Abstract
Background
Visual impairment in developing countries has both social and economic impact on individuals and communities. Understanding the subjective visual functioning of populations will allow for local policymakers to identify the need for optometric or ophthalmic services in their communities.
Methods
The authors surveyed 644 adult patients in Mwanza, Tanzania at three clinics (Buzuruga, Mwananchi and Kisesa) using a modified Visual Functioning Questionnaire 25. Responses were categorized into General health, General vision, Ocular pain, Near activities, Distance activities, Social function, Mental health, Role difficulties, Color vision, Peripheral vision and Dependency.
Results
Patients at Buzuruga reported the lowest scores on most subscales. Of 100 employed patients, 37% claimed to have at least some difficulty in performing job duties due to their eyesight. At Kisesa, 146 (246/221) patients (66.1%) had never had an eye exam, compared with 134/227 (59.0%) at Buzuruga and 69/173 (39.9%) at Mwananchi (p<0.01). Common reasons for not seeing an eye doctor were the perceived expense and lack of vision problems.
Conclusions
Due to regional differences in visual functioning in Mwanza, a national effort for vision health cannot be entirely successful without addressing the individualized needs of local communities. Reducing the cost of vision care appointments may expand vision health care utilization in Mwanza.
Keywords: global health, Mwanza, ophthalmology, optometry, Tanzania, visual functioning questionnaire
Introduction
Vision impairment remains a prominent global health concern, with unmet access to basic vision care in developing regions and limited data sets on vision in the most rural communities.1 Approximately 400 million people around the world have some degree of visual impairment, with uncorrected refractive errors, cataracts and glaucoma acting as major culprits.2,3 Tackling blindness is on the radar of global health organizations such as the WHO, which has established the VISION 2020 plan to eliminate avoidable blindness.4
Vision health affects not only individuals, but also community economics. The global annual loss of productivity from uncorrected refractive errors alone is estimated to be about 270 billion international dollars.5 These losses are particularly damaging to developing countries, where 89% of the world’s visually impaired reside.2 Poor vision health increases the risk of unemployment for both the affected individual and his/her caretakers,6 and poverty itself is a risk factor for poor vision,7 leading to a chronic cycle of poverty and vision impairment. For this reason, acting upon unmet vision health care needs has the potential for sustainable community impact.
This study assesses the subjective visual function of patients in the Mwanza region of Tanzania as a model for future studies in vision health care. The authors enlisted the perspective of patients in the Mwanza region—specifically patients visiting Buzuruga Hospital, Kisesa Health Center and Mwananchi Hospital—to learn about the effect of their vision on their daily lives. Based on a review of the literature, there are no previous studies that have assessed the vision health needs of the Mwanza region.
This study also sets the stage for replicable studies in other countries, as the primary survey instrument administered, the National Eye Institute (NEI) Visual Functioning Questionnaire 25 (VFQ-25),8 was modified to include questions regarding:
employment difficulties due to vision;
reasons why patients may not have accessed vision health care;
knowledge about glaucoma, age-related macular degeneration (AMD) and diabetic eye disease.
It is believed that this modified survey is compatible with the core questions surrounding global vision health and provides a foundation for future needs assessments in developing communities.
This paper hypothesizes that across the three participating clinics, patients visiting Kisesa (the most rural site of the three) have the worst self-reported vision problems across all subscales of the modified VFQ-25. Additionally, across all clinics, it is hypothesized that the most common reason that patients had not visited an eye doctor in at least 2 years was the lack of nearby locations to have their vision checked. Finally, it is hypothesized that patients visiting Kisesa have the least knowledge about glaucoma, AMD and diabetic eye disease, given the sparse access to health education resources and primary education.
Vision in Tanzania
In 2004, the Tanzanian Ministry of Health and Social Welfare established the National Eye Care Program (NECP) Strategic Plan, a 5-year initiative to begin an expansion of eye-care services throughout the country. Still, in 2011 there was one ophthalmologist for every 1.3 million people in Tanzania, which has an overall population of almost 53 million.9
A new Strategic Plan was created in 2011 to expand eye-care facilities, obtain equipment and train personnel. While the outcomes of this second Strategic Plan are yet to be released, it does not attempt to better characterize regional differences in vision impairment within the country, making it difficult to allocate resources to areas with a higher prevalence of disease. The Strategic Plan also does not address the patients’ perspectives on eye disease—specifically, how poor vision affects their activities of daily living, as well as if and why patients avoided seeking vision care. Because vision changes do not often have immediate life-threatening consequences, patients may forego seeking care until their disease is irreversible. Governments of developing countries, with limited funds, are less likely to allocate appropriate resources towards vision health, again due to the non-lethal nature of vision impairment.10
Methods
Setting
Study approval was granted jointly by the Institutional Review Board at the University of California, Irvine, and the Mwanza branch of the National Institute of Medical Research (NIMR). Data collection occurred in 2017 across three hospitals in the Mwanza region. Buzuruga Health Center is a public hospital near the city center of Mwanza and provides outpatient primary care. Mwananchi Hospital is a private teaching hospital in the city center that provides inpatient and outpatient primary care and minor surgical procedures. Finally, Kisesa Health Center is a public, rural hospital in the outskirts of Mwanza that acts as the only source of medical attention for patients in the region, performing inpatient and outpatient primary care, and minor surgical procedures.
Of the hospitals, Mwananchi is the only site to provide basic optometry services to its patients, and none of them has personnel or equipment for ophthalmic surgery. Mwananchi and Buzuruga are approximately 3.2 miles (5.1 km) apart, and both are about 16.3 miles from Kisesa Health Center (26.2 km). There are just two ophthalmologists in the entirety of the Mwanza region, and neither sees patients at these three facilities.
Study design
This study utilized a convenience-sampling method to recruit patients older than 18 years of age who were visiting any of the three facilities and were able to provide verbal consent to participate, and recruited patients visiting the general practice clinics at the three clinics in addition to the obstetrics/gynecology clinic at Buzuruga. The decision to recruit patients from these clinics was based on volume, as the majority of patients visiting each of these facilities were seen in the general practice clinics (or in the case of Buzuruga, the obstetrics/gynecology clinic).The Swahili-translated, modified VFQ-25 was distributed to participating patients, with several additional questions added to the original survey instrument (Table 1). The knowledge questions regarding glaucoma, AMD and diabetic eye disease were intended to assess the patients’ knowledge of the existence of each condition.
Table 1.
Additional questions added to the VFQ-25
| Question | Response options |
|---|---|
| 1. Are you currently employed? |
|
| 2. (Answer if you are not employed) Did you have to quit your job because of your eyesight? |
|
| 3. (Answer if you are not employed) Because of your eyesight, how much difficulty are you having in getting a job? |
|
| 4. (Answer if you are employed) Because of your eyesight, how much difficulty do you have in performing job duties? |
|
| 5. Because of your eyesight, have you ever been injured while working? |
|
| 6. Have you ever heard of glaucoma? |
|
| 7. Do you think vision loss from glaucoma can be prevented? |
|
| 8. Have you ever heard of age-related macular degeneration? |
|
| 9. Do you think vision loss from age-related macular degeneration can be prevented? |
|
| 10. Have you ever heard of diabetic eye disease? |
|
| 11. Do you think that vision loss from diabetic eye disease can be prevented? |
|
| 12. Has a health care provider ever told you that you have an eye condition or disease? |
|
| 13. Have you ever had your eyes checked by an eye doctor? |
|
| 14. When was the last eye examination you had by an eye doctor? |
|
| 15. If you have not seen an eye doctor recently, what was the main reason why you did not do so? |
|
Statistical analysis
Survey data were analyzed using SPSS.11 The VHQ-25 was scored as prescribed by the survey creators, categorizing the questions into the following subscales—General health (GH), General vision (GV), Ocular pain (OP), Near activities (NA), Distance activities (DA), Social function (SF), Mental health (MH), Role difficulties (RD), Color vision (CV), Peripheral vision (PV) and Dependency.12 Each subscale had a minimum score of 0 and maximum score of 100, with higher scores indicating better functioning in that category.
A one-way between-subjects ANOVA was conducted to compare the effect of the specific clinic on each of the subscale scores. For statistically significant results, post-hoc comparisons were performed using the Tukey honest significant difference (HSD) test. To compare inter-clinic differences in the time since the last eye examination, and to compare differences in knowledge regarding glaucoma, AMD and diabetic eye disease, two-sided χ2 tests were completed. Finally, descriptive statistics regarding vision difficulties in employment and reasons for not visiting an eye doctor in at least 2 years are reported.
Two-sided χ2 tests were done to compare knowledge about glaucoma, AMD and diabetic eye disease:
between men and women;
between those with and without vision aids;
between those with and without a diagnosis of diabetes.
Independent sample t-tests were conducted to compare VFQ-25 subscale scores among the same three baseline variables.
Results
In total, 644 patients across the three clinics participated in the study, but not all patients answered every question. The mean age of the sample was 37.9 years, 59.1% (207/350) (n=350) of the patients were female, and 80.6% (401/498) (n=498) wore neither contact lenses nor glasses (Table 2). There was no significant difference in age among the three groups (p=0.06), but Buzuruga had a significantly larger number of females participating than at the other two hospitals (p<0.01). Furthermore, there was a significant association between clinic and the use of a vision aid (p<0.01) and the proportion of patients with diabetes (p=0.04).
Table 2.
Demographics*
| Buzuruga | Kisesa | Mwananchi | Total | |
|---|---|---|---|---|
| Clinic | 231 (28.0%) | 226 (27.4%) | 187 (22.7%) | 644 |
| Gender (n=584) | ||||
| Male | 57 (25.9%) | 90 (46.6%) | 87 (50.9%) | 234 (40.1%) |
| Female | 163 (74.1%) | 103 (53.4%) | 84 (41.9%) | 350 (59.1%) |
| Age, mean (SD) (n=635) | 36.7 (14.3) | 39.8 (16.4) | 37.0 (14.4) | 37.9 (15.1) |
| Using vision aids (n=618) | ||||
| Glasses | 50 (22.2%) | 22 (10.3%) | 47 (26.3%) | 119 (19.3%) |
| Contact lenses | 1 (0.4%) | 0 (0%) | 0 (0%) | 1 (0.2%) |
| Both | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Neither | 174 (77.7%) | 192 (89.7%) | 132 (73.7%) | 498 (80.6%) |
| Diabetes diagnosis (n=636) | 5 (2.2%) | 8 (3.6%) | 13 (7.1%) | 26 (3.2%) |
*Note: percentages may not add up to 100% due to rounding.
On further analyses of the baseline characteristics, there was no significant difference in knowledge about AMD, glaucoma and diabetic eye disease when comparing
males vs females;
diabetes diagnoses vs no diabetes;
use of a vision aid (glasses or contact lenses) vs no vision aid (p>0.05 for all two-sided χ2 tests for each knowledge question).
Males (n=141) scored significantly higher on the GV subscale than females (n=130) (p<0.01), but there were no differences across any other subscale.
Diabetic patients (n=12) scored significantly higher on the GV score of the modified VFQ-25 than non-diabetic patients (n=268; 66.7 vs 46.0; p=0.03), but there was no significant difference between the two groups for any other VFQ-25 subscale. Patients using vision aids scored significantly higher than those without vision aids in the GV subscale (n=55 vs 220; score 58.9 vs 43.4; p<0.01) and PV subscale (n=111 vs 470; score 77.9 vs 84.4; p=0.019), with no differences in any other subscale.
Generally, patients visiting Buzuruga reported the lowest scores on many of the subscales, including GH, GV, NA, DA, SF, CV, PV and Dependency. The one-way between-subjects ANOVA showed a statistically significant effect of the hospital on all the VFQ-25 subscales (Table 3). Post-hoc comparisons using the Tukey HSD test indicated that in the GH, GV, NA and DA subscales, Buzuruga was significantly different from Kisesa and Mwananchi, but Kisesa was not significantly different from Mwananchi. For the SF, CV and PV subscales, Kisesa was significantly different from Mwananchi and Buzuruga, but Buzuruga was not significantly different from Mwananchi. For the MH, RD and Dependency subscales, Mwananchi was significantly different from Buzuruga and Kisesa, but Buzuruga was not significantly different from Kisesa. For the OP subscale, each of the clinics was significantly different from one another.
Table 3.
Descriptive statistics and one-way ANOVA results for VFQ-25 subscales
| Descriptive statistics | ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean±SD | Sum of squares | df | Mean square | F | Sig. | |||
| General health | Buzuruga | 229 | 34.9±26.8 | Between groups | 38 335.903 | 2 | 19 167.952 | 24.045 | <0.01 |
| Kisesa | 218 | 50.3±30.6 | Within groups | 493 444.643 | 619 | 797.164 | |||
| Mwananchi | 175 | 52.1±26.9 | Total | 531 780.547 | 621 | ||||
| Total | 622 | 45.2±29.3 | |||||||
| General vision | Buzuruga | 141 | 60.6±20.0 | Between groups | 34 204.468 | 2 | 17 102.234 | 53.997 | <0.01 |
| Kisesa | 117 | 83.2±16.0 | Within groups | 134 292.020 | 424 | 316.726 | |||
| Mwananchi | 169 | 74.4±17.0 | Total | 168 496.487 | 426 | ||||
| Total | 427 | 72.3±19.9 | |||||||
| Ocular pain | Buzuruga | 208 | 55.5±23.1 | Between groups | 35 099.664 | 2 | 17 549.832 | 26.534 | <0.01 |
| Kisesa | 193 | 47.2±28.1 | Within groups | 350 544.684 | 530 | 661.405 | |||
| Mwananchi | 132 | 68.4±25.9 | Total | 385 644.348 | 532 | ||||
| Total | 533 | 55.7±26.9 | |||||||
| Near activities | Buzuruga | 211 | 68.1±20.3 | Between groups | 13 306.257 | 2 | 6653.129 | 15.040 | <0.01 |
| Kisesa | 197 | 78.4±20.7 | Within groups | 245 063.482 | 554 | 442.353 | |||
| Mwananchi | 149 | 77.9±22.4 | Total | 258 369.739 | 556 | ||||
| Total | 557 | 74.3±21.6 | |||||||
| Distance activities | Buzuruga | 216 | 75.0±19.3 | Between groups | 8174.393 | 2 | 4087.197 | 10.020 | <0.01 |
| Kisesa | 206 | 83.7±19.0 | Within groups | 237 404.192 | 582 | 407.911 | |||
| Mwananchi | 163 | 80.6±22.6 | Total | 245 578.585 | 584 | ||||
| Total | 585 | 79.6±20.5 | |||||||
| Social function | Buzuruga | 224 | 86.9±20.0 | Between groups | 7947.927 | 2 | 3973.964 | 11.796 | <0.01 |
| Kisesa | 214 | 95.0±15.7 | Within groups | 202 136.546 | 600 | 336.894 | |||
| Mwananchi | 165 | 88.5±19.2 | Total | 210 084.473 | 602 | ||||
| Total | 603 | 90.2±18.7 | |||||||
| Mental health | Buzuruga | 180 | 41.8±15.1 | Between groups | 17 946.572 | 2 | 8973.286 | 26.486 | <0.01 |
| Kisesa | 159 | 39.6±20.7 | Within groups | 143 988.110 | 425 | 338.796 | |||
| Mwananchi | 89 | 56.6±20.2 | Total | 161 934.681 | 427 | ||||
| Total | 428 | 44.1±19.5 | |||||||
| Role difficulties | Buzuruga | 209 | 67.5±24.1 | Between groups | 13 476.571 | 2 | 6738.286 | 10.440 | <0.01 |
| Kisesa | 194 | 66.0±26.8 | Within groups | 349 188.870 | 541 | 645.451 | |||
| Mwananchi | 141 | 78.0±25.3 | Total | 362 665.441 | 543 | ||||
| Total | 544 | 69.7±25.8 | |||||||
| Dependency | Buzuruga | 202 | 79.6±26.5 | Between groups | 5116.721 | 2 | 2558.360 | 4.289 | 0.014 |
| Kisesa | 189 | 80.3±24.4 | Within groups | 312 593.325 | 524 | 596.552 | |||
| Mwananchi | 136 | 87.0±21.0 | Total | 317 710.046 | 526 | ||||
| Total | 527 | 81.8±24.6 | |||||||
| Color vision | Buzuruga | 228 | 86.0±22.1 | Between groups | 20 426.066 | 2 | 5213.033 | 13.430 | <0.01 |
| Kisesa | 218 | 95.4±16.2 | Within groups | 240 273.291 | 619 | 388.164 | |||
| Mwananchi | 176 | 88.6±20.3 | Total | 250 699.357 | 621 | ||||
| Total | 622 | 90.0±20.1 | |||||||
| Peripheral vision | Buzuruga | 224 | 79.2±23.4 | Between groups | 23 857.652 | 2 | 11 928.826 | 26.704 | <0.01 |
| Kisesa | 214 | 93.7±18.5 | Within groups | 269 814.094 | 604 | 446.712 | |||
| Mwananchi | 169 | 83.4±21.1 | Total | 293 671.746 | 606 | ||||
| Total | 607 | 85.5±22.0 | |||||||
All subscales scores are out of a maximum of 100, with a higher score indicating better functioning.
Of the total respondents, 111/591 (18.8%) were employed. Of the 100 employed patients who responded further, 37/100 (37%) claimed to have at least a little difficulty in performing their job due to their eyesight. Of the unemployed, 16/460 (3.5%) of respondents left their job due to eyesight problems, and 67/467 (14.3%) of respondents had at least a little difficulty in finding employment due to their eyesight. A total of 49/581 (8.4%) of respondents of the unemployed and employed patients combined reported being injured on the job in the past due to their eyesight.
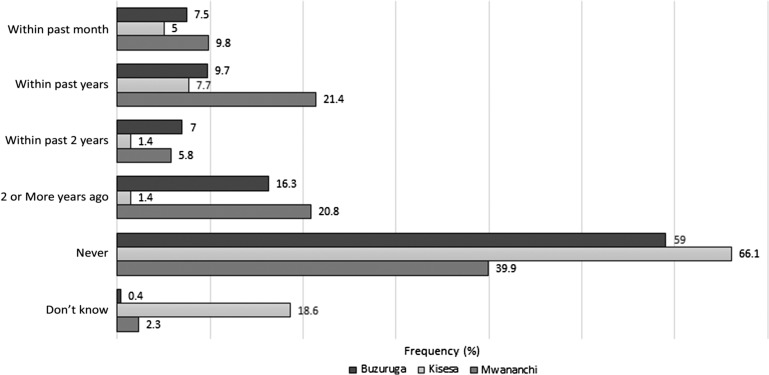
Figure 1 shows the responses of patients about their last eye exam by an eye doctor. At Kisesa, 146/221 (66.1%) of respondents have never had an eye exam, compared with 134/227 (59.0%) of respondents at Buzuruga and 69/173 (39.9%) of respondents at Mwananchi (p<0.01). Of those patients who have not seen an eye doctor in at least 2 years (including never), at Kisesa and Buzuruga the most common reason was the expense (38.3% (72/188) vs 62.1% (100/161)), while at Mwananchi the most common reason was a lack of perception of vision problems (53/102, 52%). Still, perceived expense was the second most common reason for patients at Mwananchi to not see an eye doctor (20/102, 19.6%; Table 4). Finally, patients at Kisesa scored significantly lower than patients at Buzuruga and Mwananchi in their knowledge about glaucoma, AMD and diabetic eye disease (Table 5).
Figure 1.
Last eye examination, by clinic.
Table 4.
Reasons why patients have not visited an eye doctor in at least 2 years (including ‘never’)
| Buzuruga count (%) | Kisesa count (%) | Mwananchi count (%) | |
|---|---|---|---|
| I haven’t had any problems and haven’t felt the need to see a specialist | 20 (12.4%) | 22 (11.7%) | 53 (52%) |
| I have had other, more important health problems | 7 (4.3%) | 1 (0.5%) | 3 (2.9%) |
| I can get along well enough with my eye problem | 6 (3.7%) | 0 (0%) | 3 (2.9%) |
| I don’t want to know if something is wrong | 0 (0%) | 0 (0%) | 1 (1%) |
| It might be painful | 2 (1.2%) | 2 (1.1%) | 2 (2%) |
| It is too expensive | 100 (62.1%) | 72 (38.3%) | 20 (19.6%) |
| Transportation is a problem | 0 (0%) | 9 (2%) | 0 (0%) |
| There are no places or doctors close enough to where I live or work | 22 (13.7%) | 33 (17.6%) | 7 (6.9%) |
| No time, never got around to it | 3 (1.9%) | 25 (13.3%) | 7 (6.9%) |
| Other | 1 (0.6%) | 23 (12.2%) | 6 (5.9%) |
Table 5.
Patient knowledge about glaucoma, age-related macular degeneration and diabetic eye disease
| Total count (% Yes) | Buzuruga count (%) | Kisesa count (%) | Mwananchi count (%) | Value | df | Asymptomatic ignificance two-sided) | |
|---|---|---|---|---|---|---|---|
| Have you ever heard of glaucoma? | 211 (34.6%) | 106 (48.0%) | 26 (11.8%) | 79 (47%) | 79.84 | 2 | <0.01 |
| Do you think vision loss from glaucoma can be prevented? | 167 (78.8%) | 81 (77.1%) | 16 (61.5%) | 70 (86.4%) | 7.62 | 2 | 0.02 |
| Have you ever heard of age-related macular degeneration? | 270 (44.3%) | 145 (65%) | 16 (7.2%) | 109 (65.7%) | 192.56 | 2 | <0.01 |
| Do you think vision loss from age-related macular degeneration can be prevented? | 163 (61.3%) | 66 (45.8%) | 11 (68.8%) | 86 (81.1%) | 32.46 | 2 | <0.01 |
| Have you ever heard of diabetic eye disease? | 190 (32.5%) | 95 (46.8%) | 13 (5.9%) | 82 (50.6%) | 113.54 | 2 | <0.01 |
| Do you think vision loss from diabetic eye disease can be prevented? | 118 (59%) | 38 (37.3%) | 11 (84.6%) | 69 (81.2%) | 40.75 | 2 | <0.01 |
Data reported as a percentage of patients who replied ‘yes’ to each question.
Discussion
The present study is the first to investigate self-reported vision and access to vision services in Mwanza, Tanzania using a modified VFQ-25 instrument. The VFQ-25 has previously been identified as a reliable psychometric tool for patients with a variety of chronic ocular conditions, low vision from any cause or those patients without eye diseases.8,13 Additionally, the subscale scores in the VFQ-25, particularly the GV score, have a strong positive correlation with visual acuity.14 The data presented here also seem to suggest this, as patients with vision aids had significantly higher GV and PV scores than patients not using vision aids. Therefore, while the VFQ-25 is not a replacement for a full examination of the eye, it does act as a cost-effective tool to gain an initial perspective on the vision of developing communities when personnel or instruments for examination are not available. While vision questionnaires are prone to cultural biases in responses, the VFQ-25 has shown good reliability across several different populations.15–18
As highlighted in these results, patients visiting Buzuruga collectively exhibited the lowest GV score (60.6 out of 100) compared with Kisesa (83.2) and Mwananchi (74.4). Such a low self-reported general vision profile, when compared with scores of both healthy patients and those with eye diseases, is concerning for a significant burden of ocular disease in the community. The initial study that tested the validity of the VFQ-25 in the USA8 found the mean GV score to be 83 for healthy controls, 71 for glaucoma, 62 for diabetic retinopathy, 60 for cataracts and 53 for AMD. When framed in this context, it is suspected that many patients at both Buzuruga and Mwananchi may be living with undiagnosed eye diseases. At all clinics, it appears that the effect of vision on mental health was particularly strong, suggesting that poor vision can be a source of anxiety and embarrassment for these patients.
While participants from the three clinics had no differences in age, there was a significantly higher number of female participants at Buzuruga. This is attributed to the presence of a special obstetrics and gynecology clinic at Buzuruga, which is the major service offered by the facility. Additionally, diabetic patients scored significantly higher on the GV score than non-diabetic patients; while this may be due to the very small sample size of diabetic patients who answered all questions associated with the GV subscale (n=12), this may also be a consequence of patients with known diabetes having better glucose control than undiagnosed patients, leading to reduced diabetic eye disease in this group. Finally, men scored significantly higher than women in the GV subscale despite more women using vision aids. A multifactorial gender disparity in visual impairment, wherein women suffer more from visual impairment and blindness, has been well documented in the literature,19,20 and this study suggests that Mwanza follows a similar trend.
The diabetes prevalence within this sample was small, with a significant association between clinic and prevalence. This finding is probably due to differences in the availability of diabetic screening services; Mwananchi has an on-site laboratory that the other two clinics lack. A recent study identified the prevalence of diabetes in Mwanza to be approximately 11.9%,21 so these results grossly underestimate the burden of the disease in the population. This is probably a consequence of biased sampling of these patients, as there are often significant differences in the health status of patients visiting health care centers when compared with patients who are not able to access medical services.22,23 Regardless, with a significant proportion of patients in Mwanza likely suffering from untreated diabetes, diabetic retinopathy is a preventable consequence that is important for local providers to address. While this study does not attempt to identify the prevalence of diabetic retinopathy in the population, Mwanza does not have access to retinal specialists who can screen and treat the condition. Training existing providers in the region will be critical to tackling diabetic retinopathy in the future.
Despite the proximity of Buzuruga and Mwananchi, there were significant differences in most aspects of their general and vision health profiles. Buzuruga, a government hospital with only basic primary care, serves patients of the lowest GH and GV scores of the three clinics, with most patients never receiving an eye exam. Still, despite the availability of optometry services at Mwananchi, about 40% of patients had never seen an eye doctor, leading the authors to believe that mere access to vision care does not imply utilization. It was found that the perceived cost of vision check-ups (including the actual appointment, as well as other incidental costs such as transportation) is a major reason that patients did not have frequent eye examinations. This was true even for patients at Buzuruga and Kisesa who would have to travel outside their communities for those examinations. Availability of services near patients’ homes or places of employment appears to be secondary to expense. Perceived cost of services is also likely the factor involved in this finding of a significant association between clinic and use of a vision aid, such as glasses or contact lenses.
Additionally, there was significant variability in knowledge about AMD, glaucoma and diabetic eye disease across the three clinics, a finding that can be speculated to be partially due to the young age of this sample, which may limit these patients’ knowledge of diseases (particularly AMD) that affect older populations. Unfortunately, with only two ophthalmologists in the region and neither of them at the three clinics of this study, the medical or surgical treatment of these conditions remains a difficult task in Mwanza. Kisesa, the most rural of the three facilities, exhibited the least knowledge about these. This is probably a product of poor education about eye health and disease prevention in a region already struggling with basic education.24
This study suggests that many patients in the Mwanza region have difficulty obtaining and maintaining employment due to poor vision. Therefore, the Tanzanian government may benefit from working with regional leaders to provide more optometric and ophthalmic services throughout the country. Reducing the cost of vision care appointments is likely to yield the most success in improving patient utilization of vision health care.
A successful cataract surgery model in India, the Aravind Eye Care System, may be applicable in Tanzania for even basic optometric services. By focusing on effective training to ensure high productivity and standardization of surgical procedures, the Aravind model has generated enough demand in India to supply a reduced unit cost for its surgeries.25 Importantly, the model allows patients to decide the amount they wish to pay by adding or removing certain amenities outside the surgery (like higher-quality hospital rooms), enabling higher-paying patients to indirectly subsidize surgeries for low-income patients.26 Policymakers in Tanzania should recognize the long-term benefits of establishing similar optometric and ophthalmic systems in improving the economic productivity of its individual communities.
This study has several limitations. The study design was limited to patients already visiting a hospital, and did not include individuals in the community who were not sick during data collection or who may not have had access to health care, creating a selection bias. In this study sample, many patients did not answer every question of the survey instrument. While it was not possible to identify patterns to these non-responses, this bias may impact the magnitude of the subjective visual function reported in each subscale. It is possible that, despite discussing the anonymity of responses with each patient, many still chose not to provide certain details, including gender, to ensure not being identified. Also, patients’ ocular and systemic comorbidities besides diabetes were not collected in this study, which prevents the conduction of subanalyses of VFQ-25 subscale scores by disease.
Additionally, this study only recruited patients older than 18 years of age, limiting the generalizability of these results to children who have uncorrected refractive errors and other ocular pathologies. Furthermore, sampling patients from only three hospitals prevents this study from generalizing to all patients in the Mwanza region. Finally, it was not attempted to corroborate the self-reported vision health scores with visual examination findings. Cultural differences between patients may skew their perceptions of their general health and vision strength when compared with objective measures, such as refractive error,27 even though the VFQ-25 has been reasonably reliable across different cultures, and subscores correlate well with visual acuity.
While self-reported surveys cannot replace eye examinations, they are still an inexpensive way to study subjective visual function and serve as a critical initial step in the implementation of eye health services.28 It is hoped that the modified VFQ-25 serves as a model for future assessments of vision health that local policymakers in low-income settings can use to understand the specific needs of their communities. Future extensive studies in Mwanza will focus on:
assessing the burden of vision impairment and conducting visual acuity examinations in pediatric patients;
conducting a larger scale study to correlate VFQ-25 subscales with visual acuity in the region;
assessing the need for subspecialty services (such as retina specialists for imaging and treatment of patients with diabetic retinopathy) to train local ophthalmologists and optometrists.
Acknowledgments
Authors’ contributions: SA, AMA, LAR, KPG, SAD, JET, IC, JF, DEB, SHC, OM and MCH all contributed to the study design. DJ, AMA, LAR, KPG, SAD, JET, IC, JF, DEB, SHC and OM were responsible for the study implementation. SA was responsible for the analysis and interpretation of the date. SA, DJ and MCH made a major contribution towards the writing of the paper. All authors read and approved the final version.
Acknowledgements: The authors would like to express their gratitude to Erick Oguta and Richard Machemba for their persistent efforts in forming relationships with partners with the participating hospitals in Mwanza. They would also like to thank Jackline Mueni Muthoka for her assistance in translating the survey instrument and study information sheet into Swahili.
Funding: None.
Competing interests: None declared.
Ethical approval: The study was approved by the University of California, Irvine, and the National Institute for Medical Research, Mwanza Branch. The verbal informed consent process was also approved by both research bodies.
References
- 1. Strasser R. Rural health around the world: challenges and solutions. Fam Pract 2003;20:457–63. [DOI] [PubMed] [Google Scholar]
- 2. Bourne RRA, Flaxman SR, Braithwaite T et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health 2017;5:e888–97. [DOI] [PubMed] [Google Scholar]
- 3. WHO WHO | Vision impairment and blindness. http://www.who.int/mediacentre/factsheets/fs282/en/ (accessed 20 October 2017).
- 4. Foster A, Resnikoff S. The impact of Vision 2020 on global blindness. Eye (Lond) 2005;19:1133–5. [DOI] [PubMed] [Google Scholar]
- 5. Smith T, Frick K, Holden B et al. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull World Health Org 2009;87:431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wright HR, Turner A, Taylor HR. Trachoma and poverty: unnecessary blindness further disadvantages the poorest people in the poorest countries. Clin Exp Optom 2007;90:422–8. [DOI] [PubMed] [Google Scholar]
- 7. Ulldemolins AR, Lansingh VC, Valencia LG et al. Social inequalities in blindness and visual impairment: a review of social determinants. Ind J Ophthalmol 2012;60:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mangione CM, Lee PP, Gutierrez PR et al. Development of the 25-list-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119:1050–8. [DOI] [PubMed] [Google Scholar]
- 9. United Republic of Tanzania, Ministry of Health and Social Welfare National Eye Care Strategic Plan. Dar Es Salam, MHSW, 2011. https://iapblive.blob.core.windows.net/resources/1_Final-NECP-Tanzania_2011-2016-.pdf?width=150&height=150 (accessed 20 October 2017).
- 10. Murthy P, Smith CL. Blindness and social stigma in woman and the girl-child In: Women’s global health and human rights, Murthy P (ed.). Burlington, MA: Jones & Bartlett Publishers, 2010, p. 383–391. [Google Scholar]
- 11. IBM Corp Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.
- 12.Vers 2000 VFQ-25 Manual_CM—manual_cm2000.pdf. https://www.nei.nih.gov/sites/default/files/nei-pdfs/manual_cm2000.pdf (accessed 20 October 2017).
- 13. Klein R, Moss SE, BEK Klein et al. The NEI-VFQ-25 in people with long-term type 1 diabetes mellitus: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmolol 2001;119(5):733–40. [DOI] [PubMed] [Google Scholar]
- 14. Clemons TE, Chew EY, Bressler SB et al. National Eye Institute Visual Function Questionnaire in the Age-Related Eye Disease Study (AREDS). Arch Ophthalmolol 2003;121(2):211–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McClure TM, Choi D, Becker T et al. The effect of visual impairment on vision-related quality of life in American Indian/Alaska natives. Ophthalmic Epidemiol 2009;16(2):128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kovac B, Vukosavljevic M, Djokic Kovac J et al. Validation and cross-cultural adaptation of the National Eye Institute Visual Function Questionnaire (NEI VFQ-25) in Serbian patients. Health Qual Life Outcomes 2015;13:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toprak AB, Eser E, Guler C et al. Cross-validation of the Turkish version of the 25-item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ 25). Ophthalmic Epidemiol 2005;12(4):259–69. [DOI] [PubMed] [Google Scholar]
- 18. Globe DR, Wu J, Azen SP et al. , Los Angeles Latino Eye Study Group . The impact of visual impairment on self-reported visual functioning in Latinos: the Los Angeles Latino Eye Study. Ophthalmology 2004;111(6):1141–9. [DOI] [PubMed] [Google Scholar]
- 19. Abou-Gareeb I, Lewallen S, Bassett K et al. Gender and blindness: a meta-analysis of population-based prevalence surveys. Ophthalmic Epidemiol 2001;8(1):39–56. [DOI] [PubMed] [Google Scholar]
- 20. Courtright P, Lewallen S. Why are we addressing gender issues in vision loss? Community Eye Health 2009;22(70):17–19. [PMC free article] [PubMed] [Google Scholar]
- 21. Ruhembe CC, Mosha TC, Nyaruhucha CN. Prevalence and awareness of type 2 diabetes mellitus among adult population in Mwanza city, Tanzania. Tanzan J Health Res 2014;16:89–97. [DOI] [PubMed] [Google Scholar]
- 22. Kleinbaum DG, Morgenstern H, Kupper LL. Selection bias in epidemiologic studies. Am J Epidemiol 1981;113:452–63. [DOI] [PubMed] [Google Scholar]
- 23. Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology 2004;15:615–25. [DOI] [PubMed] [Google Scholar]
- 24. Wedgwood R. Education and poverty reduction in Tanzania. Int J Educ Dev 2007; 27:383–96. [Google Scholar]
- 25. Natchiar G, Thulasiraj R, Sundaram RM. Cataract surgery at Aravind Eye Hospitals: 1988–2008. Community Eye Health 2008;21:40–2. [PMC free article] [PubMed] [Google Scholar]
- 26. Lewallen S, Thulasiraj RD. Eliminating cataract blindness—How do we apply lessons from Asia to sub-Saharan Africa? Glob Public Health 2010;5:639–48. [DOI] [PubMed] [Google Scholar]
- 27. Alonso J, Black C, Norregaard JC et al. Cross-cultural differences in the reporting of global functional capacity: an example in cataract patients. Med Care 1998;36:868–78. [DOI] [PubMed] [Google Scholar]
- 28. Hubley J, Gilbert C. Eye health promotion and the prevention of blindness in developing countries: critical issues. Br J Ophthalmol 2006;90:279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]