Abstract
While the underlying mechanisms of Parkinson’s disease (PD) are still insufficiently studied, a complex interaction between genetic and environmental factors is emphasized. Nevertheless, the role of the essential trace element zinc (Zn) in this regard remains controversial. In this study we altered Zn balance within PD models of the versatile model organism Caenorhabditis elegans (C. elegans) in order to examine whether a genetic predisposition in selected genes with relevance for PD affects Zn homeostasis. Protein-bound and labile Zn species act in various areas, such as enzymatic catalysis, protein stabilization pathways and cell signaling. Therefore, total Zn and labile Zn were quantitatively determined in living nematodes as individual biomarkers of Zn uptake and bioavailability with inductively coupled plasma tandem mass spectrometry (ICP-MS/MS) or a multi-well method using the fluorescent probe ZinPyr-1. Young and middle-aged deletion mutants of catp-6 and pdr-1, which are orthologues of mammalian ATP13A2 (PARK9) and parkin (PARK2), showed altered Zn homeostasis following Zn exposure compared to wildtype worms. Furthermore, age-specific differences in Zn uptake were observed in wildtype worms for total as well as labile Zn species. These data emphasize the importance of differentiation between Zn species as meaningful biomarkers of Zn uptake as well as the need for further studies investigating the role of dysregulated Zn homeostasis in the etiology of PD.
Keywords: Caenorhabditis elegans, zinc, zinc homeostasis, Parkinson disease, labile zinc
Introduction
Affecting 0.1 – 0.2 and 1 per 100 people at ages below and above 60, respectively, Parkinson’s disease (PD) is the second most frequent degenerative disorder of the central nervous system (CNS) [1]. PD is denoted by a progressive loss of dopaminergic (DAergic) neurons in the substantia nigra pars compacta (SNpc) of the midbrain associated with motor and cognitive deficits [2,3].
Numerous studies in the last several decades failed to conclusively clarify the underlying mechanisms of PD’s etiology, but emphasized complex interactions between genetic and environmental factors. Although the majority of cases is idiopathic, 11 genes associated to inherited forms of PD were identified so far. Especially mutations of the genes encoding for parkin (PARK2), PTEN-induced putative kinase 1 (PINK1) (PARK6) and DJ-1 (PARK7) were found to elicit autosomal-dominant early-onset forms of PD [4]. Lysosomal P-type ATPase transporter ATP13A2 (PARK9) mutations cause an early-juvenile-onset atypical form of parkinsonism, called the Kufor-Rakeb syndrome (KRS) [5]. In addition, genetics and environmental factors, such as toxic metal species or dyshomeostasis in essential metal species may also contribute to PD etiology [6].
The essential trace element zinc (Zn) serves as a structural and enzymatic cofactor and signaling molecule, and plays a crucial role in physiological processes of e.g. development, immune response and brain function [7–10]. Maintaining Zn2+ homeostasis, either as protein-bound or labile Zn2+ ions, is therefore essential for optimal neuronal function. Excessively elevated Zn is associated with vascular-type dementia (VD), Alzheimer’s disease (AD) and prion diseases [11–13]. Moreover, exposure to high Zn concentrations may be a risk factor for PD. Elevated Zn concentrations were observed in the SN region of postmortal brains of PD patients [14,15], as well as in nigrostriatal tissue accompanied with labile Zn2+ in DAergic neurons of the SN region in a Zn-induced PD rat model [16]. Contradictory results can be found in the literature regarding the relationship between circulating Zn levels and PD. Recent meta analyses have documented decreased serum Zn levels in PD patients, while other studies found no significant difference or even increased Zn levels in PD patients [17,18]. Taken together, these studies suggest that Zn imbalances may be of central importance in the underlying mechanisms of PD.
The invertebrate model organism Caenorhabditis elegans (C. elegans) is frequently used to model PD due to its several advantages as its transparent appearance, fully described nervous system, tractable genetics and pathways highly conserved across species [19]. A number of worm models of PD have been generated by reproducing the genetic defects present in inherited forms of PD with multiple characteristic phenotypic abnormalities [20,21]. Loss of function mutations of pdr-1 and catp-6 for example, C. elegans orthologues of human PD-linked genes parkin and ATP13A2, resulted in increased DAergic neuronal loss in adult worms, as well as impaired DA signaling and reduced stress resistance [22].
Genetic traits are not only a risk factor for developing PD, they also play a role in age of onset, disease progression, responsiveness to therapeutics, and importantly, sensitivity and response to environmental factors. Therefore, the role of PD-related genes in Zn homeostasis was studied herein, focusing on alterations in Zn status in the background of mutated pdr-1 and catp-6 in C. elegans. Our main objective was to determine total and labile Zn2+ levels in worms, the latter potentially serving as a biomarker of the Zn status.
Material and methods
C. elegans strains and culture
The following nematodes strains were used and maintained at 20 °C on 8P agar plates covered with the Escherichia coli (E. coli) strain NA22 as a food source, as previously described [23]: N2, wildtype, as well as the mutant strains RB2510 (W08D2.5(ok3473) IV.) and VC1024 (pdr1(gk448) III).
Eggs were isolated from gravid adults by sodium hypochlorite treatment (1 % NaOCl and 0.25 M NaOH) and seeded onto Nematode Growth Media (NGM) plates covered with the E. coli strain OP50 to obtain age-synchronous populations. To ensure the homogeneity of age even in fecund worms, adults were daily relocated onto fresh plates after segregating them from their offspring by sedimentation in M9 buffer without centrifugation until they became day 4 adults.
Solutions
For all solutions and experiments purified water provided by a water purification system (10 MΩcm, Elix®, Merck Millipore) was used, unless otherwise mentioned.
1 M ZnSO4 (ZnSO4∙7H2O, ≥ 99.5 %, Merck KGaA, Darmstadt, Germany) stock solution in 85 mM NaCl was freshly prepared weekly and stored at 4 °C. Stock solutions of 5 mM ZinPyr-1 (≥ 95 %, biomol GmbH, Hamburg, Germany) in DMSO (Invitrogen, Carlsbad, USA) and 2 mM N,N,N′,N′-Tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) (Sigma Aldrich Chemie GmbH, Steinheim, Germany) were aliquoted, stored at -20 °C and thawed right before usage.
Zn and TPEN treatment
200 μM Zn, 500 μM Zn or 200 μM TPEN diluted in OP50 were streaked onto NGM plates and allowed to dry at 25 °C, before E. coli was inactivated by irradiating the plates with 40 kJ/m2 UV-A, UV-B, UV-C using an Atlas-Suntest+ (Atlas Material Testing Technology, Bishop Court, USA) to prevent further growing and metabolizing of the bacterial lawn.
For experimental settings worms of either L4 stage or day 4 of adulthood were exposed by placing them on inactivated NGM plates covered with native or supplemented OP50 with either Zn (200 and 500 μM) or the metal chelator TPEN (200 μM) for 24 h.
Total Zn quantification in C. elegans
After treatment, either day 2 or day 5 adult worms were collected, segregated and washed in M9 buffer until no bacterial residues, eggs and larvae remained in the supernatant to determine the total Zn content via inductively coupled plasma mass spectrometry (ICP-MS/MS).
Amounts of 500 to 1000 worms were pelletized in M9, frozen in liquid nitrogen and stored at -80 °C. The worm pellet, agar or E. coli were transferred into TFA microwave vessels including a mixture of 2 mL 15 % HNO3 (65 % HNO3, suprapur, Merck KgaA), 5 % H2O2 (Sigma Aldrich Chemie GmbH) and 1 μg/L Rh (Rh stock solution 10 mg/L, Merck KgaA) as an internal standard (ISTD). Digestion was performed with the MARS 6 microwave system (CEM, Kamp-Lintfort, Germany), applying 650 W to raise the temperature up to 200 °C within 15 min and holding this temperature for further 20 min. Obtained clear fusion solutions were transferred into 15 mL tubes without further dilution. Blank samples without worms, as well as trace element references with 15 – 25 mg of certified reference materials fish muscle (ERM®-BB422) or single cell protein (BCR®−274) were treated the same way, except from an additional 1:10 dilution of digested single cell protein in purified water.
Total Zn was determined using an Agilent ICP-QQQ 8800 system (Agilent, Waldbronn, Germany) with a verified multielement on-mass technique in He mode and external calibration. Underlying ICP-MS/MS settings are listed in table 1 (see supplementary). At least six calibration standards were prepared by diluting a stock solution (multi element mix containing i.e. 100 mg/L Zn, Cu, Fe, Mn) (Spetec, Erdingen, Germany) in 15 % HNO3 containing 1 μg/L Rh in a range of 0.05 to 500 μg Zn/L.
A maximum sensitivity of the measurement was ensured by daily tuning of nebulizer gas flow, lens parameters and quadrupoles. For validation of the method limits of detection and quantification (LOD and LOQ) were determined for Zn by applying the 3σ- and 10-σ-criterion. Obtained parameters were 2.63 μg Zn/L and 8.76 μg Zn/L, respectively.
Additionally, blank samples and trace element references were measured periodically for quality assurance (recovery ≥ 95.6 %).
ZinPyr-1 loading
After Zn treatment, either day 2 or day 5 adult worms were collected, segregated and washed in incubation buffer (25 mM HEPES pH 7.35, 120 mM NaCl, 5.4 mM KCl, 5 mM Glucose, 1.3 mM CaCl2, 1 mM MgCl2, 1 mM NaH2PO4, 0.3 % BSA) until no bacterial residues, eggs and larvae remained in the supernatant to determine labile Zn content with fluorescence of the Zn-selective probe ZinPyr-1.
400 worms each were incubated with 0.5 mL of 40 μM ZinPyr-1 in incubation buffer for 3 h in the dark under mild shaking. Excessive dye was removed by 5 times washing with ZP buffer (incubation buffer without BSA).
Fluorescence microscopy
After ZinPyr-1 loading, worms were paralyzed in 5 mM levamisole (Sigma Aldrich Chemie GmbH) on 4 % agarose pads on microscope slides. Fluorescence images were taken with a Leica DM6 fluorescence microscope (Leica Camera AG, Wetzlar, Germany) by maintaining unchanged settings and exposure times.
Labile Zn quantification in C. elegans
After ZinPyr-1 loading, 400 worms per 0.2 mL ZP buffer per well were transferred into 96 well plates (CELLSTAR® black polystyrene 96-well plate with micro-clear flat bottom, Greiner Bio-one, Kremsmünster, Austria). All measurements were carried out as bottom readings using a Tecan infinite 200 Pro multidetection microplate reader (Tecan, Männedorf, Switzerland) and wavelengths of 485 nm and 535 nm for excitation and emission, respectively. The minimum fluorescence (Fmin) was determined after adding three portions of 2 mM TPEN to each well with a respective incubation time of 30 min, up to a resulting concentration of 0.86 mM TPEN. Measurement of maximal fluorescence (Fmax) was conducted after addition of two portions of 1 M ZnSO4 to each well, with a respective incubation time of 20 min each, up to a resulting concentration of 0.1 M ZnSO4. Concentrations of labile Zn per worm were calculated using the formula [24]. KD of the probe ZinPyr-1 was previously determined as 0.7 nM [25]. Worm’s viability and the number of worms were controlled using a ZEISS CL9000 LED stereomicroscope (Carl Zeiss GmbH, Jena, Germany) after the measurements.
Statistical analysis
All figures and statistical analysis were performed using GraphPad Prism 7 (GraphPad Software, La Jolla, USA). Statistics were calculated by two-way ANOVA, followed by Dunnett’s or SIDAK’s multiple comparison test. P-values < 0.05 were considered significant and are indicated as *: p < 0.05, **: p < 0.01, and ***: p < 0.001 for Dunnet’s or §: p < 0.05, §§: p < 0.01, and §§§: p < 0.001 for SIDAK’s test in the respective figure legends.
Results
Effect on the survival of C. elegans
To assess the effect of Zn supplementation on C. elegans survival, either L4 larvae or day 4 adults were exposed to native, TPEN- or Zn-enriched E. coli as food source for 24 h.
The survival data (Figure 1A) of TPEN as well as Zn exposure at day 2 (exposing L4 for 24 h) did not cause an effect in wildtype worms, nor the mutated pdr-1 and catp-6 worms. However, at day 5 catp-6 deletion mutants showed reduced survival (Figure 1B), which was not affected to a greater extent by any of the treatment conditions. Survival of wildtype worms and the pdr-1 deletion mutants was not affected by age, TPEN or Zn exposure (exposing day 4 adults for 24 h).
Figure 1:
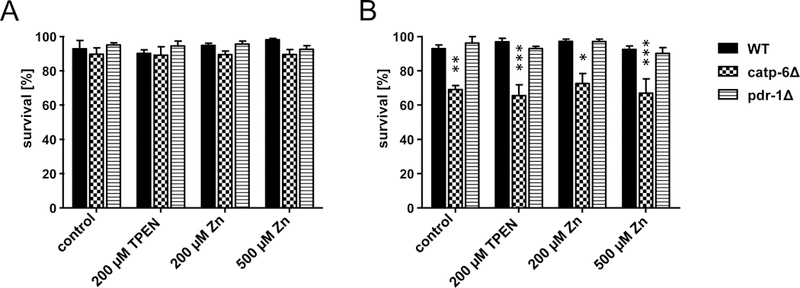
Survival of WT, catp-6Δ and pdr-1Δ after exposure to 200 μM TPEN, 200 μM or 500 μM Zn. Survival rates of [A]: day 2 adults and [B]: day 5 adults evaluated after 24 h of feeding with enriched OP50 E. coli of the respective C. elegans strains. Data are expressed as means of 4 independent determinations + SEM. *: p < 0.05, **: p < 0.01, and ***: p < 0.001 compared to WT controls.
Total Zn quantification (bioavailability)
To investigate whether the uptake of an enriched E. coli food source with either high concentrations of Zn or the divalent metal chelating agent TPEN results in altered Zn content in C. elegans, ICP-MS/MS analysis was conducted. Total Zn concentrations (Figure 2) on both, day 2 (Figure 2A) and day 5 (Figure 2B) adults of all tested strains, showed a slightly decreased trend with TPEN supplementation, as well as a dose-dependent increase following Zn supplementation. Figure 2A shows a significant increase in the Zn content in catp-6 and pdr-1 deletion mutants upon treatment with 500 μM Zn. Figure 2B establishes a moderate basal increase in Zn content with age especially in wildtype worms. 500 μM Zn exposure resulted in a significant increase in Zn levels in all worm strains at day 5 (Figure 2B). Comparing day 2 and day 5, worms showed an age-dependent Zn uptake in WT worms.
Figure 2:
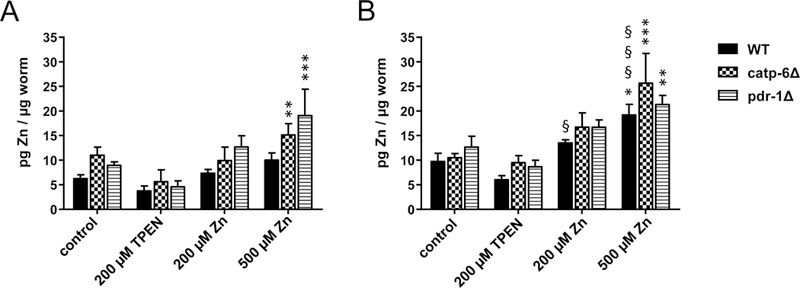
Total Zn in WT, catp-6Δ and pdr-1Δ following TPEN or Zn exposure. Zn concentrations in [A]: day 2 adults and [B]: day 5 adults of the respective C. elegans strains were determined using ICP-MS/MS after exposing L4 larvae or day 4 adults to 200 μM TPEN, 200 μM or 500 μM Zn via feeding enriched OP50 E. coli for 24 h. Data are expressed as means of at least 4 determinations + SEM normalized to worms’ weight. *: p < 0.05, **: p < 0.01, and ***: p < 0.001 compared to WT controls. §: p < 0.05, §§: p < 0.01, and §§§: p < 0.001 compared to respective same conditions in day 2 adults.
Labile Zn quantification in C. elegans by ZinPyr-1
Based on previously published work [26], a ZinPyr-1-based dye incubation protocol was utilized, allowing for [Zn]2+ visualization by fluorescence microscopy, as well as a multi-well semi-quantitative analysis of [Zn]2+ in living nematodes. Accordingly, fluorescence microscopy was performed to verify wormś uptake of ZinPyr-1 following dye loading. Figure 3 shows that the fluorescent probe is taken up by living worms and a corroborating ZinPyr-1 fluorescence signal could be attained. Optimal dye loading was achieved upon incubation of 40 μM ZinPyr-1 for 3 h, showing regular distribution and reproducible intense signals in the multi-well semi-quantitative analysis.
Figure 3:
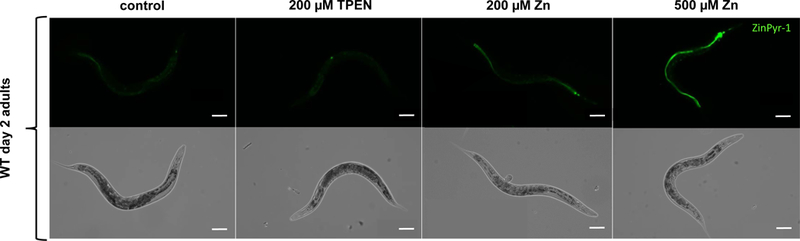
[Zn]2+ visualization after loading of C. elegans with ZinPyr-1. Fluorescence images of ZinPyr-1 signal in living WT day 2 adults after exposure to 200 μM TPEN, 200 μM and 500 μM Zn via feeding enriched OP50 E. coli for 24 h and respective bright field images. Scale bars represent 100 μm.
The semi-quantitative analysis of [Zn]2+ using a multi-well based method was further optimized. Since non-ratiometric fluorescent probes require determination of the fluorescence in the absence of metal ions (Fmin) and maximum fluorescence when the probe is saturated with Zn2+ (Fmax), the chelator TPEN was added to reach Fmin in the multi-well analysis, and Zn sulfate was added to obtain a precise Fmax [24,27].
Based on these data, [Zn]2+ levels were calculated. Exposing wildtype worms to 200 and 500 μM Zn for 24 h by feeding Zn-supplemented E. coli revealed a dose-independent 3-fold increase in [Zn]2+ in day 2 adults compared to the control. This effect was not observed in PD models (Figure 4A). However, all strains showed a TPEN-induced decreasing trend in [Zn]2+. Furthermore, after exposing day 4 adult wildtype worms for 24 h, no increase in [Zn]2+ was noted (Figure 4B), and an age-dependent decrease in [Zn]2+ in exposed WT worms was evident. Interestingly, [Zn]2+ was only increased by 500 μM Zn exposure in older pdr-1 deletion mutants.
Figure 4:
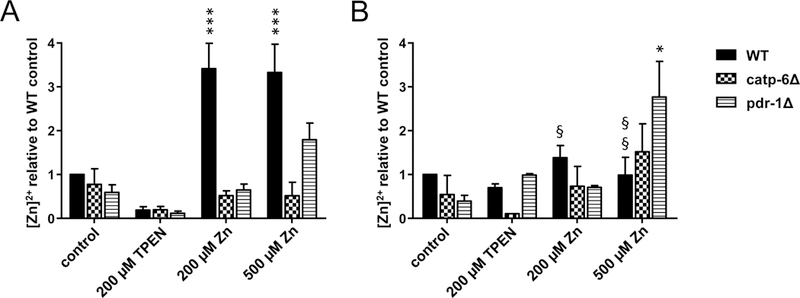
[Zn]2+ in WT, catp-6Δ and pdr-1Δ following TPEN or Zn exposure. [Zn]2+ in [A]: day 2 adults and [B]: day 5 adults of the respective C. elegans strains after exposing L4 larvae or day 4 adults to 200 μM TPEN, 200 μM or 500 μM Zn via feeding enriched OP50 E. coli for 24 h. Determination was realized by measuring [Zn]2+-dependent fluorescence as the difference between Fmax and Fmin as previously described [26]. Data are expressed as means of at least 3 determinations + SEM normalized to WT control. *: p < 0.05, **: p < 0.01, and ***: p < 0.001 compared to WT controls. §: p < 0.05, §§: p < 0.01, and §§§: p < 0.001 compared to respective same conditions in day 2 adults.
Discussion and Conclusion
The essential trace element Zn acts in various biological pathways of cell signaling, enzymatic catalysis and immune function as well as protein stabilization. Hence, dysregulation of Zn homeostasis by both, deficiency and excess may cause developmental and metabolic defects eliciting various health disorders, including neurodegenerative diseases [6].
While the involvement of several essential trace elements in PD’s etiology has been the subject of multiple investigations, Zn’s role remains controversial (see [28] as a detailed review). Protein-bound or complexed and labile zinc ions are known to affect distinct PD-associated pathways. Therefore, herein we focussed on the role of Zn homeostasis in the background of different PD models. Specifically, we quantified total and labile Zn to determine the latter’s potential to serve as a biomarker of PD.
Pdr-1 and catp-6 deletion worm mutants, which lack the orthologues of mammalian Parkin and ATP13A2 respectively, have served to study PD etiology. Parkin, an E2-dependent E3 ubiquitin ligase with four zinc-coordinating domains, is normally recruited to depolarized mitochondria promoting their autophagy by proteasomal degradation [29–31]. But further studies are needed to fill the gap of knowledge of Zn’s impact on parkin function. ATP13A2 is a cation transporter, whose physiological function remains unknown. It has been suggested to be involved in manganese (Mn) and Zn metabolism as well as mitochondrial pathways, but it is also associated with lysosomal proteolysis and α-synuclein metabolism [5,32,33]. Since ATP13A2 is highly expressed in DAergic neurons of the substantia nigra and found to be increased in sporadic forms of PD, its involvement in Zn dysregulation and PD-associated malfunctions is of particular interest [34].
While the incidence of PD increases with age, symptoms of genetically related cases, such as Parkin-associated forms of parkinsonism or KRS (ATP13A2 deficiency) have a juvenile age onset. Hence, we focussed our studies on early (L4 larvae to day 2 adults) and midlife (day 4 to day 5 adults) developmental stages in C. elegans. Zn exposure was carried out by dietary intake via feeding of bacteria supplemented with Zn. The supplemented bacteria were coated on agar plates and inactivated to avoid metabolizing processes by the bacteria. Because Zn is naturally present in the agar and peptone of the agar plates, as well as in the bacterial lawn [23,35], its amount was quantified with ICP-MS/MS and taken into account. Consequently, even the control group in this study is naturally exposed to 64.1 ± 3.3 μg Zn/kg NGM agar and 30.59 μM Zn in the E. coli strain OP50. Since high Zn exposure can affect lifespan secondary to changes in zinc burden [36,37], an effort was made to maintain Zn exposure at 500 μM or less for 24 h in early and midlife animals (Figure 1).
Catp-6 deletion mutants showed decelerated development and reduced egg-laying rates, as previously described by other groups [38–40]. Due to reduced lifespan, mutants of catp-6 showed a treatment-independent higher mortality rate in midlife age, compared to wildtype worms (Figure 1B), corroborating the important role of CATP6 in maintaining healthy development and aging. These effects remained unchanged by varying Zn exposure, confirming that acute Zn stimuli in the tested concentrations were in the subtoxic ranges [35,36]. Although Zn is being supposed as essential signaling molecule, binding of labile Zn2+ by exposing worms to the Zn selective metal chelator TPEN had no detrimental effect on survival as described elsewhere [36].
Metal dysregulation is a common feature throughout the aging process [41]. In wildtype nematodes, essential trace elements, such as iron and Mn accumulate with accelerated age, whereas Zn content only moderately increases with age [42]. Compliant to these findings our study ruled out a marked aging-dependent Zn increase in non-treated wildtypes. However, Zn supplementation increased the worms’ Zn content, whereas Zn chelation decreases total Zn, respectively [36]. It is established that C. elegans conserved Zn transporters (CDFs (cation diffusion facilitators), ZIPs (Zrt- and Irt- like proteins)) and metallothioneins that are involved in Zn metabolism, but their regulation and expression have yet to be completely understood in the nematode [35]. Interestingly, Zn exposure only significantly increased the total Zn content in middle-aged wildtype worms compared to controls. A 5-fold increase in total Zn content after 200 μM Zn exposure in young adults for 24 h as described by Roh et al. could not be affirmed, which might partly be due to different study designs [43]. TPEN supplementation caused a slightly decreasing trend in worms Zn content, which has already been observed in other studies [36]. TPEN is a membrane-permeable zinc-selective chelator that binds Zn with high affinity. The underlying mechanisms for this effect may be associated with binding of labile Zn species as signaling molecules and alteration of specific Zn importing processes [44].
Middle-aged and even young C. elegans deletion mutants of PD-related pdr-1 and catp-6 were more susceptible to Zn uptake after supplementation with higher doses. An increase in Zn content after Zn challenge has previously been observed in PD patient derived hONs cells lacking ATP13A2 [45], implicating its role in Zn homeostasis in both, importing and exporting processes. Moreover, ATP13A2 deficiency was described to alter Zn transporter expression in human cells [46]. The role of ATP13A2 in maintaining Zn homeostasis remains controversial. In addition to Zn, the ATP13A2 gene is also involved in Mn homeostasis. Altered ATP13A2 levels impact intracellular Mn levels, and modifying ATP13A2’s activity affords a therapeutic approach against Mn-induced neurotoxicity [47]. Recently, studies in C. elegans have shown that loss of pdr-1 can modulate Mn export through altered transporter expression of ferroportin [48]. Knowledge regarding the role of pdr-1 in Zn homeostasis is limited, but an impact on Zn homeostasis is likely. Parkin mutant flies exhibited PD-like abnormalities as locomotor defects and Zn supplementation ameliorated these effects [49]. Importantly, to our best knowledge, Zn uptake was not yet quantified in parkin mutants.
Accurate assessment of clinical data at an early stage of a disease is a requisite for successful therapies. Therefore, reliable biomarkers are needed. Considering the fact that changes in labile Zn2+ in biological systems can act as signaling transductors [50], monitoring of [Zn]2+ offers a promising and exciting tool. However, experimentally distinguishing between protein-bound and labile Zn2+ is challenging [51]. Applications of fluorescent probes have emerged, but were rarely applied to C. elegans. To date, [Zn]2+ sensing using different fluorescent probes in nematodes served the purpose of relative quantification of [Zn]2+ by estimating intensities of probe signals in fluorescence microscopy [36,43,52,53]. To improve the power of quantification, we aimed to establish a method to measure [Zn]2+ in C. elegans quantitatively by usage of the small molecule fluorescent probe ZinPyr-1. We based our work on a method described for E. coli to overcome typical problems of Zn determination as zinc phosphate precipitation and dye specificity [26,54]. The optimized method enables adequate intake of ZinPyr-1, as well as precise calculation of [Zn]2+ in alive worms with reference to maximal Zn saturation and autofluorescence of the probe, relying on the mass action law [24]. To our best knowledge this is the first multi well-based approach to quantitatively measure labile Zn2+ in vivo in C. elegans. Results were normalized to percentages of wildtype controls to compensate for interday variations, which commonly occur in living organisms. Levels of [Zn]2+ were markedly increased following Zn exposure in young wildtypes, but not middle-aged ones. Total Zn levels were not altered following Zn exposure, as shown in Figure 2, suggesting that ratios of bound and labile Zn2+ vary age-dependently, with lower availability of labile Zn2+ being noted in older worms. Regarding the commonly described Zn deficiency in elderly humans, this emphasizes the need for differentiated biomarkers for Zn status assessment [31,55]. Pdr-1Δ exhibit a slightly increasing trend in young age and significantly increased changes in [Zn]2+ in midlife-age after exposure to higher subtoxic Zn concentrations. The underlying mechanisms of this effect have to be clarified in future studies.
Taken together the present study in the model organism C. elegans elucidates the importance of differentiation between total and labile Zn2+ as potential biomarkers of the effective Zn status in living organisms, and offers a reliable method for quantitatively assessing labile Zn2+ in living worms by usage of a fluorescence probe. Our results revealed discerned changes in Zn2+ status following Zn exposure in wildtypes, catp-6 and pdr-1 deletion mutant models of PD. The data point out that a genetic predisposition by loss of catp-6 or pdr-1 affects Zn homeostasis in C. elegans. The consequence of such changes to worms’ healthspan will be addressed in future studies. Moreover, our results emphasize the need for further mechanistic investigations into Zn dyshomeostasis, especially in genetic disease models.
Supplementary Material
Acknowledgements
We thank the German Research Foundation (DFG) for the financial support of BO 4103/2–1, as well as the DFG Research Unit TraceAge (FOR 2558). This work was also supported by NutriAct – Competence Cluster Nutrition Research Berlin-Potsdam, funded by the Federal Ministry of Education and Research (BMBF - FKZ: 01EA1408B), as well as in part by NIH grants NIEHS R01ES10563, R01ES07331, and NIEHS R01ES020852.
We would also like to thank the Caenorhabditis Genetics Center (CGC), which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440), for providing the C. elegans strains used in this work.
Footnotes
Declarations of interest
The authors declare no conflict of interest.
References
- [1].Tysnes O-B, Storstein A, Epidemiology of Parkinson’s disease, J. Neural Transm. (Vienna) 124 (8) (2017) 901–905. 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- [2].Dawson TM, Ko HS, Dawson VL, Genetic animal models of Parkinson’s disease, Neuron 66 (5) (2010) 646–661. 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dawson TM, Golde TE, Lagier-Tourenne C, Animal models of neurodegenerative diseases, Nat. Neurosci. 21 (10) (2018) 1370–1379. 10.1038/s41593-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Corti O, Lesage S, Brice A, What genetics tells us about the causes and mechanisms of Parkinson’s disease, Physiol. Rev. 91 (4) (2011) 1161–1218. 10.1152/physrev.00022.2010. [DOI] [PubMed] [Google Scholar]
- [5].Park J-S, Blair NF, Sue CM, The role of ATP13A2 in Parkinson’s disease: Clinical phenotypes and molecular mechanisms, Mov. Disord. 30 (6) (2015) 770–779. 10.1002/mds.26243. [DOI] [PubMed] [Google Scholar]
- [6].Chen P, Miah MR, Aschner M, Metals and Neurodegeneration, F1000Res. 5 (2016). 10.12688/f1000research.7431.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Portbury SD, Adlard PA, Zinc Signal in Brain Diseases, Int. J. Mol. Sci. 18 (12) (2017). 10.3390/ijms18122506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Roohani N, Hurrell R, Kelishadi R, Schulin R, Zinc and its importance for human health: An integrative review, J. Res. Med. Sci. 18 (2) (2013) 144–157. [PMC free article] [PubMed] [Google Scholar]
- [9].Murakami M, Hirano T, Intracellular zinc homeostasis and zinc signaling, Cancer Sci. 99 (8) (2008) 1515–1522. 10.1111/j.1349-7006.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T, Zinc homeostasis and signaling in health and diseases: Zinc signaling, J. Biol. Inorg. Chem. 16 (7) (2011) 1123–1134. 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].White AR, Bush AI, Costa LG, Aschner M (Eds.), Biometals in Neurodegenerative Diseases: Mechanisms and Therapeutics, Elsevier Ltd, 2017. [Google Scholar]
- [12].Kawahara M, Tanaka K-I, Kato-Negishi M, Zinc, Carnosine, and Neurodegenerative Diseases, Nutrients 10 (2) (2018). 10.3390/nu10020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sensi SL, Paoletti P, Bush AI, Sekler I, Zinc in the physiology and pathology of the CNS, Nat. Rev. Neurosci. 10 (11) (2009) 780–791. 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- [14].Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD, Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease, J. Neurochem. 52 (6) (1989) 1830–1836. [DOI] [PubMed] [Google Scholar]
- [15].Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD, Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia, Brain 114 (Pt 4) (1991) 1953–1975. [DOI] [PubMed] [Google Scholar]
- [16].Kumar V, Singh D, Singh BK, Singh S, Mittra N, Jha RR, Patel DK, Singh C, Alpha-synuclein aggregation, Ubiquitin proteasome system impairment, and L-Dopa response in zinc-induced Parkinsonism: Resemblance to sporadic Parkinson’s disease, Mol. Cell. Biochem. 444 (1–2) (2018) 149–160. 10.1007/s11010-017-3239-y. [DOI] [PubMed] [Google Scholar]
- [17].Du K, Liu M-Y, Zhong X, Wei M-J, Decreased circulating Zinc levels in Parkinson’s disease: A meta-analysis study, Sci. Rep. 7 (1) (2017) 3902 10.1038/s41598-017-04252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sun H, Liu X, Ge H, Wang T, Wang Y, Li W, Association Between Serum Zinc Levels and the Risk of Parkinson’s Disease: A Meta-Analysis, Biol. Trace Elem. Res. 179 (1) (2017) 45–51. 10.1007/s12011-017-0941-2. [DOI] [PubMed] [Google Scholar]
- [19].Soares FA, Fagundez DA, Avila DS, Neurodegeneration Induced by Metals in Caenorhabditis elegans, Adv. Neurobiol. 18 (2017) 355–383. 10.1007/978-3-319-60189-2_18. [DOI] [PubMed] [Google Scholar]
- [20].Cooper JF, Dues DJ, Spielbauer KK, Machiela E, Senchuk MM, van Raamsdonk JM, Delaying aging is neuroprotective in Parkinson’s disease: A genetic analysis in C. elegans models, NPJ Parkinsons. Dis. 1 (2015) 15022 10.1038/npjparkd.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bornhorst J, Chakraborty S, Meyer S, Lohren H, Brinkhaus SG, Knight AL, Caldwell KA, Caldwell GA, Karst U, Schwerdtle T, Bowman A, Aschner M, The effects of pdr1, djr1.1 and pink1 loss in manganese-induced toxicity and the role of α-synuclein in C. elegans, Metallomics 6 (3) (2014) 476–490. 10.1039/c3mt00325f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cooper JF, van Raamsdonk JM, Modeling Parkinson’s Disease in C. elegans, J. Parkinsons. Dis. 8 (1) (2018) 17–32. 10.3233/JPD-171258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brenner S, The genetics of Caenorhabditis elegans 77 (1) (1974) 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Grynkiewicz G, Poenie M, Tsien RY, A new generation of Ca2+ indicators with greatly improved fluorescence properties, J. Biol. Chem. 260 (6) (1985) 3440–3450. [PubMed] [Google Scholar]
- [25].Burdette SC, Walkup GK, Spingler B, Tsien RY, Lippard SJ, Fluorescent sensors for Zn(2+) based on a fluorescein platform: Synthesis, properties and intracellular distribution, J. Am. Chem. Soc. 123 (32) (2001) 7831–7841. [DOI] [PubMed] [Google Scholar]
- [26].Haase H, Hebel S, Engelhardt G, Rink L, Application of Zinpyr-1 for the investigation of zinc signals in Escherichia coli, Biometals 26 (1) (2013) 167–177. 10.1007/s10534-012-9604-0. [DOI] [PubMed] [Google Scholar]
- [27].Haase H, Hebel S, Engelhardt G, Rink L, Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells, Anal. Biochem. 352 (2) (2006) 222–230. 10.1016/j.ab.2006.02.009. [DOI] [PubMed] [Google Scholar]
- [28].Placencia-Villa José-Yacamán, Perry Metals and Mitochondria in Neurodegeneration: Zinc Deficiency, in: White AR, Bush AI, Costa LG, Aschner M(Eds.), Biometals in Neurodegenerative Diseases: Mechanisms and Therapeutics, Elsevier Ltd, 2017, pp. 283–311. [Google Scholar]
- [29].Trempe J-F, Fon EA, Structure and Function of Parkin, PINK1, and DJ-1, the Three Musketeers of Neuroprotection, Front. Neurol. 4 (2013) 38 10.3389/fneur.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pickrell AM, Youle RJ, The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease, Neuron 85 (2) (2015) 257–273. 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hotz C, Lowe NM, Araya M, Brown KH, Assessment of the trace element status of individuals and populations: The example of zinc and copper, J. Nutr. 133 (5 Suppl 1) (2003) 1563S–8S. 10.1093/jn/133.5.1563S. [DOI] [PubMed] [Google Scholar]
- [32].Tan J, Zhang T, Jiang L, Chi J, Hu D, Pan Q, Wang D, Zhang Z, Regulation of intracellular manganese homeostasis by Kufor-Rakeb syndrome-associated ATP13A2 protein, J. Biol. Chem. 286 (34) (2011) 29654–29662. 10.1074/jbc.M111.233874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tsunemi T, Krainc D, Zn²⁺ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation, Hum. Mol. Genet. 23 (11) (2014) 2791–2801. 10.1093/hmg/ddt572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ramirez A, Heimbach A, Gründemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat A-L, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C, Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase, Nat. Genet. 38 (10) (2006) 1184–1191. 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- [35].Dietrich N, Tan C-H, Cubillas C, Earley BJ, Kornfeld K, Insights into zinc and cadmium biology in the nematode Caenorhabditis elegans, Arch. Biochem. Biophys. 611 (2016) 120–133. 10.1016/j.abb.2016.05.021. [DOI] [PubMed] [Google Scholar]
- [36].Kumar J, Barhydt T, Awasthi A, Lithgow GJ, Killilea DW, Kapahi P, Zinc Levels Modulate Lifespan through Multiple Longevity Pathways in Caenorhabditis elegans, PLoS ONE 11 (4) (2016) e0153513. 10.1371/journal.pone.0153513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang D, Shen L, Wang Y, The phenotypic and behavioral defects can be transferred from zinc-exposed nematodes to their progeny, Environ. Toxicol. Pharmacol. 24 (3) (2007) 223–230. 10.1016/j.etap.2007.05.009. [DOI] [PubMed] [Google Scholar]
- [38].Wender N, Cellular function and toxicity of the Parkinson’s disease-related genes α-synuclein and catp-6 in C. elegans. Dissertation, Göttingen, Germany, 2012. [Google Scholar]
- [39].Cooper JF, Spielbauer KK, Senchuk MM, Nadarajan S, Colaiácovo MP, van Raamsdonk JM, α-synuclein expression from a single copy transgene increases sensitivity to stress and accelerates neuronal loss in genetic models of Parkinson’s disease, Exp. Neurol. 310 (2018) 58–69. 10.1016/j.expneurol.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Trautwein C, MacKinnon N, Korvink JG, Micro-NMR elucidates altered metabolites in the Parkinson’s disease-related catp-6 genotype of Caenorhabditis elegans, Metabolomics 13 (4) (2017) e42848. 10.1007/s11306-017-1172-4. [DOI] [Google Scholar]
- [41].Malavolta M, Mocchegiani E (Eds.), Molecular basis of nutrition and aging, Academic Press, London, 2016. [Google Scholar]
- [42].Klang IM, Schilling B, Sorensen DJ, Sahu AK, Kapahi P, Andersen JK, Swoboda P, Killilea DW, Gibson BW, Lithgow GJ, Iron promotes protein insolubility and aging in C. elegans, Aging (Albany NY) 6 (11) (2014) 975–991. 10.18632/aging.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Roh HC, Collier S, Deshmukh K, Guthrie J, Robertson JD, Kornfeld K, ttm-1 encodes CDF transporters that excrete zinc from intestinal cells of C. elegans and act in a parallel negative feedback circuit that promotes homeostasis, PLoS Genet. 9 (5) (2013) e1003522. 10.1371/journal.pgen.1003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cho Y-E, Lomeda R-AR, Ryu S-H, Lee J-H, Beattie JH, Kwun I-S, Cellular Zn depletion by metal ion chelators (TPEN, DTPA and chelex resin) and its application to osteoblastic MC3T3-E1 cells, Nutr. Res. Pract. 1 (1) (2007) 29–35. 10.4162/nrp.2007.1.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kong SMY, Chan BKK, Park J-S, Hill KJ, Aitken JB, Cottle L, Farghaian H, Cole AR, Lay PA, Sue CM, Cooper AA, Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes, Hum. Mol. Genet. 23 (11) (2014) 2816–2833. 10.1093/hmg/ddu099. [DOI] [PubMed] [Google Scholar]
- [46].Park J-S, Koentjoro B, Veivers D, Mackay-Sim A, Sue CM, Parkinson’s disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction, Hum. Mol. Genet. 23 (11) (2014) 2802–2815. 10.1093/hmg/ddt623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chen P, Bornhorst J, Aschner M, Manganese metabolism in humans, Front. Biosci. (Landmark Ed) 23 (2018) 1655–1679. [DOI] [PubMed] [Google Scholar]
- [48].Chakraborty S, Chen P, Bornhorst J, Schwerdtle T, Schumacher F, Kleuser B, Bowman AB, Aschner M, Loss of pdr-1/parkin influences Mn homeostasis through altered ferroportin expression in C. elegans, Metallomics 7 (5) (2015) 847–856. 10.1039/c5mt00052a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Greene JC, Whitworth AJ, Andrews LA, Parker TJ, Pallanck LJ, Genetic and genomic studies of Drosophila parkin mutants implicate oxidative stress and innate immune responses in pathogenesis, Hum. Mol. Genet. 14 (6) (2005) 799–811. 10.1093/hmg/ddi074. [DOI] [PubMed] [Google Scholar]
- [50].Haase H, Rink L, Functional significance of zinc-related signaling pathways in immune cells, Annu. Rev. Nutr. 29 (2009) 133–152. 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- [51].Maret W, Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules, Metallomics 7 (2) (2015) 202–211. 10.1039/c4mt00230j. [DOI] [PubMed] [Google Scholar]
- [52].Roh HC, Collier S, Guthrie J, Robertson JD, Kornfeld K, Lysosome-related organelles in intestinal cells are a zinc storage site in C. elegans, Cell Metab. 15 (1) (2012) 88–99. 10.1016/j.cmet.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gong J, Li Y-H, Zhang C-J, Huang J, Sun Q, A thiazolo4,5-bpyridine-based fluorescent probe for detection of zinc ions and application for in vitro and in vivo bioimaging, Talanta 185 (2018) 396–404. 10.1016/j.talanta.2018.03.061. [DOI] [PubMed] [Google Scholar]
- [54].Ollig J, Kloubert V, Weßels I, Haase H, Rink L, Parameters Influencing Zinc in Experimental Systems in Vivo and in Vitro, Metals 6 (3) (2016) 71 10.3390/met6030071. [DOI] [Google Scholar]
- [55].Rink L, Kirchner H, Zinc-altered immune function and cytokine production, J. Nutr. 130 (5S Suppl) (2000) 1407S–11S. 10.1093/jn/130.5.1407S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


