Abstract
Neuropathic pain after spinal cord injury (SCI) is a complex condition that responds poorly to usual treatments. Cell transplantation represents a promising therapy; nevertheless, the ideal cell type in terms of neurogenic potential and effectiveness against pain remains largely controversial. Here, we evaluated the ability of fetal neural stem cells (fNSC) to relieve chronic pain and, secondarily, their effects on motor recovery. Adult Wistar rats with traumatic SCI were treated, 10 days after injury, with intra-spinal injections of culture medium (sham) or fNSCs extracted from telencephalic vesicles (TV group) or the ventral medulla (VM group) of E/14 embryos. Sensory (von Frey filaments and hot plate) and motor (the Basso, Beattie, Bresnahan locomotor rating scale and inclined plane test) assessments were performed during 8 weeks. Thereafter, spinal cords were processed for immunofluorescence and transplanted cells were quantified by stereology. The results showed improvement of thermal hyperalgesia in the TV and VM groups at 4 and 5 weeks after transplantation, respectively. Moreover, mechanical allodynia improved in both the TV and VM groups at 8 weeks. No significant motor recovery was observed in the TV or VM groups compared with sham. Stereological analyses showed that ~70% of TV and VM cells differentiated into NeuN+ neurons, with a high proportion of enkephalinergic and GABAergic cells in the TV group and enkephalinergic and serotoninergic cells in the VM group. Our study suggests that neuronal precursors from TV and VM, once implanted into the injured spinal cord, maturate into different neuronal subtypes, mainly GABAergic, serotoninergic, and enkephalinergic, and all subtypes alleviate pain, despite no significant motor recovery. The study was approved by the Animal Ethics Committee of the Medical School of the University of São Paulo (protocol number 033/14) on March 4, 2016.
Keywords: spinal cord injuries, chronic pain, neural stem cells, cell transplantation, neuronal differentiation, GABAergic neuron, serotoninergic neuron, enkephalinergic neuron
Chinese Library Classification No. R456; R364; R741
Introduction
Spinal cord injury (SCI) is a devastating neurological disease and the greatest cause of long-term physical impairment (Kumar et al., 2018). Together with motor deficits, pain is one of the most common and disabling complications experienced by individuals with SCI (Andresen et al., 2016; Muller et al., 2017; Burke et al., 2018). About 94% of patients with SCI report some kind of pain following injury, and 70% of these evolve to chronic pain (Teixeira et al., 2013). Chronic pain can be classified as nociceptive, inflammatory, dysfunctional or neuropathic (Vardeh et al., 2016). The term “neuropathic pain” refers to pain caused by a lesion or disease of the somatosensory nervous system (IASP, 2012), and is characterized by spontaneous pain, allodynia and hyperalgesia (Gilron et al., 2015; Nagoshi et al., 2015; Boadas-Vaello et al., 2016; Kramer et al., 2017).
The main cause of neuropathic pain after SCI is the hyperexcitability of wide range neurons located in the dorsal horn of the spinal cord (Kramer et al., 2017; Gwak et al., 2017). The mechanisms involved in this process include structural plasticity (e.g., dendritic spine remodeling), glutamatergic dysregulation (release, uptake and receptor expression), loss of local inhibitory tone (GABAergic), dysfunctions of descending modulatory pathways (especially serotonergic), and increased calcium channel expression, among others (Meisner et al., 2010; Boroujerdi et al., 2011; Putatunda et al., 2014; Falnikar et al., 2016; Zhao et al., 2016; Gwak et al., 2017).
Several pharmacological and non-pharmacological treatments are available, including antidepressants, antiepileptic, analgesics, intrathecal pump implantation, dorsal root entry zone lesion, spinal cord stimulation and deep brain stimulation (Baastrup and Finnerup, 2008; Previnaire et al., 2009; Ruiz-Juretschke et al., 2011; Mehta et al., 2015; Yaksh et al., 2017; Hatch et al., 2018). Nonetheless, proper treatment of neuropathic pain after SCI remains a challenge, highlighting the need for new strategies to improve the prognosis and quality of life of these patients.
While cell transplantation represents a promising option, which cell types offer the best neurogenic potential and analgesic effectiveness remains largely controversial. In previous studies with fetal neural stem cells (fNSCs) extracted from telencephalic vesicles of E14 rat embryos, our group showed neuronal differentiation in vitro even after long periods (Maciaczyk et al., 2008; Maciaczyk et al., 2009); moreover, about 60% of the mature neuronal cells obtained by in vitro differentiation presented a GABAergic phenotype in immunocytochemical stainings (Maciaczyk et al., 2008). Additionally, all immature neuroblasts in vitro presented GABAergic synaptic currents in patch clamp recordings (Lepski et al., 2013). Thus, we hypothesized that GABAergic precursor cells would eventually cause post-synaptic inhibitory potentials and consequently reverse the hyperexcitability established in the dorsal horn of the injured spinal cord.
In addition, it is known that the descending inhibitory pathways from the nucleus raphe magnus and locus coeruleus reach the dorsal horn of the spinal cord and inhibit pain transmission, as part of the pain suppressor system (Basbaum and Fields, 1978; Terman et al., 1984; Morgan et al., 1992). Thus, based on region-specific phenotype determinism (Fuentealba et al., 2015) we hypothesized that fNSCs from ventral medulla would have better analgesic effects than fNSCs harvested from other brain regions.
The purpose of this study was to evaluate the ability of transplanted fNSCs derived from telencephalic vesicles and ventral medulla to relieve chronic pain in a traumatic SCI model.
Materials and Methods
Neural progenitor/stem cell isolation and culture
All surgical procedures and handling methods with the animals were previously approved by the Animal Ethics Committee of the Medical School of the University of São Paulo (protocol number 033/14) on March 4, 2016. NSCs were isolated from E14 (gestational age: 14 days postconceptional) Wistar rat embryos. In brief, 4 time-pregnant animals were euthanized by intraperitoneal lethal injection of ketamine (200 mg/kg; Cristália Ltda) and xylazine (20 mg/kg; Syntec). After the abdominal cavity was opened and the uterus was removed, the embryos were extracted and the neural tissue was isolated from its wraps. Cells were extracted from two different regions, the telencephalic vesicles and the ventral medulla. The tissues isolated were incubated with 0.1% trypsin (Worthington, Lakewood, NJ, USA) for 12 minutes at 37°C followed by 0.05% DNase (Worthington) for 12 minutes at 37°C. Cells were plated in a proliferation medium consisting of Dulbecco’s modified eagle medium and Ham’s F12 supplement (DMEM/F12, 3:1, Sigma-Aldrich, Brazil), penicillin G/streptomycin and amphotericin (PSA 1%, Gibco), B27 supplement (2%, Gibco), basic fibroblast growth factor (bFGF, 20 ng/mL, Sigma-Aldrich), epithelial growth factor (EGF, 20 ng/mL, Sigma-Aldrich) and heparin (5 μg/mL, Sigma-Aldrich). Cells were incubated at 37°C, 5% CO2, and 20% O2. The medium was changed every 2 days and passages were performed once a week by light mechanical dissociation of the formed spheres (Lepski et al., 2011a).
BrdU labeling
5-Bromo 3′-deoxyuridine (BrdU, 10 μM; Sigma-Aldrich) was added to the culture medium after three passages and maintained for 48 hour before the transplantation sessions.
Surgical procedure
Traumatic spinal cord injury
Forty-five male Wistar rats from the University of São Paulo School of Medicine, weighing 250 to 300 g, were subjected to intraperitoneal anesthesia with ketamine (100 mg/kg) and xylazine (10 mg/kg). After hair removal and antisepsis of the surgical area, a median longitudinal skin incision was performed at levels T7–12; then, the paravertebral musculature was carefully separated from the vertebra laminae. Laminectomy was performed at levels T9 and T10, exposing the spinal cord and the intact dura mater. The SCI was applied using NYU Impactor equipment (New York University, New York, NY, USA). Animals were fixed by the upper and lower vertebrae and a moderate injury was inflicted by dropping a weight of 10 g from a 25-mm height (Gruner, 1992). A group of control animals did not undergo surgery. During the surgical procedure, saline was replaced intraperitoneally to avoid hemodynamic instability. The ideal weight to cause sustained pain throughout the experiment was determined in a pilot study (Batista et al., 2019).
At the end of the procedure, the muscle planes, subcutaneous tissue and skin were sutured in multiple layers. Immediately post-operatively, animals were warmed under bright light. The animals were housed in a temperature-controlled environment under a 12-hour light-dark cycle, with access to food and water ad libitum. All animals received subcutaneous injection of antibiotic (Flotril, 1 mg/kg) during 3 days and dipyrone (1 mg/kg) during 5 days and bladder emptying was performed twice daily until the return of function.
Immunosuppression
All animals (including controls) were immunosuppressed, starting 7 days after SCI (3 days before cell transplantation), by daily intraperitoneal injections of cyclosporine A (Sandimmun, 10 mg/kg, Novartis Pharma, Stein, Sweden) (Vanecek et al., 2012). Immunosuppression was combined with prophylactic oral administration of antibiotics (sulfamethoxazole/trimethoprim, Teuto, Anápolis, Brazil) in the drinking water.
Cell transplantation
Ten days after SCI, the animals had their spinal cord re-exposed for transplantation, which was performed by stereotactic injection of the culture medium (DMEM/F12) or fNSCs. Prior to transplantation, the expanded cells were detached from the culture flask with Trypsin-EDTA (trypsin-ethylenediamine tetraacetic acid solution, Sigma Aldrich) for 2 minutes, and then mechanically dissociated by using a 1 mL pipette to obtain a single cell suspension. Viability and cell number were controlled by trypan blue staining and counting. To minimize the trauma associated with stereotactic implantation of the cell solution, the intra-spinal injections were made with glass micropipettes (~80 μm of external diameter) obtained by a pipette puller (P-97 Flaming/Brown micropipette puller, Sutter Instrument, Novato, CA, USA). The micropipettes were coupled to a 25 μL Hamilton syringe, which in turn was connected to the infusion pump attached to the arm of the stereotaxic device. The animals received 12 μL of cells in suspension (5 × 104 cells/μL), divided into four spinal cord regions, two injections at the lesion site (bilaterally) and two injections ~3 mm above (bilaterally; Figure 1). The injection speed was 1 μL/min and after the volume injection (3 μL/point), the micropipette remained in the spinal cord for another 20 seconds before being withdrawn. The viability of telencephalic vesicle cells was ~90.36% and that of ventral medulla cells was ~74%.
Figure 1.
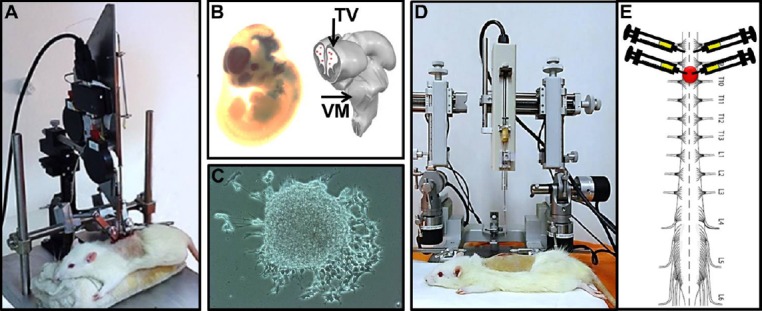
Representative picture of fetal neural stem cells (fNSC) transplantation after spinal cord injury.
(A) Animal positioned in the NYU Impactor to promote traumatic spinal cord injury at level T9–10. (B) fNSC extraction from E14 embryos. (C) fNSC culture and neurosphere formation after 1 week of extraction. (D) Animal positioned in the stereotaxic device to receive fNSC injection from telencephalic vesicles (TV) or ventral medulla (VM). Note that the infusion pump is attached to the movable arm of the stereotaxic device. (E) Representation of the injury site and the location where the transplants were performed (3 μL/point, concentration 1 × 104 cells/μL).
Experimental groups
Rats were randomized into four groups: control group (without SCI, n = 14), sham group (traumatic SCI and transplantation of culture medium [DMEM/F12], n = 11); TV group (traumatic SCI and transplantation of fNSCs from telencephalic vesicles, n = 12), and VM group (traumatic SCI and transplantation of fNSCs from ventral medulla, n = 12).
Due to weakness caused by immunosuppression, some animals died during the surgical cell transplantation procedure. Thus, the number of animals per lesion group ranged from 11 to 12 (instead of 15). This complication was computed initially and considered in the sample size calculation of the proposed study.
Vegetative function/bladder recovery index
The bladder recovery index was calculated by conducting daily latero-lateral measurements of the bladder before emptying (in the morning period). The size of the bladder indicates the percentage index of recovery: 2 cm = 0, 1.5 cm = 25%, 1.0 cm = 50%, 0.5 cm = 75% and 0 cm = 100% of recovery (Batista et al., 2019).
Mechanical allodynia/von Frey filaments
All behavioral analyses were performed 1 day before SCI, 1 day before fNSC transplantation and then weekly until the end of 8 weeks. Mechanical allodynia was assessed using von Frey filaments (Chaplan et al., 1994). For this, the rats were placed in a plastic cage with a wire mesh grid which allowed full access to the paws from below. The animals were kept for approximately 15 minutes in the cage before the experiment started, to ensure better adaptation. The forepaws and hind paws were tested. Each paw was touched with a series of 8 von Frey filaments (0.41–15.10 g). The von Frey filament was introduced perpendicular to the plantar surface with sufficient force to cause a slight deformation against the paw and held for 6–8 seconds. Stimuli were performed at random intervals lasting a few seconds. Paw withdrawal (or attempted withdrawal) after the stimulus and flinching immediately upon filament removal were considered positive responses.
Hot plate test
In the hot plate test, rats were placed on a metal surface kept at 50 ± 0.5°C. Latency to response, such as lifting or licking a hind paw, jumping, or vocalization, was recorded. A 30-second cut-off was used to minimize tissue damage. Two measurements were performed per animal with a 30-minute interval between them (Dale et al., 2006).
Motricity/Basso, Beattie, Bresnahan (BBB) locomotor rating scale
Locomotor evaluation was measured with the BBB scale (Basso et al., 1996), whose scores represent the sequential stages of recovery after SCI. The scale ranges from 0 to 21, where 0 represents no spontaneous movement of the hind paws and a score of 21 indicates normal locomotion.
Inclined plane
Muscle strength and endurance was evaluated using the inclined plane test. For this test, animals are placed face down on an adjustable inclined plane and the angle is increased from 0° to the point where the rat cannot maintain its position for 5 seconds. Two measurements were performed for each rat (Rivlin and Tator, 1977).
Immunofluorescence staining
After 8 weeks, the animals were euthanized by intraperitoneal lethal injection of ketamine (200 mg/kg), followed by transcardiac perfusion. Their spinal cords were extracted and a 2 cm segment, including the entire area of lesion (1.0 cm above and below the lesion epicenter), was frozen using isopentane and dry ice. Serial 60 μm sections were obtained from all spinal cord segments using a cryostat (CM3000, Leica, Nussloch, Germany).
The staining procedure was performed on free-floating sections (Lepski et al., 2010). In brief, the sections were washed many times with Tween 0.05% diluted in PBS and incubated in a blocking solution containing 5% of goat serum and 0.3% of Triton X-100 diluted in PBS during one hour at room temperature over a shaker. Next, the sections were incubated with the primary antibodies anti-neuronal nuclei (NeuN, a neuronal marker) (monoclonal, mouse, 1:1000, Millipore, Temecula, CA, USA), anti-glial fibrillary acidic protein antibody (GFAP, an astrocyte marker) (polyclonal, rabbit, 1:500, Millipore), anti-oligodendrocyte (monoclonal, mouse, 1:500, Millipore), anti-gamma aminobutyric acid (GABA) (polyclonal, rabbit, 1:300, Sigma, St. Louis, MO, USA), anti-serotonin (5HT) (polyclonal, rabbit, 1:500, Millipore) and anti-enkephalin (monoclonal, mouse, 1:100, Millipore), doubly labeled with anti-BrdU (polyclonal, rabbit, 1:100, Abcam, Cambridge, MA, USA; or monoclonal, mouse, 1:800, Millipore), diluted in blocking solution and maintained at 4°C for 24 hours. After this period, the sections were rinsed again in PBS-Tween and incubated for a further 2 hours at room temperature with anti-mouse and anti-rabbit Alexa Fluor 488 and Alexa Fluor 594 (Abcam) and DAPI (1:10,000, Sigma) diluted in 0.3% TritonX-100 in PBS. After another series of washes, the coverslips were mounted on slides using fluorescent mounting medium (Dako, Glostrup, Denmark) to preserve immunofluorescence.
Stereological quantification
Transplanted cell counts were performed in all sections above, below and in the lesion area, using a Zeiss Axio Imager A2 microscope (Carl Zeiss, Gottingen, Germany) and the Zen blue software (Carl Zeiss). The total number of positive BrdU cells was estimated using Abercrombie’s correction formula, as follows: P = M/(D + M)A × N, where P = total cell number, M = section thickness, D = average diameter of the positive cells, A = number of counted cells, and N = number of cut series (Furlanetti et al., 2015).
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad software, Inc, San Diego, CA, USA). We first measured means, medians, standard deviations and standard error. Mean profile graphs are shown to illustrate the differences among groups, and the bars represent the standard error, highlighting the accuracy of the measurements. Since Shapiro-Wilk and D’Agostino-Pearson tests showed that the data did not obey a normal distribution, we used the Mann-Whitney U test for two sample comparisons and the Kruskal-Wallis test followed by Dunn’s post hoc test to compare among three or more samples. Significance was set at P equal to or less than 0.05.
Results
Bladder function
Animals showed spontaneous bladder function recovery over time, with full recovery 2 weeks after surgery. No differences were observed between either of the treated groups and the sham group.
Mechanical allodynia and thermal hyperalgesia
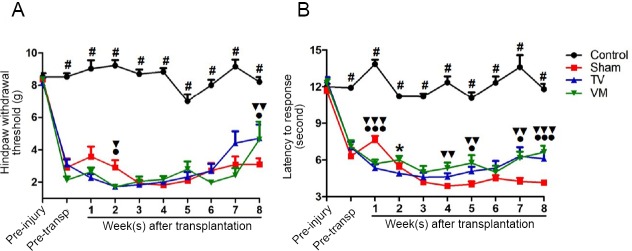
The evaluation of mechanical allodynia in the forepaws by von Frey filaments showed no difference between experimental groups. On the other hand, mechanical sensitivity of the hind paws was greater in all SCI groups relative to controls (Figure 2A), which was measured as a drop of 75% in the threshold at 1 week after injury (P < 0.001 for all groups against controls), and persisted throughout the experiment. However, TV and VM groups showed an improvement relative to sham (P < 0.05 and P < 0.01) at 8 weeks. In terms of thermal hyperalgesia measured with the hot plate test (Figure 2B), an increase in thermal sensitivity was observed in all SCI groups at the magnitude of 50% of reduction in the sensory threshold at 1 week after injury, which persisted throughout the experiment (P < 0.001 for all groups against control); however, from 4 weeks after transplantation, the TV and VM groups started to differ from sham (P < 0.05, TV vs. sham at 5 and 7 weeks, and P < 0.01, VM vs. sham at 4, 5 and 7 weeks) and the maximal difference was found at 8 weeks (P < 0.001, TV and VM groups vs. sham).
Figure 2.
Improvement of mechanical allodynia and thermal hyperalgesia in SCI rats after fetal neural stem cell transplantation.
(A) An increase in the threshold for von Frey filament stimulation in the hind paws was observed in the animals of the telencephalic vesicles (TV) group (●P < 0.05, Mann-Whitney U test) and ventral medulla (VM) group (▼▼P < 0.01, Mann-Whitney U test) compared with the sham at 8 weeks. (B) An improvement of thermal hyperalgesia in the hot plate test was observed at 5, 7 and 8 weeks in the TV group (●P < 0.05, ●●●P < 0.001, Mann-Whitney U test) and at 4, 5, 7 and 8 weeks in the VM group (▼▼P < 0.01, ▼▼▼P < 0.001, Mann-Whitney U test) compared with sham. #P < 0.05, control vs. sham, TV and VM groups (Kruskal-Wallis test followed by Dunn’s post hoc test). Error bars represent SEM.
Motor function
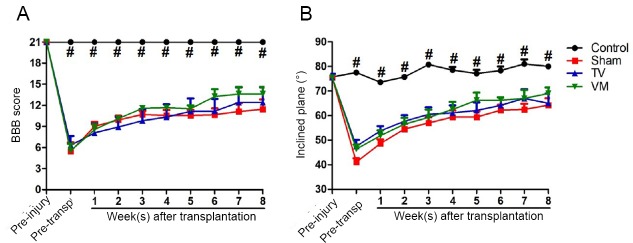
The BBB scale showed that 1 week after SCI, all animals presented severe motor deficits (approximately 26% of normal function in the sham and VM groups and 30% in the TV group). During the evaluation period, a slight spontaneous improvement was observed in all the SCI groups, with a significant difference at 8 weeks compared to 1 week after the injury (approximately 54% of normal function in the sham group, P < 0.05; 59% in TV group, P < 0.01; and 65% in VM group, P < 0.01). However, no significant differences were observed between the treated groups and sham group (Figure 3A). Similarly, the inclined plane test showed a significant loss of strength and muscular endurance in animals with traumatic SCI at 1 week after injury (approximately 54% of normal function in the sham group, 63% in the TV group and 61% in the VM group) with a partial recovery over the assessed period and an improvement in the eighth week compared to 1 week after injury (approximately 85% of normal function in the sham group, P < 0.001; 86% in the TV group, P < 0.05; and 91% in the VM group, P < 0.01), with no differences between the treated groups and the sham group (Figure 3B).
Figure 3.
Motor functions in spinal cord injury rats after fetal neural stem cell transplantation.
Motor function was assessed using the Basso, Beattie, Bresnahan (BBB) locomotor rating scale (A) and inclined plane test (B). In both tests, there was a small spontaneous motor recovery after spinal cord injury. However, no statistical differences were observed between the treated and sham groups. #P < 0.05, control vs. sham, telencephalic vesicles (TV) or the ventral medulla (VM) groups (Kruskal-Wallis test followed by Dunn’s post hoc test). Error bars represent SEM.
Stereological quantification
Stereological quantification showed that 69% of TV cells and 72% of VM cells differentiated into mature neurons, positively stained for NeuN. Moreover, a lower percentage of cells were immune positive to GFAP (9.7% of TV cells and 22.5% of VM cells) and to oligodendrocyte markers (23.2% of TV cells and 25.9% of VM cells). The counting also revealed a higher proportion of GABAergic cells in the TV group as compared with the VM group (29% of VT cells and 19% of VM cells, which comprised 42% and 26% of all neuronal cells, respectively, P < 0.05) and a high proportion of serotonergic cells in the VM group as compared with the TV group (34% of VM cells and 10% of TV cells, which comprised 47% and 14% of all neuronal cells, respectively, P < 0.001). A yield of cells in both groups differentiated into enkephalinergic cells (31% of TV cells and 36% of VM cells, which comprised 44% and 50% of all neuronal cells, respectively), but no statistically significant difference was seen between the groups (graph in Figure 4 and representative photomicrographs in Figure 5).
Figure 4.
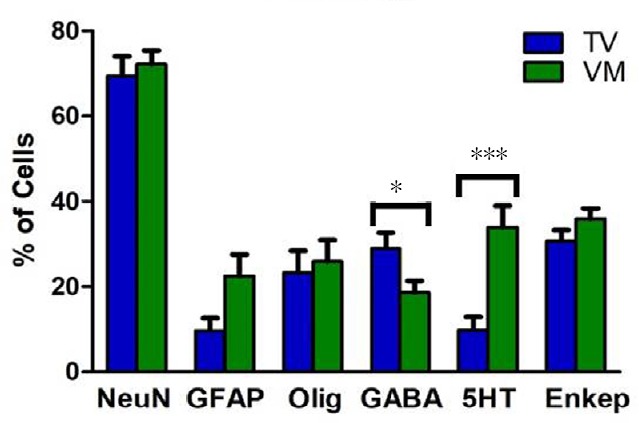
Stereological analysis of transplanted cells in spinal cord injury rats after fetal neural stem cell transplantation.
The cells were double labeled with BrdU and NeuN, GFAP, oligodendrocyte, GABA, 5HT or encephalin (Enkep). Most cells differentiated into neurons. Moreover, a higher proportion of GABAergic cells were seen in the telencephalic vesicles (TV) group and a higher proportion of serotonergic cells in the ventral medulla (VM) group. Enkephalinergic differentiation was similar between groups. *P < 0.05 and ***P < 0.001 (Mann-Whitney U test). Error bars represent SEM. BrdU: 5-Bromo 3′-deoxyuridine; NeuN: neuronal nuclei; GFAP: glial fibrillary acidic protein; GABA: gamma aminobutyric acid; 5HT: serotonin.
Figure 5.
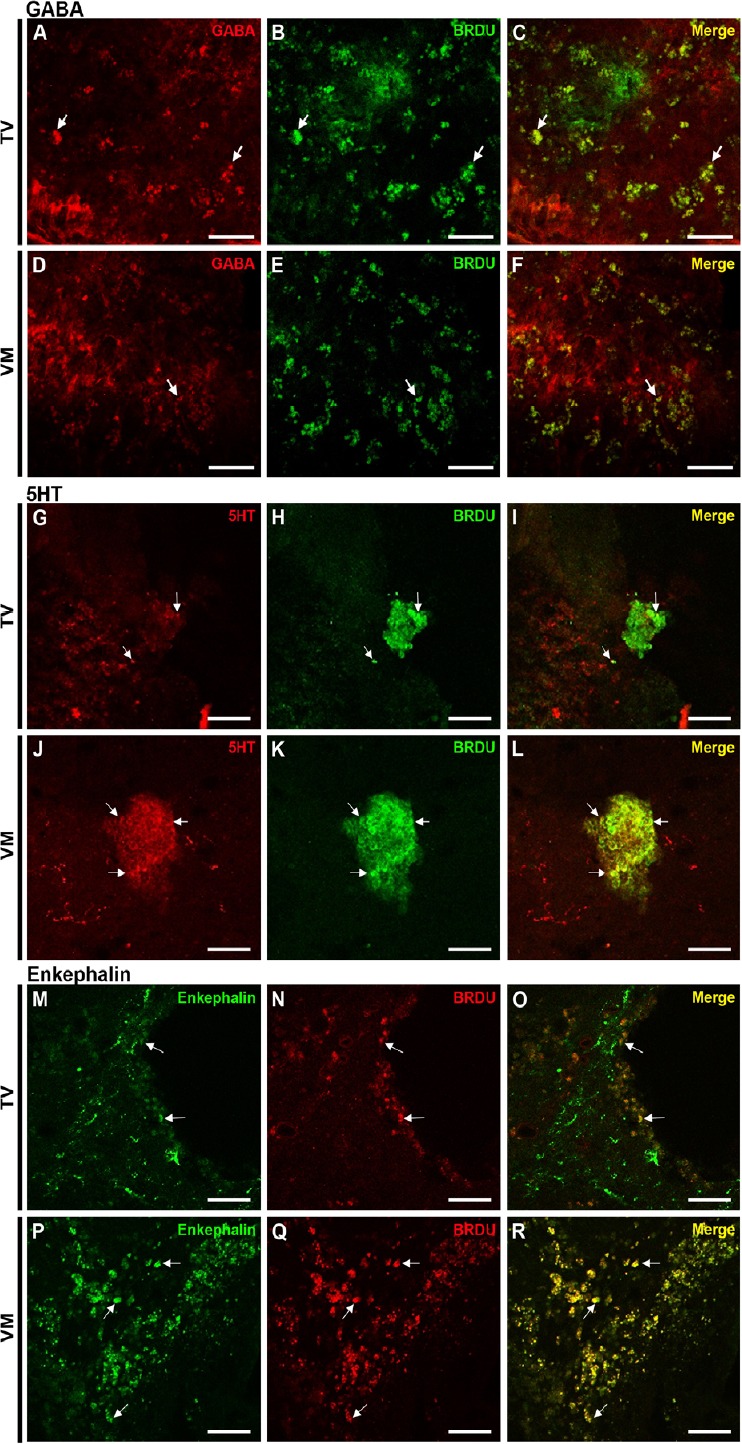
Photomicrographs showing differentiation of transplanted fetal neural stem cells into mature neurons within the injured spinal cord.
(A–C) GABA (red) and BrdU (green) in the telencephalic vesicles (TV) group. (D–F) GABA (red) and BrdU (green) in the ventral medulla (VM) group. (G–I) 5HT (red) and BrdU (green) in the TV group. (J–L) 5HT (red) and BrdU (green) in the VM group. (M–O) Enkephalin (green) and BrdU (red) in the TV group. (P–R) Enkephalin (green) and BrdU (red) in the VM group. Arrows indicate the co-localizations. Scale bars: 50 µm. BrdU: 5-Bromo 3′-deoxyuridine; GABA: gamma aminobutyric acid; 5HT: serotonin.
Discussion
Cell therapy has emerged as a potential strategy to promote repair following SCI. Several studies have reported that stem cell transplantation promotes axon regeneration and functional recovery (Tashiro et al., 2016; Assinck et al., 2017; Abbaszadeh et al., 2018; Bao et al., 2018; Miazga et al., 2018; Nori et al., 2018). However, the benefit of these cells for treating SCI-induced motor deficits is limited, and their role in controlling neuropathic pain is even less clear.
Stem cell transplantation can promote functional benefits after SCI through a variety of mechanisms, including neuroprotection, immunomodulation, axon regeneration, neuronal relay formation and myelin regeneration (Assinck et al., 2017). In this context, each candidate cell has particular characteristics that must be considered to obtain better results.
Mesenchymal stem cells (MSCs) have been shown to migrate to the injury site and have a strong ability to modulate the immune/inflammatory response (Kim and Park, 2017; Yousefifard and Nasirinezhad, 2017), which could have beneficial effects on neuropathic pain after SCI. Roh et al. (2013) investigated the therapeutic effect of human umbilical cord blood-derived MSCs (hUCB-MSCs) and amniotic epithelial stem cell (hAESCs) transplantation on central chronic pain. The cells were transplanted around the lesion site 2 weeks after SCI and only hAESC transplantation resulted in significant reduction of mechanical allodynia, while neither hUCB-MSCs or hAESCs had effects on thermal hyperalgesia. Furthermore, Watanabe et al. (2015) analyzed the effects of bone marrow-derived MSC (BMSC) transplantation on pain hypersensitivity in mice subjected to SCI. Transplantation of BMSCs three days after SCI resulted in motor function improvement and relieved mechanical and thermal hypersensitivities. Similarly, Yousefifard et al. (2016) observed mechanical allodynia and thermal hyperalgesia improvement, as well as locomotor recovery, in animals submitted to SCI and treated with bone marrow and umbilical cord-derived stem cells. Both cell types survived in the tissue for at least 8 weeks and prevented cavity formation. However, the survival rate of umbilical cord-derived stem cells was significantly higher and electrophysiological evaluations showed better results in wind up of wide dynamic range neurons compared with BMSCs.
Another alternative is the transplantation of neural stem cells (NSC). These cells have the capacity to differentiate into the three neural lineages astrocytes, oligodendrocytes and neurons, and can be found in the embryonic, neonatal and adult central nervous system. Potential uses of NSC transplantation in SCI include restitution of missing cells and activation of endogenous cells to provide “self-repair” (Gage, 2000).
Yao et al. (2015) showed that transplanting NSCs from hippocampal tissue of embryonic mice after SCI improved animals’ thermal and mechanical sensitivity. Furthermore, Luo et al. (2013) investigated the analgesic effect of the co-transplantation of NSCs with olfactory ensheathing cells (OECs) in rats submitted to complete spinal cord transection. NSC grafts recovered sensory function while OECs led to hyperalgesia and the co-transplantation reversed the hyperalgesia triggered by OECs and promoted NSC survival.
One of the challenges of using NSCs is that in severe SCI, most exogenous NSCs differentiate into astrocytes and only a small fraction differentiate into neurons (Setoguchi et al., 2004; Abematsu et al., 2006; Martino and Pluchino, 2006). Differentiation of NSCs into astrocytes has been associated with increased thermal hyperalgesia and mechanical allodynia after SCI (Macias et al., 2006). Hofstetter et al. (2005) reported that grafting adult NSCs after SCI improved motor recovery but also caused aberrant axonal sprouting associated with allodynia-like hypersensitivity in the forepaws. However, transduction of NSCs with neurogenin-2 before transplantation suppressed astrocytic differentiation and prevented graft-induced sprouting and allodynia. On the other hand, Davies et al. (2008) showed that different types of astrocytes have different effects on chronic pain after SCI. Transplantation of astrocytes derived from glial-restricted precursor cells treated with bone morphogenetic protein-4 promoted axon regeneration and functional recovery and the transplanted rats did not develop pain. In contrast, when glial-restricted precursor cell-derived astrocytes were generated by exposure to gp130 agonist ciliary neurotrophic factor, the transplantation blocked axonal regeneration and functional recovery and promoted mechanical allodynia and thermal hyperalgesia.
Considering the SCI-induced changes that contribute to the onset of neuropathic pain, GABAergic stem/progenitor cells are a strong candidate for the reversal of painful responses. As mentioned above, several mechanisms are related to the onset of SCI-induced neuropathic pain, including the downregulation of GABA-synthesizing enzymes, which, together with other alterations, can result in the disinhibition of excitatory impulses and contribute to the effect of hyperexcitability (Berrocal et al., 2014).
Fandel et al. (2016) evaluated the transplantation effects of medial ganglionic eminence-like cells derived from human embryonic stem cells. Two weeks after mice were injured at segmental level T13, the cells were transplanted into the lumbar enlargement. Six months after transplantation, cells were broadly dispersed in the injured spinal cord, extending ~10 mm rostro-caudal; furthermore, they differentiated into GABAergic neurons and the animals showed improvement in pain-related symptoms.
Similarly, Hwang et al. (2016) investigated the therapeutic potential of ESC-derived spinal GABAergic neurons to treat neuropathic pain after SCI. He observed that low doses of sonic hedgehog and retinoic acid in the culture medium induced medial ganglionic eminence-like progenitors and that cell transplantation attenuated post-injury neuropathic pain without motor recovery.
Our cell extraction protocol (fNSCs from TV of E14 embryo) and cellular culture method favored differentiation of GABAergic neurons. In fact, in a previous study we showed that 60% of the mature neuronal cells obtained from fNSCs from TV of E14 rats adopt a GABAergic phenotype (Maciaczyk et al., 2008). Additionally, patch clamp recordings revealed that 100% of cells matured according to our protocol presented GABAergic synaptic currents after 2 weeks in vitro (Lepski et al., 2013). These results led us to question whether GABAergic cells would show any potential to treat pain after SCI. In the present study we observed that these cells survived 8 weeks after transplantation, were found mainly in the periphery of lesions (no cells were found in sections above or below the lesion area), and 42% of all neuronal cells from the TV region differentiated into GABAergic neurons, as observed by stereological analyses. Moreover, animals who received intra-spinal injections of fNSCs from the TV region showed improvements in mechanical allodynia and thermal hyperalgesia after SCI.
It is known that descending monoaminergic pathways regulate the transmission of nociceptive information in the spinal cord. Descending serotoninergic pathways from the raphe nucleus and descending noradrenergic pathways from the locus coeruleus reach the spinal cord and inhibit the 2nd order neurons from the pain pathways, either through direct connections or interneurons, in the substantia gelatinosa of Rolando (Kandel et al., 2012). Thus, we hypothesized that transplanting cells from the ventral region of the metencephalon and myelencephalon, which will later give rise to the pons and medulla, could inhibit painful transmissions after SCI.
The fNSCs from the VM also survived 8 weeks after transplantation and were found mainly in the border of lesion. However, a higher proportion of serotonergic cells were observed in the VM group (i.e., 47% of all neuronal cells, as shown by stereological analyses). The sensory and motor analyses for the two groups were very similar: animals from the VM group also had mechanical allodynia and thermal hyperalgesia improvement after SCI. However, the statistical difference between that group and sham was higher than that observed in the TV group. Furthermore, no significant motor recovery was observed in either the TV or VM groups.
Based on previous studies (Lepski et al., 2011a, b), we considered 8 weeks of analysis time to be sufficient for the transplanted cells to differentiate and integrate and to be able to determine the effectiveness of this intervention. Moreover, as shown by the stereological analysis, the extraction of fNSCs from different brain regions of E14 yielded mature neurons with different phenotypes. These events can be explained by the theory of early embryonic regional specification of postnatal NSC, as proposed by Fuentealba et al. (2015). As showed in his study, most adult neural stem/progenitor cells produced between embryonic days 13.5 and 15.5 remained largely quiescent until reactivated in the postnatal period.
Despite the observed effects in pain control, some limitations of cell transplantation should be considered: only a low rate of transplanted cells survived and were able to differentiate and integrate into the system. Moreover, we did not observe any improvement in the motor pattern of the transplanted animals. This likely reflects the fact that system complexity affects transplant success, and since our transplants were focal, they may not have reached the motor system. An efficient cell manipulation from the regenerative point of view should perhaps address each of these processing segments and in an interconnected way. However, the cellular intervention against pain proposed here is based only on the local inhibition of second-order neurons in deafferentated spinal cord segments. Although the sensory system is no less complex than the motor system, the cellular intervention proposed here is non-physiological (since that the transplanted cells are not found naturally in that region), local and restricted, aiming to restore the balance between excitation and inhibition in a specific part of the hyper-excited neural tissue after injury.
In terms of clinical perspectives, a realistic scenario would be to combine stem cell transplantation with electrical stimulation, since recent studies have shown the beneficial effects of electric current on synaptic plasticity induction and cell maturation.
Taken together, our results suggest that neuronal precursors from TV and VM generate distinct populations of mature neuron phenotypes (mainly GABA, 5HT and enkephalin), and both of them reduce pain after SCI without significant motor recovery.
Additional file: Open peer review report 1 (98.3KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that there is no conflict of interest regarding the publication of this paper.
Financial support: This work was supported by FAPESP - Fundação de Amparo à Pesquisa do Estado de São Paulo, No. 2013/12056-0 (to GL) and No. 2015/02154-0 (to CMB).
Institutional review board statement: The study was approved by the Animal Ethics Committee of the Medical School of the University of São Paulo (protocol number 033/14) on March 4, 2016.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Gentian Vyshka, Faculty of Medicine, University of Medicine in Tirana, Albania.
Funding: This work was supported by FAPESP - Fundação de Amparo à Pesquisa do Estado de São Paulo, No. 2013/12056-0 (to GL) and No. 2015/02154-0 (to CMB).
P-Reviewer: Vyshka G; C-Editors: Zhao M, Li CH; T-Editor: Jia Y
References
- 1.Abbaszadeh HA, Niknazar S, Darabi S, Ahmady Roozbahany N, Noori-Zadeh A, Ghoreishi SK, Khoramgah MS, Sadeghi Y. Stem cell transplantation and functional recovery after spinal cord injury: a systematic review and meta-analysis. Anat Cell Biol. 2018;51:180–188. doi: 10.5115/acb.2018.51.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abematsu M, Smith I, Nakashima K. Mechanisms of neural stem cell fate determination: extracellular cues and intracellular programs. Curr Stem Cell Res Ther. 2006;1:267–277. doi: 10.2174/157488806776956887. [DOI] [PubMed] [Google Scholar]
- 3.Andresen SR, Biering-Sorensen F, Hagen EM, Nielsen JF, Bach FW, Finnerup NB. Pain, spasticity and quality of life in individuals with traumatic spinal cord injury in Denmark. Spinal Cord. 2016;54:973–979. doi: 10.1038/sc.2016.46. [DOI] [PubMed] [Google Scholar]
- 4.Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20:637–647. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 5.Baastrup C, Finnerup NB. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008;22:455–475. doi: 10.2165/00023210-200822060-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bao CS, Li XL, Liu L, Wang B, Yang FB, Chen LG. Transplantation of Human umbilical cord mesenchymal stem cells promotes functional recovery after spinal cord injury by blocking the expression of IL-7. Eur Rev Med Pharmacol Sci. 2018;22:6436–6447. doi: 10.26355/eurrev_201810_16056. [DOI] [PubMed] [Google Scholar]
- 7.Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- 8.Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- 9.Batista CM, Mariano ED, Onuchic F, Dale CS, Dos Santos GB, Cristante AF, Otoch JP, Teixeira MJ, Morgalla M, Lepski G. Characterization of traumatic spinal cord injury model in relation to neuropathic pain in the rat. Somatosens Mot Res. 2019 doi: 10.1080/08990220.2018.1563537. doi: 10.1080/08990220.2018.1563537. [DOI] [PubMed] [Google Scholar]
- 10.Berrocal YA, Almeida VW, Puentes R, Knott EP, Hechtman JF, Garland M, Pearse DD. Loss of central inhibition: implications for behavioral hypersensitivity after contusive spinal cord injury in rats. Pain Res Treat. 2014;2014:178278. doi: 10.1155/2014/178278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boadas-Vaello P, Castany S, Homs J, Alvarez-Perez B, Deulofeu M, Verdu E. Neuroplasticity of ascending and descending pathways after somatosensory system injury: reviewing knowledge to identify neuropathic pain therapeutic targets. Spinal Cord. 2016;54:330–340. doi: 10.1038/sc.2015.225. [DOI] [PubMed] [Google Scholar]
- 12.Boroujerdi A, Zeng J, Sharp K, Kim D, Steward O, Luo ZD. Calcium channel alpha-2-delta-1 protein upregulation in dorsal spinal cord mediates spinal cord injury-induced neuropathic pain states. Pain. 2011;152:649–655. doi: 10.1016/j.pain.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke D, Lennon O, Fullen BM. Quality of life after spinal cord injury: The impact of pain. Eur J Pain. 2018;22:1662–1672. doi: 10.1002/ejp.1248. [DOI] [PubMed] [Google Scholar]
- 14.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 15.Dale CS, Pagano Rde L, Paccola CC, Pinotti-Guirao T, Juliano MA, Juliano L, Giorgi R. Effect of the C-terminus of murine S100A9 protein on experimental nociception. Peptides. 2006;27:2794–2802. doi: 10.1016/j.peptides.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Davies JE, Proschel C, Zhang N, Noble M, Mayer-Proschel M, Davies SJ. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falnikar A, Hala TJ, Poulsen DJ, Lepore AC. GLT1 overexpression reverses established neuropathic pain-related behavior and attenuates chronic dorsal horn neuron activation following cervical spinal cord injury. Glia. 2016;64:396–406. doi: 10.1002/glia.22936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fandel TM, Trivedi A, Nicholas CR, Zhang H, Chen J, Martinez AF, Noble-Haeusslein LJ, Kriegstein AR. Transplanted human stem cell-derived interneuron precursors mitigate mouse bladder dysfunction and central neuropathic pain after spinal cord injury. Cell Stem Cell. 2016;19:544–557. doi: 10.1016/j.stem.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Fuentealba LC, Rompani SB, Parraguez JI, Obernier K, Romero R, Cepko CL, Alvarez-Buylla A. Embryonic origin of postnatal neural stem cells. Cell. 2015;161:1644–1655. doi: 10.1016/j.cell.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furlanetti LL, Cordeiro JG, Cordeiro KK, Garcia JA, Winkler C, Lepski GA, Coenen VA, Nikkhah G, Dobrossy MD. Continuous high-frequency stimulation of the subthalamic nucleus improves cell survival and functional recovery following dopaminergic cell transplantation in rodents. Neurorehabil Neural Repair. 2015;29:1001–1012. doi: 10.1177/1545968315581419. [DOI] [PubMed] [Google Scholar]
- 21.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 22.Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 2015;90:532–545. doi: 10.1016/j.mayocp.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–128. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- 24.Gwak YS, Hulsebosch CE, Leem JW. Neuronal-glial interactions maintain chronic neuropathic pain after spinal cord injury. Neural Plast. 2017;2017:2480689. doi: 10.1155/2017/2480689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatch MN, Cushing TR, Carlson GD, Chang EY. Neuropathic pain and SCI: Identification and treatment strategies in the 21st century. J Neurol Sci. 2018;384:75–83. doi: 10.1016/j.jns.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisen J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- 27.Hwang I, Hahm SC, Choi KA, Park SH, Jeong H, Yea JH, Kim J, Hong S. Intrathecal transplantation of embryonic stem cell-derived spinal GABAergic neural precursor cells attenuates neuropathic pain in a spinal cord injury rat model. Cell Transplant. 2016;25:593–607. doi: 10.3727/096368915X689460. [DOI] [PubMed] [Google Scholar]
- 28.IASP (2012). IASP Terminology updated from: Merskey H, Bogduk N (1994). Classification of chronic pain. Part III: Pain terms, a current list with definitions and notes on usage. 2nd ed. IASP Press, Seattle; pp. 209–214. Website: https://www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673&navItemNumber=677 . [Google Scholar]
- 29.Kandel E, Schwartz J, Jessell T, Siegelbaum S, Hudspeth A. 5th ed. New York: McGraw-Hill; 2012. Principles of neural science. [Google Scholar]
- 30.Kim HJ, Park JS. Usage of human mesenchymal stem cells in cell-based therapy: advantages and disadvantages. Dev Reprod. 2017;21:1–10. doi: 10.12717/DR.2017.21.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer JL, Minhas NK, Jutzeler CR, Erskine EL, Liu LJ, Ramer MS. Neuropathic pain following traumatic spinal cord injury: Models, measurement, and mechanisms. J Neurosci Res. 2017;95:1295–1306. doi: 10.1002/jnr.23881. [DOI] [PubMed] [Google Scholar]
- 32.Kumar R, Lim J, Mekary RA, Rattani A, Dewan MC, Sharif SY, Osorio-Fonseca E, Park KB. Traumatic spinal injury: global epidemiology and worldwide volume. World Neurosurg. 2018;113:e345–363. doi: 10.1016/j.wneu.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 33.Lepski G, Jannes CE, Nikkhah G, Bischofberger J. cAMP promotes the differentiation of neural progenitor cells in vitro via modulation of voltage-gated calcium channels. Front Cell Neurosci. 2013;7:155. doi: 10.3389/fncel.2013.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lepski G, Jannes CE, Strauss B, Marie SK, Nikkhah G. Survival and neuronal differentiation of mesenchymal stem cells transplanted into the rodent brain are dependent upon microenvironment. Tissue Eng Part A. 2010;16:2769–2782. doi: 10.1089/ten.TEA.2009.0686. [DOI] [PubMed] [Google Scholar]
- 35.Lepski G, Jannes CE, Wessolleck J, Kobayashi E, Nikkhah G. Equivalent neurogenic potential of wild-type and GFP-labeled fetal-derived neural progenitor cells before and after transplantation into the rodent hippocampus. Transplantation. 2011a;91:390–397. doi: 10.1097/TP.0b013e3182063083. [DOI] [PubMed] [Google Scholar]
- 36.Lepski G, Maciaczyk J, Jannes CE, Maciaczyk D, Bischofberger J, Nikkhah G. Delayed functional maturation of human neuronal progenitor cells in vitro. Mol Cell Neurosci. 2011b;47:36–44. doi: 10.1016/j.mcn.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Luo Y, Zou Y, Yang L, Liu J, Liu S, Zhou X, Zhang W, Wang T. Transplantation of NSCs with OECs alleviates neuropathic pain associated with NGF downregulation in rats following spinal cord injury. Neurosci Lett. 2013;549:103–108. doi: 10.1016/j.neulet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Maciaczyk J, Singec I, Maciaczyk D, Nikkhah G. Combined use of BDNF, ascorbic acid, low oxygen, and prolonged differentiation time generates tyrosine hydroxylase-expressing neurons after long-term in vitro expansion of human fetal midbrain precursor cells. Exp Neurol. 2008;213:354–362. doi: 10.1016/j.expneurol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Maciaczyk J, Singec I, Maciaczyk D, Klein A, Nikkhah G. Restricted spontaneous in vitro differentiation and region-specific migration of long-term expanded fetal human neural precursor cells after transplantation into the adult rat brain. Stem Cells Dev. 2009;18:1043–1058. doi: 10.1089/scd.2008.0346. [DOI] [PubMed] [Google Scholar]
- 40.Macias MY, Syring MB, Pizzi MA, Crowe MJ, Alexanian AR, Kurpad SN. Pain with no gain: allodynia following neural stem cell transplantation in spinal cord injury. Exp Neurol. 2006;201:335–348. doi: 10.1016/j.expneurol.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 41.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 42.Mehta S, Guy S, Lam T, Teasell R, Loh E. Antidepressants are effective in decreasing neuropathic pain after SCI: a meta-analysis. Top Spinal Cord Inj Rehabil. 2015;21:166–173. doi: 10.1310/sci2102-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meisner JG, Marsh AD, Marsh DR. Loss of GABAergic interneurons in laminae I-III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J Neurotrauma. 2010;27:729–737. doi: 10.1089/neu.2009.1166. [DOI] [PubMed] [Google Scholar]
- 44.Miazga K, Fabczak H, Joachimiak E, Zawadzka M, Krzemien-Ojak L, Bekisz M, Bejrowska A, Jordan LM, Slawinska U. Intraspinal grafting of serotonergic neurons modifies expression of genes important for functional recovery in paraplegic rats. Neural Plast. 2018;2018:4232706. doi: 10.1155/2018/4232706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan MM, Heinricher MM, Fields HL. Circuitry linking opioid-sensitive nociceptive modulatory systems in periaqueductal gray and spinal cord with rostral ventromedial medulla. Neuroscience. 1992;47:863–871. doi: 10.1016/0306-4522(92)90036-2. [DOI] [PubMed] [Google Scholar]
- 46.Muller R, Landmann G, Bechir M, Hinrichs T, Arnet U, Jordan X, Brinkhof MWG. Chronic pain, depression and quality of life in individuals with spinal cord injury: mediating role of participation. J Rehabil Med. 2017;49:489–496. doi: 10.2340/16501977-2241. [DOI] [PubMed] [Google Scholar]
- 47.Nagoshi N, Kaneko S, Fujiyoshi K, Takemitsu M, Yagi M, Iizuka S, Miyake A, Hasegawa A, Machida M, Konomi T, Asazuma T, Nakamura M. Characteristics of neuropathic pain and its relationship with quality of life in 72 patients with spinal cord injury. Spinal Cord. 2015;54:656–661. doi: 10.1038/sc.2015.210. [DOI] [PubMed] [Google Scholar]
- 48.Nori S, Khazaei M, Ahuja CS, Yokota K, Ahlfors JE, Liu Y, Wang J, Shibata S, Chio J, Hettiaratchi MH, Fuhrmann T, Shoichet MS, Fehlings MG. Human oligodendrogenic neural progenitor cells delivered with chondroitinase ABC facilitate functional repair of chronic spinal cord injury. Stem Cell Reports. 2018;11:1433–1448. doi: 10.1016/j.stemcr.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Previnaire JG, Nguyen JP, Perrouin-Verbe B, Fattal C. Chronic neuropathic pain in spinal cord injury: efficiency of deep brain and motor cortex stimulation therapies for neuropathic pain in spinal cord injury patients. Ann Phys Rehabil Med. 2009;52:188–193. doi: 10.1016/j.rehab.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Putatunda R, Hala TJ, Chin J, Lepore AC. Chronic at-level thermal hyperalgesia following rat cervical contusion spinal cord injury is accompanied by neuronal and astrocyte activation and loss of the astrocyte glutamate transporter, GLT1, in superficial dorsal horn. Brain Res. 2014;1581:64–79. doi: 10.1016/j.brainres.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg. 1977;47:577–581. doi: 10.3171/jns.1977.47.4.0577. [DOI] [PubMed] [Google Scholar]
- 52.Roh DH, Seo MS, Choi HS, Park SB, Han HJ, Beitz AJ, Kang KS, Lee JH. Transplantation of human umbilical cord blood or amniotic epithelial stem cells alleviates mechanical allodynia after spinal cord injury in rats. Cell Transplant. 2013;22:1577–1590. doi: 10.3727/096368912X659907. [DOI] [PubMed] [Google Scholar]
- 53.Ruiz-Juretschke F, Garcia-Salazar F, Garcia-Leal R, Fernandez-Carballal C, Iza B, Garbizu JM, Garcia-Duque S, Panadero T. Treatment of neuropathic deafferentation pain using DREZ lesions; long-term results. Neurologia. 2011;26:26–31. doi: 10.1016/j.nrl.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Setoguchi T, Nakashima K, Takizawa T, Yanagisawa M, Ochiai W, Okabe M, Yone K, Komiya S, Taga T. Treatment of spinal cord injury by transplantation of fetal neural precursor cells engineered to express BMP inhibitor. Exp Neurol. 2004;189:33–44. doi: 10.1016/j.expneurol.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Tashiro S, Nishimura S, Iwai H, Sugai K, Zhang L, Shinozaki M, Iwanami A, Toyama Y, Liu M, Okano H, Nakamura M. Functional recovery from neural stem/progenitor cell transplantation combined with treadmill training in nice with chronic spinal cord injury. Sci Rep. 2016;6:30898. doi: 10.1038/srep30898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teixeira MJ, Paiva WS, Assis MS, Fonoff ET, Bor-Seng-Shu E, Cecon AD. Neuropathic pain in patients with spinal cord injury: report of 213 patients. Arq Neuropsiquiatr. 2013;71:600–603. doi: 10.1590/0004-282X20130103. [DOI] [PubMed] [Google Scholar]
- 57.Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebeskind JC. Intrinsic mechanisms of pain inhibition: activation by stress. Science. 1984;226:1270–1277. doi: 10.1126/science.6505691. [DOI] [PubMed] [Google Scholar]
- 58.Vanecek V, Zablotskii V, Forostyak S, Ruzicka J, Herynek V, Babic M, Jendelova P, Kubinova S, Dejneka A, Sykova E. Highly efficient magnetic targeting of mesenchymal stem cells in spinal cord injury. Int J Nanomedicine. 2012;7:3719–3730. doi: 10.2147/IJN.S32824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vardeh D, Mannion RJ, Woolf CJ. Toward a mechanism-based spproach to pain diagnosis. J Pain. 2016;17:T50–69. doi: 10.1016/j.jpain.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe S, Uchida K, Nakajima H, Matsuo H, Sugita D, Yoshida A, Honjoh K, Johnson WE, Baba H. Early transplantation of mesenchymal stem cells after spinal cord injury relieves pain hypersensitivity through suppression of pain-related signaling cascades and reduced inflammatory cell recruitment. Stem Cells. 2015;33:1902–1914. doi: 10.1002/stem.2006. [DOI] [PubMed] [Google Scholar]
- 61.Yaksh TL, Fisher CJ, Hockman TM, Wiese AJ. Current and future issues in the development of spinal agents for the management of pain. Curr Neuropharmacol. 2017;15:232–259. doi: 10.2174/1570159X14666160307145542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao ZG, Sun XL, Li P, Liu HL, Wu HL, Xi ZQ, Zheng ZH. Neural stem cells transplantation alleviate the hyperalgesia of spinal cord injured (SCI) associated with down-regulation of BDNF. Int J Clin Exp Med. 2015;8:404–412. [PMC free article] [PubMed] [Google Scholar]
- 63.Yousefifard M, Nasirinezhad F. Review of cell therapy in spinal cord injury; effect on neuropathic pain. J Med Physiol. 2017;2:34–44. [Google Scholar]
- 64.Yousefifard M, Nasirinezhad F, Shardi Manaheji H, Janzadeh A, Hosseini M, Keshavarz M. Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model. Stem Cell Res Ther. 2016;7:36. doi: 10.1186/s13287-016-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao P, Hill M, Liu S, Chen L, Bangalore L, Waxman SG, Tan AM. Dendritic spine remodeling following early and late Rac1 inhibition after spinal cord injury: evidence for a pain biomarker. J Neurophysiol. 2016;115:2893–2910. doi: 10.1152/jn.01057.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.