Keywords: nerve regeneration, etomidate, retinal ganglion cells, optic nerve transection, anti-oxidative stress, nitric oxide, malonaldehyde, glutathione, neural regeneration
Abstract
Our previous studies revealed that etomidate, a non-barbiturate intravenous anesthetic agent, has protective effects on retinal ganglion cells within 7 days after optic nerve transection. Whether this process is related to anti-oxidative stress is not clear. To reveal its mechanism, we established the optic nerve transection injury model by transecting 1 mm behind the left eyeball of adult male Sprague-Dawley rats. The rats received an intraperitoneal injection of etomidate (4 mg/kg) once per day for 7 days. The results showed that etomidate significantly enhanced the number of retinal ganglion cells retrogradely labeled with Fluorogold at 7 days after optic nerve transection. Etomidate also significantly reduced the levels of nitric oxide and malonaldehyde in the retina and increased the level of glutathione at 12 hours after optic nerve transection. Thus, etomidate can protect retinal ganglion cells after optic nerve transection in adult rats by activating an anti-oxidative stress response. The study was approved by the Animal Ethics Committee at Air Force Medical University, China (approval No. 20180305) on March 5, 2018.
Chinese Library Classification No. R453; R362; R741
Introduction
Optic nerve (ON) injury can lead to the death of retinal ganglion cells (RGCs) and the loss of visual function (Vigneswara and Ahmed, 2019). Promoting the survival of RGCs is essential for repairing ON injury, and drug therapy is an effective method to protect injured RGCs. Etomidate (ET), an imidazole, non-barbiturate intravenous anesthetic agent, selectively acts on gamma-aminobutyric acid A receptors containing β2 and β3 subunits. It promotes the influx of chloride ions, thus serves as an anesthetic through hyperpolarization of the cell membrane. Recent reports indicated that neuroprotection by ET acted by attenuating the injury of hippocampal neurons in rats with forebrain ischemia (Watson et al., 1992) and damage to hippocampal neurons from kainic acid-induced injury (Lee et al., 2000). Immediate ET treatment after spinal cord injury can also promote the recovery of spinal cord function (Cayli et al., 2006). Our previous studies demonstrated that ET had protective effects on RGCs within 7 days after ON transection (ONT) (Xu et al., 2015) and that the protein kinase C/nuclear factor-kappa B signaling pathway may be involved in the protective mechanism of ET (Xu et al., 2015).
Apoptosis is the leading cause of RGC death caused by ON injury (Weishaupt et al., 2003; Cheng et al., 2018). There are several possible mechanisms underlying the onset of apoptosis in this context, oxidative damage being one of the most im-portant putative causes. Oxidative stress comprises the excessive production of reactive oxygen species (ROS), reactive nitrogen species and other similar reactive species. This process results in the untimely cleavage of oxides in the body when exposed to various harmful stimuli, leading to physiological and pathological responses such as cell and tissue damage. The ON is part of central nervous system that has a high lipid content. Moreover, the central nervous system exhibits a high metabolic rate because of its high oxygen consumption and a high level of adenosine triphosphate synthesis, and is therefore susceptible to oxidative stress (Tezel, 2006; Yamamoto et al., 2014). The death of injured RGCs is also closely related to oxidative stress. The survival of axotomized RGCs is sensitive to the reduction-oxidation state and reduced formation of ROS can promote the RGC survival after ONT (Geiger et al., 2002). Superoxide content in RGCs increases significantly 24 hours after ONT and the intravitreal injection of superoxide dismutase could block the formation of superoxide in RGCs, resulting in delayed RGC death (Kanamori et al., 2010). The above findings prompted the purpose of the present study to investigate any possible relationship between the protective effect of ET on injured RGCs and the anti-oxidative stress response.
Materials and Methods
Animals
Two hundred and thirty-four specific-pathogen-free, adult male Sprague-Dawley rats, aged 8–10 weeks, weighing 200–250 g, were obtained from the Laboratory Animal Center of Air Force Medical University, China [license No. SCXK (Shann) 2014-002]. The rats were housed in a temperature- and humidity-controlled room with a 12-hour light: 12-hour dark cycle. Food and water were freely available. This study was performed in accordance with the National Institutes of Health (USA) guidelines for care and use of laboratory animals (NIH Publication No. 85-23, revised 1996). The Animal Ethics Committee at Air Force Medical University approved all animal protocols (approval No. 20180305) on March 5, 2018.
Labeling and counting of retinal ganglion cells
Eighteen rats were randomly divided into normal control, ONT and ET groups (ONT + ET) (n = 6 for each group). All animals were anesthetized with an intraperitoneal injection of 1% pentobarbital sodium (Sigma-Aldrich, St. Louis, MO, USA) and the intraorbital segment of the ON was exposed under an operating microscope behind the left eyeball of each animal. The dorsal sheath of the ON was then cut longitudinally along the long axis of the ON, and the ON was transected 1 mm from the eyeball, without any injury to the central retinal artery located in the ventral sheath. A small piece of gelfoam impregnated with 5% FluoroGold (Fluorochrome LLC., Denver, CO, USA) was placed onto the orbital end of transected ON to achieve retrograde labeling of RGCs. The rats in the normal control group were allowed to survive for 2 days without any intervention. The animals in the ONT and ET groups were injected intraperitoneally with either normal saline (2 mL/kg) or ET (4 mg/kg; ET injectable emulsion, license No. H20020511, Jiangsu Nhwa Pharmaceutical Co. Ltd., Xuzhou, Jiangsu Province, China), respectively, immediately after ONT. The injections were repeated once per day for 7 days. All the animals were killed with an overdose of pentobarbital sodium at the appropriate time after ONT. The left eyeballs were immediately isolated and immersed in 4% paraformaldehyde solution. The retinas were then removed and divided into four quadrants, supra-temporal, infra-temporal, supra-nasal and infra-nasal, of similar size. After postfixation in 4% paraformaldehyde solution for 1 hour, each retina was rinsed three times (5 minutes per wash) with phosphate-buffered saline (0.01 M, pH 7.4). The retinas were then placed on glass slides, sealed with 50% glycerol and observed with an eyepiece grid of 500 µm × 500 µm at 500-µm intervals under a BX51 fluorescence microscope (Olympus Corporation, Tokyo, Japan) using a 355–425 nm ultraviolet filter. The sample area was defined by an ocular grid (0.5 mm × 0.5 mm) and respective sample regions were selected from 1, 2, and 3 mm to the edge of the optic disk along the center line of each quadrant. Using the ultraviolet excitation mode, the FluoroGold-labeled surviving RGCs in each sample region were counted and the average density of the surviving RGCs was calculated.
Detection of biochemical indicators related to oxidative stress injury
The other 216 rats were randomly divided into normal control, ONT and ET groups (n = 72 for each group). Immediately after the rats were killed using an overdose of pentobarbital sodium, the left retinas of rats in the normal control group (n = 72) were removed and stored at −80°C. Some of the rats in the ONT group (n = 54) and ET group (n = 54) received an intraperitoneal injection of normal saline (2 mL/kg) or ET (4 mg/kg) immediately after the left intraorbital ONT and survived for 6 (n = 18 per group), 12 (n = 18 per group), and 24 hours (n = 18 per group), respectively. The remaining animals in the ONT (n = 18) and ET (n = 18) groups received repeated injections of the same amount of saline or ET once per day until they were sacrificed on postoperative day 7. The left retinas were isolated and stored at −80°C. The levels of nitric oxide (NO; Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China), malonaldehyde (MDA; Wanleibio, Shenyang, Liaoning Province, China) and glutathione (GSH; Wanleibio) in the retinas of rats in each group were determined in accordance with the instructions of the respective measurement kits.
Statistical analysis
The mean densities of RGCs and the levels of NO, MDA and GSH in the retinas were statistically analyzed with SPSS 13.0 software (SPSS Inc., Chicago, IL, USA) using one-way analysis of variance and the differences between groups were compared using Tukey’s test. Data were expressed as the mean ± SD. P < 0.05 was considered to indicate significant differences.
Results
Etomidate increases the retinal ganglion cell densities in rats with injured optic nerves
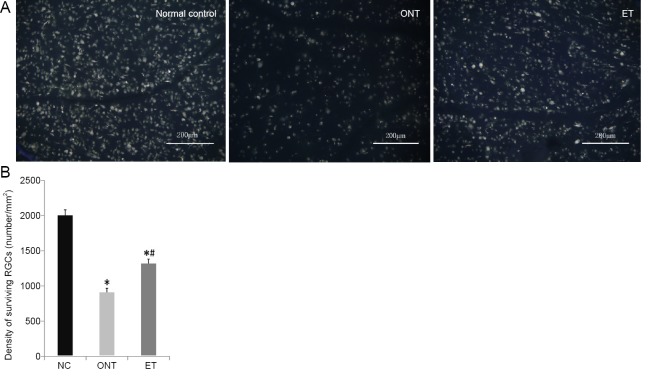
FluoroGold-labeled RGCs were observed in the retinas of rats in the normal control, ONT and ET groups. Most RGCs were oval and some were polygonal in shape. The fluorescent substances were bright and evenly distributed in these RGCs (Figure 1A). The mean densities of surviving RGCs in the ONT and ET groups decreased significantly in comparison with the normal control group at 7 days after ONT (P < 0.05). However, the RGC density in the ET group was significantly higher than that in the ONT group (P < 0.05, Figure 1B).
Figure 1.
Etomidate promots retinal ganglion cells (RGCs) survival at 7 days after optic nerve transection.
(A) Surviving RGCs with FluoroGold-labeled. Compared with the normal control (NC) group, surviving RGCs of the ONT group were decreased. Etomidate significantly reduced the loss of RGCs. Scale bars: 200 µm. (B) Quantitative result of surviving RGCs densities. There were significantly more surviving RGCs in the ET group than in the ONT group; however, the average density of RGCs in both groups was significantly lower than that in the normal control group. The data are expressed as the mean ± SD (n = 6 per group). *P < 0.05, vs. normal control group; #P < 0.05, vs. ONT group (one-way analysis of variance followed by Tukey’s test). ONT: Optic nerve transection; ET: ONT + etomidate.
Etomidate affects biochemical indexes related to oxidative stress in rats with injured optic nerves
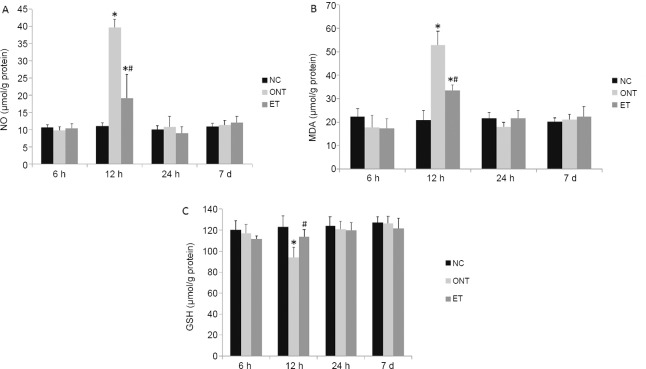
Compared with the normal control group, the NO and MDA levels were significantly higher (P < 0.05), and the GSH level in ONT group significantly lower at 12 hours after ONT (P < 0.05). ET markedly reduced the changes in NO, MDA and GSH levels when compared with the ONT group at 12 hours after ONT (P < 0.05). No significant differences in the NO, MDA and GSH levels could be detected among the three groups at 6, 24 hours and 7 days after ONT (P >0.05; Figure 2).
Figure 2.
Etomidate affects the retinal NO (A), MDA (B), and GSH (C) levels in rats with optic nerve transection.
The data are expressed as the mean ± SD (n = 6 per group). *P < 0.05, vs. normal control (NC) group; #P < 0.05, vs. ONT group (one-way analysis of variance followed by Tukey’s test). NO: Nitric oxide; MDA: malonaldehyde; GSH: glutathione; ONT: optic nerve transection; ET: ONT + etomidate; h: hours; d: days.
Discussion
ONT is a commonly used model for ON injury (Rovere et al., 2019). There was no significant reduction in the number of surviving RGCs in the retina up to 3 days after intraorbital ONT. Therefore, the number of RGCs at 2 days after intraorbital ONT was used as a normal control. The number of RGCs significantly decreased to about 50% of the normal amount at 7 days after ONT and only about 10% of the RGCs had survived by day 14 post-injury (Cheung et al., 2004). In this study, the mean RGC density at 7 days after ONT in the ONT group was 47% of that in the normal control group, similar to the RGC density observed in previous studies. Successive injections of ET for 7 days after ONT protected the RGCs showing a density in ET group to 66% of that in normal control group, a significantly higher RGC density than that observed in the ONT group. These results showed that ET had a significant protective effect on axotomized RGCs at 7 days after ONT in adult rats.
ROS are normal by-products of cell metabolism. However, harmful stimulation can cause an excessive production of ROS in the body, resulting in cell damage when the intrinsic antioxidant capacity is overwhelmed. Such a state of damage is known as oxidative stress. Control of ROS has been shown to reduce apoptosis of RGCs after ONT (Geiger et al., 2002) Excessive ROS production can directly damage proteins, DNA and lipids in cells, leading to apoptosis of RGCs in our ONT model. ROS can also enhance the formation of advanced glycation end products and induce apoptosis through direct cytotoxicity or receptor-mediated signaling pathways (Tezel et al., 2007). RGC apoptosis is induced by ROS-induced protein oxidative modification promoting secretion of tumor necrosis factor-α by glial cells (Tezel and Yang, 2004). ROS induce RGC apoptosis by upregulating major histocompatibility complex-II molecules in glial cells, promote T-cell activation and increase tumor necrosis factor-α secretion by T cells, suggesting that regulation of the immune response by ROS can also affect the RGC survival (Tezel and Wax, 2004).
Reactive nitrogen species are chemically reactive substances derived from NO and superoxides. NO is a type of reactive nitrogen species and retinal NO is primarily produced by astrocytes. Under pathological conditions, a large quantity of superoxide anions (O2 –) accumulate in mitochondria, resulting in the formation of a strong free radical peroxynitrite (ONOO–) which, together with NO, can directly induce RGC apoptosis. NO, O2 – and ONOO– occur together and reinforce each other, forming a vicious cycle that propagates RGC damage (Sato et al., 2010).
MDA is an end metabolite of free radical lipid peroxidation and a marker of lipid peroxides. Determination of MDA levels can enable assessment of the degree of lipid peroxidation in the body and indirectly indicate the degree of free radical damage to tissues and cells (Tsikas, 2017). GSH is an important endogenous antioxidant, which can react with O2 –, •OH and O2 to protect cells from ROS damage (He et al., 2017). Therefore, we measured the levels of retinal NO, MDA and GSH in this study to determine the degree of oxidative stress after ONT and the effect of ET on oxidative stress. Our results demonstrated that the retinal tissues contained similar minimal NO and MDA in normal control and ONT groups at 6 hours after ONT. At 12 hours, however, the levels of NO and MDA in the ONT group had significantly increased, 4- and 2.5-fold higher than those in normal control group. The high levels of ON and MDA later dropped until they were similar to those in the normal control group at 24 hours and 7 days after ONT. The GSH level in the ONT group decreased significantly to 76% of that in the normal control group at 12 hours, but had returned to the level of the normal control group at 24 hours and 7 days. These results suggest that oxidative stress exists transiently for only 12 hours after ONT.
An intraperitoneal injection of ET immediately after ONT significantly inhibited oxidative stress within 12 hours after injury. The NO and MDA levels in the ET group were significantly lower at 48% and 63%, respectively, and the GSH level in the ET group was significantly higher (similar to that in the normal control group) than those observed in the ONT group 12 hours after ONT. However, repeated injections of ET had no further impact on the levels of retinal NO, MDA and GSH because these had returned to the normal control levels at 24 hours and 7 days after ONT. Similar previous findings have also revealed that the neuroprotective effect of ET is related to anti-oxidative stress responses: Streptomycin was used to induce hyperglycemia in rats, resulting in oxidative stress in the central nervous system. After successive intraperitoneal ET injections for 6 weeks, the levels of MDA and total nitrite were reduced, whereas the GSH level was increased, in the cerebral cortex, hippocampus, cerebellum, brainstem and spinal cord of hyperglycemic rats (Ates et al., 2006). Immediate intraperitoneal ET injection after spinal cord injury in rats could reduce the intra-spinal NO and MDA levels and increase the GSH level 24 hours after injury (Cayli et al., 2006). Because the peaked oxidative stress and the anti-oxidative stress effect of ET were only detected at 12 hours after ONT, the time window within which ET can provide a neuroprotective effect is rather short. Therefore, immediate ET administration is necessary after ON injury. In conclusion, our study provides an experimental basis for ET to treat ON injury. However, the mechanism of ET action in anti-oxidative stress remains unclear and requires further investigation.
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81670846 (to MMW) and 81470631 (to SWY); the Natural Science Foundation of Shaanxi Province of China, No. 2016SF-171 (to MMW), and the National Basic Research Program of China, No. 2014CB542202 (to SWY). The funders had no involvement in the study design, data collection and analysis; paper writing; the decision to publish.
Institutional review board statement: The study was approved by the Animal Ethics Committee at Air Force Medical University, China (approval No. 20180305) on March 5, 2018.
Copyright license agreement: The Copyright License Agreement hasbeen signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Steven Levy, MD Stem Cells, USA.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81670846 (to MMW) and 81470631 (to SWY); the Natural Science Foundation of Shaanxi Province of China, No. 2016SF-171 (to MMW), and the National Basic Research Program of China, No. 2014CB542202 (to SWY).
P-Reviewer: Levy S; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Ates O, Yucel N, Cayli SR, Altinoz E, Yologlu S, Kocak A, Cakir CO, Turkoz Y. Neuroprotective effect of etomidate in the central nervous system of streptozotocin-induced diabetic rats. Neurochem Res. 2006;31:777–783. doi: 10.1007/s11064-006-9076-0. [DOI] [PubMed] [Google Scholar]
- 2.Cayli SR, Ates O, Karadag N, Altinoz E, Yucel N, Yologlu S, Kocak A, Cakir CO. Neuroprotective effect of etomidate on functional recovery in experimental spinal cord injury. Int J Dev Neurosci. 2006;24:233–239. doi: 10.1016/j.ijdevneu.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Cheng HH, Ye H, Peng RP, Deng J, Ding Y. Inhibition of retinal ganglion cell apoptosis: regulation of mitochondrial function by PACAP. Neural Regen Res. 2018;13:923–929. doi: 10.4103/1673-5374.232489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung ZH, Chan YM, Siu FK, Yip HK, Wu W, Leung MC, So KF. Regulation of caspase activation in axotomized retinal ganglion cells. Mol Cell Neurosci. 2004;25:383–393. doi: 10.1016/j.mcn.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Geiger LK, Kortuem KR, Alexejun C, Levin LA. Reduced redox state allows prolonged survival of axotomized neonatal retinal ganglion cells. Neuroscience. 2002;109:635–642. doi: 10.1016/s0306-4522(01)00493-6. [DOI] [PubMed] [Google Scholar]
- 6.He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 7.Kanamori A, Catrinescu MM, Kanamori N, Mears KA, Beaubien R, Levin LA. Superoxide is an associated signal for apoptosis in axonal injury. Brain. 2010;133:2612–2625. doi: 10.1093/brain/awq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Kim D, Hong H, Han S, Kim J. Protective effect of etomidate on kainic acid-induced neurotoxicity in rat hippocampus. Neurosci Lett. 2000;286:179–182. doi: 10.1016/s0304-3940(00)01118-6. [DOI] [PubMed] [Google Scholar]
- 9.Rovere G, Nadal-Nicolás FM, Sobrado-Calvo P, Villegas-Pérez MP, Vidal-Sanz M, Agudo-Barriuso M. Topical bromfenac transiently delays axotomy-induced retinal ganglion cell loss. Exp Eye Res. 2019;182:156–159. doi: 10.1016/j.exer.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Sato T, Oku H, Tsuruma K, Katsumura K, Shimazawa M, Hara H, Sugiyama T, Ikeda T. Effect of hypoxia on susceptibility of RGC-5 cells to nitric oxide. Invest Ophthalmol Vis Sci. 2010;51:2575–2586. doi: 10.1167/iovs.09-4303. [DOI] [PubMed] [Google Scholar]
- 11.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tezel G, Yang X. Caspase-independent component of retinal ganglion cell death, in vitro. Invest Ophthalmol Vis Sci. 2004;45:4049–4059. doi: 10.1167/iovs.04-0490. [DOI] [PubMed] [Google Scholar]
- 13.Tezel G, Wax MB. The immune system and glaucoma. Curr Opin Ophthalmol. 2004;15:80–84. doi: 10.1097/00055735-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Tezel G, Luo C, Yang X. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest Ophthalmol Vis Sci. 2007;48:1201–1211. doi: 10.1167/iovs.06-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Vigneswara V, Ahmed Z. Pigment epithelium-derived factor mediates retinal ganglion cell neuroprotection by suppression of caspase-2. Cell Death Dis. 2019;10:102. doi: 10.1038/s41419-019-1379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson JC, Drummond JC, Patel PM, Sano T, Akrawi W, U HS. An assessment of the cerebral protective effects of etomidate in a model of incomplete forebrain ischemia in the rat. Neurosurgery. 1992;30:540–544. doi: 10.1227/00006123-199204000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Weishaupt JH, Diem R, Kermer P, Krajewski S, Reed JC, Bähr M. Contribution of caspase-8 to apoptosis of axotomized rat retinal ganglion cells in vivo. Neurobiol Dis. 2003;13:124–135. doi: 10.1016/s0969-9961(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 19.Xu ZX, Hu JM, Xu GZ, Ma LT. Etomidate promoting retinal ganglion cells survival after optic nerve transaction in adult rats via inhibition of PKC/NF-κB signaling. Yanke Xin Jinzhan. 2015;35:1121–1125. [Google Scholar]
- 20.Yamamoto K, Maruyama K, Himori N, Omodaka K, Yokoyama Y, Shiga Y, Morin R, Nakazawa T. The novel Rho kinase (ROCK) inhibitor K-115: a new candidate drug for neuroprotective treatment in glaucoma. Invest Ophthalmol Vis Sci. 2014;55:7126–7136. doi: 10.1167/iovs.13-13842. [DOI] [PubMed] [Google Scholar]