Abstract
Background & objectives:
In contrast to Caucasians of European origin, the aetiology of diabetes mellitus (DM) in young adults in other ethnic groups, including Indians is likely to be heterogeneous and difficult to determine. This study was undertaken to determine the aetiology of diabetes in young Indian adults using a protocol-based set of simple clinical and investigation tools.
Methods:
In this prospective study, 105 Indian young adults with diabetes (age at onset 18-35 yr; duration <2 yr) were studied for a period of 1-3 years. Pancreatic imaging, fasting C-peptide, islet antibodies (against glutamic acid decarboxylase, tyrosine phosphatase and zinc transporter-8) and mitochondrial A3243G mutational analysis were performed in all patients. Four patients were screened for maturity-onset diabetes of the young (MODY) using next-generation sequencing.
Results:
Type 1 and type 2 diabetes mellitus (T1DM and T2DM) were equally frequent (40% each), followed by fibrocalculous pancreatic diabetes (FCPD, 15%). Less common aetiologies included MODY (2%), mitochondrial diabetes (1%) and Flatbush diabetes (2%). There was considerable phenotypic overlap between the main aetiological subtypes. Elevated islet antibodies were noted in 62 per cent of T1DM patients [positive predictive value (PPV) 84%; negative predictive value (NPV) 78%] while low plasma C-peptide (<250 pmol/l) was present in 56 per cent of T1DM patients [PPV 96% (after excluding FCPD), NPV 72%]. Using these tests and observing the clinical course over one year, a final diagnosis was made in 103 (99%) patients, while the diagnosis at recruitment changed in 23 per cent of patients.
Interpretation & conclusions:
The aetiology of diabetes in young adults was heterogeneous, with T1DM and T2DM being equally common. FCPD was also frequent, warranting its screening in Indian patients. Testing for islet antibodies and C-peptide in this age group had good PPV for diagnosis of T1DM.
Keywords: Aetiological heterogeneity, C-peptide, early-onset diabetes, fibrocalculous pancreatic diabetes, islet antibodies
Diabetes mellitus (DM) increasingly affects young adults1,2. This is especially true for low- and middle-income countries where diabetes occurs at an earlier age than in more affluent nations1,2. In a nation-wide study, the age-specific prevalence of diabetes in young adults between 30-34 years was 4 per cent in males 3 per cent among females3. Both type 1 and type 2 diabetes mellitus (T1DM and T2DM) are prevalent in this age group, with their relative frequency varying with the ethnic group studied4,5,6,7,8. In addition, secondary diabetes and genetic forms of diabetes such as maturity-onset diabetes of the young (MODY) and mitochondrial diabetes need to be considered. In the Indian subcontinent, chronic pancreatitis may present with minimal abdominal pain or exocrine insufficiency and thus be misclassified as either T1DM or T2DM9. While it is necessary to accurately determine the aetiology of diabetes, an overlap of clinical features makes this difficult to achieve.
There are considerable differences in the aetiology of diabetes in young adults in different regions of the world. While T1DM is the predominant phenotype in Caucasians of European origin4,5, T2DM forms the major subtype in several Asian countries6,7. Results suggesting heterogeneity among early-onset T2DM were noted in studies from UK10 and Mexico11. Monogenic diabetes (MODY and mitochondrial diabetes) has also been diagnosed among patients diagnosed as T1DM or T2DM10,11,12,13,14.
Aetiological heterogeneity has been reported among early-onset diabetes from different regions of India8,15,16. However, there were some limitations in these studies. First, malnutrition-modulated diabetes mellitus (MMDM) constituted a major subset of diabetes in one study8, the existence of which is controversial and no longer proposed by the World Health Organization. Nearly, a quarter of the patients with MMDM in this study were positive for glutamic acid decarboxylase (GAD) antibodies8, suggesting that a large proportion of them had T1DM. Second, these studies relied mostly on clinical criteria and abdominal imaging [to diagnose fibrocalculous pancreatic diabetes (FCPD)]. In the absence of other investigations, such as islet antibodies and the common mitochondrial gene mutation, it is likely that a proportion of patients might have been misdiagnosed. Finally, despite the fact that in a proportion of patients with diabetes, the determination of the aetiology of diabetes may require a short follow up, none of the earlier reports have studied their patients over time.
In view of the paucity of information regarding the aetiology of diabetes in young adults in India and the utility of different investigations in accurately classifying these patients, this prospective study was conducted to determine the aetiology of diabetes in young adults using protocol-based set of clinical features and laboratory investigations. The utility of different variables in predicting the aetiology of diabetes was also estimated.
Material & Methods
This prospective study was conducted between July 2013 and June 2015. The patients were recruited from the Endocrinology clinic of Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), a tertiary care centre located in Lucknow, India. The inclusion criteria were an age at onset between 18 and 35 yr and duration of diabetes less than two years. Patients with gestational diabetes, diabetes secondary to drugs and endocrinopathies and those with chronic diseases were excluded. All patients with a prior diagnosis of chronic pancreatitis were also excluded. After initial exclusion, 105 patients were found eligible in the final study (Fig. 1). There were 74 males with a median age of diagnosis of 27 [interquartile range (IQR) 22-31] yr and duration of diabetes seven (IQR 1-18) months. All patients were seen in follow up for at least one year (median 18 months, range 12-36 months), and 45 patients completed two years of follow up. Informed written consent was obtained from each patient, and the study was approved by the Institutional Ethics Committee.
Fig. 1.
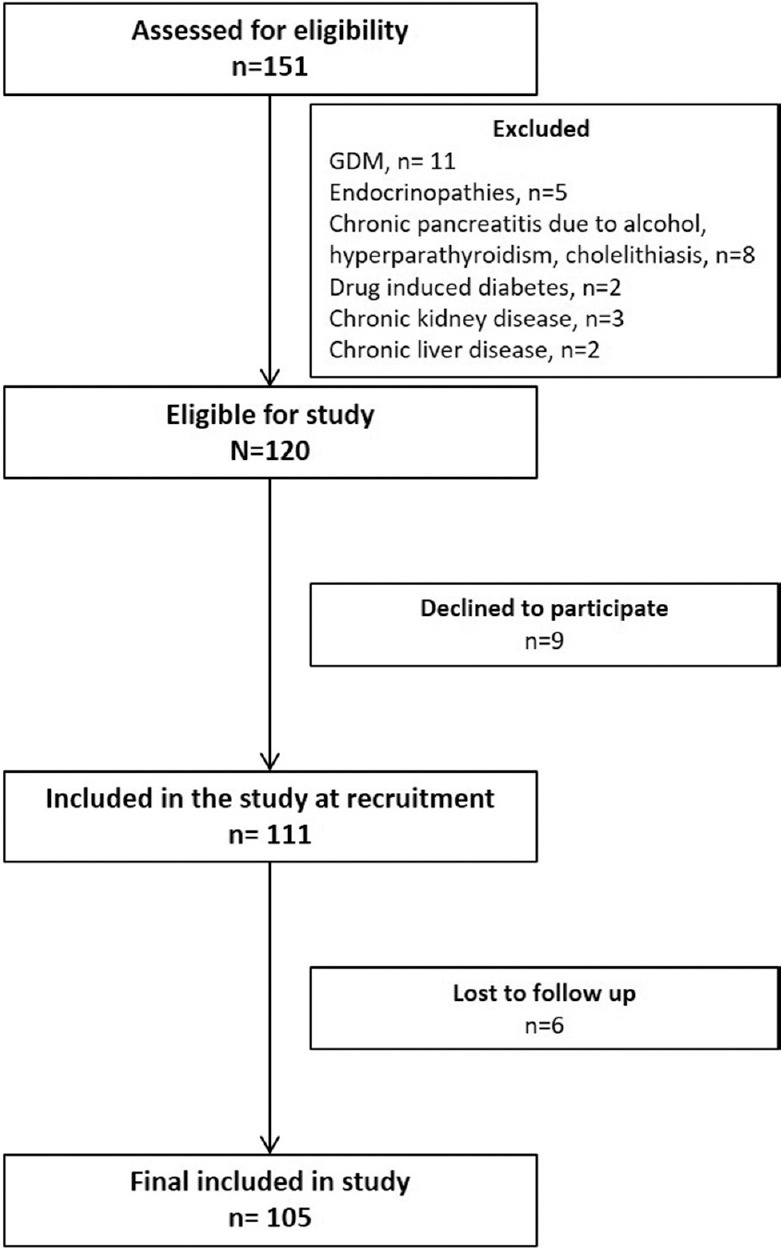
Flow chart demonstrating recruitment of patients. GDM, gestational diabetes mellitus.
Patient evaluation: All patients were subjected to a thorough clinical evaluation and an abdominal ultrasound and/or computed tomography (CT) scan for pancreatic morphology. All patients were tested for plasma glucose, lipids, glycated haemoglobin (HbA1c), C-peptide and islet antibodies [against GAD, tyrosine phosphatase (IA-2) and zinc transporter-8 (ZnT8)] and mitochondrial A3243G mutation. Screening for MODY gene mutations was performed in four patients who fulfilled the following criteria: absence of diabetic ketoacidosis (DKA), lack of metabolic syndrome (MetS), a positive family history of early-onset diabetes (age <35 yr) in at least two generations, and absent GAD, IA-2 and ZnT8 antibodies. All patients were treated with medical nutrition therapy (MNT), along with oral hypoglycaemic agents (OHAs) or insulin, as required. They were seen as part of their routine follow up at three and six months and then at six monthly intervals. Any episodes of DKA were noted and insulin requirement was assessed. HbA1c was measured at each visit. Fasting plasma C-peptide was measured at two years from diagnosis in 56 and between one and two years in 48 patients. The sample for C-peptide from one patient with T1DM was not available.
Investigations: All biochemical investigations and measurement of autoantibodies were done in the department of Endocrinology, SGPGIMS, except ZnT8 antibody which was assayed at University of Colorado. Sequencing for MODY genes, done in the department of Endocrinology at Christian Medical College, Vellore. A fasting venous sample (7 ml) was obtained at presentation. Plasma glucose and lipids were measured within four hours, using an autoanalyzer (Technicon RX-XT; Bayer, Tarrytown, NY, USA). Serum and plasma were preserved at −80°C, while genomic DNA was extracted using columns (Qiagen, Hilden, Germany) and stored at −20°C. HbA1c (normal range 4-6%, http://www.ngsp.org/ifccngsp.asp) was measured by HPLC (Bio-Rad Variant II, Hercules, CA, USA). The measuring range was 3.1-18.5 per cent, and the inter- and intra-assay coefficients of variation (CV) were 0.46-0.64 and 0.59-0.9 per cent, respectively. C-peptide was measured by immunoradiometric assay (Beckman Coulter, Prague, Czech Republic). The intra- and inter-assay CV were 3.1 and 5.2 per cent, respectively, while the analytic sensitivity was 3.6 pmol/l.
Antibodies against GAD, IA-2 an d ZnT8 were measured using radioligand binding assay using immunoprecipitation of35 S-methionine-labelled human recombinant antigens14. The cut-off for positivity was ≥47 and ≥3.7 units for GAD and IA-2 antibodies, respectively. The assay had a sensitivity and specificity of 74 and 98 per cent for GAD antibody and 76 and 99 per cent for IA-2 antibody in the Islet Autoantibody Standardization Program (IASP), 201517. The cut-off for positivity for ZnT8 antibody was 0.02 units with a sensitivity and specificity of 66 and 100 per cent, respectively, in the 2017, IASP workshop17. Transglutaminase (TTG) antibody was measured by ELISA (Diesse Chorus tTg-A, Siena, Italy). A value of >18 units/ml was considered positive. The measuring range was 3-100 units/ml, and the inter- and intra-assay CV were 1.3-7.7 per cent and 3.7-8.5 per cent, respectively, across different range of measurements. Thyroid peroxidase (TPO) antibody was measured by chemiluminescent assay (Immulite 1000, Erlangen, Germany); >35 IU/ml was considered as positive (analytic sensitivity 7 IU/ml, inter-assay CV 7.8 -10.5% and intra-assay CV 3.5-5.6%).
Abdominal imaging with ultrasonography and/or CT scan was performed for pancreatic ductal dilatation and calcification in all patients. All patients were tested for mitochondrial A3243G mutation by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis14. Screening for 13 MODY gene mutations was performed using next-generation sequencer (Ion Personal Genome Machine System, Thermo Fisher Scientific, Life Technologies Corporation, Carlsbad, CA, USA)18. The data were analyzed using Ion torrent suit software (Life Technologies Corporation) and DNAStar (DNAStar Inc., Madison, WI, USA) software. All mutations and rare variants were validated by Sanger DNA sequencing18.
Definitions: T1DM: continuous insulin requirement within six months of diagnosis, fasting plasma C-peptide <250 pmol/l at one year (or later) or positive GAD, IA-2 and/or ZnT8 antibody19; FCPD: pancreatic duct dilatation/calcification on imaging and absence of established risk factors for pancreatitis; T2DM: clinical features of insulin resistance (acanthosis, overweight and/or hypertension), family history of T2DM, adequate glycaemic control with OHA, fasting C-peptide >400 pmol/l at one year (or later) and absent islet antibodies; MODY or mitochondrial diabetes: mutation in any MODY genes or mitochondrial A3243G gene. MetS was defined as per International Diabetes Federation criteria20.
Statistical analysis: The required sample size was calculated using results from a previous north Indian study16, in which the proportion of T1DM and T2DM in ages 19-25 yr was 63 and 26 per cent, respectively. At a confidence interval of 95 per cent with 90 per cent power, a minimum of 38 patients were required in each of the T1DM and T2DM groups. Using another study15, where the proportions of T1DM and T2DM were 17 and 66 per cent, respectively, in the 20-30 yr age group, the minimum sample size was estimated to be 18 in each group. Data were presented as mean±standard deviation or median (IQR). To compare the means between the groups, ANOVA and Kruskal-Wallis test were used for parametric and non-parametric data, respectively. For the significant variables, post hoc analyses using Tukey test and Kruskal-Wallis one-way ANOVA were done for parametric and non-parametric data, respectively. The Chi-square test was used to assess differences in the proportions, and Fisher's exact test was carried out where the cell frequency was <5. Diagnostic value of various clinical and biochemical factors in T1DM and T2DM were calculated with their 95 per cent confidence intervals. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) software version 20.0 (IBM, Armonk, NY, USA).
Results
Patient characteristics are described in Table I. The mean body mass index (BMI) was 21.9 (range 10-36.7) kg/m2 and 37 (35%) had central obesity. Initial treatment included MNT alone in five (5%), OHAs in 61 (58%) and insulin in 39 (37%) patients.
Table I.
Characteristics of patients and major subtypes of diabetes mellitus
| Parameters | Whole group (n=105)† | T1DM (n=42) | T2DM (n=42) | FCPD (n=16) |
|---|---|---|---|---|
| Age at diagnosis (yr) | 27 (22-31) | 23 (20-27) | 31 (28-33)*** | 24 (19-28)††† |
| Plasma glucose at diagnosis (mmol/l) | 22.2±7.4 | 26.4±7.4 | 18.4±5.2*** | 21.7±6.9* |
| HbA1c at diagnosis | ||||
| NGSP (%) | 11.7±3.2 | 12.4±3.8 | 10.3±2.1 | 12.3±2.7 |
| IFCC (mmol/mol) | 104±11 | 112±18 | 89±9 | 111±9 |
| DKA | 25 (24) | 22 (52) | 0*** | 1 (6)*** |
| Symptomatic | 82 (78) | 40 (95) | 23 (55)*** | 15 (94)†† |
| Acanthosis nigricans | 18 (17) | 1 (2) | 15 (36)*** | 0†† |
| Associated autoimmunity | 19 (18) | 12 (29) | 3 (7)** | 2 (13) |
| Family history of T2DM | 45 (43) | 11 (26) | 25 (60)** | 6 (38) |
| Metabolic syndrome | 34 (32) | 4 (10) | 27 (64)*** | 1 (6)††† |
| BMI (kg/m2) | 21.9±5.6 | 19.0±4.0 | 25.9±4.9*** | 18.7±3.9††† |
| Waist circumference (cm) | 80 (74-91) | 76 (71-80) | 92 (86-97)*** | 75 (72-82)††† |
| Overweight | 44 (41) | 9 (21) | 30 (71)*** | 3 (19)††† |
| SBP (mmHg) | 120 (110-130) | 110 (100-120) | 130 (116-140)*** | 113 (110-129) |
| DBP (mmHg) | 76 (70-80) | 70 (68-80) | 80 (70-90)*** | 80 (70-80) |
| Triglyceride (mmol/l) | 1.50 (1.05-1.98) | 1.10 (0.94-1.62) | 1.66 (1.37-2.18)*** | 1.51 (0.97-1.76) |
| HDL cholesterol (mmol/l) | 0.98±0.26 | 0.98±0.31 | 0.96±0.21 | 1.01±0.23 |
| Total cholesterol (mmol/l) | 4.07 (3.37-4.84) | 3.81 (3.13-4.51) | 4.56 (3.88-5.39)** | 3.73 (3.37-4.79) |
| LDL cholesterol (mmol/l) | 2.36 (1.84-2.95) | 2.10 (1.63-2.95) | 2.54 (2.07-3.00)* | 2.28 (1.84-2.72) |
| Fasting C-peptide (pmol/l) | 619 (191-1130) (n=104) | 228 (115-546) (n=41) | 1137 (832-1647)*** | 209 (165-637)††† |
| Islet antibodies (against GAD, IA-2 and ZnT8) | 31 (30) | 26 (62) | 3 (7)*** | 1 (6)*** |
| Current treatment, n(MNT alone/OHAs/Insulin) | 1/41/63 | 0/0/42 | 1/40/1 | 0/0/16 |
P *<0.05, **<0.01, ***<0.001 compared to T1DM, ††<0.01 †††<0.001 compared to T2DM. Categorical data expressed as n (%); continuous data expressed as mean+SD or median (interquartile range). Analyses were based on the final diabetes subtype at the end of follow up. †The cohort also included 2 patients with maturity-onset diabetes in the young, 1 with mitochondrial diabetes and 2 of unclassified aetiology; ‡BMI ≥23 kg/m2. BMI, body mass index; DBP, diastolic blood pressure; DKA, diabetic ketoacidosis; FCPD, fibrocalculous pancreatic diabetes; MNT, medical nutrition therapy; OHAs, oral hypoglycaemic agents; SBP, systolic blood pressure; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; GAD, glutamic acid decarboxylase; NGSP, National Glycohemoglobin Standardization Program; IFCC, International Federation of Clinical Chemistry and Laboratory Medicine; HDL, high-density lipoprotein; LDL, low-density lipoprotein
Aetiology of diabetes: At recruitment, based on their clinical features and results of abdominal imaging, 37 (35%) patients were classified as T1DM, 34 (32%) as T2DM and 14 (13%) as FCPD, while 20 (19%) individuals could not be classified (Fig. 2). After completing the investigations and a follow up, all 20 unclassified patients could be placed in specific categories (8 each as T1DM and T2DM, 2 as FCPD and one each as HNF1A-MODY and mitochondrial diabetes). In addition, the earlier diagnosis was changed in four patients (Fig. 2). In the end, the aetiology was confirmed in 103 patients, and only two patients remained unclassified.
Fig. 2.
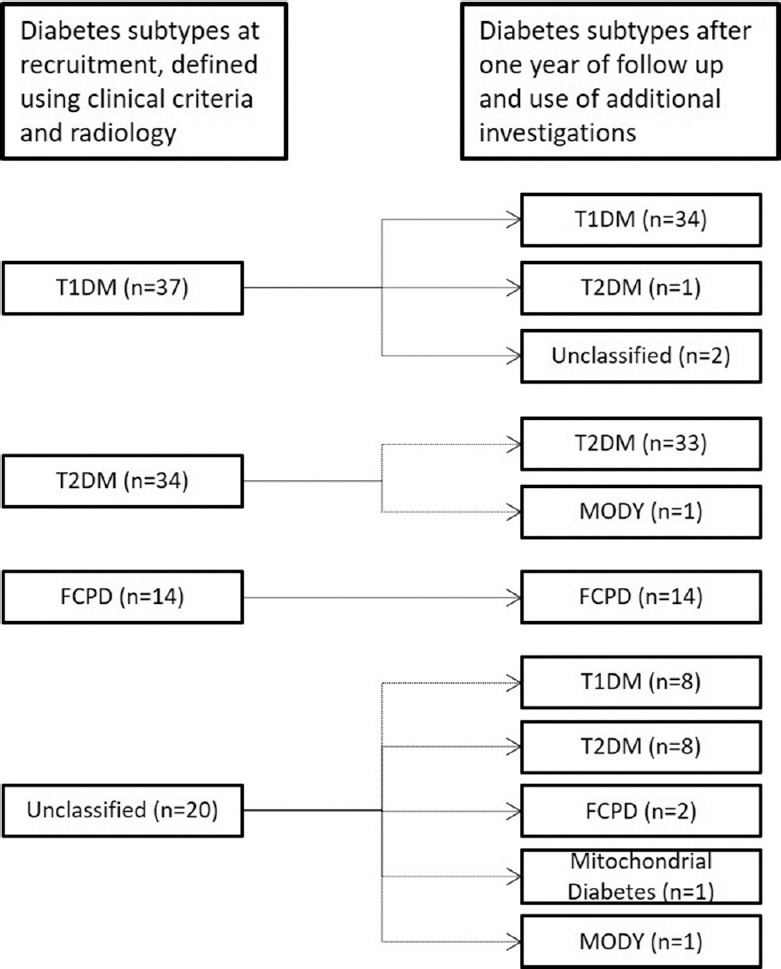
Etiological subtypes of diabetes at recruitment and final follow up. Subtypes were defined using clinical criteria and abdominal imaging at initial visit and after one year of follow up and additional investigations i.e. fasting plasma C-peptide, islet antibodies and genetic testing. T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; FCPD, fibrocalculous pancreatic diabetes; MODY, maturity-onset diabetes of the young.
Compared to patients with T2DM, T1DM were younger, had higher plasma glucose at diagnosis, higher prevalence of ketosis and associated autoimmune diseases, lower prevalence of acanthosis, obesity and hypertension and lower fasting C-peptide (Table I). However, there was considerable overlap in clinical features. Among patients with T1DM, nine (21%) were overweight (BMI ≥23 kg/m2) while MetS was present in four (10%). Among patients with T2DM, nine (21%) had a plasma glucose of >22.2 mmol/l at diagnosis, 12 (29%) had a normal BMI, while 27 (64%) lacked acanthosis. Three (7%) patients with T2DM had detectable islet antibodies, although in low titres (met the criteria for diagnosis of latent autoimmune diabetes in adults). All T2DM patients with islet antibody had preserved C-peptide (826-1041 pmol/l) and good glycaemic control (HbA1c< 7%) on metformin alone after more than one year of follow up.
Of the 16 patients with FCPD, five (31%) had no history of abdominal pain and 10 (62%) lacked evidence of pancreatic exocrine insufficiency. Three (19%) patients were overweight. Eight patients were treated with OHA but required insulin within one year, while the remainder required insulin from diagnosis. Two patients who had a normal CT scan at presentation were subsequently detected to have pancreatic calcification after one year. Pancreatic calcification was present in all patients with FCPD, while ductal dilation (50%) and atrophic pancreas (50%) was seen in a subset of them.
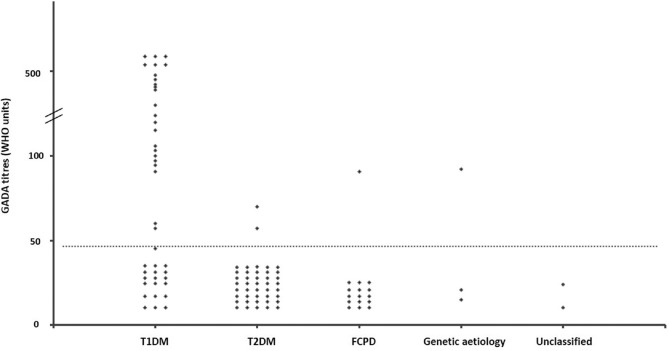
Autoantibodies and plasma C-peptide: Islet antibodies were elevated in 31 (30%) patients. Among patients with T1DM, GAD, IA-2 and ZnT8 antibodies were elevated in 23 (55%), two (5%) and five (12%), respectively, while any one antibody was present in 26 (62%) patients. For diagnosis of T1DM, addition of IA-2 and ZnT8 to GAD antibodies increased the yield in one and two patients, respectively. Low titre GAD antibody was detected in two patients with T2DM and one patient each with FCPD and HNF1A-MODY (Fig. 3). Taking twice the upper limit (94 units) as cut-off, GAD antibody was elevated in 18 (43%) patients with T1DM but was absent in all other subtypes. IA-2 antibody was only detected in T1DM, while ZnT8 antibody (in low titre) was present in one patient with T2DM. For the diagnosis of T1DM, the positive predictive value (PPV) of islet antibodies was 84 per cent, while the negative predictive value (NPV) was 78 per cent (Table II).
Fig. 3.
Glutamic acid decarboxylase antibody (GADA) titres among different aetiological subtypes of early-onset diabetes mellitus. Cut-off for glutamic acid decarboxylase antibody positivity ≥47 units (shown as horizontal dotted line). Genetic aetiology included two patients with MODY and one with mitochondrial diabetes. Abbreviations are as given in Fig. 2.
Table II.
Utility of different parameters in diagnosis of type 1 and type 2 diabetes
| Parameters | 95% CI | ||||
|---|---|---|---|---|---|
| PPV (%) | NPV (%) | Sensitivity (%) | Specificity (%) | AUC | |
| Islet autoantibodies (against GAD, IA-2 and ZnT8)a | 84 (69-93) | 78 (71-84) | 62 (46-76) | 92 (82-97) | * |
| Low fasting plasma C-peptide (<250 pmol/l)a | 70 (55-81) | 75 (67-81) | 56 (40-72) | 84 (73-92) | 0.82 (0.73-0.90) |
| Low fasting plasma C-peptide in absence of pancreatic calcificationa | 96 (77-99) | 72 (64-78) | 56 (40-72) | 98 (89-99) | 0.91 (0.85-0.97) |
| AN and/or MetSb | 71 (59-81) | 81 (72-87) | 71 (55-84) | 81 (69-90) | * |
aFor diagnosis of T1DM; bFor diagnosis of T2DM. *AUC could not be calculated as these are categorical variables. AN, acanthosis nigricans; AUC, area under curve; MetS, metabolic syndrome; NPV, negative predictive value; PPV, positive predictive value; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; CI, confidence interval
Six (6%) patients were positive for TTG antibody, of whom five (83%) had T1DM. Only one patient had evidence of coeliac disease on duodenal biopsy, while others were asymptomatic and had no histological changes of coeliac disease. Similarly, 16 (15%) were positive for TPO antibody, of whom 12 (75%) belonged to the group with T1DM. Six TPO-positive patients had primary hypothyroidism, while another one had subclinical Grave's disease (all with T1DM).
Fifty six per cent of patients with T1DM (23/41) and FCPD (9/16) had a low fasting C-peptide (<250 pmol/l) in follow up, while no patient with T2DM had a low C-peptide. The PPV and NPV for a low C-peptide as a predictor of T1DM were 70 per cent (96% after excluding FCPD patients) and 75 per cent, respectively. The presence of either acanthosis or MetS had a PPV of 71 per cent and NPV of 81 per cent for the diagnosis of T2DM (Table II).
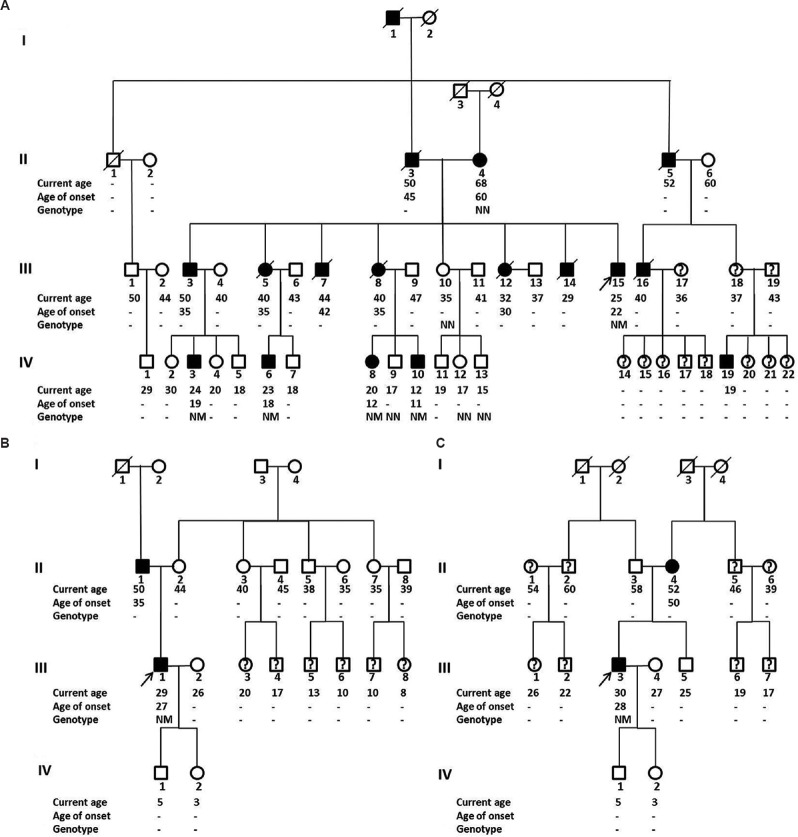
Maturity-onset diabetes of the young (MODY) and mitochondrial diabetes: Two patients had MODY gene variants. One patient, with BMI 18.8 kg/m2 and preserved C-peptide (1193 pmol/l), carried a known heterozygous variant in the HNF1A gene, c.788G>A (p.R263H). This mutation co-segregated with diabetes in the paternal side of the family (Fig. 4A). It was noted to be consistently linked with the variant, c.1729C>G (p.H577D), which was also absent in family members without diabetes. Despite the presence of low-titre GAD antibody, this patient was screened for MODY in view of a family history of early-onset diabetes in four generations. A PDX1 gene variant (c.302C>T; p.P101L) was detected in the second MODY patient (BMI 18.8 kg/m2, absent islet antibodies, A1c 6.4% on glimepiride) (Fig. 4B). The family did not consent to genetic testing. The mitochondrial A3243G mutation was detected in one patient. The patient had low BMI (15 kg/m2) but normal hearing on audiometry (Fig. 4C). The mother had onset of her diabetes at 60 yr and had a normal hearing.
Fig. 4.
Family pedigree of patients with MODY and mitochondrial diabetes. Family pedigree of patients with MODY (A: HNF1A mutations and B: PDX1 mutation) and mitochondrial diabetes (C). Filled squares and circles, diabetes; open squares and circles, normal glucose tolerance; squares and circles with a question mark, not tested for diabetes. N denotes wild-type allele, and M denotes mutations (A, both p.R263H and p.H577D mutations; B, p.P101L mutation; C, mitochondrial A3243G mutation). Age of patient and age at onset of diabetes in years is written below each symbol. Proband is depicted with an arrow. MODY, maturity-onset diabetes in the young.
Two patients remained unclassified at the end of follow up. Both had mixed features of T1DM (high glucose at diagnosis, poor response to OHA and DKA without a precipitating event) and T2DM [overweight (BMI 27.6 and 26.4 kg/m2), T2DM in multiple family members, acanthosis, high plasma C-peptide (1451 and 2241 pmol/l) and absent islet antibodies] and appeared to fit the description of Flatbush diabetes.
Discussion
In this prospective study, considerable heterogeneity and overlap in clinical features in patients with early-onset diabetes were noted. While T1DM and T2DM formed 80 per cent of patients and had a similar frequency, a surprisingly high rate of FCPD was also detected. In addition, patients with MODY and mitochondrial diabetes, who were likely to have been missed without additional screening tests, were also identified. Both islet antibodies and plasma C-peptide had a high predictive value for determining the aetiology of diabetes.
The aetiology of diabetes in young adults varies in different ethnic groups. For example, T1DM is the predominant aetiology in European Caucasians4,5, while T2DM forms the major subtype in many Asian populations6,7. In the current study, T1DM and T2DM were present with equal frequency (40% each). Unexpectedly, FCPD was also common (15%) but was missed or asymptomatic at the time of referral. There was substantial phenotypic overlap among the main subtypes of diabetes. In previous studies from India, the proportion of different types of diabetes in young adults has varied widely8,15,16. T1DM formed the major subtype in two multicentric studies involving patients under 20 and 25 yr, respectively21,22. However, these studies have not used all relevant investigations (islet antibodies, C-peptide and genetic testing) or followed their patients over time to arrive at a firm diagnosis.
Among the different clinical variables and investigations, features of insulin resistance i.e. acanthosis or MetS had a moderate predictive value in the diagnosis of T2DM. Islet antibodies and fasting plasma C-peptide (especially in the absence of pancreatic calcification) had moderate sensitivity, but high PPV for diagnosing T1DM. The protocol of testing for islet antibodies, plasma C-peptide and follow up for 1-2 years allowed to provide a diagnosis to the 20 patients in whom we were unable to assign an aetiology after initial clinical assessment. In addition, the initial diagnosis was altered in another four patients. Thus, these investigations should be considered in cases where the initial diagnosis is unclear or where the clinical features or course suggest a different type of diabetes.
Among patients with T1DM, the frequency of islet antibody (62%) was lower than European patients of similar age group (antibody positivity - 67-90%)23,24. In particular, the frequency of IA-2 antibody (5%) was far lower than reported among European patients (prevalence - 30-45%)23,24. IA-2 antibody prevalence has previously been reported to be decreased in Indian children with T1DM, possibly due to the low frequency of DRB1*04.DQA1*03.DQB1*0302 in this population25. Similarly, the frequency of ZnT8 antibody among T1DM patients in this study (12%) was lower compared to European patients of the same age group (19%)26. The low prevalence of ZnT8 antibody may be ascribed to the lower frequency of DQB1*0302 genotype in Indians. TPO and TTG antibodies were primarily detected in patients with T1DM. The frequency of TPO27 and TTG28 positivity in our patients was similar to that mentioned in previous Indian studies.
MODY-related variants were identified in two patients after genetic analysis. In one patient, two heterozygous mutations in the HNF1A gene were detected. While the mutation p.R263H has been reported to be pathogenic29, the significance of the p.H577D has not been studied earlier30. Since p.H577D mutation did not co-segregate with diabetes in a previous study30, it may be considered as a variant of unknown significance. The second patient with MODY carried a heterozygous variant in the PDX1 gene (p.P101L), which has been reported in ExAC database (http://exac.broadinstitute.org/). In the absence of family screening, this can only be classified as a variant with unknown clinical significance. While homozygous PDX1 gene mutations result in neonatal diabetes and pancreatic agenesis, heterozygous mutations are a rare cause of MODY31. The low prevalence of mitochondrial diabetes in our patients was similar to that noted in our previous study14.
Two patients had mixed features of T1DM and T2DM and could not finally be classified. They most resembled atypical diabetes (ketosis-prone diabetes or Flatbush diabetes)32. Flatbush diabetes is characterized by a presentation with DKA or unprovoked ketosis but without a phenotype of autoimmune T1DM. β-cell function is preserved, and after an initial period of insulin therapy, these patients can be managed with oral agents. While it is most commonly reported among patients of American-African and Hispanic origin, it has also been described in Chinese and Japanese patients32. This entity has previously been described in a single Indian-origin patient33.
There were certain limitations in our study. The sample size was estimated based on a limited number of available Indian studies. The patients in our referral clinic may not reflect the true frequency of subtypes of diabetes in the community since more severe forms such as T1DM and FCPD may be overrepresented. Further, there are likely to be differences in the aetiology in different regions of the country, as well as between government and private hospitals.
In conclusion, our findings showed that among young Indian adults, T1DM and T2DM were equally frequent, while FCPD was also present in a significant proportion of patients. Other genetic aetiologies such as MODY and mitochondrial diabetes were infrequent but would have been missed if specific tests were not performed. A standardized protocol, including tests for islet antibodies and C-peptide, along with a follow up of one year, allowed accurate classification of nearly all patients whose aetiology could not be delineated on the initial visit.
Footnotes
Financial support & sponsorship: Authors acknowledge the Indian Council of Medical Research, New Delhi, for providing financial support (5/3/1/100/2011-RCH).
Conflicts of Interest: None.
References
- 1.Alberti G, Zimmet P, Shaw J, Bloomgarden Z, Kaufman F, Silink M, et al. Type 2 diabetes in the young: The evolving epidemic: The International Diabetes Federation consensus workshop. Diabetes Care. 2004;27:1798–811. doi: 10.2337/diacare.27.7.1798. [DOI] [PubMed] [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.India state-level disease burden initiative diabetes collaborators. The increasing burden of diabetes and variations among states of India: the Global Burden of Disease Study 1990-2016. Lancet Glob Health. 2018;6:e1352–62. doi: 10.1016/S2214-109X(18)30387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laakso M, Pyörälä K. Age of onset and type of diabetes. Diabetes Care. 1985;8:114–7. doi: 10.2337/diacare.8.2.114. [DOI] [PubMed] [Google Scholar]
- 5.Borg H, Arnqvist HJ, Björk E, Bolinder J, Eriksson JW, Nyström L, et al. Evaluation of the new ADA and WHO criteria for classification of diabetes mellitus in young adult people (15-34 yrs) in the diabetes incidence study in Sweden (DISS) Diabetologia. 2003;46:173–81. doi: 10.1007/s00125-002-1021-4. [DOI] [PubMed] [Google Scholar]
- 6.Pan CY, So WY, Khalid BA, Mohan V, Thai AC, Zimmet P, et al. Metabolic, immunological and clinical characteristics in newly diagnosed Asian diabetes patients aged 12-40 years. Diabet Med. 2004;21:1007–13. doi: 10.1111/j.1464-5491.2004.01287.x. [DOI] [PubMed] [Google Scholar]
- 7.Katulanda P, Shine B, Katulanda GW, Silva A, Asfir EL, Sheriff R, et al. Diabetes mellitus among young adults in Sri Lanka – Role of GAD antibodies in classification and treatment: The Sri Lanka young diabetes study. Diabetologia. 2008;51:1368–74. doi: 10.1007/s00125-008-1052-6. [DOI] [PubMed] [Google Scholar]
- 8.Singh AK, Bhatia E, Dabadghao P, Bhatia V, Gellert SA, Colman PG, et al. Role of islet autoimmunity in the aetiology of different clinical subtypes of diabetes mellitus in young North Indians. Diabet Med. 2000;17:275–80. doi: 10.1046/j.1464-5491.2000.00267.x. [DOI] [PubMed] [Google Scholar]
- 9.Mittal N, Mehrotra R, Agarwal G, Rajeswari, Choudhuri G, Sikora S, et al. The clinical spectrum of fibrocalculous pancreatic diabetes in North India. Natl Med J India. 2002;15:327–31. [PubMed] [Google Scholar]
- 10.Owen KR, Stride A, Ellard S, Hattersley AT. Etiological investigation of diabetes in young adults presenting with apparent type 2 diabetes. Diabetes Care. 2003;26:2088–93. doi: 10.2337/diacare.26.7.2088. [DOI] [PubMed] [Google Scholar]
- 11.Aguilar-Salinas CA, Reyes-Rodríguez E, Ordóñez-Sánchez ML, Torres MA, Ramírez-Jiménez S, Domínguez-López A, et al. Early-onset type 2 diabetes: Metabolic and genetic characterization in the Mexican population. J Clin EndocrinolMetab. 2001;86:220–6. doi: 10.1210/jcem.86.1.7134. [DOI] [PubMed] [Google Scholar]
- 12.Thanabalasingham G, Pal A, Selwood MP, Dudley C, Fisher K, Bingley PJ, et al. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care. 2012;35:1206–12. doi: 10.2337/dc11-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehto M, Wipemo C, Ivarsson SA, Lindgren C, Lipsanen-Nyman M, Weng J, et al. High frequency of mutations in MODY and mitochondrial genes in Scandinavian patients with familial early-onset diabetes. Diabetologia. 1999;42:1131–7. doi: 10.1007/s001250051281. [DOI] [PubMed] [Google Scholar]
- 14.Sahu RP, Aggarwal A, Zaidi G, Shah A, Modi K, Kongara S, et al. Etiology of early-onset type 2 diabetes in Indians: Islet autoimmunity and mutations in hepatocyte nuclear factor 1alpha and mitochondrial gene. J Clin Endocrinol Metab. 2007;92:2462–7. doi: 10.1210/jc.2006-2467. [DOI] [PubMed] [Google Scholar]
- 15.Zargar AH, Bhat MH, Laway BA, Masoodi SR. Clinical and aetiological profile of early onset diabetes mellitus: Data from a tertiary care centre in the Indian subcontinent. J Postgrad Med. 2001;47:27–9. [PubMed] [Google Scholar]
- 16.Jevalikar G, Kohli C, Bansal B, Mishra SK, Wasir JS, Singh S, et al. Childhood and youth onset diabetes: A single centre experience. Indian J Pediatr. 2016;83:792–8. doi: 10.1007/s12098-015-2009-7. [DOI] [PubMed] [Google Scholar]
- 17.Törn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ Participating Laboratories. Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia. 2008;51:846–52. doi: 10.1007/s00125-008-0967-2. [DOI] [PubMed] [Google Scholar]
- 18.Chapla A, Mruthyunjaya MD, Asha HS, Varghese D, Varshney M, Vasan SK, et al. Maturity onset diabetes of the young in India – A distinctive mutation pattern identified through targeted next-generation sequencing. Clin Endocrinol (Oxf) 2015;82:533–42. doi: 10.1111/cen.12541. [DOI] [PubMed] [Google Scholar]
- 19.Bruno G, Runzo C, Cavallo-Perin P, Merletti F, Rivetti M, Pinach S, et al. Incidence of type 1 and type 2 diabetes in adults aged 30-49 years. Diabetes Care. 2005;28:2613–19. doi: 10.2337/diacare.28.11.2613. [DOI] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome - a new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 21.Unnikrishnan AG, Bhatia E, Bhatia V, Bhadada SK, Sahay RK, Kannan A, et al. Type 1 diabetes versus type 2 diabetes with onset in persons younger than 20 years of age. Ann N Y Acad Sci. 2008;1150:239–44. doi: 10.1196/annals.1447.056. [DOI] [PubMed] [Google Scholar]
- 22.Praveen PA, Madhu SV, Mohan V, Das S, Kakati S, Shah N, et al. Registry of youth onset diabetes in India (YDR): Rationale, recruitment, and current status. J Diabetes Sci Technol. 2016;10:1034–41. doi: 10.1177/1932296816645121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham J, Hagopian WA, Kockum I, Li LS, Sanjeevi CB, Lowe RM, et al. Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes. 2002;51:1346–55. doi: 10.2337/diabetes.51.5.1346. [DOI] [PubMed] [Google Scholar]
- 24.Gorus FK, Goubert P, Semakula C, Vandewalle CL, De Schepper J, Scheen A, et al. IA-2-autoantibodies complement GAD65-autoantibodies in new-onset IDDM patients and help predict impending diabetes in their siblings. The Belgian diabetes registry. Diabetologia. 1997;40:95–9. doi: 10.1007/s001250050648. [DOI] [PubMed] [Google Scholar]
- 25.Kelly MA, Alvi NS, Croft NJ, Mijovic CH, Bottazzo GF, Barnett AH, et al. Genetic and immunological characteristics of type I diabetes mellitus in an Indo-Aryan population. Diabetologia. 2000;43:450–6. doi: 10.1007/s001250051328. [DOI] [PubMed] [Google Scholar]
- 26.Andersen MK, Härkönen T, Forsblom C, Groop PH, Knip M, Tuomi T, et al. Zinc transporter type 8 autoantibodies (ZnT8A): Prevalence and phenotypic associations in latent autoimmune diabetes patients and patients with adult onset type 1 diabetes. Autoimmunity. 2013;46:251–8. doi: 10.3109/08916934.2012.741155. [DOI] [PubMed] [Google Scholar]
- 27.Goswami R, Marwaha RK, Goswami D, Gupta N, Ray D, Tomar N, et al. Prevalence of thyroid autoimmunity in sporadic idiopathic hypoparathyroidism in comparison to type 1 diabetes and premature ovarian failure. J Clin Endocrinol Metab. 2006;91:4256–9. doi: 10.1210/jc.2006-1005. [DOI] [PubMed] [Google Scholar]
- 28.Bhadada SK, Kochhar R, Bhansali A, Dutta U, Kumar PR, Poornachandra KS, et al. Prevalence and clinical profile of celiac disease in type 1 diabetes mellitus in North India. J Gastroenterol Hepatol. 2011;26:378–81. doi: 10.1111/j.1440-1746.2010.06508.x. [DOI] [PubMed] [Google Scholar]
- 29.Radha V, Ek J, Anuradha S, Hansen T, Pedersen O, Mohan V, et al. Identification of novel variants in the hepatocyte nuclear factor-1alpha gene in South Indian patients with maturity onset diabetes of young. J Clin Endocrinol Metab. 2009;94:1959–65. doi: 10.1210/jc.2008-2371. [DOI] [PubMed] [Google Scholar]
- 30.Lambert AP, Ellard S, Allen LI, Gallen IW, Gillespie KM, Bingley PJ, et al. Identifying hepatic nuclear factor 1alpha mutations in children and young adults with a clinical diagnosis of type 1 diabetes. Diabetes Care. 2003;26:333–7. doi: 10.2337/diacare.26.2.333. [DOI] [PubMed] [Google Scholar]
- 31.Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17:138–9. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- 32.Balasubramanyam A, Nalini R, Hampe CS, Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev. 2008;29:292–302. doi: 10.1210/er.2007-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jha S, Waghdhare S, Siddiqui S, Srivastava K, Bhargava A. First identification of Flatbush diabetes in patients of Indian origin. Diabetes Care. 2015;38:e164–5. doi: 10.2337/dc15-1209. [DOI] [PubMed] [Google Scholar]