health using the agency’s self-management support tools. The aim of this brief report is to delineate the process for accomplishing institutional review board (IRB) approval for the INSTTEPP project.
In the spirit of accelerating PBRN research, 4 participating Meta-LARC research teams — the Oregon Rural Practice-based Research Network (ORPRN), Iowa Research Network (IRENE), State Networks of Colorado Ambulatory Practices and Partners (SNOCAP), and Wisconsin Research and Education Network (WREN) — endeavored to establish a cooperative research agreement for INSTTEPP wherein the participating PBRNs (Table 1) would cede to the lead institution’s IRB. Through ceding, the lead IRB has the authority for review and oversight of the project delegated by all participating sites’ IRBs and becomes the IRB of record. The goal of the ceding process was to make human subjects protection review of research across sites as efficient and timely as possible while still recognizing the importance of each participating IRB’s responsibility for ensuring the safety, rights, and welfare of research subjects. Practice-based research focuses on research conducted typically in offices of clinicians practicing in the community,3 and this particular study involved 16 family physician offices in four states.
Table 1.
Original PBRN Locations, Institutional Affiliations, Investigator and Principal
| Network | Location | Institutional Affiliation | PBRN Director |
|---|---|---|---|
| IRENE* | Iowa City, IA | University of Iowa | Barcey Levy |
| ORPRN* | Portland, OR | Oregon Health & Science University | Lyle Fagnan |
| SNOCAP*† | Denver, CO | University of Colorado | Donald Nease |
| WREN* | Madison, WI | University of Wisconsin | David Hahn |
Practice-based research network (PBRN) participating in research study.
Lead team for the INSTTEPP study.1
Cooperative Research
The U.S. Department of Health and Human Services and Food and Drug Administration provide federal regulations (45 CFR 46.114 and 21 CFR 56.114, respectively) that allow cooperative research projects involving more than one institution to use reasonable methods of cooperative IRB review4 to cede authority for review and oversight of the project to a single lead IRB, as multiple IRB applications would be redundant and cause unnecessary work.5 Each family physician office may have its own IRB or may have no IRB oversight. Cooperative research would minimally involve one PBRN with multiple physician offices.6
To avoid duplication of review efforts, an IRB may choose to conduct joint reviews, rely on the review of another qualified IRB, or make other arrangements to establish oversight responsibilities. In making this decision, an IRB must determine if an institution is engaged in human subjects research. Once an IRB determines that an outside institution’s IRB can provide oversight, an IRB Authorization Agreement or other equivalent agreement is signed. In the conduct of cooperative research projects, each institution or primary care office site is still responsible for safeguarding the rights and welfare of human subjects and for complying with applicable regulations.
Ceding Process for the INSTTEPP Project
The lead research team for this study was based at the University of Colorado (home of the SNOCAP PBRN). SNOCAP’s research activity falls under the auspices of the Colorado Multiple Institutional Review Board (COMIRB). The lead investigator and 3 collaborating investigators agreed to use a cooperative research project review agreement. All investigators initiated conversations with their respective IRB chairs about any particular factors to check. The lead investigator met with the COMIRB director to discuss this study and determine if it was appropriate for COMIRB to serve as the IRB of record. Upon approval through a modification, COMIRB agreed to be the oversight IRB for the other 3 PBRNs. For INSTTEPP, each PBRN was expected to recruit 4 family physician offices to participate in the study. At the time of approval, COMIRB determined that the 4 SNOCAP participating physician offices were not conducting human subjects research, only the research team itself was conducting human subjects research.
Upon approval to serve as the IRB of record, COMIRB provided approval documentation for the lead investigator to send to the collaborating investigators. The lead investigator shared the COMIRB application, approval documentation, and IRB Authorization Agreement (Appendix A) with each of the 3 participating PBRN investigators. In turn, the collaborating investigators submitted the approved application material to their institutions’ respective IRBs. The IRB submission process varied by each PBRN as described hereinafter.
IRENE Ceding
At the University of Iowa (home of the IRENE PBRN), the collaborating investigator submitted via email a description of the study, the COMIRB-approved application, the approval letter, and the IRB Authorization Agreement. In addition to those materials, the university’s IRB asked for associated study materials, such as recruitment handouts and questionnaires, and provided its version of an authorization agreement for COMIRB to populate. The IRB deemed the project as human subjects research for the research staff.
The IRENE PBRN recruited 4 family physician offices to participate in the study. The first was a University of Iowa office, in which office staff would provide potential patients with study information and, if authorization granted, would provide the potential patients’ names and contact information to the IRENE PBRN research staff to recruit for the study. The office staff person was deemed to be engaged in research, as she was providing personal information to another person. The university’s IRB signed the IRB Authorization Agreement and ceded to COMIRB.
Two other participating IRENE offices each had their own IRB. Those office contacts were sent the COMIRB-approved application, approval letter, associated materials, and IRB Authorization Agreement. They submitted the materials to their respective IRBs, each of which signed the IRB Authorization Agreement. The fourth participating IRENE office did not have an IRB, and no IRB paperwork was completed. Once each authorization agreement was signed by a respective site and after the COMIRB chair signature was obtained, each IRB and office contact was given a copy of the fully signed document.
ORPRN Ceding
ORPRN research is reviewed by the Oregon Health & Science University Research Integrity Office, which has established cooperative agreements to cede or accept ceded oversight from institutions. For INSTTEPP, a new IRB application was created as a request to waive oversight to another IRB. The office reviewed the application, and the request was approved for three practices not having a local IRB and the ORPRN research staff; this process took about 28 days. The same office deemed the research staff engaged in research but not the staff at participating practices.
A fourth practice had a local health system IRB that had not ceded oversight to another IRB before. However, to maintain a consistent process across all PBRNs, ceding oversight was considered a requirement for participation. After review of ceding materials, that health system IRB determined that it would be appropriate to cede oversight to COMIRB and submitted an IRB Authorization Agreement, which was accepted by COMIRB.
WREN Ceding
At the University of Wisconsin (home of the WREN PBRN), there is a formal online application to defer IRB review to another institution. In this submission, an application for research had to be completed. The university’s IRB reviewed the application, consent document, and study protocol approved by the proposed COMIRB. After reviewing the application and modifying the information sheets and consent forms to include WREN contacts, the university’s IRB agreed to defer to COMIRB.
One of the 4 participating WREN physician offices had its own IRB; the other 3 did not. The university’s IRB deemed that the research staff was engaged in human subjects research and that the office staff was not. The participating office’s IRB reviewed the application from COMIRB and talking points from WREN research staff and also determined that its staff was not engaged in human subjects research. WREN’s memorandum of understanding provided information about ceding to COMIRB for the 3 offices without IRBs and why their clinics were not engaged in human subjects research but rather WREN was engaged in human subjects research.
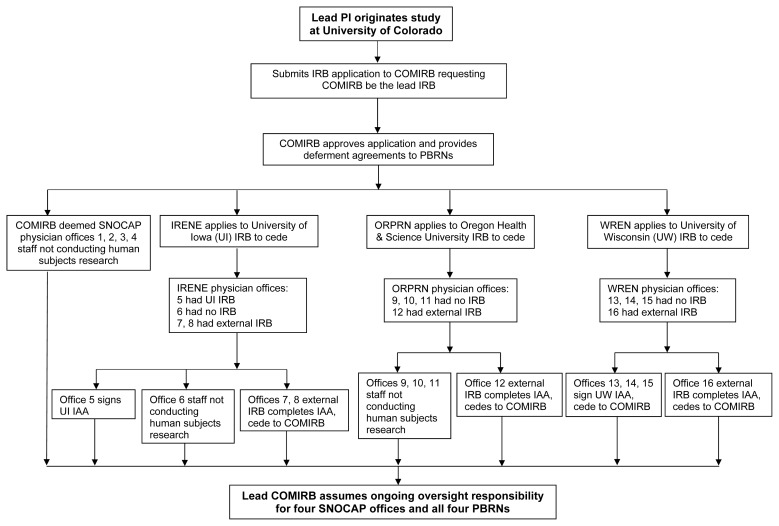
A summary of ceding results from all IRB applications is shown in Figure 1.
Figure 1.
Overview of practice-based research networks (PBRNs) and 16 office practices ceding to a lead institutional review board (IRB). COMIRB, Colorado Multiple Institutional Review Board; IAA, IRB Authorization Agreement; IRENE, Iowa Research Network; ORPRN, Oregon Rural Practice-based Research Network; PI, principal investigator; SNOCAP, State Networks of Colorado Ambulatory Practices and Partners; WREN, Wisconsin Research and Education Network.
Causes for Delays in the Ceding Process
Delays and exorbitant time drains are an inherent part of most research projects and may be especially true for IRB submissions. The COMIRB project submission and final approval of all ceding to COMIRB took 145 days, with an additional 45 days of cooperative research work done by the lead principal investigator (PI) prior to submission. Studying the effects of local IRB review on participation in national PBRN studies, researchers found the median days to obtain approval was 81 days for the Child Abuse Recognition Experience Study and 109 days for the Safety Check Study.6 In another review of a 43-site study, IRB approval took a median of 286 days with a range of 52 to 798 days.7
Some causes of delays in the ceding process for this INSTTEPP project:
First time a PBRN participated in a ceding process.
Online IRB application program isn’t capable of accepting a ceding application.
Back and forth between the IRB(s) and study team because of unclear or insufficient information.
Different interpretations of the research protocol.
Different role of office staff in recruiting subjects, such as giving patients the study contact information or giving patient names to study staff.
Different IRB Authorization Agreement forms for each institution.
The process for final approval itself was lengthy.
Any IRB process involving study review implicates time and cost. For IRENE involvement in INSTTEPP, the study was reviewed by the University of Iowa IRB and 2 private physician office IRBs after COMIRB approval. For ORPRN and WREN, the study was reviewed by their respective university IRBs and 2 external IRBs for participating physician offices. A total of 8 IRB reviews were completed for this one study in 4 states.
The National Institutes of Health (NIH) released a policy in 2016 (becoming effective in 2018) maintaining that, when NIH studies are conducted at multiple sites, a single primary IRB of record will be used.8 The goal is to streamline IRB review, reduce administrative burden, and reduce systemic inefficiencies. In 2017 the U.S. Department of Health and Humans Services released the Common Rule, which stated that institutions engaging in cooperative research use a single primary IRB. The cooperative research portion of the revised Common Rule becomes effective in 2020.9 With this new policy and rule, the ceding process will become common to many IRBs and investigators, lending the process to becoming more efficient over time.
Regardless of the ceding process, each PI and research team member is responsible for ensuring that the rights and welfare of study participants are protected. Safeguarding participants from undue risk is the ethical responsibility of everyone involved in the research. The required IRB review process should provide an external review of the study, ensuring all steps meet the principles of justice, autonomy, and beneficence.
Identifying strategies to facilitate readiness for multicenter projects, Blustein and colleagues found that making a plan for early involvement in the IRB process was helpful to facilitate 11 acute care hospitals in a study.10 Other effective strategies were provision of IRB application templates, modular approach to study description, and reliance on conference calls to collectively engage prospective investigators, local IRB members, and the program office team.10
Continual communication among investigators and project managers from the 4 PBRNs was crucial in realizing the success of the IRB cooperative research. The success of the ceding process for this project was enhanced by the researchers and project coordinators having worked together on other projects, having networked at national PBRN meetings, and being part of a PBRN research center (ie, Meta-LARC). In addition, the lead COMIRB’s application was sent to each of the PBRNs to use for each of their own individual IRB reviews, which ensured continuity across sites and sped the application process.10
Suggestions for enhancing multisite, multi-PBRN research include 1) determine during the writing of the proposal if the lead PI will have his or her respective institution be the lead IRB and allow ceding from other IRBs, 2) determine who is responsible for the IRB application at each site, 3) schedule routine conference calls regarding IRB application on a regular basis until final approval is received, 4) include site PIs and coordinators and a local IRB member on each conference call, 5) provide the lead IRB’s application template to each non-lead site, 6) notify potential sites that IRB review is necessary for the study, and 7) identify early on which participating PBRN physician office sites have an IRB.
Conclusions
After the IRB approval process was completed at each institution and all participating physician offices, the Colorado Multiple Institutional Review Board became the IRB of record for the INSTTEPP study. It is responsible for continuing review as well as review of subsequent modifications, amendments, and any adverse events and unanticipated problems. For 15 of the 16 physician offices, on-site staff were determined to be not engaged in research for handing the study information sheet to prospective subjects and not trying to recruit the patient (Figure 1). If the potential subject was interested, he or she was to call research staff to inquire about the study.
A total of 8 IRB reviews were completed for this 1 study in 4 states. The entirety of project submission and joint approval took 145 days. For this project, the lead investigator and research team had the foresight to start the IRB application 45 days prior to the project’s start date. Continual communication between the 4 PBRN investigators was crucial in successfully realizing IRB approval of this cooperative research.
Undertaking this cooperative research endeavor taught all involved — PBRN investigators, staff, participating clinicians, and IRB members — that the process is doable. It does require one lead person, probably at the lead PBRN site, to keep communication flowing in a timely manner. Learning about the cooperative research process during the writing of a proposal and when obtaining letters of support from participants would enhance a project once ultimately funded.
Patient-Friendly Recap.
Research conducted in health systems and physician offices carries subject protections that are monitored by review boards.
Multiple physician offices collaborating on a research project may coordinate their review process by ceding oversight to one institutional review board (IRB).
Although the review process for human subject research is detailed and time-consuming to ensure protection of patients, ceding to a single primary IRB can help curtail redundant regulatory hurdles and ease the burden on researchers.
Acknowledgments
Mayo Clinic Health System (Tomah, WI); Milwaukee Health Services, Inc. (Milwaukee, WI); Northlakes Community Clinic (Iron River, WI); Richland Medical Center (Richland Center, WI); Family Medical Group, NE, P.C. (Portland, OR); Northwest Primary Care Sellwood Clinic (Portland, OR); Springfield Family Physicians (Springfield, OR); Winding Waters Clinic (Enterprise, OR); Akron Mercy Medical Clinic (Akron, IA); Red Haw Family Medical Center, P.C. (Chariton, IA); Family Medicine Clinic, University of Iowa Hospitals and Clinics (Iowa City, IA); Family Care Southwest (Littleton, CO); MidValley Family Practice (Basalt, CO); Westminster Medical Clinic (Westminster, CO); Yuma Clinic, (Yuma, CO).
Appendix A.
Footnotes
Implementing Networks Self-management Tools Through Engaging Patients and Practices
The Meta-Network Learning and Research Center is comprised of Duke Primary Care Research Consortium, Iowa Research Network, Oregon Rural Practice-based Research Network, Quebec Practice-Based Research Network, State Networks of Colorado Ambulatory Practices and Partners, and Wisconsin Research and Education Network.
Author Contributions
Study design: Daly, Weiner Harrod, Judge, Michaels. Data acquisition or analysis: Daly, Weiner Harrod, Judge, Michaels. Manuscript drafting: Daly, Weiner Harrod, Judge, Michaels. Critical revision: Levy, Hahn, Fagnan, Nease.
Conflicts of Interest
None.
Funding Sources
This research was funded by Agency for Healthcare Research and Quality (AHRQ) grant 1R18HS022491-01. Infrastructure support (ie, Meta-LARC) was funded by AHRQ grant IP30HS021639-01.
References
- 1.ClinicalTrials.gov. Implementing Networks Self-management Tools Through Engaging Patients and Practices (INSTTEPP) [Accessed July 31, 2018]. (Last update posted 2016 Jun 28) https://clinicaltrials.gov/ct2/show/NCT02815020.
- 2.Norman N, Bennett C, Cowart S, et al. Boot camp translation: a method for building a community of solution. J Am Board Fam Med. 2013;26:254–63. doi: 10.3122/jabfm.2013.03.120253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf LE, Croughan M, Lo B. The challenges of IRB review and human subjects protections in practice-based research. Med Care. 2002;40:521–9. doi: 10.1097/00005650-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. Regulatory information. Cooperative research – information sheet. [Accessed July 31, 2018]. (Last updated 2018 Jul 12) http://www.fda.gov/RegulatoryInformation/Guidances/ucm126422.htm.
- 5.Koski G, Aungst J, Kupersmith J, Getz K, Rimoin D. Cooperative research ethics review boards: a win-win solution? IRB. 2005;27(3):1–7. doi: 10.2307/3564071. [DOI] [PubMed] [Google Scholar]
- 6.Finch SA, Barkin SL, Wasserman RC, Dhepyasuwan N, Slora EJ, Sege RD. Effects of local institutional review board review on participation in national practice-based research network studies. Arch Pediatr Adolesc Med. 2009;163:1130–4. doi: 10.1001/archpediatrics.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green LA, Lowery JC, Kowalski CP, Wyszewianski L. Impact of institutional review board practice variation on observational health services research. Health Serv Res. 2006;41:214–30. doi: 10.1111/j.1475-6773.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health. Final NIH policy on the use of a single institutional review board for multi-site research. [Accessed June 8, 2018]. (Policy notice released 2016 Jul 21) https://grants.nih.gov/grants/guide/notice-files/NOT-OD-16-094.html.
- 9.U. S. Department of Health and Human Services. Federal policy for the protection of human subjects (45 CFR Part 46) Fed Regist. 2017;82(12):7149–274. [PubMed] [Google Scholar]
- 10.Blustein J, Regenstein M, Siegel B, Billings J. Notes from the field: jumpstarting the IRB approval process in multicenter studies. Health Serv Res. 2007;42:1773–82. doi: 10.1111/j.1475-6773.2006.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]