The presence of adult neurogenesis in the mammalian brain has been a theme of intense controversy for a long time since the original report by Altman and Das (1965). The scientific community, for about 30 years, has difficulties to accept that progenitor cells give rise to new neurons in some specific regions of the mammalian adult brain, the neurogenic niches (Kuhn et al., 1996; Doetsch et al., 1997, 1999).
The lack of techniques suitable enough to prove that mitotic cells (originally called “microneurons”) present in the hippocampus and other central nervous system regions were really neurons and not glia, was the main reason for the obtusity of the scientific community. This long-lasting dogma was also influenced by a famous quote in a book published by the Physiology and Medicine Nobel prize-awarded (1906) Spanish neuroanatomist Santiago Ramon y Cajal who stated that “Once development was ended, the fonts of growth and regeneration of axons and dendrites dried up irrevocably. In the adult centers, the neural paths are something fixed and immutable: everything may die, nothing may be regenerated” (Ramon y Cajal, 1928). Nevertheless, Cajal also wrote that “(…) It is a duty for future generations find a way to overcome the intrinsic failure of adult brain to regenerate (…)”. In 1990s, future generation of scientists partially accomplished this monumental task by finding that new neurons are continuously produced in the adult rodent brain, namely in the hippocampal dentate gyrus subgranular zone (Kuhn et al., 1996) and subventricular zone (SVZ) located at the wall of lateral ventricles (Doetsch et al., 1997). This was soon expanded to the brain of several species, including Homo sapiens (Eriksson et al., 1998). The cytoarchitecture of SVZ, the main central nervous system neurogenic niche, was described in 1997 using electron microscopy (Doetsch et al., 1997) and the identity of SVZ neural stem cells, as a special kind of astrocytes (B cells) soon (Doetsch et al., 1999).
The first report of hippocampal neurogenesis in human adult brain was provided by Eriksson et al. (1998). These researchers described production of new neurons in the human hippocampus dentate gyrus, a limbic region important for some kind of spatial and episodic memories and other cognitive functions (Luna et al., 2019; Toda et al., 2019). The researchers used brain samples from patients with cancer who had received 5-bromo-2′-deoxyuridine, a thymidine analogue and marker of cell proliferation (Eriksson et al., 1998).
Spalding et al. (2013) proposed an innovative method to evaluate neurogenesis in the human hippocampus. They used 14C incorporation into human DNA, the amount of which was increased in the atmosphere between 1955 and 1963 due to tests of nuclear bombs during cold war. The excess of 14C in the atmosphere, reacting with oxygen, forms carbon dioxide, which is used by plants during photosynthesis. People who consumed these vegetables, or animals that fed on plants (herbivores) at that time, had high levels of 14C in their DNA until 1963, when nuclear tests were banned. Analyzing the presence of 14C in hippocampal cells of people born between 1955 and 1963, authors calculated that about 1/3 of hippocampal neurons can undergo replacement even in adult humans, and that about 700 new neurons were daily added to hippocampus. This study suggested a considerable role for adult neurogenesis on the replacement of human hippocampal neurons.
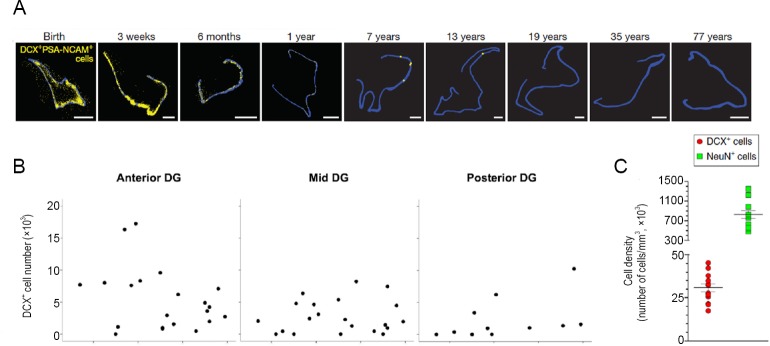
However, the results described by Spalding et al. (2013) were not confirmed by a recent report by Sorrells et al. (2018), who reported that the rate of human hippocampal neurogenesis considerably declines in the first year and virtually disappears after 7 years of age in humans (Figure 1A). They immunolabeled brain samples containing hippocampus of 59 people who had died or had part of the hippocampus removed for surgical treatment of epilepsy, and used brain samples from people with age ranging from newborns to 77 years old (Sorrells et al., 2018).
Figure 1.
Polemic on the existence of adult neurogenesis in the human hippocampus.
Neurogenesis drops in children to negligible levels in adult humans (A, adapted from Sorrels et al., 2018). Endogenous neurogenesis persists in the hippocampus of adult humans, even in elderly people (B, adapted from Boldrini et al., 2018; C, adapted from Moreno-Jimènez et al., 2019). Methodological differences, including tissue overfixation, likely explains the discrepant results. DG: Dentate gyrus; DCX: doublecortin; NeuN: neuronal nuclear antigen.
Boldrini et al. (2018) surprised the scientific community reporting that there is neurogenesis in the human hippocampus (Figure 1B), even in elderly individuals. They used a variety of markers to identify neural progenitors, neuroblasts (immature neurons), adult neurons, and glial cells in hippocampal samples of 28 people without neurological disorders, who died at the age of 14–79 years. The researchers found thousands of immature neurons in the dentate gyrus, even in elderly people, and a comparable number of glial cells and granular neurons in the hippocampus, although older individuals showed less neuroplasticity (ability of the nervous system to adapt to new situations) and formation of new blood vessels (angiogenesis). The findings suggested that adult neurogenesis continues in the hippocampus of adult humans, even in the elderly ones (Boldrini et al., 2018).
It was clear that these contradicting findings would be likely explained by methodological differences between the two studies. For example, in the paper by Sorrells et al. (2018), most of the tissue was obtained from people who had chronic epilepsy, which may influence the amount of neuroblasts in the hippocampus. In the paper by Boldrini et al. (2018), they were careful enough to choose tissue samples from people who, at the time of death, had no psychiatric disorders, drug addiction, vascular lesions or chronic neurodegenerative diseases, conditions that could influence neurogenesis in adult humans. Boldrini et al. (2018) also used more sophisticated techniques for cell countings, such as “stereology”. The use of such a different methodological approach makes it difficult to compare the numbers of new neurons reported in the two studies, but considering the methodological aspects, it was clear that a high accuracy seemed to be present in the results presented by Boldrini et al. (2018). This has been recently confirmed by Moreno-Jiménez et al. (2019).
Moreno-Jiménez et al. (2019) reported the presence of thousands of new neurons (43,000 neuroblasts/mm2, depending on age) in the hippocampus (Figure 1C) of healthy people aged 43–87 years. Tissue samples from people who died and had Alzheimer’s disease presented less neuroblasts in the dentate hippocampal gyrus, indicating that even the new neurons formed by adult neurogenesis die in this type of dementia. This seminal paper confirmed previous studies reported by Boldrini et al. (2018) as well as the initial findings by Spalding et al. (2013).
According to the Spanish neuroscientists, the likely reason for the discrepancies between their results and the ones reported by Sorrells et al. (2018) was the fixation protocol for the brain samples. In order to be preserved, brain tissue must be immersed in fixatives, such as paraformaldehyde. Overfixation in 4% paraformadehyde may inhibit immunolabeling for doublecortin, a microtubule-associated protein commonly used as a specific marker for neuroblasts. Moreno-Jiménez et al. (2019) showed that a 24-hour postfixation time is well suitable for neuroblast immunolabeling using different markers, including doublecortin, but longer fixation times considerably impair immunolabeling. They argued that brain samples were immersed into the fixative for very long periods, which totally inhibits neuroblast labeling.
The study by Moreno-Jiménez et al. (2019) brings great hope that one day the knowledge about adult neurogenesis in the hippocampus and other brain regions can be transformed into a therapy to minimize neuronal loss that occurs in both acute and chronic neural disorders. It has been recently shown that adult-born neuroblasts play a fundamental role on the modulation of entorhinal inputs to dentate gyrus in rodents, which has a tremendous influence on the hippocampal function (Luna et al., 2019).
As pattern separation is a fundamental role played by hippocampal adult-born cells, loss of this function may underlie psychiatric disorders, including depression, anxiety, post-traumatic stress disorder and panic syndrome (Toda et al., 2019). Future pharmacological approaches and cognitive therapies that increase hippocampal adult neurogenesis and assure a proper integration of new-born cells into functional circuits may be useful treatments for those psychiatric disorders (Toda et al., 2019).
The initial claim for the non-existence of adult neurogenesis in the mammalian brain came from Spain (Ramon y Cajal, 1928), but the final redemption and confirmation of adult hippocampal neurogenesis (Figure 1C) seems to come from the sunny Cajal’s land, as well Moreno-Jiménez et al. (2019).
The work was supported by Organização não governamental (ONG) Iluminando A Vida (AIV/2019).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Lei Chen, University of Kentucky, USA.
P-Reviewer: Chen L; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Altman J, Das GD. Autoradiographic and histological evidence of post-natal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–599 e585. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 4.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luna VM, Anacker C, Burghardt NS, Khandaker H, Andreu V, Millette A, Leary P, Ravenelle R, Jimenez JC, Mastrodonato A, Denny CA, Fenton AA, Scharfman HE, Hen R. Adult-born hippocampal neurons bidirectionally modulate entorhinal inputs into the dentate gyrus. Science. 2019;364:578–583. doi: 10.1126/science.aat8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, Ávila J, Llorens-Martín M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 9.Ramon y Cajal S. New York: Hafner; 1928. Degeneration and regeneration of the nervous system. edn. [Google Scholar]
- 10.Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisén J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toda T, Parylak SL, Linker SB, Gage FH. The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry. 2019;24:67–87. doi: 10.1038/s41380-018-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]