Toll-like receptor signaling in brain is emerging as an important contributor to the pathology of stress and alcohol use disorders (AUDs). Toll-like receptor 4 (TLR4), a member of the interleukin-1 receptor/toll-like receptor (TLR) superfamily is expressed in brain. Microglia have basal expression, whereas neuronal and astrocyte expression increases the following priming by stress or alcohol. The endotoxin-LPS receptor discovered to be TLR4 in the late 20th century, more recently, has been found to respond to the endogenous agonist, high mobility group box 1 (HMGB1). Studies in rodents find ethanol exposure increases brain expression of both HMGB1 and TLR4 as well as other neuroimmune genes in association with increased ethanol drinking (Blednov et al., 2012) and neurodegeneration (Coleman and Crews, 2018). Further, human studies find increases of TLR4 and HMGB1 in post-mortem brain of individuals with AUD that positively correlate with life-time alcohol consumption (see Fig. 1; Crews et al., 2017).
Fig. 1.
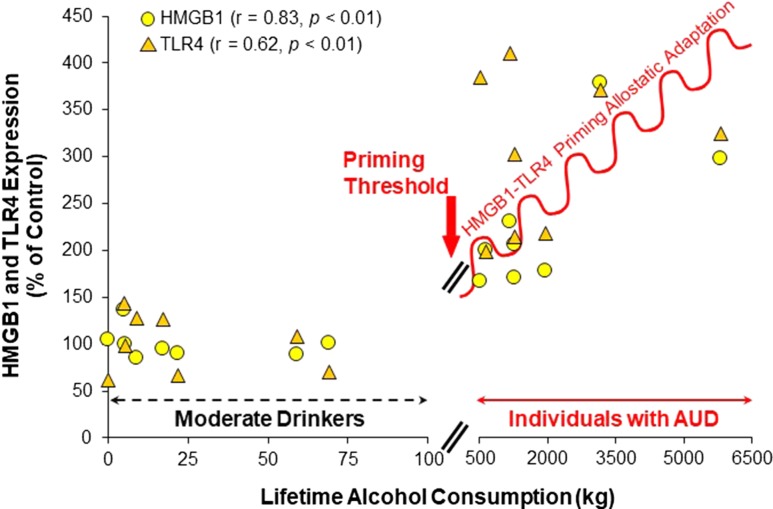
HMGB1 and TLR4 expressions in post-mortem PFC of moderate drinkers and individuals with AUD. Shown are cellular expressions in orbital frontal cortex determined using immunohistochemistry and the life-time alcohol consumption in kilograms of absolute alcohol as determined by New South Wales Brain Research Center for moderate-drinking controls and individuals with AUD. Note: Individuals with AUD consume from 5 to 50 fold more alcohol than moderate drinkers. A priming threshold of about 100 kg life-time consumption represents less than one drink per day and controls show no induction of HMGB1 or TLR4. In contrast, individuals with AUD have progressive increases with increasing alcohol consumption. These findings are consistent with multiple cycles of binge drinking contributing to the increased HMGB1-TLR4 found in AUD that likely primes neurocircuitry contributing to subjective responses to alcohol and the accumulation of stress- and alcohol-induced withdrawal anxiety (adapted from Crews et al., 2013).
Evidence suggests that ethanol priming of HMGB1-TLR4 signaling occurs through multiple mechanisms including direct ethanol induction in brain cells as well as brain responses to systemic immune signals. Excessive alcohol and stress can increase gut ‘leakage’ of endotoxin into the blood that increases circulating immune signals. These signals may both prime brain HMGB1-TLR4 signaling as well as increase craving for alcohol in individuals with AUD (Temko et al., 2017; Leclercq et al., 2018). Cycles of binge drinking and stress have been suggested to increase the risk for development of AUDs through sensitization or priming of synaptic circuitry increasing craving and risks for AUD. Alcohol- and stress-induced increases in TLR4 and HMGB1 may sensitize specific neurocircuits through autocrine and paracrine signaling across neurons and glia within a brain region. HMGB1-TLR4 signaling is emerging as a mechanism of innate immune memory, which in brain is plasticity related to experience that could be a mechanism of allosteric adaptation that is proposed to drive alcohol seeking (see Fig. 1). Innate immune signals in brain lead to persistent and progressive synaptic changes, most often enhancing excitability.
Seizures provide an example of the persistent and progressive changes due to increased HMGB1-TLR4 expression in hippocampus. Epilepsy and hippocampal seizures provide an example of HMGB1-TLR4 synaptic plasticity. An initial seizure is known to cause a reduction in seizure threshold that increases risk of subsequent seizures and which persists and progresses with each additional seizure. Hippocampal seizures increase HMGB1-TLR4 expression that endures and lowers seizure threshold through excitation-induced neuronal release of HMGB1 triggering TLR4 glutamate release increasing excitability (Maroso et al., 2010). Similarly, exposure to stress and alcohol causes modest changes in HMGB1 and TLR4 expressions that persist, and with repeated exposures progressively sensitize responses of neurocircuits.
HMGB1 and TLR4 expressions in post-mortem prefrontal cortex (PFC) of individuals with AUD are increased and correlated with life-time alcohol consumption, but moderate-drinking controls do not show a relationship with life-time alcohol consumption; perhaps a seizure-like threshold of binge drinking or stress are needed to induce plasticity (see Fig. 1). Individuals with AUD show a broad range of consumption that is considerably greater than control moderate drinkers. The progressive and persistent development of AUD is consistent with binge drinking- and stress-induced progressive and persistent increases in HMGB1-TLR4 signaling. However, there are multiple innate immune signaling molecules, many of which are increased by HMGB1-TLR4 signaling that could also contribute to the development of AUD. Further, cycles of moderate ethanol exposure have been found to prime anxiety-like behavior through this signaling (see below). How this signaling contributes to changes in synaptic circuitry that might contribute to AUD is poorly understood and needs further investigation. In this issue of Alcohol and Alcoholism, two articles explore TLR4 interactions, one rat study focused on amygdala GABA–corticotropin-releasing factor (CRF) responses to repeated stress and one human study on how TLR4 gene methylation impacts subjective responses to alcohol.
The study by Karolya et al. (2018) finding interactions between TLR4 methylation and alcohol subjective and physiological responses extends previous studies reporting hyper-methylation of the TLR4 receptor DNA in individuals with AUD. In this study over 200 subjects were divided into heavy drinkers, defined as greater than 14 drinks per week or light-moderate drinkers, and the level of TLR4 DNA CpG site methylation, a known regulator of gene expression, was assessed. Individuals were also assessed for subjective and physiological responses to i.v. alcohol exposure to blood alcohol levels up to 0.06 g/dl (i.e. a moderate, but not binge drinking blood alcohol concentration). Heavy drinkers had positive associations between TLR4 DNA methylation and subjective stimulation, tension and arousal. Light drinkers had the opposite response. These studies suggest a relationship between TLR4 methylation and subjective physiological responses to acute alcohol intoxication. Acute ethanol can release HMGB1 from neurons that can trigger TLR4 signaling impacting subjective responses to alcohol. However, preclinical studies find HMGB1-TLR priming occurs at blood alcohol concentrataions (BACs) higher than those that can be easily studied in humans. Karoly et al. infused alcohol to 0.06 g/dl while determining subjective responses. One would expect subtle changes at BACs below the binge drinking level and they cleverly use regression analysis to disclose subtle differences in arousal and positive feelings with TLR4 methylation. TLR4 methylation and subjective responses to alcohol were found to be dependent upon regular drinking levels, with higher regular drinking being associated with arousal and more positive subjective responses to alcohol. Studies of individuals with AUD find increased DNA methylation of multiple genes, but this study focused on a single gene (i.e. TLR4) in non-alcoholic drinkers that is consistent with TLR4 signaling contributing to subjective responses to alcohol in all individuals.
Varodayan et al. (2018) assessed synaptic processes underlying the effects of CRF on the central nucleus of the amygdala (CeA) GABAA-mediated spontaneous inhibitory post-synaptic currents (sIPSCs). Wild-type (WT) and TLR4-KO rats were used to test the hypothesis that TLR4 plays a role in CRF modulation of GABA in the CeA. No differences in baseline responses were found between WT and TLR4-KO in baseline sIPSCs or CRF increased sIPSCs. Restraint stress did not cause a change in either WT or TLR4-KO. The lack of difference in responses is consistent with the idea that priming of HMGB1-TLR4 signaling by stress or alcohol is needed to observe the role of TLR4. Interestingly, although single or repeated stress did not alter sIPSCs in WT or TLR4-KO, it did change the CRF-induced increase in sIPSCs following repeated stress. CRF increases IPSCs, but repeated stress led to a loss of the CRF potentiation in the TLR4-KO animals. CRF in amygdala is primed by stress and alcohol. These findings are consistent with repeated stress priming HMGB1-TLR4 signaling that sustains CRF increased GABAergic sIPSCs. Breese and colleagues (2016) have reported that multiple cycles of moderate ethanol consumption and stress in rats increase amygdalar excitability and ethanol withdrawal anxiety-like behavior. HMGB1, other neuroimmune signaling molecules, and CRF increase with cycles of exposure that shows parallel increases in ethanol withdrawal anxiety-like behavior. They found that injection of TLR4 agonists, other immune signaling molecules, or CRF mimics a cycle of moderate ethanol exposure in the priming of anxiety consistent with immune-CRF signaling increasing anxiety-like behavior. Further, ethanol withdrawal anxiety is blocked by CRF and HMGB1 antagonists (Whitman et al., 2013). This is consistent with the findings presented by Varodayan et al. that the TLR4-KO did not alter sIPSPs after a single stress, but did alter sIPSPs after multiple stress exposures. These studies suggest that TLR4-CRF interactions in amygdala require priming to observe a change. Controls show no effect since they are not primed by cycles. These studies further support the role of cycles of stress or alcohol contributing to increases in alcohol withdrawal-induced anxiety. Thus, the studies reported here extend the link between amygdala HMGB1-TLR4 signaling, CRF and GABA synaptic sIPSCs that likely contribute to withdrawal anxiety and possibly craving for alcohol.
The above mentioned two papers in this issue of Alcohol and Alcoholism on TLR4 receptors relating human subjective responses to alcohol and rat stress synaptic circuitry continue to support the role of HMGB1-TLR4 signaling in stress and alcohol-induced pathologies.
REFERENCES
- Blednov YA, Ponomarev I, Geil C, et al. (2012) Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol 17:108–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ (2016) Persistent adaptation by chronic alcohol is facilitated by neuroimmune activation linked to stress and CRF. Alcohol 52:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Crews FT (2018) Innate immune signaling and alcohol use disorders. In Handbook of Experimental Pharmacology. Berlin: Springer, Heidelberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, et al. (2013) High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry 73:602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Walter TJ, Coleman LG Jr., et al. (2017) Toll-like receptor signaling and stages of addiction. Psychopharmacology (Berl) 234:1483–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolya HC, Ellingsona JM, Hutchison KE, et al. (2018) Interactions between TLR4 methylation and alcohol consumption on subjective responses to an alcohol infusion Hollis C. Alcohol and Alcoholism 53:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Starkel P, Delzenne NM, et al. (2018) The gut microbiota: a new target in the management of alcohol dependence? Alcohol. doi:10.1016/j.alcohol.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, et al. (2010) Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 16:413–9. [DOI] [PubMed] [Google Scholar]
- Temko JE, Bouhlal S, Farokhnia M, et al. (2017) The micobiota, the gut and the brain in eating and alcohol use disorders: a ‘Ménage à Trois’? Alcohol and Alcoholosm 52:403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Khom S, Patel RR, et al. (2018) Role of TLR4 in the modulation of central amygdala GABA transmission by CRF following restraint stress. Alcohol and Alcoholism 54:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman BA, Knapp DJ, Werner DF, et al. (2013) The cytokine mRNA increase induced by withdrawal from chronic ethanol in the sterile environment of brain is mediated by CRF and HMGB1 release. Alcohol Clin Exp Res 37:2086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]