Abstract
Background
Few perioperative studies have assessed subjective cognitive complaint (SCC) in combination with neuropsychological testing. New nomenclature guidelines require both SCC and objective decline on cognitive testing. The objective of our study was to compare SCC and neuropsychological testing in an elderly surgical cohort.
Methods
This was a secondary analysis of a prospective cohort trial at a single urban medical centre. We included patients older than 65 yr, undergoing major non-cardiac surgery with general anaesthesia. Those with dementia or inability to consent were excluded, as were those undergoing emergency, cardiac, or intracranial procedures. Patients completed a neuropsychiatry battery before and 3 months after surgery. SCC was defined utilising the single question: ‘do you feel that surgery and anaesthesia have impacted your clarity of thought?’ Objective cognitive decline was defined as 1 standard deviation decline from the baseline of the cohort.
Results
Of the 120 patients who completed assessments, 16/120 (13%) had SCC after surgery, and 41/120 (34%) had objective decline. The sensitivity of SCC in relation to objective decline was 24% and specificity was 92%. Of the patients with SCC, 43.8% were screened positive for depression after surgery compared with 4.9% without SCC; P=0.001.
Conclusions
Many patients with objective cognitive decline did not report SCC. There appears to be a relationship between SCC and depression. The use of SCC in surgical patients to define postoperative neurocognitive disorders needs to be better delineated.
Clinical trial registration
Keywords: cognition, cognitive decline, depression, general anaesthesia, neurocognitive testing, perioperative neurocognitive disorders
Editor's key points.
-
•
Cognitive decline after surgery is a controversial determination, and structured cognitive tests as well as patients' subjective perceptions might be informative.
-
•
In this study of 120 older adults undergoing major non-cardiac surgery, arbitrary criteria were specified to indicate subjective and objective postoperative cognitive decline.
-
•
At 3 months after surgery, 13% of patients fulfilled the arbitrary definition of subjective cognitive decline and 34% met the arbitrary criteria for objective cognitive decline, but only 25% of those with objective decline also reported subjective complaints.
-
•
According to the arbitrary criteria used in this study, there is a considerable disagreement between subjective and objective determination of postoperative cognitive decline.
Few perioperative studies have assessed subjective cognitive complaint (SCC) in conjunction with neuropsychological testing.1 We do not know which older surgical patients are likely to have SCC after surgery, and whether there is an agreement between SCC and cognitive decline measured by testing in surgical patients. Recently, members of the International Perioperative Neurotoxicity Working Group have proposed a nomenclature system to align postoperative cognitive decline (POCD) with the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) definition of neurocognitive disorders (NCDs).2 The DSM-5 states that both a cognitive concern by the individual, informant, or clinician, and objective evidence are required for a diagnosis of NCD.3 The Nomenclature Consensus Panel endorsed this definition, and defined postoperative NCD as occurring more than 30 days and less than 1 yr after surgery. Patients were further classified by whether they maintained previous activities of daily living (ADL) (minor NCD) or had impaired ADL (major NCD). The proposed new nomenclature, if adopted, will change which patients are classified as having a cognitive problem, because it requires both objective decline on cognitive testing (1–2 standard deviation [sd] negative change) and SCC by the individual or an informant (caregiver or physician).
There are many reasons that SCC and objective neuropsychological testing may not agree. The current practice of defining patients as impaired or unimpaired based on objective testing may not capture the experience of patients who notice subtle changes, but still perform well within the normal range for their age. This is most often the case for patients who were high performers before the possible cognitive decline. Also, low performers might decline cognitively, but without exceeding a test threshold for cognitive change. Not all tests within a battery will agree with SCC; for example, patients may notice memory issues more than processing speed. SCC may also vary with level of impairment; in patients with Alzheimer's disease, as the impairment increases, individuals may not be aware of their deteriorating cognition.4, 5 The relationship between SCC and objective cognitive performance in the perioperative period may differ because of acute changes related to surgery rather than insidious changes as a result of neurodegenerative disease.
The aim of this study was to examine the relationship between SCC and objective testing in surgical patients. The hypothesis was that patients with objective cognitive decline would also have SCC.
Methods
The study was a secondary analysis of a prospective cohort trial (optimizing postoperative cognition in the elderly: PRESERVE) registered with ClinicalTrials.gov (NCT02650687) at the Icahn School of Medicine at Mount Sinai, New York, NY, USA, which is an urban academic medical centre. In brief, we recruited English-speaking patients over the age of 65 yr having major non-cardiac surgery with general anaesthesia. Major surgery was defined as at least a 2-day hospital stay, but patients with history of dementia or inability to consent for themselves, and cardiac, intracranial, or emergency surgery were excluded. The study was approved by the Mount Sinai Institutional Review Board, and all subjects provided written informed consent.
The primary purpose of the parent PRESERVE study was to perform cognitive testing, including analysis of domain-based change, and to measure intraoperative raw EEG and postoperative cognition. (There was no intervention.) The aims were to examine (i) cognitive dysfunction after surgery by domain, and (ii) the relationships between processed EEG indices, burst suppression, and cognitive dysfunction. We performed a secondary analysis of 120 patients with in-depth cognitive testing before and after surgery. To assess SCC, patients were asked a single, simple subjective question: ‘did surgery impact your clarity of thought?’ We compared SCC to objective testing after surgery, reporting both different thresholds of ‘impairment’ and looking at clusters of tests as functional ‘domains’.
Cognitive testing
All patients were assessed with a neuropsychological battery before surgery (within 30 days, but at least 24 h prior) and again at 3 months after surgery. We used the Uniform Data Set Battery II from the Alzheimer's Disease Research Centers.6 The test battery included Trails A and B, digit span forward and backward, Wechsler Adult Intelligence Scale (WAIS), Logical Memory Story A, immediate and delayed recall, animal and vegetable lists, and Boston naming. We also added the California Verbal Learning Test (CVLT).7 Testing was performed by trained research coordinators, and all efforts were made to ensure that the patients were in a quiet environment, in most cases in their homes. Patients also completed the Mini-Mental State Examination before surgery and at 3 months after surgery.8, 9 The question regarding subjective cognition was taken from the Postoperative Quality of Recovery Scale, as described by Royse and colleagues.10 The question posed was: ‘do you feel that the surgery negatively impacted your clarity of thought?’ The response options for the question were not at all, minimally, moderately, and severely. We dichotomised these to report as no SCC (response: not at all) vs SCC (responses of minimally, moderately, or severely).
Anaesthesia and surgery
Patients had major non-cardiac surgery with general anaesthesia. Anaesthesiologists received minimal directions regarding anaesthetic choice, as most previous studies have not found evidence that anaesthetic technique or choice (e.g. total i.v. anaesthesia vs volatile-agent-based anaesthesia) significantly impacts cognitive outcomes.11, 12, 13, 14, 15 Anaesthesiologists were instructed to not administer midazolam, nitrous oxide, ketamine, and etomidate. Intraoperative variables were recorded, including blood pressure, measured anaesthetic concentrations, drugs administered (e.g. opioids), and duration of surgery and anaesthesia. Patients were generally extubated at the end of surgery and transferred to the recovery area or the ICU.
Researchers interviewed patients and noted their characteristics and co-morbidities. These details were also extracted from the medical record. Pain was assessed using the Geriatric Pain Measure,16 which is reliable in older patients with multiple medical problems. Depression and anxiety symptomatology were measured using the Hospital Anxiety and Depression Scale (HADS),17, 18 which identifies emotional distress and has sub-scores for anxiety and depression. Data were stored in a Research Electronic Data Capture (Nashville, TN, USA) database.19
Analysis
Baseline characteristics (e.g. age, sex, and ASA physical status score) and the HADS17 were compared between patients with and without SCC, using parametric or non-parametric tests. Continuous variables are presented as mean (sd), and categorical variables as count (%).
Cluster analysis
For each cognitive test, the individual scores obtained before operation and 3 months after surgery were normalised to create z-scores. (The difference between the individual score and the preoperative average was divided by the preoperative sd.) Before normalisation, logarithmic transformation was performed for Trails A and B, and the sign was corrected so that when change scores were created, higher values were always ‘better’. The group comparison of changes in cognitive score from preoperative to 3 months was adjusted for their respective preoperative score using least-squares regression.
Cluster analysis was used to perform grouping of the cognitive tests in the manner presented by Price and colleagues.20 The variable cluster analysis procedure in SAS (version 9.4, SAS Institute, Cary, North Carolina, USA) was implemented to find clusters of cognitive tests that were correlated as closely as possible within the group and not with tests in other clusters. The algorithm was binary and divisive; at the beginning of the analysis, all cognitive tests start in one cluster and splitting continues until a stopping criterion (based on eigenvalues) is reached. The factor analysis generated factor structures similar to the clustering generated by the cluster analysis procedure.
Identification of decline
We calculated the change in z-score from preoperative to 3 months. Change z-scores within a cluster were added to obtain the cluster-specific score. The practice effect was calculated as the average of the domain-specific change scores across all individuals.20 The difference between an individual's domain-specific change score combined with the practice effect was compared with the average score of the study cohort before operation. Patients with a decline of 1 or more sd were defined as having cognitive decline in that cluster. We also reported a group characterised by a decline in any cluster. We chose to use this surgical cohort as the normative group because they were lower scoring before surgery in almost every test relative to age normative data.6 This was true despite the fact that the patients were overall highly educated. Additionally, we repeated the analysis using –1.5 sd and –2 sd thresholds for classification of decline; the former is the cut-off for mild cognitive impairment (MCI)21 and allows assessment of more substantial decline. We calculated the percentage of patients that had objective or subjective evidence of postoperative cognitive decline, and the percentage that would be classified as having the objective and subjective components of DSM-5 NCD.
Finally, we performed a test of agreement between the SCC and objective testing, and reported the Cohen's kappa statistic for all clusters and 1, 1.5, and 2 sd.
Results
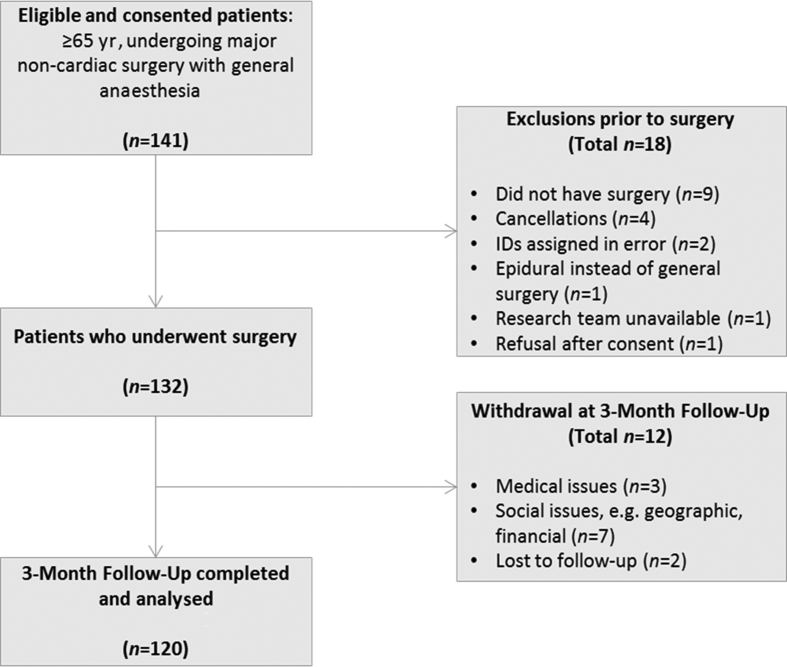
In total, 141 patients met the inclusion criteria, of whom 132 proceeded to surgery and 12 patients dropped out of the study at 3 months post-surgery (9%). The most common reason for lack of follow-up was social (e.g. need to be a caregiver and moved out of area), followed by medical illness that precluded testing (see Fig. 122). The average age was 71.4 (66.3–76.5) yr old; 81.5% were white, 55% were female, and the average years of education was 15.8 (3.8). Of the patients, 60% were classified as ASA physical status, the most common surgery was spine surgery (42.5%), the second most common was general surgery (29.2%), and the average surgical duration was 188.4 (96) min (Table 1).
Fig 1.
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) diagram.
Table 1.
Comparison of patients with and without subjective cognitive complaint (SCC) at baseline. Continuous variables are presented as mean (standard deviation) or median (Q1, Q3), and categorical variables as count (%). Univariate comparisons used Fisher's exact test, Wilcoxon rank sum test, or χ2 test as appropriate. CVLT, California Verbal Learning Test; MMSE, Mini-Mental State Examination; WAIS, Wechsler Adult Intelligence Scale.
| n | Total, n=120 | No SCC, n=104 | SCC, n=16 | P-value | |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age (yr) | 120 | 71.43 (76.52, 66.3) | 71.34 (76.81, 65.96) | 72.06 (74.58, 69.54) | 0.19 |
| Education (yr) | 120 | 15.83 (3.80) | 15.94 (3.55) | 15.06 (5.18) | 0.57 |
| Race/ethnicity | 118 | 0.74 | |||
| Black Hispanic | 2 (1.7) | 2 (1.9) | 0 | ||
| White Hispanic | 6 (5.1) | 5 (4.8) | 1 (6.7) | ||
| Black non-Hispanic | 20 (16.9) | 17 (16.5) | 3 (20) | ||
| White non-Hispanic | 90 (76.3) | 79 (76.7) | 11 (73.3) | ||
| Sex | 120 | 0.33 | |||
| Male | 54 (45) | 45 (43.3) | 9 (56.3) | ||
| Female | 66 (55) | 59 (56.7) | 7 (43.7) | ||
| Preoperative mood | |||||
| Depression score >8 | 120 | 8 (6.7) | 7 (6.7) | 1 (6.3) | 0.94 |
| Depression score ≤8 | 112 | 97 (93.3) | 15 (93.8) | ||
| Anxiety score >8 | 27 (23) | 21 (20.2) | 6 (37.5) | 0.12 | |
| Anxiety score ≤8 | 93 (77.5) | 83 (79.8) | 10 (62.5) | ||
| History of depression | 120 | 0.05 | |||
| No | 103 (85.8) | 92 (88.5) | 11 (68.7) | ||
| Yes | 17 (14.2) | 12 (11.5) | 5 (31.3) | ||
| History of anxiety | 120 | 0.49 | |||
| No | 98 (81.7) | 86 (82.7) | 12 (75.0) | ||
| Yes | 22 (18.3) | 18 (17.3) | 4 (25.0) | ||
| Geriatric pain measure | 120 | 39.17 (29.94) | 38.58 (30.28) | 42.99 (28.25) | 0.63 |
| ASA physical status | 120 | 0.51 | |||
| 2 | 42 (35) | 36 (34.6) | 6 (37.5) | ||
| 3 | 74 (61.7) | 65 (62.5) | 9 (56.3) | ||
| 4 | 4 (3.3) | 3 (2.9) | 1 (6.2) | ||
| Surgery category | 120 | 0.75 | |||
| Spine | 51 (42.5) | 43 (41.4) | 8 (50.0) | ||
| Thoracic | 12 (10) | 10 (9.6) | 2 (12.5) | ||
| Urologic | 22 (18.3) | 19 (18.3) | 3 (18.7) | ||
| General | 35 (29.2) | 32 (30.8) | 3 (18.7) | ||
| Postoperative status | |||||
| Surgery duration (min) | 120 | 188.40 (95.98) | 186.34 (95.05) | 201.81 (104.04) | 0.61 |
| Baseline test scores | |||||
| CVLT immediate | 120 | 7.64 (2.73) | 7.73 (2.72) | 7.06 (2.82) | 0.34 |
| CVLT delayed | 120 | 7.61 (2.92) | 7.69 (2.86) | 7.06 (3.36) | 0.59 |
| CVLT recognition | 120 | 12.98 (2.21) | 12.88 (2.23) | 13.56 (2.06) | 0.31 |
| Logical Memory immediate recall | 120 | 12.33 (3.83) | 12.35 (3.93) | 12.25 (3.24) | 0.99 |
| Logical Memory delayed recall | 120 | 10.92 (3.83) | 10.92 (3.86) | 10.88 (3.76) | 0.95 |
| Cat fluency (animals) | 120 | 18.8 (5.71) | 19.02 (5.72) | 17.38 (5.61) | 0.22 |
| Cat fluency (vegetables) | 120 | 13.93 (4.43) | 14.38 (4.47) | 11.00 (2.90) | 0.003 |
| Digit span forward | 120 | 6.43 (1.14) | 6.46 (1.16) | 6.25 (1.00) | 0.45 |
| Digit span backward | 120 | 4.38 (1.29) | 4.48 (1.29) | 3.75 (1.13) | 0.04 |
| Trail A time | 120 | 36 (28, 43.5) | 34.5 (27, 43) | 38 (33.5, 46.5) | 0.093 |
| Trail B time | 118 | 100 (72, 133) | 97 (69, 123) | 142.5 (95, 265.5) | 0.003 |
| WAIS digit symbol | 120 | 43.24 (12.78) | 44.57 (11.90) | 34.63 (15.23) | 0.02 |
| Boston naming | 120 | 26.56 (2.92) | 26.62 (2.85) | 26.19 (3.43) | 0.82 |
| MMSE | 120 | 28.56 (1.82) | 28.67 (1.70) | 27.81 (2.40) | 0.08 |
Table 2 shows the normalised objective change scores adjusted for baseline performance and composite cluster scores, as described in the Methods section. The cluster analysis divided the z-score data from the cognitive testing battery into three clusters. The memory/language cluster included category fluency (animals and vegetables) and logical memory (immediate and delayed recall). The executive function cluster included Trails A and B, digit span forward and backward, Boston naming, and WAIS. The CVLT (immediate and delayed), which is a measure of episodic verbal learning, was its own cluster.23 The incidence of cognitive decline at the 1 sd level was 34% (41/120), 15.8% (19/120) of patients at 1.5 sd, and 8.3% (10/120) at 2 sd. Among the 120 patients tested at both preoperative and 3 months post-surgery, 16/120 (13%) endorsed SCC 3 months after surgery, 104 felt their cognition was ‘not at all affected’, and 16 felt they were ‘impacted by surgery’, of whom nine endorsed ‘minimally’ and seven ‘moderately’ impacted (Table 3).
Table 2.
Comparison of patients with and without subjective cognitive complaint (SCC) at 3 months, including normalised test and cluster change scores. ∗Continuous variables are presented as mean (standard deviation) or median (Q1, Q3), and categorical variables as count [%]. ƗFor log-transformed change scores, log transformation was taken first, the sign was reversed, and then the difference was taken. CVLT, California Verbal Learning Test; MMSE, Mini-Mental State Examination; WAIS, Wechsler Adult Intelligence Scale.
| n | Total | No SCC, n=104 | SCC, n=16 | P-value | |
|---|---|---|---|---|---|
| CVLT composite | 0.001 | ||||
| Decline | 20 | 12 (11.5) | 8 (50)* | ||
| No | 100 | 92 (88.5) | 8 (50)* | ||
| Tests | |||||
| CVLT immediate | 120 | 1.28 (2.53) | 1.41 (2.44) | 0.44 (3.01) | 0.10 |
| CVLT delayed | 120 | 1.29 (2.45) | 1.53 (2.05) | –0.25 (3.97) | 0.002 |
| CVLT recognition | 120 | 0.88 (2.04) | 1.07 (2.03) | –0.38 (1.67) | 0.02 |
| Memory Language composite | 0.7 | ||||
| Decline | 19 | 16 (15.3) | 3 (18.7) | ||
| No | 101 | 88 (84.6) | 13 (81.3) | ||
| Tests | |||||
| Logical Memory immediate recall | 120 | 0.91 (3.06) | 0.98 (3.19) | 0.44 (2.03) | 0.39 |
| Logical Memory delay recall | 120 | 1.02 (3.29) | 1.08 (3.42) | 0.63 (2.31) | 0.55 |
| Cat fluency (animals) | 120 | –0.36 (4.65) | –0.30 (4.71) | –0.75 (4.33) | 0.38 |
| Cat fluency (vegetables) | 120 | –0.63 (3.19) | –0.70 (3.30) | –0.19 (2.34) | 0.38 |
| Executive function composite | 0.04 | ||||
| Decline | 16 | 11 (10.6) | 5 (31.2) | ||
| No | 104 | 93 (89.4) | 11 (68.8) | ||
| Tests | |||||
| Digit span forward | 120 | –0.03 (1.02) | –0.03 (0.99) | 0.00 (1.21) | 0.85 |
| Digit span backward | 120 | 0.06 (1.15) | 0.03 (1.18) | 0.25 (0.93) | 0.90 |
| Log Trail AƗ | 119 | 0.00 (0.32) | 0.01 (0.32) | –0.10 (0.35) | 0.18 |
| Log Trail BƗ | 116 | 0.02 (0.35) | 0.01 (0.36) | 0.06 (0.31) | 0.83 |
| WAIS digit symbol | 119 | –0.80 (5.97) | –0.53 (5.92) | –2.50 (6.22) | 0.15 |
| Boston naming | 119 | 0.40 (1.83) | 0.44 (1.85) | 0.19 (1.80) | 0.607 |
| MMSE score | 120 | –0.27 (1.66) | –0.17 (1.55) | –0.88 (2.22) | 0.04 |
| Decline in any composite | 0.01 | ||||
| Decline | 41 | 31 (29.8) | 10 (62.5) | ||
| No | 79 | 73 (70.2) | 6 (37.5) | ||
| Postoperative mood | |||||
| Depression score >8 | 119 | 12 (10)* | 5 (4.9) | 7 (43.8) | 0.001 |
| Depression score ≤8 | 107 (89.9) | 98 (95.2) | 9 (56.3) | ||
| Anxiety score >8 | 119 | 14 (11.8) | 10 (9.7) | 4 (25)* | 0.08 |
| Anxiety score ≤8 | 105 (88.2) | 93 (90.3) | 12 (75)* | ||
| Geriatric pain measure | 120 | 30.03 (28.72) | 28.06 (28.25) | 42.84 (29.41) | 0.04 |
Table 3.
Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of subjective cognitive complaint for types of cognitive decline.
| Any cognitive decline (%) | California verbal learning test decline (%) | Executive decline (%) | Memory language decline (%) | |
|---|---|---|---|---|
| Prevalence | 34.0 | 16.0 | 13.0 | 15.8 |
| Sensitivity | 24.0 | 60.0 | 31.0 | 15.8 |
| Specificity | 92.0 | 92.0 | 90.0 | 87.1 |
| NPV | 70.0 | 92.0 | 90.0 | 84.6 |
| PPV | 62.5 | 60.0 | 31.0 | 18.8 |
More of the patients with SCC had a history of depression before surgery (31%) compared with patients without SCC (11%), although the proportion of patients who screened positive for depression before surgery was not significantly different between patients who did and did not go on to endorse SCC (6.7% vs 6.3%). After surgery, a much larger proportion of patients with SCC screened positive for depression (43.8%) compared with patients who did not have SCC (4.9); P=0.001. Anxiety after surgery was more prevalent in patients with SCC (25%) compared with those without (9.7%), although this difference was not statistically significant (P=0.08). Furthermore, patients with SCC had higher pain scores at 3 months after surgery and on average were in moderate-to-severe pain (Table 2). The preoperative cognitive testing scores of category fluency (vegetables), digit span backward, and WAIS digit symbol were all worse in patients who went on to have SCC.
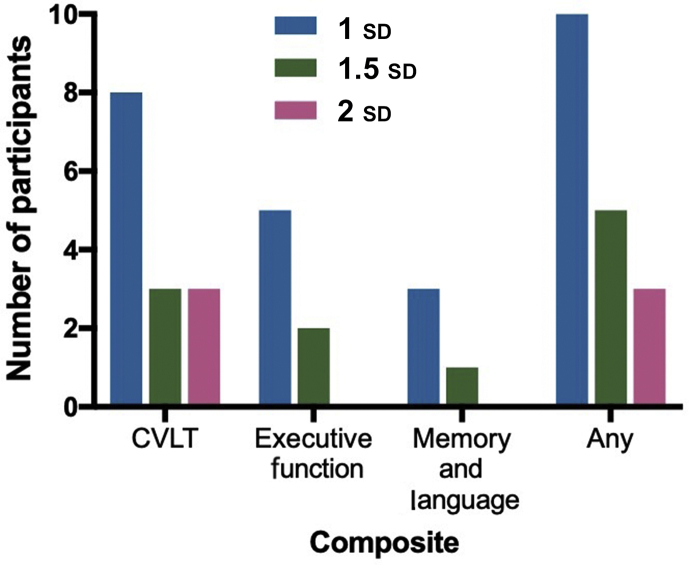
Of patients who reported SCC, 10/16 (62.5%) had cognitive decline in at least one cluster. At the 1.5 sd level, of patients who reported SCC, only 5/16 (30%) had decline, and at the 2 sd level of patients who reported SCC, only three/16 (20%) had decline. Of patients with >1 sd decline in any test from baseline, only 10/41 (24%) endorsed SCC. Only five/16 patients with executive dysfunction reported SCC, eight/20 patients with CVLT decline had SCC, and three/19 patients with memory/language dysfunction had SCC (Fig. 2).
Fig 2.
Number of participants with decline at 1, 1.5, and 2 standard deviation (sd) and reporting subjective cognitive complaint. CVLT, California Verbal Learning Test.
The percentage of all patients who had both objective and subjective evidence of postoperative cognitive decline (i.e. the percentage who would be classified as having DSM-5 NCD) was 10/120 (8.3%).
At the 1 sd level, the overall sensitivity of the SCC for any cognitive change was 24%, the specificity was 92%, the negative predictive value was 70%, and the positive predictive value was 62.5%. The CVLT or the executive function cluster had sensitivities of 60% and 31%, respectively, and both together had specificity and negative predictive value of >90%. SCC had 60% positive predictive value for change in CVLT, and 31% for the executive function cluster. Taken together, the sensitivity of SCC was not very high, but the specificity and negative predictive value were much higher.
The kappa statistics relating SCC to decline in cognitive measures were all very low, with none exceeding 0.35 (Supplementary Appendix), consistent with the low sensitivity of SCC for objective cognitive change.
Discussion
This study found a substantial disagreement between SCC and decline based on objective cognitive test performance at 3 months post-surgery. Over a third of the patients (41/120) had objective cognitive decline of 1 sd or more relative to their own baseline cognitive performance on one or more cognitive domains, but only about 25% of these had SCC. Therefore, the answer to the question: ‘do you know you have POCD?’ appears most often to be ‘no’. These findings have an implication for the proposed nomenclature in that it will exclude a lot of patients who previously would have been defined as having ‘dysfunction’. An argument can be made that, if the patient or family does not notice the impairment, it may not be significant. However, without additional studies, which include functional outcomes, we do not know whether this is correct.
There is evidence suggesting that SCC in the absence of measurable cognitive decline is one of the signs of preclinical dementia.24 About a third of the patients in the current study with SCC did not have measurable decline. However, studies suggest that patients with SCC are at greater risk for decline than those without SCC.25 In patients with MCI, SCCs are closely related to instrumental ADL decline.26 Therefore, it seems that patients (i) with SCC and no objective cognitive decline, or (ii) with objective cognitive decline without SCC need further evaluation. Referral to a neuropsychologist, particularly if symptoms persist, can help clarify the type of impairment and its implications.27
SCCs are related most closely to episodic memory in patients with MCI.28 We also found that some types of decline were more closely related to SCC than others. For example, the CVLT was related to SCC, whereas the memory/language cluster composed of other memory tests and tests of category fluency and naming was not related. This differential awareness of impairment suggests that patients notice decline on tests that place a greater demand on attentional resources, as in the CVLT, with interleaved distractor lists.
Community-based studies have found that SCC is related to depressive symptoms.29 In the current study, patients with SCC more often had a history of depression before surgery and screened in for depression after surgery. Whether surgical patients are depressed after surgery or have medical problems with symptomatology, which overlaps with depression (e.g. slowing and sleep disturbance) is unclear. In any case, depression may be an important factor in cognitive recovery after surgery, has mechanistic implications, and is potentially treatable. However, anaesthesia clinicians may be reluctant to screen for depression, as it is generally outside of their scope of practice. It is unclear whether depression in the perioperative period is a marker of somatic problems or an independent feature that predicts the development of cognitive impairment. Regarding the latter, there is extensive literature showing that geriatric depression often presents as complaints about memory and cognitive function, but also can accompany MCI.30, 31, 32 Additionally, some of the signs of depression are also markers of a physical problem (e.g. fatigue and frailty).18 Furthermore, patients with SCC had higher pain scores at 3 months after surgery and, on average, were in moderate-to-severe pain. Pain may have impacted their mood, ability to perform on objective testing, and SCC. Future studies should focus on the role of chronic pain after surgery in cognitive recovery for older adults.
Limitations
The way that we assessed SCC may have underestimated the number of patients who noticed a negative change in their cognition. In the current study, the SCC question was: ‘did the surgery negatively impact your clarity of thought?’ The term ‘clarity’ may not signify cognitive performance to patients. A previous study in middle-aged surgical patients used a more in-depth questionnaire and found that half the patients with decline had SCC.1 In our study, patients may have attributed their ‘clarity of thought’ to other parts of the recovery process, such as sleep changes, medication use, or illness. Additionally, cognition may not be at the forefront of patients' concerns because other recovery priorities, such as physical therapy, are paramount. The new nomenclature for postoperative cognitive changes2 allows the subjective informer to be the patient, family, or even the patient's clinician. We did not solicit impressions of SCC in our patients from caregivers or physicians.
It is possible that the patients' awareness of their cognitive battery performance influenced the report of SCC, but this is unlikely because an agreement between the two was low. It is likely that patients noticed tests where they truly performed worse, rather than tests where they failed to demonstrate a practice effect (see, e.g. CVLT vs executive function in Table 2). Failure to demonstrate a practice effect may not be a marker of cognitive injury, and therefore, it will be important to understand whether true decline vs lack of practice effect has the same implications for postoperative function. The perioperative cognition literature has focused on decline; however, resilience and improvement are an important area for future studies. We see that a subset of patients demonstrate improved test scores at 3 months after surgery. Simply ascribing improvement to ‘practice effects’ may not capture the salient features of patients or techniques that support recovery. Another limitation of the study was that a small proportion of patients (16/120) reported SCC at 3 months after operation. Although some patients dropped out of the study between the preoperative assessment and 3 months follow-up, the dropout rate was less than 10%, and most dropouts were related to social or medical issues. An analysis of the preoperative cognitive tests of the dropouts suggests that they were not consistently lower performers. We did not use control subjects, but rather compared patients to the sd of the cohort at baseline. Surgical patients tend to score lower than age- and education-matched community-dwelling older adults, and have confounding conditions, such as depression and intrusive pain. Therefore, it is likely that a comparison to non-surgical controls would classify more patients as ‘impaired’. Although the lack of a control group for a direct comparison precludes the use of a reliable change index or similar approaches, our method is generally more conservative and, if anything, would miss patients with smaller or more subtle change.
Future directions
In light of the nomenclature recommendations,2 it is important to understand the significance of SCC in surgical patients. SCC may be a harbinger of impairment; a longitudinal follow-up of patients with complaints, but not objective change, could demonstrate whether there is an abnormal decline over subsequent years, analogous to patients with Alzheimer's disease.33 A translational study could investigate whether patients with SCC also demonstrate higher concentrations of biomarkers of Alzheimer's or neural injury, or to see if there is evidence of Alzheimer's pathology on imaging. The role of depression for older adults on post-surgical recovery in the perioperative period is unexamined. Even descriptive information regarding the incidence and trajectory of depression in the context of postoperative cognition would be helpful, as depression is often treatable. This would give insight into whether mitigation of depressed mood also leads to the resolution of SCC or impaired performance on neuropsychological tests. Finally, patients with SCC had moderate-to-severe pain 3 months after surgery. Future studies could focus on the role of chronic pain after surgery in cognitive recovery for older adults.
Summary
In conclusion, this study suggests that the use of SCC in surgical patients to define NCD needs to be better delineated. Many patients do not know they meet the criteria for objective cognitive decline after surgery. There is a high prevalence of depression in patients who feel impaired. More studies are needed to better understand the role of depression and potentially anxiety in cognitive recovery after surgery. Correlation with functional and longer-term outcomes may shed some light on which whether SCC alone, objective testing alone, or the combination is most useful. In any case, patients with persistent cognitive complaints after surgery should be referred for evaluation to help better understand the nature of their impairment and create a supportive environment to promote recovery.
Authors' contributions
Study design: SD, KB, FS, MS, H-ML.
Patient recruitment: SD.
Data analysis: SD, MGB, H-ML, XL, MS.
Manuscript preparation: all authors.
Declarations of interest
SD has provided expert witness testimony, Merck consultation, and Covidien product support. MS is a consultant for VTV, Hoffman-La Roche Ltd, Biogen Idec, CogRx, Brackett, Eisai Inc., and Eli Lilly and Company; member of the Data and Safety Monitoring Board for AZTherapies and for the National Institute on Aging's Aspirin in Reducing Events in Elderly; and adjudicator for Trial Endpoint for Takeda, Takeda Pharmaceutical Company Ltd. The other authors declare that they have no conflicts of interest.
Funding
National Institute on Aging and the American Federation for Aging Research, Optimizing Postoperative Cognition in the Elderly (K23AG048332 to SD); 2P50AG005138-31 (to MS); Alzheimer's Association (to MS); 1I01HX001563-01A2 (to KB); 5P30AG028741-09 (to KB); R01AG033615 (to FS); R01AG046634 (to MGB).
Acknowledgements
The authors would like to acknowledge the support of National Institute on Aging Beeson Program, American Federation for Aging Research, Icahn School of Medicine at Mount Sinai, Department of Anesthesiology, and Ms Rachelle Jacoby.
Handling editor: M. Avidan
Editorial decision: 17 February 2019
Footnotes
This work was presented at the ASA Meeting 2018, San Francisco, CA, USA, and Beeson Retreat 2018, Charlotte, NC, USA.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.02.027.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Johnson T., Monk T., Rasmussen L.S. Postoperative cognitive dysfunction in middle-aged patients. Anesthesiology. 2002;96:1351–1357. doi: 10.1097/00000542-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Evered L., Silbert B., Knopman D.S. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery—2018. Br J Anaesth. 2018;129:872–879. doi: 10.1097/ALN.0000000000002334. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association . 5th Edn. American Psychiatric Association Publishers; Washington, DC: 2013. Diagnostic and statistical manual of mental disorders: DSM-5. [Google Scholar]
- 4.Koppara A., Wagner M., Lange C. Cognitive performance before and after the onset of subjective cognitive decline in old age. Alzheimer’s Dement (Amst) 2015;1:194–205. doi: 10.1016/j.dadm.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmonds E.C., Weigand A.J., Thomas K.R. Increasing inaccuracy of self-reported subjective cognitive complaints over 24 months in empirically derived subtypes of mild cognitive impairment. J Int Neuropsychol Soc. 2018;24:842–853. doi: 10.1017/S1355617718000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weintraub S., Salmon D., Mercaldo N. The Alzheimer’s disease Centers’ Uniform data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elwood R.W. The California Verbal Learning Test: psychometric characteristics and clinical application. Neuropsychol Rev. 1995;5:173–201. doi: 10.1007/BF02214761. [DOI] [PubMed] [Google Scholar]
- 8.Petrini L., Matthiesen S.T., Arendt-Nielsen L. The effect of age and gender on pressure pain thresholds and suprathreshold stimuli. Perception. 2015;44:587–596. doi: 10.1068/p7847. [DOI] [PubMed] [Google Scholar]
- 9.Folstein M.F., Folstein S.E., McHugh P.R. ‘Mini-mental state’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Royse C.F., Newman S., Chung F. Development and feasibility of a scale to assess postoperative recovery: the post-operative quality recovery scale. Anesthesiology. 2010;113:892–905. doi: 10.1097/ALN.0b013e3181d960a9. [DOI] [PubMed] [Google Scholar]
- 11.Brown C., Deiner S. Perioperative cognitive protection. Br J Anaesth. 2016;117 doi: 10.1093/bja/aew361. iii52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deiner S., Silverstein J.H. Anesthesia for geriatric patients. Minerva Anestesiol. 2011;77:180–189. [PubMed] [Google Scholar]
- 13.Deiner S., Lin H.M., Bodansky D., Silverstein J., Sano M. Do stress markers and anesthetic technique predict delirium in the elderly? Dement Geriatr Cogn Disord. 2014;38:366–374. doi: 10.1159/000363762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Royse C.F., Andrews D.T., Newman S.N. The influence of propofol or desflurane on postoperative cognitive dysfunction in patients undergoing coronary artery bypass surgery. Anaesthesia. 2011;66:455–464. doi: 10.1111/j.1365-2044.2011.06704.x. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen L.S., Johnson T., Kuipers H.M. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47:260–266. doi: 10.1034/j.1399-6576.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 16.Ferrell B.A., Stein W.M., Beck J.C. The geriatric pain measure: validity, reliability and factor analysis. J Am Geriatr Soc. 2000;48:1669–1673. doi: 10.1111/j.1532-5415.2000.tb03881.x. [DOI] [PubMed] [Google Scholar]
- 17.Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 18.Cosco T.D., Doyle F., Ward M., McGee H. Latent structure of the hospital anxiety and depression scale: a 10-year systematic review. J Psychosom Res. 2012;72:180–184. doi: 10.1016/j.jpsychores.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price C.C., Garvan C.W., Monk T.G. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology. 2008;108:8–17. doi: 10.1097/01.anes.0000296072.02527.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winblad B., Palmer K., Kivipelto M. Mild cognitive impairment—beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 22.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delis D.C., Freeland J., Kramer J.H., Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. J Consult Clin Psychol. 1988;56:123–130. doi: 10.1037//0022-006x.56.1.123. 1988. [DOI] [PubMed] [Google Scholar]
- 24.Molinuevo J.L., Rabin L.A., Amariglio R. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement. 2017;296–311 doi: 10.1016/j.jalz.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jessen F., Amariglio R.E., van Boxtel M. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu S.Y., Lee S.B., Kim T.W., Lee T.J. Subjective memory complaints, depressive symptoms and instrumental activities of daily living in mild cognitive impairment. Int Psychogeriatrics. 2016;28:487–494. doi: 10.1017/S1041610215001945. [DOI] [PubMed] [Google Scholar]
- 27.Buckley R.F., Maruff P., Ames D. Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer’s disease. Alzheimers Dement. 2016;12:796–804. doi: 10.1016/j.jalz.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Gifford K.A., Liu D., Damon S.M. Subjective memory complaint only relates to verbal episodic memory performance in mild cognitive impairment. J Alzheimers Dis. 2015;44:309–318. doi: 10.3233/JAD-140636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markova H., Andel R., Stepankova H. Subjective cognitive complaints in cognitively healthy older adults and their relationship to cognitive performance and depressive symptoms. J Alzheimers Dis. 2017;59:871–881. doi: 10.3233/JAD-160970. [DOI] [PubMed] [Google Scholar]
- 30.Richard E., Reitz C., Honig L.H. Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol. 2013;70:374–382. doi: 10.1001/jamaneurol.2013.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pellegrino L.D., Peters M.E., Lyketsos C.G., Marano C.M. Depression in cognitive impairment. Curr Psychiatry Rep. 2013;15:384. doi: 10.1007/s11920-013-0384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panza F., Frisardi V., Capurso C. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry. 2010;18:98–116. doi: 10.1097/JGP.0b013e3181b0fa13. [DOI] [PubMed] [Google Scholar]
- 33.Reid L.M., MacLullich A.M.J. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22:471–485. doi: 10.1159/000096295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.