Abstract
In chronic phase of spinal cord injury, functional recovery is more untreatable compared with early intervention in acute phase of spinal cord injury. In the last decade, several combination therapies successfully improved motor dysfunction in chronic spinal cord injury. However, their effectiveness is not sufficient. We previously found a new effective compound for spinal cord injury, matrine, which induced axonal growth and functional recovery in acute spinal cord injury mice via direct activation of extracellular heat shock protein 90. Although our previous study clarified that matrine was an activator of extracellular heat shock protein 90, the potential of matrine for spinal cord injury in chronic phase has not been sufficiently evaluated. Thus, this study aimed to investigate whether matrine ameliorates chronic spinal cord injury in mice. Once daily intragastric administration of matrine (100 μmol/kg per day) to spinal cord injury mice were starte at 28 days after injury, and continued for 154 days. Continuous matrine treatment improved hindlimb motor function in chronic spinal cord injury mice. In injured spinal cords of the matrine-treated mice, the density of neurofilament-H-positive axons was increased. Moreover, matrine treatment increased the density of bassoon-positive presynapses in contact with choline acetyltransferase-positive motor neurons in the lumbar spinal cord. These findings suggest that matrine promotes remodeling and reconnection of neural circuits to regulate hindlimb movement. All protocols were approved by the Committee for Animal Care and Use of the Sugitani Campus of the University of Toyama (approval No. A2013INM-1 and A2016INM-3) on May 7, 2013 and May 17, 2016, respectively.
Keywords: matrine, chronic spinal cord injury, axonal growth, synaptogenesis, hindlimb locomotor, presynapse, immunohistochemistry, Basso Mouse Scale, Body Support Score, Sophora flavescens
Chinese Library Classification No. R453; R364; R741
Introduction
In spinal cord injury (SCI), traumatic damage and acute inflammation cause cell death and disruption of tissue structure, including neural tracts. Aberrant histological changes at the lesion site, such as formation of glial scar and deposition of chondroitin sulphate proteoglycans, limit spontaneous growth of axons and reconstruction of neural connection, leading to the long-lasting functional deficits (Cregg et al., 2014). Although the promising treatments effective for SCI patients have not been established yet, various approaches succeed to enhance spontaneous recovery by early intervention following injury on the animal model of SCI. In contrast, those interventions give no functional recovery in case of delayed treatment over long periods of time after injury. Successful treatments against acute SCI are not necessarily effective for chronic SCI, such as tamoxifen (Guptarak et al., 2014), Rho-associated protein kinase inhibitor (Nishio et al., 2006) and chondroitinase ABC (Bradbury et al., 2002; Karimi-Abdolrezaee et al., 2010; Wang et al., 2011). Therefore chronic SCI is considered to be more refractory than acute SCI. In the last decade, several combination therapies have been reported to achieve improvement of motor function even in chronic SCI, such as chondroitinase ABC injection to lesion site for 7 or 10 days and continuous rehabilitation (Wang et al., 2011; Shinozaki et al., 2016), neural stem/progenitor cells transplantation and injection of chondroitinase ABC and growth factor cocktail (epidermal growth factor, fibroblast growth factor, and plateletderived growth factor) to the lesion site for 7 days (Karimi-Abdolrezaee et al., 2010), neural stem/progenitor cells transplantation and continuous rehabilitation (Tashiro et al., 2016). However, the effectiveness of these therapies is not sufficient.
Matrine (molecular weight: 248) is a lupin alkaloid and abundantly contained in roots of Sophora flavescens Aiton (Li and Wang, 2004). Our previous study demonstrated that matrine promotes axonal growth of cultured cortical neurons under an inhibitory circumstance (Tanabe et al., 2015). Matrine has been shown to enhance functional recovery and extension of 5-hydroxytryptamine-positive tracts beyond the lesion site in acute SCI mice (Tanabe et al., 2018). Furthermore, we found that extracellular heat shock protein 90 (HSP90) is the direct target molecule of matrine to induce axonal growth and SCI amelioration (Tanabe et al., 2018), and matrine is an activator of chaperon function of HSP90.
Although our previous study clarified that matrine was an activator of extracellular HSP90, the potential of matrine for SCI in chronic phase has not been sufficiently evaluated.
Materials and Methods
Ethics statement
All experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Sugitani Campus of the University of Toyama. All protocols were approved by the Committee for Animal Care and Use of the Sugitani Campus of the University of Toyama (approval No. A2013INM-1 and A2016INM-3) on May 7, 2013 and May 17, 2016, respectively. All efforts were made to minimize the number of animals used.
SCI and drug treatment
A mouse model of weight drop-induced contusive SCI was established, which is a major experimental model of SCI (Zhang et al., 2014). The model mice were produced using a stereotaxic instrument (Narishige, Tokyo, Japan) and several customized impact devices. ddY-strain was a closed-colony outbred mouse strain, and was established in Japan. ddY mice (female, 8 weeks old, 28–33 g) were purchased from Japan SLC (Hamamatsu, Japan) and housed in a constant environment (22 ± 2°C, 50 ± 5% humidity, 12-hour light cycle starting at 07:00) with free access to food and water. The mice were anesthetized with the mixture of three anesthetics 230–280 μL [75 μg/mL medetomidine (Nippon Zenyaku Kogyo, Koriyama, Japan), 400 μg/mL midazolam (Sandoz, Tokyo, Japan), 500 μg/mL butorphanol (Meiji Seika Pharma, Tokyo, Japan)], laminectomized and set on a stereotaxic instrument (Narishige, Tokyo, Japan). Exposed spinal cord at the T10–11 level was contused by dropping a 6.5-g rod (the tip diameter; 1 mm) through a vertical cylinder from a 3-cm height. This method can control severity of injury by dropping height and weight of the rod. We set the condition of weight drop to produce severe SCI model (Basso Mouse Scale (BMS) (Basso et al., 2006) score is plateaued approximately point 2 in chronic phase. During and after surgery, the mice were placed on a hotplate to maintain body temperature. The locomotor function of hindlimbs of the SCI mice was assessed using the BMS (0 – 8 points) which evaluates hindlimb movement comprehensively and the Body Support Score (BSS) (Teshigawara et al., 2013) (0 – 4 points) which focuses on the ability of body support in an open field (black color box, 50.0 cm × 42.5 cm × 15.0 cm under 500-lux illumination at 1, 7, 14, 21 and 28 days after SCI. High scores of BMS and BSS indicate functional recovery of hindlimbs, such as hindlimb movement and the ability of body support, respectively. Sham-operated mice maintained full scores in BMS (8 points) and BSS (4 points) (data not shown).
At 28 days after injury, mice were allocated to two groups, vehicle (BMS = 2.50 ± 0.27, n = 5 mice, 10 hindlimbs) and matrine (BMS = 2.50 ± 0.23, n = 6 mice, 12 hindlimbs), by order of BMS scores (Figure 1). It was for equalization of motor function in two groups before matrine treatment. The average score of two groups was almost similar. Once daily administration of matrine (Figure 2) (100 μmol/kg per day; Tokyo Chemical Industry, Tokyo, Japan) or vehicle solution (saline) were started at 28 days after injury, and continued for 154 days. The dose of matrine was decided based on our previous study (Tanabe et al., 2018). The drugs were intragastrically administered using a feeding tube (Cat# 20G-70, Natsume Seisakusho, Tokyo, Japan). The assessment of motor function using the BMS and BSS were performed once a week and before drug administration.
Figure 1.

Experiment flow chart.
Matrine (100 μmol/kg) or vehicle solution (saline) was constitutively administered orally for 154 days from 28 days post-injury.
Figure 2.
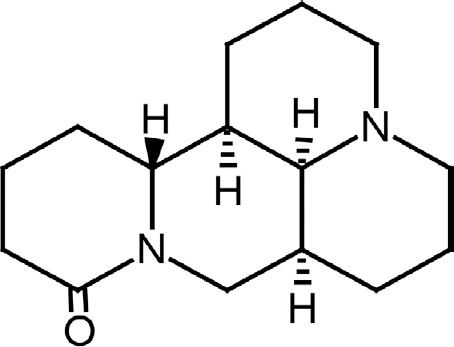
Chemical structure of matrine.
Immunohistological analysis
At 182 days after SCI, the mice were anesthetized with the mixture of three anesthetics, and were transcardially perfused with ice-cold saline and 4% paraformaldehyde (PFA). Spinal columns were isolated and kept in 4% PFA at 4°C. Spinal cord tissues around the lesion were separated and fixed further with 4% PFA overnight. The spinal cords were immersed in 10, 20, and 30% sucrose-phosphate buffered saline (PBS) successively at 4°C, and embedded in Tissue-Tek Optimal Cutting Temperature Compound (Sakura Finetek Japan, Tokyo, Japan). The spinal cords were cut into 14-μm thick successive sagittal sections using a CM3050S cryostat (Leica, Heidelberg, Germany). The slices were post-fixed with 4% PFA for 60 minutes at room temperature and immunostained with a chicken anti-neurofilament-H (NF-H, a marker for axons) polyclonal antibody (1:1000; Merck, Kenilworth, NJ, USA; Cat#AB5539), a rabbit anti-glial fibrillary acidic protein (GFAP, a marker for reactive astrocytes) polyclonal antibody (1:1000; Merck; Cat#AB5804), a goat anti-choline acetyltransferase (ChAT, a marker for motor neurons) polyclonal antibody (1:200; Merck; Cat#AB144P), and/or a mouse anti-bassoon (a marker for presynapses) monoclonal antibody (clone# SAP7F407; 1:2000; abcam, Cambridge, UK; Cat#ab82958). As secondary antibodies, Alexa Fluor 568-conjugated goat anti-chicken IgY (1:400; Thermo Fisher Scientific, Waltham, MA, USA), Alexa Fluor 350-conjugated goat anti-rabbit IgG (1:400; Thermo Fisher Scientific), Alexa Fluor 488-conjugated donkey anti-mouse IgG (1:400; Thermo Fisher Scientific), and/or Alexa Fluor 594-conjugated donkey anti-goat IgG (1:400; Thermo Fisher Scientific) were used. The secondary antibodies were incubated for 2 hours at room temperature. Counterstaining with 4′,6-diamidino-2-phenylindole (DAPI, a nuclei marker; 0.1 μg/mL; Enzo Life Science, Farmingdale, NY, USA) was also performed.
Image capture and analysis
Sagittal sections of injured spinal cord were immunostained for NF-H and GFAP. Fluorescence images were captured using an inverted microscope system (BZ-X710; Keyence, Osaka, Japan) with a 20 × NA 0.75 objective lens (CFI Plan Apo-λ; Nikon Instech, Tokyo, Japan). Successive images were obtained from lesion site to the rostral and caudal site, and were connected using BZ-X Analyzer (Keyence). The connected images were cleared by reducing haze using BZ-X Analyzer. The lesion site was discerned by a GFAP-stained image, because reactive astrocytes form a glial scar on the lesion site. To detect NF-H-positive fibers, NF-H-positive areas were binarized and quantified using ImageJ software (NIH, Rockville, MD, USA). Ratio of NF-H-positive area to total analyzed area was quantified in gray mature 2–3 mm rostral and caudal from the lesion center. One section per mouse was selected and analyzed. The criterion of the slice selection was the lateral position (the closest to the central canal). Five vehicle-treated SCI mice and six matrine-treated SCI mice were analyzed.
Sagittal sections of injured spinal cord were immunostained for bassoon and ChAT. Bassoon is a protein composing active zoon in presynapses, and used as a maker of presynapses (Wootz et al., 2013; Jacobi et al., 2015; Yokota et al., 2015). Fluorescence images were captured using an inverted microscope (BZ-X710) with a 40× NA 0.95 objective lens (CFI Plan Apo-λ). We obtained the images of motor neuron locating on L1-3 spinal cord and reduced haze of the images using BZ-X Analyzer to clear. Image analysis was performed using ImageJ software. The plasma membrane of motor neuron’s cell body was traced with a thick line (2.3 μm width) one by one, and the area of bassoon-positive puncta overlaid on the line was measured. The bassoon-positive area per unit length of the plasma membrane in each motor neuron was calculated. One slice per mouse was selected and 18–27 motor neurons located on one slice were analyzed. Five vehicle-treated SCI mice and five matrine-treated SCI mice were analyzed. Then presynaptic termination to motor neurons in the lumbar spinal cord innervating hindlimbs was evaluated.
Statistical analysis
Statistical comparisons were performed using two-way repeated measures analysis of variance (ANOVA) with post hoc Bonferroni tests and unpaired two-tailed t-test using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). A P < 0.05 was considered statistically significant. The data are presented as the mean ± standard error of the mean (SE).
Results
Matrine improves motor function of hindlimbs in chronic SCI mice
There were no significant differences in spontaneous recovery before drug treatment between groups (at 28 days after SCI, vehicle-treated group: BMS = 2.50 ± 0.27, BSS = 0.70 ± 0.15; matrine-treated group: BMS = 2.50 ± 0.23, BSS = 0.67 ± 0.14) (Figure 3A and B). At 28 days after SCI, F and P values of the two-way repeated measures ANOVA were as follows: the drug × day interaction: F(4, 80) = 0.9208, P = 0.4561 in BMS; F(4, 80) = 0.3688, P = 0.8302 in BSS. Continuous administrations of vehicle solution for 154 days did not improve hindlimbs movement (at 128 days, BMS = 2.00 ± 0.33, BSS = 0.50 ± 0.17). In contrast, matrine-treated group gradually improved locomotor ability (at 128 days after SCI, BMS = 3.83 ± 0.39, BSS = 1.41 ± 0.19). During 28–182 days after SCI, F and P values of the two-way repeated measures ANOVA were as follows: the drug × day interaction between both groups: F(19, 380) = 4.525, P < 0.0001 in BMS; F(19, 380) = 2.738, P = 0.0001 in BSS. At the end of observation (182 days after SCI), vehicle-treated mice hardly placed their paw on the ground, and could not lift up their trunk (Figure 4A and Additional Video 1). In contrast, matrine-treated mice walked with paw placing and occasional trunk support (Figure 4B and Additional Video 2). Matrine-treated SCI mice showed no side effects such as obvious abnormal behaviors, body weight loss (Figure 3C), or death.
Figure 3.
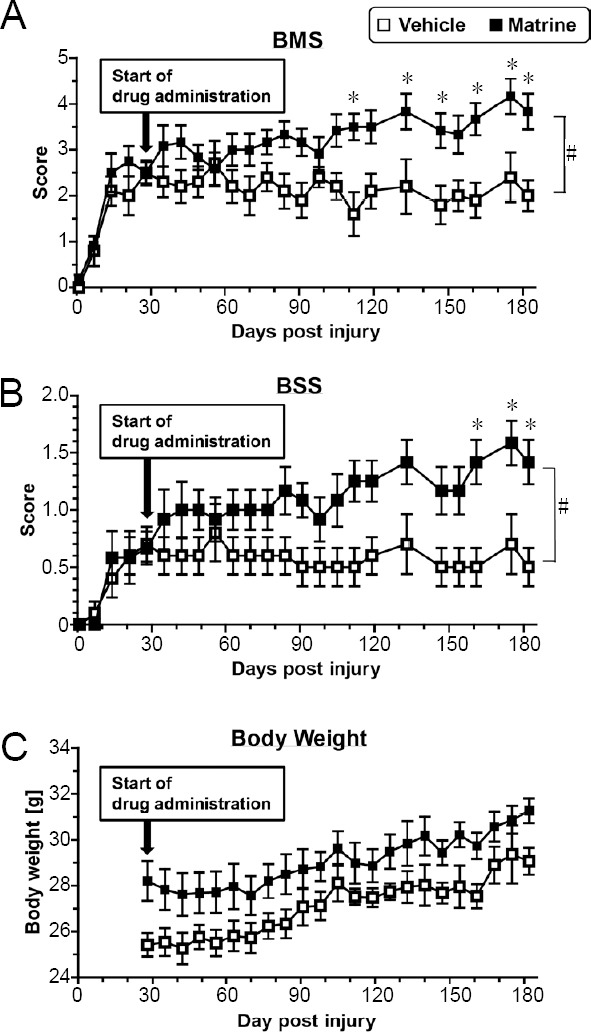
Matrine improves motor function of hindlimbs in chronic spinal cord injury mice.
The Basso Mouse Scale (BMS, A), Body Support Score (BSS, B), and body weight (C) were measured. #P < 0.05, vs. vehicle, drug × day interaction analyzed by two-way repeated measures analysis of variance. *P < 0.05, vs. vehicle, post hoc Bonferroni test. n = 10 and 12 hindlimbs in the vehicle and matrine groups, respectively.
Figure 4.
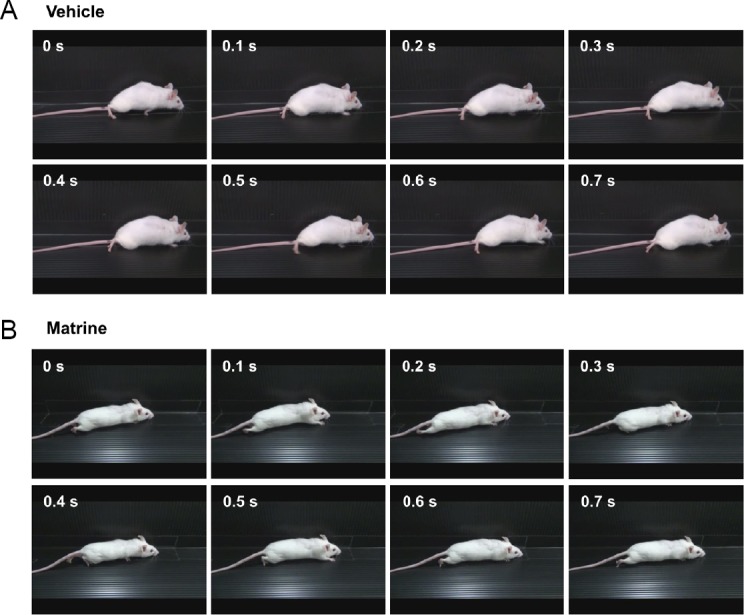
Matrine improves motor function of hindlimbs in chronic spinal cord injury (SCI) mice.
The movement of a representative SCI mouse treated with vehicle (A) or matrine (B) was captured at 182 days after injury. The images are sequential for 0.7 seconds. Vehicle-treated mouse hardly touched soles on the floor, whereas matrine-treated mouse walked with touching soles on the floor.
Matrine increases the density of total axons and synapses on motor neurons in injured spinal cord of chronic SCI mice
At 182 days after SCI, axonal densities were measured inside of glial scar and rostral and caudal sites 2 mm apart from the lesion center. Glial scar was defined as the area surrounded by GFAP-positive reactive astrocyte. Axons were stained with an anti-NF-H antibody to detect all types of axons. Since spinal cord tissue inside the glial scar was cavity in both groups by severe damage (Additional Figure 1 (443.6KB, tif) ), analysis of axon inside the glial scar was impossible. In the rostral and caudal sites, tissue loss was not observed. Vehicle-treated group showed dot-like axon structure (Figure 5A). In contrast, fiber-like axon structures were observed in matrine-treated group (Figure 5A). Matrine treatment increased NF-H-positive area in both rostral and caudal sites compared with vehicle treatment (Figure 5C and D).
Figure 5.
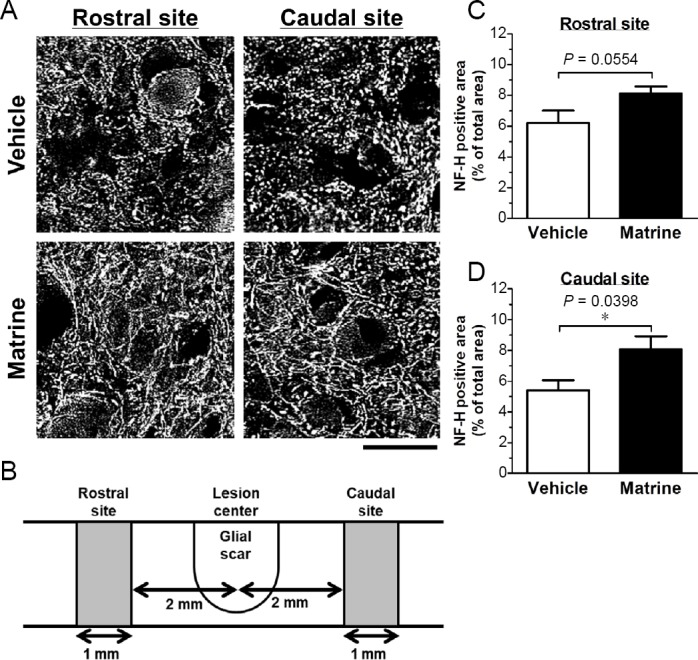
Matrine increases neurofilament-H (NF-H)-positive axons in injured spinal cord.
Sagittal sections of the spinal cords of vehicle-treated or matrine-treated were immunostained for NF-H. (A) Representative images at rostral and caudal sits are shown. Scale bar: 25 μm. (B) The diagram of analyzed area is shown. The measured areas in rostral and caudal sites were defined as the area 2.0–3.0 mm apart from the lesion center. (C, D) Ratio of NF-H-positive area to measured area was quantified at the rostral (C) and the caudal site (D). *P < 0.05, unpaired t-test (two-tailed). n = 5 mice (vehicle) or 6 mice (matrine).
Bassoon-positive puncta were densely located on cell surface of ChAT-positive motor neurons, and matrine treatment increased the presynaptic density on the plasma membrane of motor neurons (Figure 6).
Figure 6.
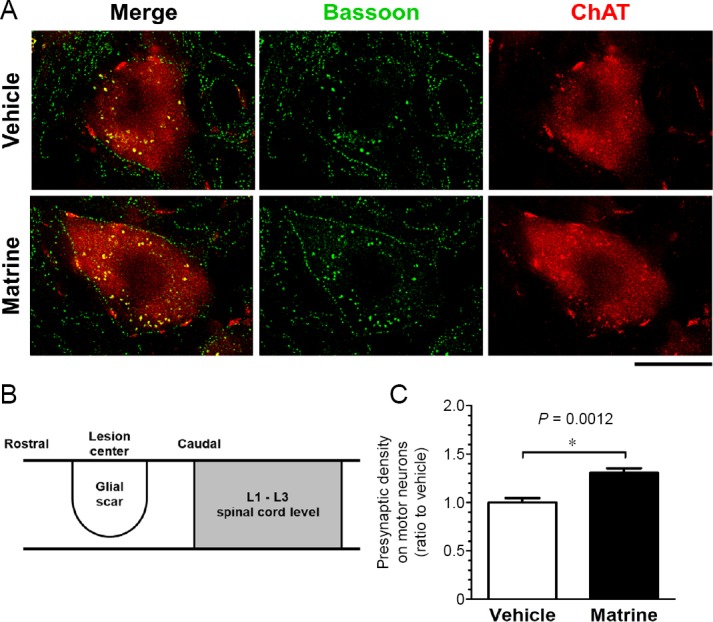
Matrine increases presynaptic density on motor neurons in injured spinal cord.
Sagittal sections of the spinal cords of vehicle-treated or matrine-treated were immunostained for choline acetyltransferase (ChAT) and bassoon. (A) Representative stains of single motor neuron on lumbar spinal cord are shown. Immunohistological images for ChAT (red) and bassoon (green) are merged. Scale bar: 20 μm. (B) The diagram of analyzed area. Motor neurons in L1–3 segments of the spinal cord were analyzed. (C) Density of bassoon-positive presynapses was quantified on the contour of motor neurons. *P < 0.05, unpaired t-test (two-tailed). n = 5 mice (vehicle) or 5 mice (matrine).
Discussion
Our previous study demonstrated that matrine directly activated the chaperone function of HSP90, and enhanced axonal growth and functional recovery in acute SCI mice via extracellular HSP90 (Tanabe et al., 2018). In this study, the effectiveness of matrine in chronic SCI was proved, which is more refractory than acute SCI. Continuous administrations of matrine could improve motor function in chronic SCI mice. Matrine increased axonal density in the spinal cord and presynaptic density on motor neurons in the lumbar spinal cords. These findings suggest that matrine remodels spinal circuits including connection to motor neurons, resulting in improvement of locomotor function in chronic SCI mice. Oral bioavailability of matrine is 17.1 ± 5.4% in rats (Yang et al., 2010). Matrine was detected in the brain after oral administration in rats (Gao and Law, 2009).
Spinal motor neurons receive neural projections from brainstem nuclei (Esposito et al., 2014), propriospinal neurons (Levine et al., 2014), and spindle proprioceptive sensory neurons (Shneider et al., 2009; Imai and Yoshida, 2018) in rodents, resulting that activity of motor neurons is controlled complicatedly. SCI decreases the number of synapses on motor neurons locating below the lesion site, and the synaptic loss is gradually recovered but partially remains in chronic stage (Satkunendrarajah et al., 2016; Freria et al., 2017). Several effective treatments for SCI models increase presynaptic density around the motor neurons, which is accompanied by functional recovery in acute and chronic phases (Satkunendrarajah et al., 2016; Tashiro et al., 2016; Freria et al., 2017). Thus, increase in presynapses on the lumbar motor neurons by matrine treatment should be an important histological change that contributes to improvement of hindlimbs motor function.
Axonal extension is essential to reconstruct neural relay regulating motor function after SCI. The signals are transmitted from brain to muscles through descending tracts, propriospinal neurons and motor neurons. Raphespinal tract (Liu and Jordan, 2005) and corticospinal tract (Whishaw et al., 1998) are descending tracts regulating motor function. Extension of these tracts is considered to contribute to functional recovery in acute SCI (Bradbury et al., 2002; Ruschel et al., 2015) and chronic SCI (Wang et al., 2011; Tashiro et al., 2016). Raphespinal tracts is known to project to propriospinal neurons that compose central pattern generator, which regulate coordination of locomotor movements (Slawinska et al., 2013; Gackiere and Vinay, 2014). Corticospinal tract also transmits a signal to motor neurons indirectly, via propriospinal neurons (Alstermark et al., 2004). When spontaneous recovery after SCI, both corticospinal tracts and propriospinal neurons sprout and construct new neural circuits that contribute functional recovery (Bareyre et al., 2004). Matrine extended NF-H-positive axons in the spinal cord of chronic SCI mice, in which these descending tracts and the propriospinal neurons may be contained. As indicated in the previous report, matrine enhanced axonal growth via extracellular HSP90 activation (Tanabe et al., 2018). The expression property of extracellular HSP90 in the spinal cord of chronic SCI should be investigated in the future.
In conclusion, this study demonstrated that matrine promoted neural circuit remodeling, resulting in improvement of motor function in chronic SCI mice. Matrine has high-potential as anti-SCI agent, and is a promising drug against chronic SCI. Although matrine-induced functional recovery in chronic SCI is still insufficient, the therapeutic effect of matrine may be enhanced by combination with other therapies, such as rehabilitation and cell transplantation.
Additional files:
Additional Figure 1 (443.6KB, tif) : Representative images at lesion center in injured spinal cords.
Representative images at lesion center in injured spinal cords.
Sagittal sections of the spinal cords of vehicle-treated (A) or matrine-treated (B) were immunostained for neurofilament-H (NF-H) and glial fibrillary acidic protein (GFAP). Left side of the images is rostral, and right side is caudal. Scale bars: 1 mm.
Additional video 1: The representative ambulation of a vehicle-treated SCI mouse at 182 days.
Additional video 2: The representative ambulation of a matrine-treated SCI mouse at 182 days.
Additional file 1: Open peer review reports 1 (109.8KB, pdf) and 2 (118.7KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no conflicts of interest regarding the publication of this article.
Financial support: This work was supported by a Grant-in-Aid for Challenging Exploratory Research (No. 26670044) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to CT), a Grant-in-Aid for a Cooperative Research Project from the Institute of Natural Medicine, University of Toyama, in 2014 and 2015 (to CT), and discretionary funds of the President of the University of Toyama, in 2014, 2015, and 2016 (to CT), and the Natural Medicine and Biotechnology Research of Toyama Prefecture, Japan (to CT).
Institutional review board statement: All experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Sugitani Campus of the University of Toyama. All protocols were approved by the Committee for Animal Care and Use of the Sugitani Campus of the University of Toyama (approval No. A2013INM-1 and A2016INM-3) on May 7, 2013 and May 17, 2016, respectively.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Wei Wu, Indiana University School of Medicine-South Bend, USA; Rodolfo Gabriel Gatto, University of Illinois at Chicago, USA.
Funding: This work was supported by a Grant-in-Aid for Challenging Exploratory Research (No. 26670044) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to CT), a Grant-in-Aid for a Cooperative Research Project from the Institute of Natural Medicine, University of Toyama, in 2014 and 2015 (to CT), and discretionary funds of the President of the University of Toyama, in 2014, 2015, and 2016 (to CT), and the Natural Medicine and Biotechnology Research of Toyama Prefecture, Japan (to CT).
P-Reviewers: Wa W, Gatto RG; C-Editors: Zhao M, Li CH; T-Editor: Jia Y
References
- 1.Alstermark B, Ogawa J, Isa T. Lack of monosynaptic corticomotoneuronal EPSPs in rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J Neurophysiol. 2004;91:1832–1839. doi: 10.1152/jn.00820.2003. [DOI] [PubMed] [Google Scholar]
- 2.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 3.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 4.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 5.Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito MS, Capelli P, Arber S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature. 2014;508:351–356. doi: 10.1038/nature13023. [DOI] [PubMed] [Google Scholar]
- 7.Freria CM, Hall JC, Wei P, Guan Z, McTigue DM, Popovich PG. Deletion of the fractalkine receptor, CX3CR1, improves endogenous repair, axon sprouting, mand synaptogenesis after spinal cord injury in mice. J Neurosci. 2017;37:3568–3587. doi: 10.1523/JNEUROSCI.2841-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gackiere F, Vinay L. Serotonergic modulation of post-synaptic inhibition and locomotor alternating pattern in the spinal cord. Front Neural Circuits. 2014;8:102. doi: 10.3389/fncir.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao G, Law FC. Physiologically based pharmacokinetics of matrine in the rat after oral administration of pure chemical and ACAPHA. Drug Metab Dispos. 2009;37:884–891. doi: 10.1124/dmd.108.023788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guptarak J, Wiktorowicz JE, Sadygov RG, Zivadinovic D, Paulucci-Holthauzen AA, Vergara L, Nesic O. The cancer drug tamoxifen: a potential therapeutic treatment for spinal cord injury. J Neurotrauma. 2014;31:268–283. doi: 10.1089/neu.2013.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai F, Yoshida Y. Molecular mechanisms underlying monosynaptic sensory-motor circuit development in the spinal cord. Dev Dyn. 2018;247:581–587. doi: 10.1002/dvdy.24611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobi A, Loy K, Schmalz AM, Hellsten M, Umemori H, Kerschensteiner M, Bareyre FM. FGF22 signaling regulates synapse formation during post-injury remodeling of the spinal cord. EMBO J. 2015;34:1231–1243. doi: 10.15252/embj.201490578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine AJ, Hinckley CA, Hilde KL, Driscoll SP, Poon TH, Montgomery JM, Pfaff SL. Identification of a cellular node for motor control pathways. Nat Neurosci. 2014;17:586–593. doi: 10.1038/nn.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li K, Wang H. Simultaneous determination of matrine, sophoridine and oxymatrine in Sophora flavescens Ait. by high performance liquid chromatography. Biomed Chromatogr. 2004;18:178–182. doi: 10.1002/bmc.308. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J Neurophysiol. 2005;94:1392–1404. doi: 10.1152/jn.00136.2005. [DOI] [PubMed] [Google Scholar]
- 17.Nishio Y, Koda M, Kitajo K, Seto M, Hata K, Taniguchi J, Moriya H, Fujitani M, Kubo T, Yamashita T. Delayed treatment with Rho-kinase inhibitor does not enhance axonal regeneration or functional recovery after spinal cord injury in rats. Exp Neurol. 2006;200:392–397. doi: 10.1016/j.expneurol.2006.02.123. [DOI] [PubMed] [Google Scholar]
- 18.Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, Bates M, Sliwinski C, Brook G, Dobrindt K, Peitz M, Brustle O, Norenberg MD, Blesch A, Weidner N, Bunge MB, Bixby JL, Bradke F. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 2015;348:347–352. doi: 10.1126/science.aaa2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satkunendrarajah K, Nassiri F, Karadimas SK, Lip A, Yao G, Fehlings MG. Riluzole promotes motor and respiratory recovery associated with enhanced neuronal survival and function following high cervical spinal hemisection. Exp Neurol. 2016;276:59–71. doi: 10.1016/j.expneurol.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Shinozaki M, Iwanami A, Fujiyoshi K, Tashiro S, Kitamura K, Shibata S, Fujita H, Nakamura M, Okano H. Combined treatment with chondroitinase ABC and treadmill rehabilitation for chronic severe spinal cord injury in adult rats. Neurosci Res. 2016;113:37–47. doi: 10.1016/j.neures.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Shneider NA, Mentis GZ, Schustak J, O’Donovan MJ. Functionally reduced sensorimotor connections form with normal specificity despite abnormal muscle spindle development: the role of spindle-derived neurotrophin 3. J Neurosci. 2009;29:4719–4735. doi: 10.1523/JNEUROSCI.5790-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slawinska U, Miazga K, Cabaj AM, Leszczynska AN, Majczynski H, Nagy JI, Jordan LM. Grafting of fetal brainstem 5-HT neurons into the sublesional spinal cord of paraplegic rats restores coordinated hindlimb locomotion. Exp Neurol. 2013;247:572–581. doi: 10.1016/j.expneurol.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Tanabe N, Kuboyama T, Tohda C. Matrine directly activates extracellular heat shock protein 90, resulting in axonal growth and functional recovery in spinal cord injured-mice. Front Pharmacol. 2018;9:446. doi: 10.3389/fphar.2018.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanabe N, Kuboyama T, Kazuma K, Konno K, Tohda C. The Extract of Roots of Sophora flavescens Enhances the Recovery of Motor Function by Axonal Growth in Mice with a Spinal Cord Injury. Front Pharmacol. 2015;6:326. doi: 10.3389/fphar.2015.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tashiro S, Nishimura S, Iwai H, Sugai K, Zhang L, Shinozaki M, Iwanami A, Toyama Y, Liu M, Okano H, Nakamura M. Functional recovery from neural stem/progenitor cell transplantation combined with treadmill training in mice with chronic spinal cord injury. Sci Rep. 2016;6:30898. doi: 10.1038/srep30898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teshigawara K, Kuboyama T, Shigyo M, Nagata A, Sugimoto K, Matsuya Y, Tohda C. A novel compound, denosomin, ameliorates spinal cord injury via axonal growth associated with astrocyte-secreted vimentin. Br J Pharmacol. 2013;168:903–919. doi: 10.1111/j.1476-5381.2012.02211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci. 2011;31:9332–9344. doi: 10.1523/JNEUROSCI.0983-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whishaw IQ, Gorny B, Sarna J. Paw and limb use in skilled and spontaneous reaching after pyramidal tract, red nucleus and combined lesions in the rat: behavioral and anatomical dissociations. Behav Brain Res. 1998;93:167–183. doi: 10.1016/s0166-4328(97)00152-6. [DOI] [PubMed] [Google Scholar]
- 29.Wootz H, Fitzsimons-Kantamneni E, Larhammar M, Rotterman TM, Enjin A, Patra K, Andre E, Van Zundert B, Kullander K, Alvarez FJ. Alterations in the motor neuron-renshaw cell circuit in the Sod1(G93A) mouse model. J Comp Neurol. 2013;521:1449–1469. doi: 10.1002/cne.23266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Z, Gao S, Yin T, Kulkarni KH, Teng Y, You M, Hu M. Biopharmaceutical and pharmacokinetic characterization of matrine as determined by a sensitive and robust UPLC-MS/MS method. J Pharm Biomed Anal. 2010;51:1120–1127. doi: 10.1016/j.jpba.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokota K, Kobayakawa K, Kubota K, Miyawaki A, Okano H, Ohkawa Y, Iwamoto Y, Okada S. Engrafted neural stem/progenitor cells promote functional recovery through synapse reorganization with spared host neurons after spinal cord injury. Stem Cell Reports. 2015;5:264–277. doi: 10.1016/j.stemcr.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang N, Fang M, Chen H, Gou F, Ding M. Evaluation of spinal cord injury animal models. Neural Regen Res. 2014;9:2008–2012. doi: 10.4103/1673-5374.143436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative images at lesion center in injured spinal cords.
Sagittal sections of the spinal cords of vehicle-treated (A) or matrine-treated (B) were immunostained for neurofilament-H (NF-H) and glial fibrillary acidic protein (GFAP). Left side of the images is rostral, and right side is caudal. Scale bars: 1 mm.


