Abstract
Tangential excision and autologous split-thickness skin grafting is the standard management of the burn wound, but autograft has limitation of donor-site availability and morbidity. Human skin allograft is an alternate option of wound coverage when autograft is not available. Various synthetic skin substitute dressings are now available in the market, and thus use of human skin allograft has decreased. This case report explores the role of human skin allograft in burn wound management. Allograft facilitates excision of burn wounds during acute phase of burn injury in pediatric patients. It is cost-effective, reduces pain and risk of infection, and avoids frequent dressing changes. Availability of allograft and risk of infection are the two main constraints in its regular use.
Keywords: Allograft, burns, human, skin
Keymessages: 1. Human skin allograft facilitates excision of burn wounds during acute phase of burn injury in pediatric patients., 2. Human skin allograft cannot be replaced by synthetic skin substitutes at present.
Introduction
Use of skin allograft in burn wound management was first described by Bettman in 1938.[1] Skin allograft is obtained from a human donor (deceased or healthy) and used as a temporary cover for burn wounds. It can be classified into the following:
-
Viable:
a. Fresh (freshly harvested from donor or refrigerated)
b. Cryopreserved
-
Nonviable:
a. Lyophilized (glycerol)
b. Irradiated (gamma irradiation)[2]
Full-thickness burn wounds should be excised and covered with split-thickness autograft. However, autograft has limitation of donor-site availability and morbidity. When autograft is not available, allograft can be used. It can also be used to cover partial-thickness burn wounds till it gets epithelized.
Allografts are preserved in skin bank. After the advent of commercially available biological dressings (various skin substitutes), use of human skin allograft has decreased. Biological dressing materials also serve all the functions of allograft, but there are certain advantages of allograft. Through this case report, we have revisited the viability of human skin allograft as an option in the armamentarium of surgeon for burn wound management.
Case Report
A 5-year-old girl was admitted to tertiary burn care center of our institute with 30% body surface area flame burn injury over face, shoulders, and trunk on anterior and posterior aspect. On clinical examination, it was found that the wound had mixed areas of second-degree superficial and deep burn injury [Figure 1]. The patient was in hypovolemic shock at the time of presentation. She was provided intravenous fluid resuscitation and supportive treatment after which she recovered from shock. On 2nd post-burn day, it was decided to carry out tangential excision and biological coverage of the second-degree deep burn portion (around 10% of the total body surface area) of the wound. It was decided to put fresh human skin allograft for wound coverage. Option of skin allograft was explained to the parents, and the patient’s father agreed to donate skin allograft. Written informed consent was obtained from both the parents. The donor was taken to the operation theatre and under regional anesthesia, a single sheet of split-thickness skin graft was harvested with aseptic precautions from the anterolateral aspect of his left thigh. The graft was kept in tissue culture medium at 4°C and transferred to the recipient operation theatre. Fresh skin allograft was applied over the patient’s wounds after tangential excision [Figure 2]. Only second-degree deep burn portion of the wound was excised and grafted. Allograft was minimally meshed (hand meshing) to cover the excised wound completely. The graft was taken well initially and then started showing signs of rejection from 10th postoperative day. It got completely peeled off on 14th day and the wound was fully healed by that time. The patient responded well to the allografting and did not develop hypovolemia, dyselectrolytemia, or any wound infection during the course of treatment.
Figure 1.
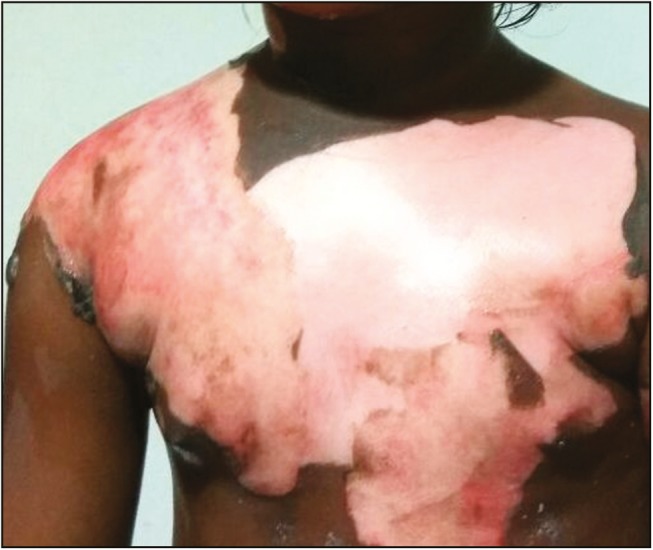
Burn wound before debridement
Figure 2.
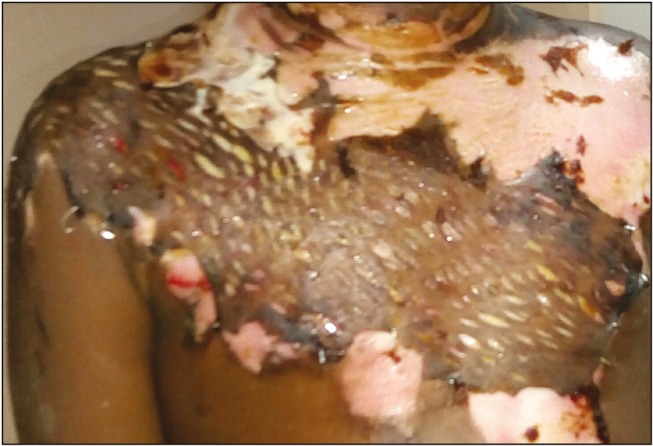
Wound debrided and allograft applied (postoperative day 3)
Discussion
Clinical indications of skin allograft use in burns are the following:
Coverage of extensive full-thickness wounds
Coverage of widely meshed skin autografts
Healing of partial-thickness wounds
Wound bed preparation and testing for later acceptance of autograft[1]
Pediatric patients have limited body reserves and develop severe shock and dyselectrolytemia with surgical stress. Fluids and protein loss from the deep wounds further contributes in disturbing body homeostasis. Harvesting autograft from the patient with burns produces another wound and it has the risk of further deteriorating the patient’s condition, especially in pediatric patients during acute phase of burn injury. Option of allograft makes the early wound excision possible in such situations. Human skin allograft effectively reduces water, electrolyte, and protein loss from the wound and reduces energy requirements of the body.[1] In our patient, we have used allograft for the same reason.
Many skin substitutes are available for the coverage of burn wounds [Table 1].[2,3] Allograft is compared with synthetic skin substitutes in Table 2.[1,2,3]
Table 1.
Skin substitutes
| Skin substitutes |
|---|
| Biological |
| Amnion |
| Xenograft (porcine) |
| Allograft |
| Synthetic |
| Acellular |
| ®Biobrane |
| ®Integra |
| ®Matriderm |
| ®Renoskin |
| ®Alloderm |
| Various forms of collagen (sheet/gel/flakes) |
| Cellular allogeneic |
| ®Dermagraft |
| ®Apligraft (Graftskin) |
| ®Orcel |
| ®Hyalomatrix |
| ®TransCyte |
| Cellular autologous |
| Cultured epidermal autograft (CEA) |
Table 2.
Comparison between allograft and synthetic skin substitutes
| Allograft | Synthetic skin substitutes |
|---|---|
| Advantages | Advantages |
| Presence of a basement membrane | Controlled composition of scaffold |
| More intact and natural extracellular matrix composition | Growth factors and matrix components can be added as required |
| Provides growth factors and cytokines (helps in wound bed preparation) | Low risk of infection |
| Excellent reepithelialization rate | Reduces pain |
| Reduces pain | Reduces water, electrolyte, and protein loss from the wound |
| Reduces water, electrolyte, and protein loss from the wound | Less frequent dressing change |
| Less frequent dressing change | |
| Relatively less expensive | |
| Disadvantages | Disadvantages |
| Antigenicity (rejection) | Lack of basement membrane |
| Risk of infection | Less stable scaffold |
| Availability of donor | High cost |
| Antigenicity (foreign body reaction) | |
| In case of collagen: frequent dressing changes lead to more pain and risk of infection | |
| Reepithelialization and engraftment rates are similar or less than allograft (inconclusive evidence) |
Collagen is the most commonly available and comparatively economical synthetic skin substitute. Both allograft and collagen dressing reduce the incidence of infection and wound pain and accelerate the reepithelialization of the wound.[4] But collagen dressing gets absorbed in 3–6 days. Pain and risk of infection increase with frequent dressing changes. Skin allograft remains over the wound for 2–3 weeks before its rejection.[4,5] Allograft relieves pain and controls infection more effectively than collagen dressing. Allograft was rejected in 14 days in our patient.
Allografts are cost-effective compared to commercially available collagen dressings because collagen dressings are costly and require to be applied multiple times before the wound heals. Other synthetic skin substitutes have more cost and less availability.[2,3] Ability of allograft to prevent tissue desiccation and promote epithelialization reduces hospital stay.
Fresh (freshly harvested or refrigerated) skin allografts are better than cryopreserved or nonviable allografts. Fresh allograft gets revascularized rapidly and adheres to the wound. It provides better control of microbial growth.[1] We used fresh skin allograft and it was taken up well in the beginning.
One interesting indication of skin allograft is the social indication. This is common in pediatric patients with burn, especially in girls. Parents usually do not like extra scarring over patient’s body, rather they are ready to sacrifice and get scar over their own body. In such situations, allograft from one of the parents is a viable option for burn wound coverage. Although skin allograft can be obtained from any donor after initial screening, but if available, a donor who is first-degree relative of the recipient is preferred to delay the rejection.
Allograft should not be meshed because allogeneic epidermis does not epithelialize into the interstices.[1] We have minimally expanded the allograft to avoid harvesting one more sheet of skin from the healthy donor.
Skin allograft can be harvested from the torso, hips, thighs, and upper calves of the diseased donor.[1] But in case of healthy donor, allograft retrieval should be limited to minimum possible. Written informed consent should be obtained and the donor should be well explained about the procedure, postoperative pain, healing time of the donor site, and expected scarring.
There are concerns regarding disease transmission through skin allograft.[1,6] Skin donor should be screened for human immunodeficiency virus (HIV)-1, HIV-2, hepatitis B, hepatitis C, hepatitis C virus, human T-cell lymphotrophic virus-1, and syphilis serology status. In case of stored skin, microbial and fungal culture should be performed before donor skin release from the tissue bank. In case of fresh skin, the donor site should be examined to rule out any infection.[1] Hence, all the preoperative screening should be performed before transferring allograft to the patient.
Conclusion
Human skin allograft facilitates the excision of burn wounds during acute phase of burn injury in pediatric patients. Allograft avoids pain and risk of infection from frequent dressing changes. Availability of allograft and risk of infection are the two main constraints in its regular use. Within its indications, human skin allograft is an effective method of burn wound coverage and it cannot be replaced by synthetic skin substitutes at present.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This study was supported by the Department of Plastic Surgery, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kagan RJ, Winter R, Robb EC. The skin bank. In: Herndon DN, editor. Total burn care. Edinburgh, Scotland: Elsevier; 2012. pp. 199–208. [Google Scholar]
- 2.Halim AS, Khoo TL, Yussof SJ. Biologic and synthetic skin substitutes: an overview. Indian J Plast Surg. 2010;43:23–8. doi: 10.4103/0970-0358.70712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alrubaiy L, Al-Rubaiy KK. Skin substitutes: a brief review of types and clinical applications. Oman Med J. 2009;24:4. doi: 10.5001/omj.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leon-Villapalos J, Eldardiri M, Dziewulski P. The use of human deceased donor skin allograft in burn care. Cell Tissue Bank. 2010;11:99–104. doi: 10.1007/s10561-009-9152-1. [DOI] [PubMed] [Google Scholar]
- 5.Qaryoute S, Mirdad I, Hamail AA. Usage of autograft and allograft skin in treatment of burns in children. Burns. 2001;27:599–602. doi: 10.1016/s0305-4179(00)00152-2. [DOI] [PubMed] [Google Scholar]
- 6.Kealey GP, Aguiar J, Lewis RW, Rosenquist MD, Strauss RG, Bale JF. Cadaver skin allografts and transmission of human cytomegalovirus to burn patients. J American Col Surg. 1996;182:201–5. [PubMed] [Google Scholar]


