Keywords: cerebral ischemia, paired associative stimulation, cognitive function, long-term potentiation, synaptic plasticity, Morris water maze, synaptic structure, N-methyl-D-aspartic acid receptor, brain-derived neurotrophic factor, multi-electrode array, neural regeneration
Abstract
Paired associative stimulation is a relatively new non-invasive brain stimulation technique that combines transcranial magnetic stimulation and peripheral nerve stimulation. The effects of paired associative stimulation on the excitability of the cerebral cortex can vary according to the time interval between the transcranial magnetic stimulation and peripheral nerve stimulation. We established a model of cerebral ischemia in rats via transient middle cerebral artery occlusion. We administered paired associative stimulation with a frequency of 0.05 Hz 90 times over 4 weeks. We then evaluated spatial learning and memory using the Morris water maze. Changes in the cerebral ultra-structure and synaptic plasticity were assessed via transmission electron microscopy and a 64-channel multi-electrode array. We measured mRNA and protein expression levels of brain-derived neurotrophic factor and N-methyl-D-aspartate receptor 1 in the hippocampus using a real-time polymerase chain reaction and western blot assay. Paired associative stimulation treatment significantly improved learning and memory in rats subjected to cerebral ischemia. The ultra-structures of synapses in the CA1 area of the hippocampus in rats subjected to cerebral ischemia were restored by paired associative stimulation. Long-term potentiation at synapses in the CA3 and CA1 regions of the hippocampus was enhanced as well. The protein and mRNA expression of brain-derived neurotrophic factor and N-methyl-D-aspartate receptor 1 increased after paired associative stimulation treatment. These data indicate that paired associative stimulation can protect cognition after cerebral ischemia. The observed effect may be mediated by increases in the mRNA and protein expression of brain-derived neurotrophic factor and N-methyl-D-aspartate receptor 1, and by enhanced synaptic plasticity in the CA1 area of the hippocampus. The animal experiments were approved by the Animal Ethics Committee of Tongji Medical College, Huazhong University of Science & Technology, China (approval No. TJ-A20151102) on July 11, 2015.
Chinese Library Classification No. R454; R364; R741
Introduction
Stroke is the second leading cause of death and the third most frequent cause of physical disability worldwide, representing a substantial burden in terms of morbidity and mortality (Sousa et al., 2017). Ischemic stroke accounts for approximately 80% of all strokes (Truelsen et al., 2003). Cerebral ischemia results in complex pathophysiological changes that include excitotoxity, ionic imbalances, and oxidative stress. It eventually leads to the loss of neurons and synaptic dysfunction (Quillinan et al., 2016). As a result, most stroke survivors exhibit persistent motor deficits and cognitive disorders that seriously affect their quality of life and carry a heavy economic burden for families and society (Cumming et al., 2013). Effective treatments to improve the functional recovery of stroke patients are urgently needed.
In recent years, a series of non-invasive brain stimulation (NIBS) techniques have been developed to modulate cortical excitability, neural plasticity, and human behaviour (Takeuchi and Izumi, 2012; Wessel et al., 2015; Gunduz et al., 2017; Henrich-Noash et al., 2017; Faulkner and Wright, 2018). Among them, the most common and widely used are transcranial direct current stimulation, tran-scranial magnetic stimulation (TMS), and paired associative stimulation (PAS). As NIBS can help decrease or increase the excitability of the contralesional or ipsilesional hemisphere, depending on the parameters of the NIBS protocol, NIBS may be particularly efficacious in restoring balance in individuals with impaired interhemispheric inhibition after stroke (Boddington and Reynolds, 2017).
PAS is a relatively new NIBS technique, developed by Stefan et al. (Stefan et al., 2000). The original PAS protocol involved two paired stimuli: low frequency electrostimulation of a peripheral nerve and TMS of the motor cortex. When the two stimuli are timed so that the sensory input from the peripheral nerve reaches the primary motor area shortly before TMS, PAS potentiates regional cortical excitability that outlasts the stim-ulation period. Since the after-effects of PAS are timing-dependent, long-lasting, and input-specific, and that they can be blocked by N-methyl-D-aspartate receptor (NMDAR) antagonists, PAS is likely to reflect systemic long term potentiation (LTP)-like plasticity (Stefan et al., 2002). LTP has been acknowledged as a cellular basis for learning and memory (Bear and Malenka, 1994). Some researchers have demonstrated that PAS can accelerate the recovery of motor function after stroke (Shin et al., 2008; Castel-Lacanal et al., 2009; Rogers et al., 2011). Experimental treatments using PAS have often concentrated on reducing motor injury, however cognitive impairment is a common stroke sequela that has received less scholarly attention. Studies have shown that the changes induced by repetitive TMS (rTMS) are not limited to the stimulated region, but also arise in distant areas through functional anatomical connections (Zhang et al., 2015). PAS targeting M1 has also been found to affect the remote premotor area, insula, and perhaps other regions via altered neurotransmission (Michou et al., 2015).
In the present study, we investigated the effects of PAS on the hippocampus, as this area is most vulnerable to ischemia and neurodegeneration (Lee et al., 2010). We evaluated PAS-induced changes in spatial learning and memory in rats exposed to a model of cerebral ischemia. We also examined synaptic plasticity in the hippocampus, including structural and functional plasticity, as well as the expression of NMDAR1 and brain-derived neurotrophic factor (BDNF) to explore the underlying mechanisms.
Materials and Methods
Animals
All of the animal experiments were approved by the Animal Ethics Committee of Tongji Medical College, Huazhong University of Science & Technology, China (approval No. TJ-A20151102) on July 11, 2015. Forty-five 7-week-old male Sprague-Dawley (SD) rats weighing 230 ± 10 g each were purchased from Hunan SJA Laboratory Animal Co., Ltd., Changsha, China (licence No. SCXK (Xiang) 2016-0002) and housed under specified pathogen-free (SPF) conditions. The rats were randomly divided into a sham operation group (sham, n = 15), a transient middle cerebral artery occlusion group (tMCAO, n = 15), and a PAS-treated group (tMCAO + PAS, n = 15). All of the rats received tMCAO except those in the sham group. We conducted PAS in the PAS group 24 hours after the surgery, and administered no special interventions in the sham and tMCAO groups. The overall schedule of the experiments is depicted in Figure 1A.
Figure 1.
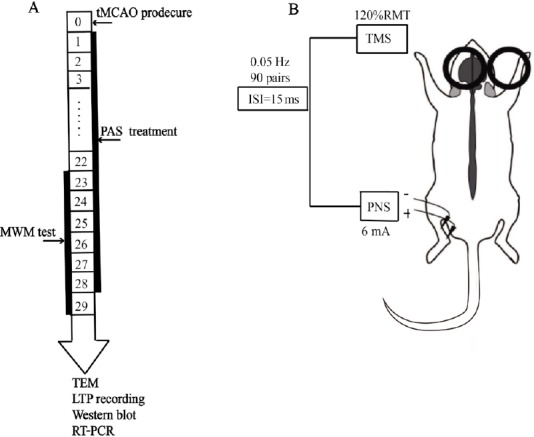
The experimental schedules and the design of the paired associative stimulation (PAS) treatment.
(A) The overall schedule of the experiments. (B) The experimental protocol: We delivered 90 pairs of stimuli, consisting of electrical stimuli delivered to the left tibial nerve, followed by TMS at 0.05 Hz applied over the right hemisphere at the optimal site for activating the soleus muscle. The interpair interval was 20 seconds and the inter-stimulus interval (ISI) was 15 ms. The intensity of the electrical stimulation was 6 mA, and the intensity of the TMS was 120% of the resting motor threshold (RMT). The experiment was always conducted between 9:00 a.m. and 11:00 a.m. tMCAO: Transient middle cerebral artery occlusion; TEM: transmission electron microscopy; LTP: long term potentiation; RT-PCR: real-time polymerase chain reaction.
Transient middle cerebral artery occlusion procedure
The rats were anesthetized via an intraperitoneal injection containing 10% chloral hydrate (300 mg/kg) (Si-nopharm Chemical Reagent Co., Ltd., Beijing, China). The right middle cerebral artery was then occluded for 90 minutes followed by reperfusion in accordance with Longa’s method (Longa et al., 1989). After re-covering from anaesthesia, each rat was returned to its cage and given free access to food and water. During the operation, rectal temperature was maintained at 37 ± 0.5oC with a heating lamp. For the sham group, only the external carotid artery was ligated. An observer blind to the experimental conditions assessed the neurological status of each rat 24 hours after surgery using the methods described by Longa et al. (1989). The total score ranged from 0 to 4, and only rats in the tMCAO group (excluding those in the sham operation group) with scores ranging from 1 to 3 were included in the experiments. Those displaying signs of subarachnoid haemorrhage or meningeal irritation were excluded from subsequent experiments.
Paired associative stimulation intervention
The rats in the PAS group were immobilized 24 hours after the operation in a fixation apparatus appropriate to their size, with their heads, limbs, and tails outside of the apparatus. Ninety pairs of stimuli were delivered. These consisted of an electrical stimulation delivered to the left tibial nerve followed by TMS at 0.05 Hz over the right hemisphere at the optimal site for activating the soleus muscle. The paired stimulation was repeated once every 24 hours for 4 weeks. TMS was delivered using a customized YRDCCY-IV magnetic stimulator (Yiruide Medical Equipment Co. Ltd., Wuhan, China) with a figure-eight coil. The inter-pair and inter-stimulus intervals were 20 seconds and 15 ms, respectively, as these have been found in previous experiments to increase cortical excitability in control rats (Zhang et al., 2018). The intensity of the electrical stimulation was 6 mA and the intensity of the TMS was 120% of the resting motor threshold, as per previous studies by Stefan et al. (2000, 2002). The resting motor threshold was defined as the min-imum stimulus intensity needed to obtain a motor evoked potential with an amplitude ≥ 20 μV in at least 5 of 10 consecutive trials. The intensity was measured before the PAS treatment and kept constant throughout the experiment. To avoid any diurnal variations in the efficacy of PAS, we conducted all experiments between 9:00 a.m. and 11:00 a.m. The experimental protocol is summarized in Figure 1B.
Spatial learning and memory testing
We used a Morris water maze to quantify spatial learning and memory abilities (Morris, 1984). A black, circular water pool (150 cm in diameter with walls 50 cm high) (Chinese Academy of Sciences, Beijing, China) was filled to a depth of 20 cm with water kept at 23 ± 2°C. The water pool was divided into four quadrants, and a cylindrical platform was placed in the centre of the target quadrant such that it was submerged 2 cm beneath the surface. The testing procedures have been previously described (Xia et al., 2017). On days 23–28, we subjected each rat to 2 daily four-place navigation trials (6 consecutive days). The time required to find the platform (escape latency) was recorded, with a limit of 60 seconds. If a rat failed to reach the platform within 60 seconds, it was guided to the platform and permitted to stay there for 10 seconds, and the escape latency was recorded as 60 seconds. We considered long escape latencies to represent weak learning ability. We also conducted a spatial probe test. On day 29, the platform was removed and the rats were released in the quadrant opposite the quadrant where the platform had been. We recorded the number of times the rats crossed the quadrant where the platform had been previously located within a 60-second period. We considered a low frequency of crossing the former platform quadrant to indicate weak memory ability. All data were automatically recorded by a computerized video system (Chengdu Technology & Market Co., Chengdu, China).
Long-term potentiation assay
After 4 weeks of behavioural testing, as described above, the rats were sacrificed, their brains were resected, and slices of the hippocampus were prepared using standard techniques described previously (Oka et al., 1999). The rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (350 mg/kg) and the entire brain was quickly and carefully removed from the skull. The brains were immediately immersed for approximately 1 minute in ice-cold, oxygenated (95%O2–5%CO2) artificial cerebrospinal fluid (pH 7.4) containing (in mM concentrations) NaCl 125, NaHCO3 26, KCl 3, MgCl2·6H2O 1.0, NaH2PO4·2H2O 1.2, CaCl2 2.0, and glucose 10. The brains were then sectioned with a DTK-1500 vibratome (Dosaka, Kyoto, Japan). The thickness of each tissue slice was 300 μm. The slices from the hippocampus were incubated in oxygenated artificial cerebrospinal fluid for a minimum of 1 hour at room temperature before undergoing testing with a multi-electrode array.
For the multi-electrode array, 64 microelectrodes (60 μm × 60 μm each) were arranged in an 8 × 8 array with an inter-polar distance of 150 μm. Procedures for preparing the MED64 multi-electrode dish (Panasonic, MED probe, Japan) were as described previously (Oka et al., 1999). The surface of the dish was treated with 0.1% polyethylenimine in 25 mM borate buffer (pH 8.4) overnight at room temperature before use to ensure sufficient adhesion of the slice to the surface of the probe. A slice was placed upon the MED64 probe in such a manner that the CA3 and CA1 areas of the hippocampus were covered by the recording dish installed upon the stage of an inverted image microscope. Once the slice was settled, a fine-mesh anchor was carefully positioned to stabilize the slice during recording. During the whole process of electrophysiological recording, the slice was continuously perfused with oxygenated fresh artificial cerebrospinal fluid at 2–3 mL/min via a peristaltic pump. A microphotograph of one hippocampal slice positioned on the MED64 probe is shown in Figure 2A.
Figure 2.
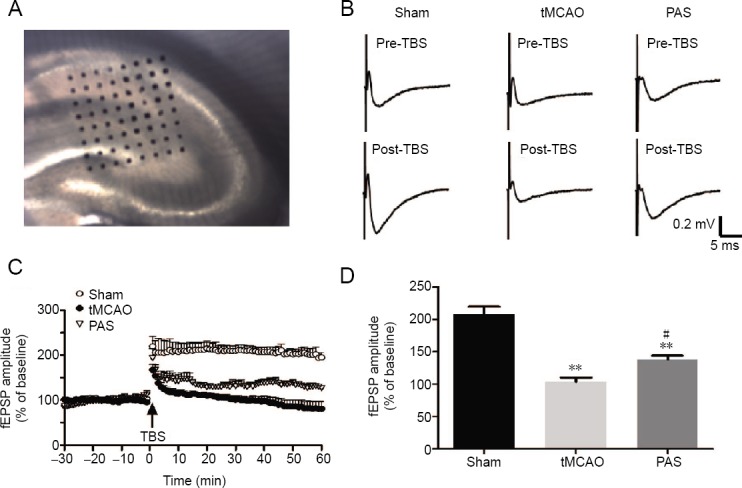
Effects of paired associative stimulation (PAS) on the recovery of long-term potentiation.
(A) Light photomicrograph showing the location of the MED probe on a slice. (B) Typical field excitatory postsynaptic potential (fEPSP) traces at 5 min pre-theta burst stimulation (TBS) and at 55 min post-TBS in each group. (C) The normalized fEPSP amplitudes before and after the TBS. (D) The average percent change in fEPSP amplitude within 60 min after TBS. Each value represents mean ± SD of the normalized fEPSP amplitudes (n = 5 slices from 5 rats for each group). **P < 0.01, vs. sham group; #P < 0.05, vs. tMCAO group (one-way analysis of variance followed by Fisher’s least significant difference test). tMCAO: Transient middle cerebral artery occlusion; min: minute(s).
Twenty minutes after positioning a slice, we selected one of the 64 available planar microelectrodes to stimulate the Schaffer collateral axons in the CA3 region. The microelectrodes monitoring the hippocampal CA1 region were used to record extracellular field potentials. The stimulus intensity was set to elicit field excitatory postsynaptic potentials (fEPSPs) with a slope that was approximately 30% of the maximum de-termined from the input-output curve. After obtaining a stable fEPSP recording for 30 minutes, we used theta burst stimulation (TBS) (10 bursts at 5 Hz, with 4 pulses at 100 Hz in each burst) to induce LTP (He et al., 2009). We then continuously recorded fEPSPs for 60 minutes after the stimulation. There were five rats in each group and we recorded from one brain slice from each rat. The data were analysed off line using the MED64 system (Alpha MED Sciences, Tokyo, Japan). To quantify any long-term potentiation, the amplitudes of the field potentials were normalized and expressed as a percentage of the averaged baseline value.
Ultrastructure of the hippocampal CA1 area
We used Pellegrino’s three-dimensional map of the rat brain as a guide when resectioning about 1 mm × 1 mm × 1 mm pieces of the hippocampal CA1 area on the ischemic side of each hippocampus (n = 5 each group). The samples were immediately fixed in a solution of 4% formaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 2 hours at room temperature and then post-fixed in 2.5% osmium tetroxide in 0.1 M phosphate buffer for 1 hour. After that, they were dehydrated in graded ethanol and embedded in Epon 812 resin. Sections 70 to 80 nm thick were sliced using an LKB-V microtome (Bromm, Sweden). The slices were double-stained with uranyl acetate for 15–30 minutes and then lead citrate for 3–15 minutes and photographed under an H-300 transmission electron microscope (TEM) (Hitachi Electronic Instruments, Japan). The brain tissue from each rat was photographed at six non-overlapping areas. We assessed the synaptic ultrastructure with the help of version 6.0 of Image Pro Plus image analysis software (Media Cybernetics, MD, USA). We observed the curvature of synaptic interfaces, the width of synaptic clefts, the length of the synaptic active zones, and the thickness of the zone of post-synaptic density. All of these features are commonly interpreted as reflecting changes in synaptic structure (Xia et al., 2017).
Protein expression assay
To further study the molecular mechanisms of PAS in improving learning and memory function and en-hancing synaptic plasticity, we examined the expression of BDNF and NMDAR1 via western blot assay. Total protein was extracted from the ipsilateral hippocampi of the different groups of rats (n = 5 in each group). Briefly, 500 mg of hippocampal tissue was lysed in ice-cold lysis buffer containing 50 mM Tris-HCl (pH 7.5), 1% Triton X-100, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, and phosphatase inhibitors (Sigma, St. Louis, MO, USA). The lysates (50 μg) were separated using 8% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, transferred to a polyvinylidene difluoride membrane, and blocked with 5% bovine serum albumin for 1 hour at room temperature. Primary antibodies in the blocking buffer were poured onto the polyvinylidene difluoride membrane and held overnight at 4°C. We used rabbit anti-BDNF (1:2000; Abcam, Cambridge, MA, USA) and rabbit anti-NMDAR1 (1:1000; Abcam). Blots were then washed five times in tris-buffered saline Tween-20 buffer and incubated with a horseradish peroxi-das-labelled goat anti-rabbit secondary antibody (1:5000; Bioworld, Wuhan, China) for 2 hours at 37°C. Finally, the polyvinylidene difluoride was washed again with tris-buffered saline Tween-20 five times and protein bands were visualized using an enhanced chemiluminescence system (Thermo, Waltham, MA, USA) with imaging capability. The levels were quantified with the help of Gel-Pro Analyzer 4.0 software (Media Cybernetics, Rockville, MD, USA). The results were normalized to the quantity of β-actin (1:200; Bioworld, Wuhan, China) in each sample lane. All assays were performed at minimum in triplicate.
mRNA expression assay
The total RNA was extracted from the ipsilateral hippocampal tissue samples using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. 1 μg of the total RNA was then reverse-transcribed into cDNA with the M-MLV First-Strand cDNA Synthesis Kit (Invitrogen). The details of the polymerase chain reaction technique have been previously described (Ma et al., 2014). The reactions were carried out in triplicate using the StepOne Real-Time polymerase chain reaction system (Life Tech-nologies, Grand Island, NY, USA) in a 20 μL reaction mixture. The polymerase chain reaction primers were synthesized by Sangon Biotech in Shanghai. The primer nucleotide sequences are shown in Table 1. The β-actin gene served as an internal reference. We determined the relative expression of BDNF and NMDAR1 via the 2–ΔΔCT method, as described previously (Livak and Schmittgen, 2001).
Table 1.
Primers for β-actin, BDNF, and NMDAR1 genes in a real-time polymerase chain reaction
| Gene name | Primer sequences (5′–3′) | Product length (bp) |
|---|---|---|
| β-Actin | F: 5′-CAC GAT GGA GGG GCC GGA CTC ATC-3′ | 240 |
| R: 5′-TAA AGA CCT CTA TGC CAA CAC AGT-3′ | ||
| BDNF | F: 5′- CTG GAT GAG GAC CAG AAG GTT-3′ | 278 |
| R: 5′-GGA TTG CAC TTG GTC TCG TAG C-3′ | ||
| NMDAR1 | F: 5′-AAG TAT GCG GAT GCG GTG ACT-3′ | 413 |
| R: 5′-CAA AAG CCG TAG CAT CAC TGA-3′ |
BDNF: Brain-derived neurotrophic factor; NMDAR1: N-methyl-D-aspartate receptor 1; F: forward; R: reverse.
Statistical analysis
All of the data are presented as the mean ± standard deviation (SD). Statistical significance was analysed using version 18.0 of the SPSS statistical software package (SPSS, Chicago, IL, USA). The results were analyzed via a one-way analysis of variance followed by Fisher’s least significant difference test in which all groups were compared with the sham group. A value of P < 0.05 was considered statistically significant.
Results
Paired associative stimulation improves spatial cognition in rats with cerebral ischemia
Compared with the sham group, the mean escape latency to reach the hidden platform was significantly prolonged and the platform quadrant crossing frequency was markedly reduced in the tMCAO group (P < 0.01). This indicates that the rats in the tMCAO group were cognitively impaired. Following PAS treatment, the mean escape latency significantly decreased (P < 0.01, vs. tMCAO group) and the platform quadrant crossing frequency significantly increased (P < 0.01, vs. tMCAO group), although these values were still lower than those in the sham group. These results indicate that PAS treatment can significantly ameliorate impaired learning and memory after cerebral ischemia in rats (Figure 3).
Figure 3.
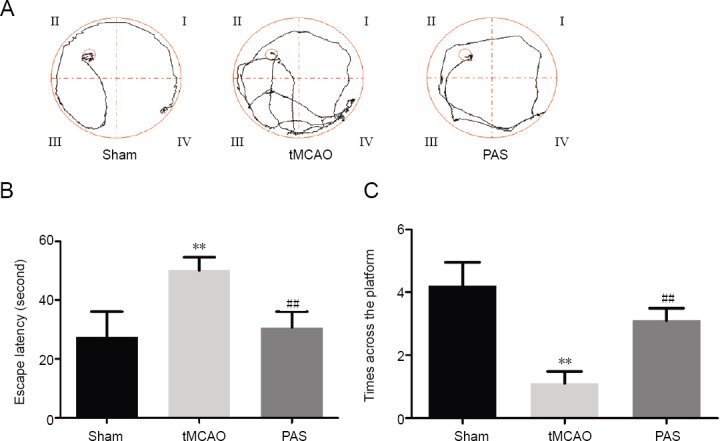
Effects of paired associative stimulation (PAS) on cognitive impairment in rats with cerebral ischemia.
(A) Typical traces from the Morris water maze tests. (B) The average time taken for the rats to reach the platform (average escape latency). (C) The number of times the rats crossed the area where the platform had been previously located within 60 seconds in the spatial probe test. Data are expressed as the mean ± SD (n = 10 rats for each group). **P < 0.01, vs. sham group; ##P < 0.01, vs. tMCAO group (one-way analysis of variance followed by Fisher’s least significant difference test). tMCAO: Transient middle cerebral artery occlusion.
Paired associative stimulation alters the ultrastructure of hippocampal neurons in rats with cerebral ischemia
We examined ultrastructural changes in hippocampal CA1 area neurons in each group using transmission electron microscopy (Figure 4). In the tMCAO group, we observed synapses that had a damaged structure, decreased number of synaptic vesicles, and a higher number of vacuoles compared with the sham group. This damage appeared to have been alleviated in the PAS group compared with the tMCAO group as the synapses had more regular forms. The synaptic structure parameters of the three groups are shown in Figure 4. Compared with the sham group, the curvature of synaptic interfaces, the lengths of synaptic active zones, and the thickness of the zones of post-synaptic density in the tMCAO group were significantly lower (P < 0.05). After the PAS treatment, the lengths of the synaptic active zones and the thicknesses of the zones of post-synaptic density were significantly higher (P < 0.05). However, we found no difference in the average width of the synaptic cleft among the three groups (P > 0.05).
Figure 4.
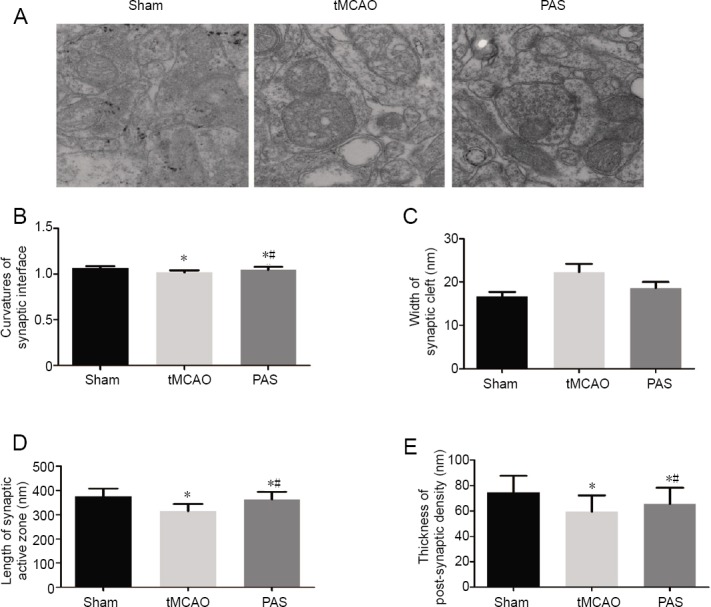
Effects of paired associative stimulation (PAS) on the ultra-structure and synaptic structure parameters in the hippocampal CA1 area of rats with cerebral ischemia.
(A) Ultra-structure of hippocampal CA1 area (transmission electron, ×15,000). (B–E) Synaptic structure parameters in the hippocampal CA1 area. The results are expressed as the mean ± SD (n = 30 pieces in each group). *P < 0.05, vs. sham group; #P < 0.05, vs. tMCAO group (one-way analysis of variance followed by Fisher’s least significant difference test). tMCAO: Transient middle cerebral artery occlusion.
Paired associative stimulation alleviates impaired long-term potentiation in rats with cerebral ischemia
We tested the effects of PAS on tMCAO-induced LTP impairment via hippocampal slices and the 64-channel multi-electrode array. After stabilization of the baseline, we recorded activity during a 30-minute control period and then delivered TBS to induce LTP. In the sham group, the TBS resulted in a notable increase in fEPSP amplitude, which then remained stable during a further 60-minute period (208 ± 11.6%). Schaffer collateral-CA1 synapses were severely impaired in the tMCAO group (103 ± 6.4% post-TBS during 60 minutes vs. the sham group, P < 0.01). We found that although TBS induced an immediate significant increase in fEPSP (179 ± 7.7%), the amplitudes showed a constant rate of decay over time and had reached the pre-stimulation level by the end of the recording session (81 ± 15.4% at 55 to 60 minutes post-TBS vs. 96 ± 1.1% pre-TBS, P < 0.05). Notably, PAS restored the LTP impaired by the surgery (138 ± 6.0% post-TBS during 60 minutes vs. tMCAO group, P < 0.05), although LTP never reached control levels (PAS group vs. sham group, P < 0.01; Figure 2B–D).
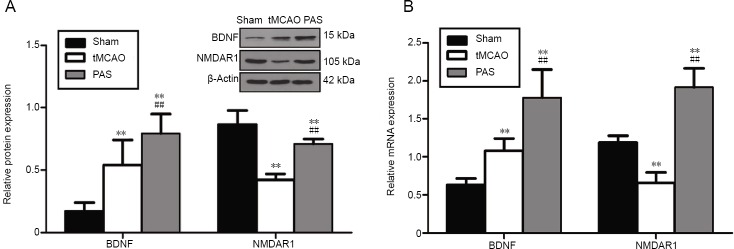
Paired associative stimulation upregulates protein and gene expression of hippocampal brain-derived neurotrophic factor and N-methyl-D-aspartate receptor 1 in rats with cerebral ischemia
The protein expression of BDNF in the tMCAO group was significantly higher than that in the sham group (P < 0.01), and NMDAR1 protein expression was lower than that in the sham group (P < 0.01; Figure 5A). Rats in the PAS group exhibited higher average BDNF and NMDAR1 expression levels than those in the tMCAO group (P < 0.01). To examine whether the observed altered expression levels were due to changes at the mRNA level, we performed real-time polymerase chain reaction analysis on samples of hippocampal tissue. The mRNA expression levels of BDNF and NMDAR1 in the different groups were consistent with those of the corresponding proteins. Those results show that the mRNA level of BDNF in the tMCAO group was higher than that in the sham group (P < 0.01) and that the NMDAR1 mRNA level was lower than that in the sham group (P < 0.01; Figure 5B). Thus, PAS significantly upregulated mRNA expression of BDNF and NMDAR1 in the hippocampus.
Figure 5.
Effect of paired associative stimulation (PAS) on the protein (A) and mRNA (B) expression of brain-derived neurotrophic factor (BDNF) and N-methyl-D-aspartate receptor 1 (NMDAR1) in the hippocampus of rats with cerebral ischemia.
Protein and mRNA expression measured in terms of relative optical density to β-actin. The results are expressed as the mean ± SD (n = 5 rats in each group). **P < 0.01, vs. sham group; ##P < 0.01, vs. tMCAO group (one-way analysis of variance followed by Fisher’s least significant difference test). tMCAO: Transient middle cerebral artery occlusion.
Discussion
Ischemic stroke normally impacts both sensorimotor and cognitive function. tMCAO is considered to be a convenient, reproducible, and reliable way to model the effects of ischemic stroke in rodents, and the technique is widely used to investigate the characteristics of ischemic stroke and to explore potential therapeutic interventions (Mhairi Macrae, 1992). Previous studies have shown that tMCAO can induce progressive impairments in spatial learning and memory (Li et al., 2013). After PAS treatment, we found that the escape latency decreased significantly and the number of target quadrant crossings increased in the water maze test. This indicates that spatial learning and memory ability improved following PAS.
Synaptic plasticity is the cellular basis of memory formation and cognition. It involves morphological plasticity and changes in transmission efficacy. When we measured morphological changes using a transmission electron microscope, we found a decrease in synaptic strength and a reduction in neuronal activity after tMCAO. This suggests that cognitive decline that occurs as a result of cerebral ischemia is correlated with a decrease in the activity of hippocampal neurons. The efficacy of synaptic transmission can be evaluated according to changes in electrical activity both in vivo and in vitro. LTP in the hippocampus is considered to be a major electrophysiological indicator of the efficacy of synaptic transmission, and thus cognition. Multiple electrode arrays, first described by Thomas et al. (Thomas et al., 1972), allow extracellular electrophysio-logical recording (Hofmann and Bading, 2006; Steidl et al., 2006). Compared with conventional electro-physiology techniques, the use of multiple electrode arrays is advantageous in that it causes less damage to neurons, thus allowing longer-term observation, and it also enables steady recording of electrophysiological activity at many sites within one slice (Morin et al., 2005; Hofmann and Bading, 2006; Steidl et al., 2006). Such electrode arrays have been widely used in recent LTP studies (Liu et al., 2016). In the present study, our data indicate that focal cerebral ischemia impairs synaptic plasticity in the hippocampus. This is consistent with the observations of numerous other researchers (Li et al., 2012). Thus, our data suggest that PAS can partially rescue LTP impairment, at least in rats after tMCAO.
Synaptic plasticity and cognitive ability rely on specific proteins, neurotransmitters, receptors, and mes-senger molecules in neurons as well as in synapses (Sheng and Kim, 2002). BDNF plays a crucial part in the activity-dependent regulation of synaptic structure and synaptic transmission (Waterhouse and Xu, 2009). It plays a major role in inducing LTP and is implicated in learning and memory (Guo et al., 2014). BDNF-gene knockout or knockdown mice display damaged spatial learning and memory (Heldt et al., 2007). Several studies have confirmed that transient ischemia can rapidly upregulate BDNF expression in the hippocampus, a phenomenon thought to participate in protection and the recovery of function after stroke (Dmitrieva et al., 2016). Intravenous BDNF treatment after ischemia was shown to improve long-term functional neurological outcomes (Schabitz et al., 2007). NMDARs are glutamate-gated cation channels important for learning and memory formation (Bliss and Collingridge, 1993). NMDAR1, an NMDAR subtype, has the full functionality of NMDARs and is distributed intensively in the hippocampus (Behe et al., 1995; Rebola et al., 2010). NMDARs play a dual role in cerebral ischemia (Wang et al., 2010). In the acute phase, they mediate amino acid cytotoxicity and neuron damage that impairs cognition. In the chronic phase, when amino acid cytotoxicity is no longer observed, further damage may be related to a reduction in NMDARs, thus inducing dysfunctional synaptic transmission. The mechanisms underlying the loss of NMDARs in the chronic phase are not fully understood. Some researchers believe that the reduction may be a neuroprotective response against glutamate neurotoxicity (Li et al., 2013). In any case, the NMDAR agonist D-cycloserine was found to significantly improve somatosensory and cognitive function in a rat model of stroke 24 hours after tMCAO (Dhawan et al., 2011).
In this study, we investigated the possible molecular mechanisms by which PAS influences LTP. Specifically, we measured mRNA as well as BDNF and NMDAR1 protein levels in the hippocampus. Our findings are consistent with those of several previous studies showing that BDNF protein and gene expression increase significantly after tMCAO, while NMDAR1 decreases significantly (Dhawan et al., 2010; Dmitrieva et al., 2016). After PAS, BDNF and NMDAR1 expression was reinforced, and this was accompanied by restoration of LTP and enhanced cognitive ability. PAS also increased levels of BDNF and NMDAR1 mRNA in the hip-pocampus, suggesting that PAS modulates BDNF and NMDAR expression via a mechanism that regulates transcription. BDNF is mediated by glutamate receptors including NMDA and non-NMDA receptors. When activation of NMDAR increases BDNF gene expression, BDNF can then increase the frequency with which NMDA receptor channels open to phosphorylate the NMDAR1 subunit of NMDA (Yamada and Nabeshima, 2004). When NMDARs are activated, calcium ion permeation into cells increases. Calcium ions further ac-tivate intracellular calcium-dependent enzymes, which lead to a series of biochemical processes that pro-mote LTP, change the efficiency of synaptic transmission, and improve cognition. In summary, our data suggest that the therapeutic effects of PAS on cognition might partially be due to enhanced synaptic plas-ticity and increases in the expression of BDNF and NMDAR1 in the hippocampus of rats. To our knowledge, this is the first study to investigate the effect of PAS on cognitive ability and synaptic plasticity. Our findings represent a theoretical basis for the development of clinical PAS in the treatment of poststroke cognitive dysfunction. However, our study had several limitations. Firstly, factors other than BDNF and NMDAR may also have contributed to the therapeutic effect of PAS on cognitive dysfunction, and were not explored in this study. Secondly, the detailed signalling pathway that regulates BDNF and NMDAR1 expression was not explored. Thirdly, as learning and memory is a complex process, it still remains a problem to fully translate our experimental results to human conditions. In future work, we plan to investigate synaptic plasticity after PAS more precisely on the molecular level, and the additional underlying mechanisms.
Additional file: Open peer review reports 1 (104.3KB, pdf) and 2 (105.2KB, pdf) .
Footnotes
Conflicts of interest: There were no conflicts of interest in this experiment
Financial support: This work was supported by the National Natural Science Foundation of China, No. 81272156 (to TCG). The funding body played no role in the study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Institutional review board statement: The study was approved by the Animal Ethics Committee of the Tongji Medical College, Huazhong University of Science & Technology, China (approval number: TJ-A20151102) on July 11, 2015.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Giovanni Casini, Universita degli Studi di Pisa, Italy; Jonathan M. Borkum, University of Maine, USA.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81272156 (to TCG).
P-Reviewers: Casini G, Borkun JM; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Koke S, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 2.Behe P, Stern P, Wyllie DJ, Nassar M, Schoepfer R, Colquhoun D. Determination of NMDA NR1 subunit copy number in recombi-nant NMDA receptors. Proc Biol Sci. 1995;262:205–213. doi: 10.1098/rspb.1995.0197. [DOI] [PubMed] [Google Scholar]
- 3.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 4.Boddington LJ, Reynolds JNJ. Targeting interhemispheric inhibition with neuromodulation to enhance stroke rehabilitation. Brain Stimul. 2017;10:214–222. doi: 10.1016/j.brs.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Castel-Lacanal E, Marque P, Tardy J, de Boissezon X, Guiraud V, Chollet F, Loubinoux I, Moreau MS. Induction of cortical plastic changes in wrist muscles by paired associative stimulation in the recovery phase of stroke patients. Neurorehabil Neural Repair. 2009;23:366–372. doi: 10.1177/1545968308322841. [DOI] [PubMed] [Google Scholar]
- 6.Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. Int J Stroke. 2013;8:38–45. doi: 10.1111/j.1747-4949.2012.00972.x. [DOI] [PubMed] [Google Scholar]
- 7.Dhawan J, Benveniste H, Nawrocky M, Smith SD, Biegon A. Transient focal ischemia results in persistent and widespread neuroinflammation and loss of glutamate NMDA receptors. Neuroimage. 2010;51:599–605. doi: 10.1016/j.neuroimage.2010.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhawan J, Benveniste H, Luo Z, Nawrocky M, Smith SD, Biegon A. A new look at glutamate and ischemia: NMDA agonist improves long-term functional outcome in a rat model of stroke. Future Neurol. 2011;6:823–834. doi: 10.2217/fnl.11.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dmitrieva VG, Stavchansky VV, Povarova OV, Skvortsova VI, Limborska SA, Dergunova LV. Effects of ischemia on the expression of neurotrophins and their receptors in rat brain structures outside the lesion site, including on the opposite hemisphere. Mol Biol (Mosk) 2016;50:775–784. doi: 10.7868/S002689841603006X. [DOI] [PubMed] [Google Scholar]
- 10.Faulkner J, Wright A. Using robotic-assisted technology to improve lower-limb function in people with stroke. Clin Trials Degener Dis. 2018;3:111–114. [Google Scholar]
- 11.Guo W, Ji Y, Wang S, Sun Y, Lu B. Neuronal activity alters BDNF-TrkB signaling kinetics and downstream functions. J Cell Sci. 2014;127:2249–2260. doi: 10.1242/jcs.139964. [DOI] [PubMed] [Google Scholar]
- 12.Gunduz A, Rothwell J, Vidal J, Kumru H. Non-invasive brain stimulation to promote motor and functional recovery following spinal cord injury. Neural Regen Res. 2017;12:1933–1938. doi: 10.4103/1673-5374.221143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Y, Liu MG, Gong KR, Chen J. Differential effects of long and short train theta burst stimulation on LTP induction in rat anterior cingulate cortex slices: multi-electrode array recordings. Neurosci Bull. 2009;25:309–318. doi: 10.1007/s12264-009-0831-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henrich-Noack P, Sergeeva EG, Sabel BA. Non-invasive electrical brain stimulation: from acute to late-stage treatment of central nervous system damage. Neural Regen Res. 2017;12:1590–1594. doi: 10.4103/1673-5374.217322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann F, Bading H. Long term recordings with microelectrode arrays: studies of transcription-dependent neuronal plasticity and axonal regeneration. J Physiol Paris. 2006;99:125–132. doi: 10.1016/j.jphysparis.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Lee CH, Yoo KY, Choi JH, Park OK, Hwang IK, Kim SK, Kang IJ, Kim YM, Won MH. Neuronal damage is much delayed and micro-gliosis is more severe in the aged hippocampus induced by transient cerebral ischemia compared to the adult hippocampus. J Neurol Sci. 2010;294:1–6. doi: 10.1016/j.jns.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Huang R, Shetty RA, Thangthaeng N, Liu R, Chen Z, Sumien N, Rutledge M, Dillon GH, Yuan F, Forster MJ, Simpkins JW, Yang SH. Transient focal cerebral ischemia induces long-term cognitive function deficit in an experimental ischemic stroke model. Neurobiol Dis. 2013;59:18–25. doi: 10.1016/j.nbd.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Pang L, Fang F, Zhang G, Zhang J, Xie M, Wang L. Resveratrol attenuates brain damage in a rat model of focal cerebral ischemia via up-regulation of hippocampal Bcl-2. Brain Res. 2012;1450:116–124. doi: 10.1016/j.brainres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Liu MG, Li HS, Li WG, Wu YJ, Deng SN, Huang C, Maximyuk O, Sukach V, Krishtal O, Zhu MX, Xu TL. Acid-sensing ion channel 1a contributes to hippocampal LTP inducibility through multiple mechanisms. Sci Rep. 2016;6:23350. doi: 10.1038/srep23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 23.Ma J, Zhang Z, Kang L, Geng D, Wang Y, Wang M, Cui H. Repetitive transcranial magnetic stimulation (rTMS) influences spatial cognition and modulates hippocampal structural synaptic plasticity in aging mice. Exp Gerontol. 2014;58:256–268. doi: 10.1016/j.exger.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Mhairi Macrae I. New models of focal cerebral ischaemia. Br J Clin Pharmacol. 1992;34:302–308. doi: 10.1111/j.1365-2125.1992.tb05634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michou E, Williams S, Vidyasagar R, Downey D, Mistry S, Edden RA, Hamdy S. fMRI and MRS measures of neuroplasticity in the pharyngeal motor cortex. Neuroimage. 2015;117:1–10. doi: 10.1016/j.neuroimage.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morin FO, Takamura Y, Tamiya E. Investigating neuronal activity with planar microelectrode arrays: achievements and new per-spectives. J Biosci Bioeng. 2005;100:131–143. doi: 10.1263/jbb.100.131. [DOI] [PubMed] [Google Scholar]
- 27.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 28.Oka H, Shimono K, Ogawa R, Sugihara H, Taketani M. A new planar multielectrode array for extracellular recording: application to hippocampal acute slice. J Neurosci Methods. 1999;93:61–67. doi: 10.1016/s0165-0270(99)00113-2. [DOI] [PubMed] [Google Scholar]
- 29.Quillinan N, Herson PS, Traystman RJ. Neuropathophysiology of Brain Injury. Anesthesiol Clin. 2016;34:453–464. doi: 10.1016/j.anclin.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebola N, Srikumar BN, Mulle C. Activity-dependent synaptic plasticity of NMDA receptors. J Physiol. 2010;588:93–99. doi: 10.1113/jphysiol.2009.179382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers LM, Brown DA, Stinear JW. The effects of paired associative stimulation on knee extensor motor excitability of individuals post-stroke: a pilot study. Clin Neurophysiol. 2011;122:1211–1218. doi: 10.1016/j.clinph.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, Kuhn HG. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- 33.Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- 34.Shin HI, Han TR, Paik NJ. Effect of consecutive application of paired associative stimulation on motor recovery in a rat stroke model: a preliminary study. Int J Neurosci. 2008;118:807–820. doi: 10.1080/00207450601123480. [DOI] [PubMed] [Google Scholar]
- 35.Sousa LVA, Paiva LDS, Figueiredo FWDS, Almeida TCDC, Oliveira FR, Adami F. Trends in stroke-related mortality in the ABC region, Sao Paulo, Brazil: an ecological study between 1997 and 2012. Open Cardiovasc Med J. 2017;11:111–119. doi: 10.2174/1874192401711010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 37.Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steidl EM, Neveu E, Bertrand D, Buisson B. The adult rat hippocampal slice revisited with multi-electrode arrays. Brain Res. 2006;1096:70–84. doi: 10.1016/j.brainres.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi N, Izumi S. Noninvasive brain stimulation for motor recovery after stroke: mechanisms and future views. Stroke Res Treat. 2012;2012:584727. doi: 10.1155/2012/584727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas CA, Jr, Springer PA, Loeb GE, Berwald-Netter Y, Okun LM. A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp Cell Res. 1972;74:61–66. doi: 10.1016/0014-4827(72)90481-8. [DOI] [PubMed] [Google Scholar]
- 41.Truelsen T, Mahonen M, Tolonen H, Asplund K, Bonita R, Vanuzzo D WHO MONICA Project. Trends in stroke and coronary heart disease in the WHO MONICA Project. Stroke. 2003;34:1346–1352. doi: 10.1161/01.STR.0000069724.36173.4D. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Geng X, Tao HY, Cheng Y. The restoration after repetitive transcranial magnetic stimulation treatment on cognitive ability of vascular dementia rats and its impacts on synaptic plasticity in hippocampal CA1 area. J Mol Neurosci. 2010;41:145–155. doi: 10.1007/s12031-009-9311-7. [DOI] [PubMed] [Google Scholar]
- 43.Waterhouse EG, Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol Cell Neurosci. 2009;42:81–89. doi: 10.1016/j.mcn.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wessel MJ, Zimerman M, Hummel FC. Non-invasive brain stimulation: an interventional tool for enhancing behavioral training after stroke. Front Hum Neurosci. 2015;9:265. doi: 10.3389/fnhum.2015.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia WG, Zheng CJ, Zhang X, Wang J. Effects of “nourishing liver and kidney” acupuncture therapy on expression of brain derived neurotrophic factor and synaptophysin after cerebral ischemia reperfusion in rats. J Huazhong Univ Sci Technol Med Sci. 2017;37:271–278. doi: 10.1007/s11596-017-1727-7. [DOI] [PubMed] [Google Scholar]
- 46.Yamada K, Nabeshima T. Interaction of BDNF/TrkB signaling with NMDA receptor in learning and memory. Drug News Perspect. 2004;17:435–438. doi: 10.1358/dnp.2004.17.7.863702. [DOI] [PubMed] [Google Scholar]
- 47.Zhang N, Xing M, Wang Y, Tao H, Cheng Y. Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF-NMDAR pathways in a rat model of vascular dementia. Neuroscience. 2015;311:284–291. doi: 10.1016/j.neuroscience.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 48.Zhang XY, Sui YF, Guo TC, Wang SH, Hu Y, Lu YS. Effect of paired associative stimulation on motor cortex excitability in rats. Curr Med Sci. 2018;38:903–909. doi: 10.1007/s11596-018-1960-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.