Abstract
The prevalence of methicillin-resistant Staphylococcus aureus (MRSA) colonization among nursing home residents is high. Health-care workers (HCWs) often serve as a vector in MRSA transmission. The ability to identify residents who are likely to transmit MRSA to HCWs’ hands and clothing during clinical care is important so that infection control measures, such as Contact Precautions, can be employed. Using data on demographic and clinical characteristics collected from residents of community nursing homes in Maryland and Michigan between 2012 and 2014, we developed a clinical prediction rule predicting the probability of MRSA transmission to HCWs’ gowns. We externally validated this model in a cohort of Department of Veterans Affairs nursing home residents from 7 states between 2012 and 2016. The prediction model, which included sex, race, resident dependency on HCWs for care, the presence of any medical device, diabetes mellitus, and chronic skin breakdown, showed good performance (C statistic = 0.70; sensitivity = 76%, specificity = 49%) in the development set. The decision curve analysis indicated that this model has greater clinical utility than use of a nares surveillance culture for MRSA colonization, which is current clinical practice for placing hospital inpatients on Contact Precautions. The prediction rule demonstrated less utility in the validation cohort, suggesting that a separate rule should be developed for residents of Veterans Affairs nursing homes.
Keywords: decision curve analysis, disease transmission, external validation, methicillin-resistant Staphylococcus aureus, nursing homes, prediction rule
Person-to-person transmission of multiple-drug–resistant organisms, such as methicillin-resistant Staphylococcus aureus (MRSA), is a major problem in nursing homes. The prevalence of MRSA colonization among nursing home residents has been reported to be between 25% and 50% (1). Colonization is the presence of the organisms at a body site without signs or symptoms of infection (2). Residents can be colonized with MRSA at 1 or more body sites, with the anterior nares being the most common site (2). Importantly, colonized residents can serve as a source of transmission to others (3, 4). Nursing-home residents colonized with MRSA are at higher risk of developing MRSA infection, which entails substantial morbidity and mortality (5, 6).
Health-care workers (HCWs) serve as vectors for MRSA transmission when their hands and clothing become contaminated with the bacterium. In hospitals, MRSA-colonized inpatients are placed on Contact Precautions (the use of gloves and gowns for all known colonized patients with a multiple-drug–resistant organism) to prevent transmission to other patients (7). However, the standard of care for nursing home residents colonized with MRSA is the use of Standard Precautions (e.g., the use of gowns and gloves for anticipated contact with blood, body fluids, skin breakdown, or mucous membranes) (7, 8). Prior research on MRSA acquisition among nursing home residents suggests that Standard Precautions do not sufficiently reduce transmission (5, 6, 9–11). Further, risk factors for MRSA transmission to HCWs’ gloves and gowns include many care activities for which Standard Precautions are not indicated, such as changing linens, dressing residents, and transferring residents into and out of bed (3, 4).
Nursing homes have been resistant to adopting the use of gloves and gowns for all MRSA-colonized residents because of financial and logistical concerns (7, 8). Active surveillance for MRSA colonization among new and existing residents is impractical because nursing homes lack in-house laboratories (8). Nares cultures must be sent to outside laboratories, which can delay the availability of test results, rendering surveillance effectively useless for short-stay residents. Further, test results can be falsely negative due to imperfect sensitivity or colonization at other body sites (2, 3). Identifying resident characteristics that can be used to predict transmission in the absence of surveillance cultures could lead to more efficient targeting of Contact Precautions when resources are limited. Targeted precautions could be an important step in reducing MRSA transmission throughout nursing homes.
We developed a clinical prediction rule using resident characteristics associated with transmission of MRSA to HCWs’ gowns in a previously assembled cohort of community nursing home residents. We chose to focus on MRSA transmission to HCWs’ gowns only, since this may represent a higher level of transmission and a more intense level of care provided to the resident than glove transmission. HCWs’ use of gowns in nursing home settings is uncommon, making transmission to clothing a risk factor for transmission of MRSA to other residents. We then used a similar cohort of Department of Veterans Affairs (VA) nursing home residents to externally validate our clinical prediction rule.
METHODS
Study design
We identified 2 cohorts of nursing home residents that had been previously assembled to estimate the frequency of transmission of and risk factors for MRSA transmission from residents to HCWs’ gloves and gowns while providing care. For each recruited patient, multiple encounters with HCWs were observed. The protocol was approved by the institutional review boards of the University of Maryland, Baltimore (Baltimore, Maryland), the University of Michigan (Ann Arbor, Michigan), and the VA (Washington, DC).
Participants
Both the community and the VA-based nursing home cohorts have been described elsewhere (3, 4). Briefly, in the former, cohort residents were sampled from 13 community-based nursing homes in Maryland and Michigan, ranging in size from 62 beds to 209 beds, between March 26, 2012, and May 1, 2014 (3). The VA cohort was sampled from 7 nursing homes in Maryland, Massachusetts, New York, Texas, and Washington, DC, between September 19, 2012, and January 6, 2016 (4). In both studies, eligible residents had an expected length of stay of at least 1 week, were not identified by nursing staff as having behavioral problems, and were enrolled after receipt of written informed consent from them or their legally authorized representative. HCWs were enrolled with verbal consent.
Data collection
In both cohorts, data on resident demographic characteristics, type of long-term care (rehabilitation vs. residential care), comorbidity, recent hospitalizations, activities of daily living, current antibiotic use, skin breakdown, use of medical devices, and secretions were abstracted from medical records and nursing home staff. Residents were cultured for MRSA colonization at enrollment. MRSA cultures were obtained from the residents’ anterior nares, perineal skin, and wound (if present) using separate nylon-flocked swabs (Copan ESwabs; Copan Diagnostics, Inc., Murrieta, California). In this analysis, a resident was considered MRSA-positive if the culture from the anterior nares grew MRSA.
During the study period, HCWs wore gowns and gloves during every resident interaction, regardless of the type of care provided. For each care activity, a researcher observed and recorded the type and duration of care delivered. After the delivery of care, the HCW’s gown and gloves were swabbed with a dual-tipped rayon-flocked swab (BBL CultureSwab; Becton, Dickinson and Company, Sparks, Maryland) (as described previously) (3, 4). In the community cohort, all HCW interactions with residents positive for MRSA and a random sample of MRSA-negative residents were selected for HCW glove and gown testing. In the VA cohort, all HCW glove and gown swabs were tested for the presence of MRSA.
Laboratory procedures
Resident specimens and HCWs’ gowns and gloves were cultured for the presence of MRSA at a central laboratory; procedures have been described elsewhere (3, 4). Briefly, the swabs were vortexed, and 50 μL of Amies Transport Medium (Becton, Dickinson and Company) was plated onto CHROMagar S. aureus (Becton, Dickinson and Company). The swabs were enriched in Tryptic soy broth (Remel, Inc., Lenexa, Kansas) with 6.5% sodium chloride and incubated for 24 hours at 37°C, after which 50 μL of broth was plated onto CHROMagar S. aureus. Mauve-colored colonies on CHROMagar S. aureus that were suspicious for S. aureus were confirmed by the detection of coagulase (Staphaurex; Remel, Inc.). Susceptibility testing was performed on all S. aureus isolates using disk diffusion following Clinical and Laboratory Standards Institute guidelines (12).
Predictors
We used the community-based nursing home cohort (referred to throughout as the “development set”) to develop the prediction rule. We built 2 models where the outcome of interest was transmission of MRSA from a resident to HCWs’ gowns during resident care activities. These models included: 1) a model where the MRSA nares culture was the only fixed predictor and 2) a clinical prediction model that excluded the MRSA nares culture as a predictor. Candidate predictors for the prediction model included the following resident-level variables on which data were collected at baseline: age (years; continuous variable) and the following binary predictors: sex, race (white vs. nonwhite), the presence of a medical device (indwelling catheter, etc.), diabetes mellitus, renal failure, chronic skin breakdown (any skin breakdown that was not a surgical wound (e.g., pressure ulcer, foot infection, diabetic foot ulcer, open lesions, and burns)), being hospitalized in the last 3 months, having a blood transfusion while hospitalized, receipt of tracheotomy care while in the nursing home, receipt of antibiotics within the last 7 days, and dependency on HCWs. To define the residents’ level of dependency on HCWs, we used performance scores from the minimum data set for activities of daily living (13). The performance scores ranged from 0 (totally independent) to 4 (totally dependent). A resident was considered dependent on HCWs if he or she scored a 3 (requiring extensive assistance) or higher for any one of the following activities: dressing, hygiene, toileting, or transfer. Residents requiring this level of assistance were considered dependent on HCWs (14).
There were missing data on renal failure for 11% of the residents in the community cohort. On the basis of prior experience and clinical knowledge, missing data for renal failure were assumed to indicate the absence of that comorbid condition. Because fewer than 2% of the data for the other variables were missing in both data sets, we used complete-case analysis after recoding the renal failure variable.
Development and internal validation of the clinical prediction rule
We used a logistic regression mixed model with a random intercept for nursing home resident. The prediction rule was developed by including all candidate predictors in the model and then using LASSO regression (“least absolute shrinkage and selection operator”) to select the predictors and shrink the coefficients. The tuning parameter that controls shrinkage and variable selection was determined with cross-validation (14).
The predicted probabilities from the fixed effects for both the MRSA nares-culture-only model and our clinical prediction rule were used to estimate the C statistic and its 95% confidence interval. For the clinical prediction rule, the median predicted probability was used as the cutpoint to calculate sensitivity, specificity, positive predictive value, and negative predictive value and their associated 95% confidence intervals.
The calibration slope was estimated, and a calibration curve was constructed by dividing the sample into deciles of predicted risk. Within each decile, the observed transmission proportion was plotted against the predicted probability from the model. A 45-degree line was added to the plot to visually inspect how closely the predicted risk agreed with the observed proportion of transmission. A perfectly calibrated model has a slope of 1.0, and the decile points in the curve will rest exactly on the 45-degree line, implying that the predicted risks are equal to the observed proportion (15).
External validation of the clinical prediction rule
We validated the model in an external data set (the VA nursing home cohort, hereafter referred to as the “validation set”). We applied the regression coefficients from our clinical prediction rule to the validation set to estimate the predicted risk of MRSA transmission to HCWs’ gowns and calculated the same performance measures as above. Further, we built a model with MRSA nares-culture results as the only predictor in the model to compare this test’s accuracy in predicting transmission with the performance of our model in the validation data set.
Decision curve analysis
We used decision curve analysis, a method that incorporates clinical consequences, to compare the clinical utility of placing residents on Contact Precautions under each model (16, 17). To construct the decision curve analysis, we followed the steps outlined by Vickers et al. (16). First, we calculated several threshold probabilities (pt) from our prediction rule model. Then we calculated the net benefit for each value of pt using the formula
We repeated the net benefit calculation for 3 other scenarios: 1) the net benefit of putting no resident on Contact Precautions (current policy), which was set at 0; 2) putting all nursing home residents on Contact Precautions; and 3) putting all MRSA nares culture-positive residents on Contact Precautions. These 4 scenarios were used to create a decision curve in which the net benefit of each model was plotted on the y-axis against the range of threshold probabilities on the x-axis.
The net benefit calculated by this formula provides a measure of benefit of the strategy of placing only those residents above a specific threshold of risk, as estimated by the prediction model, on Contact Precautions. This threshold is one that a rational person would use to treat someone, given the benefits of Contact Precautions for those likely to transmit MRSA and the costs of treating a person who will not transmit MRSA. The decision curve shows the net benefit of the prediction model at many different threshold levels (17). Thus, it provides the benefit at different levels of the relative value of the rational threshold for treatment.
All analyses were conducted and the calibration and decision curve analysis plots were created (18) in R Studio, version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria). The LASSO regression was performed using the R package “glmmLasso.” Model development and external validation were performed in accordance with the statement on transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (see Web Table 1, available at https://academic.oup.com/aje) (19).
RESULTS
There were 280 nursing home residents in the development data set who had both MRSA cultures and HCW swabs analyzed by the laboratory. Each resident had 1–22 (median, 5) HCW interactions, for a total of 2,200 HCW observations in the development set. From the validation cohort, there were 200 residents with 1–25 (median, 8) HCW interactions, for a total of 3,011 HCW interactions in the validation set.
The characteristics of each resident cohort are shown in Table 1. The average age of residents in the development set was 77.8 (standard deviation, 16) years; 66% were female, 80% were white, and 59% were short-stay residents; and the median length of stay prior to enrollment was 21 (interquartile range, 6–223) days. In the validation set, the average age of residents was 71.3 (standard deviation, 13) years; 96% were male, 65% were white, and 70% were long-stay residents; and the median length of stay prior to enrollment was 217 (interquartile range, 44–607) days.
Table 1.
Characteristics of Residents of Community-Based Nursing Homes Recruited From March 26, 2012, to May 1, 2014 (Development Set) and Residents of Department of Veterans Affairs–Based Nursing Homes Recruited From September 19, 2012, to January 6, 2016 (Validation Set) for a Study of Prediction of Methicillin-Resistant Staphylococcus aureus Transmission in Nursing Homes, United States, 2012–2016
| Overall | Development Set (n = 280) | Validation Set (n = 200) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, yearsa | 77.8 (16) | 71.3 (13) | ||
| Sex | ||||
| Female | 186 | 66 | 8 | 4 |
| Male | 94 | 34 | 192 | 96 |
| Race | ||||
| Asian | 1 | 0.5 | 1 | 0.5 |
| Black | 52 | 19 | 66 | 33 |
| Native Hawaiian or Pacific Islander | 1 | 0.5 | 1 | 0.5 |
| White | 222 | 80 | 132 | 66 |
| Patient type | ||||
| Rehabilitation | 164 | 59 | 60 | 30 |
| Residential | 116 | 41 | 140 | 70 |
| Length of stay prior to enrollment, daysb | 21 (6–223) | 217 (44–607) | ||
| Dependency on HCWc | ||||
| No | 47 | 17 | 110 | 55 |
| Yes | 233 | 83 | 90 | 45 |
| Use of any medical device | ||||
| No | 244 | 87 | 143 | 71 |
| Yes | 36 | 13 | 57 | 29 |
| Diabetes mellitus | ||||
| No | 176 | 63 | 140 | 70 |
| Yes | 103 | 37 | 60 | 30 |
| Chronic skin breakdownd | ||||
| No | 229 | 82 | 143 | 73 |
| Yes | 50 | 18 | 54 | 27 |
| Antibiotic use in past 7 days | ||||
| No | 211 | 77 | 150 | 75 |
| Yes | 63 | 23 | 50 | 25 |
| Hospitalized in the last 3 months | ||||
| No | 101 | 36 | 140 | 70 |
| Yes | 178 | 64 | 60 | 30 |
| MRSA colonization in anterior nares | ||||
| No | 178 | 64 | 114 | 57 |
| Yes | 102 | 36 | 86 | 43 |
| Transmission of MRSA to HCWs’ gowns | ||||
| No | 179 | 64 | 130 | 65 |
| Yes | 100 | 36 | 70 | 35 |
Abbreviations: HCW, health-care worker; MRSA, methicillin-resistant Staphylococcus aureus.
a Values are expressed as mean (standard deviation).
b Values are expressed as median (interquartile range).
c Dependency on an HCW was defined as having a dependency score of 3 (requiring extensive assistance) or 4 (total dependence) for any of the following activities: dressing, hygiene, toileting, or transfer.
d Any type of skin breakdown, excluding surgical wounds.
In the development set, 190 (9%) resident-HCW interactions led to transmission of MRSA to HCWs’ gowns. The predictors included in the clinical prediction rule were sex, race, HCW dependency, the presence of any medical device, diabetes, and chronic skin breakdown. The shrunken regression coefficients for each parameter in this model are shown in Table 2.
Table 2.
Parameters Included in a Prediction Modela for Transmission of Methicillin-Resistant Staphylococcus aureus in Nursing Homes, United States, 2012–2016
| Parameter | β Coefficient From Development Modelb |
|---|---|
| Intercept | −1.42 |
| Use of any medical device | 0.59 |
| Chronic skin breakdownc | 0.21 |
| Dependency on HCWd | 0.61 |
| Diabetes mellitus | 0.06 |
| Male sex | 0.54 |
| White race | −0.94 |
Abbreviation: HCW, health-care worker.
a The model was developed among residents of community-based nursing homes recruited from March 26, 2012, to May 1, 2014 (development set) and applied to residents of Department of Veterans Affairs–based nursing homes recruited from September 19, 2012, to January 6, 2016 (validation set).
b The logit of the probability that a resident would transmit methicillin-resistant Staphylococcus aureus to an HCW was estimated by summing the β coefficients from the model (including the intercept) multiplied by the value of each variable for each resident. The risk of transmission for each resident was then calculated as 1/(1 + e−logit).
c Any type of skin breakdown, excluding surgical wounds.
d Dependency on an HCW was defined as having a dependency score of 3 (requiring extensive assistance) or 4 (total dependence) for any of the following activities: dressing, hygiene, toileting, or transfer.
Table 3 shows the performance measures of the clinical prediction rule and the MRSA culture model in the development set. The C statistic for the clinical prediction rule was 0.70 (95% confidence interval (CI): 0.66, 0.74) and that for the MRSA nares culture was 0.62 (95% CI: 0.59, 0.66). The median value of the predicted probabilities from the prediction model was used to calculate sensitivity as 76% (95% CI: 69, 82) and specificity as 49% (95% CI: 47, 51), as compared with the MRSA culture model, where sensitivity was 61% (95% CI: 53, 67) and specificity was 64% (95% CI: 62, 66). The models had comparable positive and negative predictive values. The prediction model’s calibration slope was 1.30, and the calibration curve is shown in Figure 1A. The slope and the calibration curve indicated that the model was well calibrated in that the predicted risk was roughly equal to the observed transmission in the sample.
Table 3.
Performance Metrics for a Clinical Prediction Rule and a MRSA-Culture-Only Model Created to Predict MRSA Transmission in Nursing Homes Using Development and Validation Data Sets, United States, 2012–2016
| Modela | C Statistic | 95% CI | Sensitivity, % | 95% CI | Specificity, % | 95% CI | Positive Predictive Value | 95% CI | Negative Predictive Value | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| Development set | ||||||||||
| Prediction rule | 0.70 | 0.66, 0.74 | 76 | 69, 82 | 49 | 47, 51 | 12 | 10, 14 | 96 | 94, 97 |
| MRSA positivity in anterior nares culture | 0.62 | 0.59, 0.66 | 61 | 53, 67 | 64 | 62, 66 | 14 | 11, 16 | 95 | 93, 96 |
| Validation set | ||||||||||
| Prediction rule | 0.48 | 0.44, 0.53 | 50 | 43, 58 | 49 | 47, 51 | 6 | 5, 8 | 94 | 92, 95 |
| MRSA positivity in anterior nares culture | 0.67 | 0.63, 0.70 | 78 | 72, 84 | 55 | 53, 57 | 10 | 9, 12 | 97 | 97, 98 |
Abbreviations: CI, confidence interval; MRSA, methicillin-resistant Staphylococcus aureus.
a The model was developed among residents of community-based nursing homes recruited from March 26, 2012, to May 1, 2014 (development set) and applied to residents of Department of Veterans Affairs–based nursing homes recruited from September 19, 2012, to January 6, 2016 (validation set).
Figure 1.
Calibration curves for a clinical rule predicting transmission of methicillin-resistant Staphylococcus aureus (MRSA) to health-care workers’ gowns in a community-based nursing home cohort (the “development cohort”; 2012–2014) (A) and a Department of Veterans Affairs nursing home cohort (the “validation cohort”; 2012–2016) (B), United States, 2012–2016. The graph shows the observed proportion of MRSA transmission plotted on the y-axis against the risk of MRSA transmission predicted by the model on the x-axis. The triangles represent the observed proportion in each model-defined decile of risk, the vertical lines show the 95% confidence intervals, and the dotted line is the locally weighted scatterplot smoothing (LOESS) smooth regression line. The dashed 45-degree line represents perfect model calibration.
To validate the model, we applied the shrunken β coefficients from the clinical prediction rule to the validation data set. In this cohort, 189 (6%) HCW-resident interactions led to HCW gown contamination. The performance of the clinical prediction rule and the MRSA culture in the validation data is shown in Table 3. The clinical prediction rule had a C statistic of 0.48 (95% CI: 0.44, 0.53), and the MRSA culture model C statistic was 0.67 (95% CI: 0.63, 0.70). Using the median value of the predicted probabilities as the cutpoint, we calculated the sensitivity of the prediction rule as 50% (95% CI: 43, 58) and the specificity as 49% (95% CI: 47, 51), whereas the MRSA model’s sensitivity was 78% (95% CI: 72, 84) and its specificity was 55% (95% CI: 53, 57). The positive and negative predictive values for the clinical prediction rule were lower than those of the MRSA-culture-only model. The prediction model’s calibration slope was 0.16, and the curve is shown in Figure 1B. The slope and the calibration curve were indicative of poor model calibration in the validation set, as the model overestimated the risk of transmission for much of the sample.
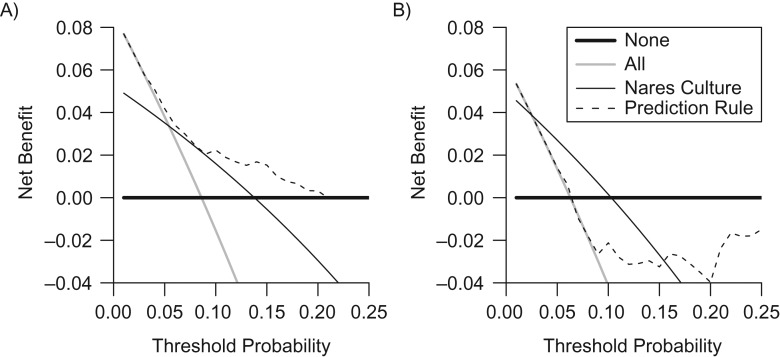
The results of the decision curve analysis in the development and validation cohorts are shown in Figures 2A and 2B, respectively. In both cohorts, the net benefit of each model was plotted against a range (5%–25%) of pt values. In the development set, the net benefit of the clinical prediction rule was higher than that of the nares-culture model at all levels of risk but lower than requiring Contact Precautions for all residents. In the validation set, the MRSA nares-culture model had a higher net benefit than the clinical prediction rule at every level of risk.
Figure 2.
Decision curve showing the net benefit of use of Contact Precautions for the prevention of methicillin-resistant Staphylococcus aureus (MRSA) transmission in nursing homes at varying threshold probabilities, United States, 2012–2016. A) Development cohort (a community-based nursing home cohort; 2012–2014); B) validation cohort (a Department of Veterans Affairs nursing home cohort; 2012–2016). Threshold probabilities: 1) none (heavy black line)—the net benefit of putting no resident on Contact Precautions (current policy), which is set at 0; 2) all (gray line)—putting all nursing home residents on Contact Precautions; 3) prediction rule (dashed line)—putting residents who meet a specified threshold of risk according to the prediction rule on Contact Precautions; or 4) MRSA-positive nares culture (light black line)—putting all MRSA nares culture-positive residents on Contact Precautions.
DISCUSSION
If all nursing homes were to screen residents for MRSA colonization using a nares culture to determine who should be placed on Contact Precautions, they would miss residents who tested falsely negative or were colonized at other body sites. More importantly, they would miss residents with the potential to transmit MRSA to HCWs and others. Indeed, 36% and 30% (in the development and validation sets, respectively) of the HCW interactions with MRSA culture-negative residents led to transmission of MRSA to HCWs’ gowns. We developed a clinical prediction rule that has better predictive accuracy in identifying residents who will transmit MRSA to HCWs' gowns than a MRSA nares culture. The model’s predictive ability was greatly reduced in the VA cohort, where the MRSA nares culture was a better predictor of MRSA transmission to HCWs. These findings suggest that a prediction rule could identify a large proportion of patients likely to transmit MRSA in community nursing homes (though not in the VA setting) independently of a nares culture for MRSA.
The risks and benefits of any policy concerning the implementation of Contact Precautions must be weighed carefully. Comparing prediction rules and choosing an appropriate cutoff can often be difficult because of the trade-offs between sensitivity and specificity. Decision curve analysis provides a visual method of comparing the clinical utility of several different models using the net benefit equation. This method has been used to assess the costs and benefits of prediction rules in other clinical settings (14, 17). We plotted the net benefit of 4 possible policy decisions: 1) use no Contact Precautions (current policy); 2) use Contact Precautions on everyone; 3) use a MRSA nares culture to identify residents likely to transmit MRSA; and 4) use a clinical prediction rule to identify residents likely to transmit MRSA. If no Contact Precautions were used in the community nursing homes, the potential risk of transmission of MRSA from a resident to an HCW would be 9%, and the net benefit of this policy would be 0. On the other hand, the implementation of universal Contact Precautions had the highest net benefit at low threshold probabilities, though this policy would also require unnecessary glove and gown use for 91% of HCW-resident interactions. Starting at threshold probabilities of around 5%, our clinical prediction rule begins to show a greater net benefit than the policy of universal Contact Precautions or use of a MRSA nares culture to screen residents. The decision curve analysis balances the benefits of identifying true-positive individuals (a resident who will transmit MRSA) with the harms of placing a false-positive individual (a resident who will not transmit MRSA) on Contact Precautions. Conversely, in the validation cohort, the MRSA nares culture had better clinical utility than either universal Contact Precautions or our prediction rule.
To our knowledge, we are the first investigators to have developed a prediction rule to predict transmission of a multiple-drug–resistant organism within a health-care setting. This area of research has previously focused on predicting colonization or infection, usually in inpatient settings. Haley et al. (20) developed a prediction rule for identifying hospitalized patients colonized or infected with MRSA in the absence of a MRSA nares culture. They found that history of a nursing home stay, previous MRSA infection, and a history of homelessness, jail stay, drug use, or risky sexual behavior were predictive of the presence of MRSA at admission (20). This model had better sensitivity (78%) and specificity (90%) than surveillance cultures (46% and 58%) (20). Furuno et al. (9) found that self-report of a previous hospital stay in the past year was predictive of MRSA acquisition during the current stay, with a sensitivity of 76% and a specificity of 35%. Interestingly, prior hospitalization was not a risk factor for MRSA transmission in either of our populations. However, the sensitivity of Furuno et al.’s prediction rule decreased when the rule was applied to inpatients in a VA medical system (sensitivity = 70% and specificity = 51%) (21). Because of reduced sensitivity, the authors concluded that this prediction rule would miss an unacceptably high number of colonized patients (21).
Prediction rules for MRSA colonization and infection have also been developed in the VA setting. Prior research in one VA medical center found 8 clinical risk factors associated with MRSA infection independent of a nares culture: homelessness, residence in a long-term care facility, incarceration, use of immunosuppressive medications, skin or soft tissue infection, spinal cord injury, previous MRSA colonization or infection, end-stage renal disease, and diabetes (22). Application of this rule identified 93% of patients who would go on to develop MRSA infection. Chronic skin breakdown and diabetes were risk factors for transmission in our development data set, but diabetes was not predictive of transmission in our validation set. In another VA inpatient population, Morgan et al. (23) found that antibiotic use in the past year predicted MRSA colonization at hospital admission and resulted in a sensitivity and specificity of 75% and 45%, respectively. While the performance of this model was similar to ours, we did not find that antibiotic exposure was associated with transmission of MRSA in the VA population.
Our analysis had some limitations. We did not focus on predicting which residents would acquire MRSA in our research, for several reasons. First, these cohort studies were not designed to detect incident MRSA acquisition. Second, while MRSA colonization is necessary for transmission to HCWs, colonization itself does not predict transmission. The prevalence of MRSA colonization was high in our cohorts (36% in the development cohort and 35% in the validation cohort), but transmission to HCWs’ gowns was low (9% and 6%, respectively), and not all MRSA-colonized residents transmitted MRSA to HCWs.
While our prediction model showed better clinical utility than MRSA nares culture in the development set, the C statistic was only moderate. This suggests that we were missing other, and perhaps stronger, predictors of MRSA transmission. While the proportions of HCW-resident interactions that led to transmission were similar between the development and validation sets, our prediction model had less-than-ideal predictive accuracy in the latter cohort. The patient population used to develop the prediction rule may have underperformed in the validation set because of underlying differences between the resident populations. The VA nursing home residents were younger, were largely male, had longer lengths of stay, and were less dependent on HCWs than residents in the community facilities. Access to nursing home care in civilian populations is different than access among eligible veterans, because of differences in financing (24). The results of the internal validation we conducted for the clinical prediction rule indicated that our model could work well in another community-based setting. However, the results of the validation study suggested that a separate clinical prediction rule should be developed for the VA setting.
As multiple-drug–resistant organisms such as MRSA become increasingly prevalent in health-care settings, the need for HCWs to don gowns and gloves to stop MRSA transmission becomes more important. Policy decisions regarding the use of Contact Precautions should be based on evidence from nursing homes, as these are resource-limited settings in comparison with acute-care hospitals (8). Future transmission studies in nursing homes are warranted to identify whether targeting gown-and-glove use toward residents with risk factors for MRSA transmission decreases MRSA acquisition and to validate the results of our clinical prediction rule in another community setting.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology and Public Health, School of Medicine, University of Maryland, Baltimore, Baltimore, Maryland (Sarah S. Jackson, Laurence S. Magder, Alison D. Lydecker, Mary-Claire Roghmann); and Veterans Affairs Maryland Health Care System, Baltimore, Maryland (Alison D. Lydecker, Mary-Claire Roghmann).
This project (clinical trial registration no. NCT01350479) was funded under grant 1R18HS019979-01A1 from the Agency for Healthcare Research and Quality, US Department of Health and Human Services, and was supported by Merit Review Award IIR 10-154 from the Health Services Research and Development Service, US Department of Veterans Affairs.
This paper was presented in part at the 51st Annual Meeting of the Society for Epidemiology Research, Baltimore, Maryland, June 19–22, 2018.
The contents of this article do not represent the official views of the US Department of Veterans Affairs or the US government.
Conflict of interest: none declared.
Abbreviations
- CI
confidence interval
- HCW
health-care worker
- MRSA
methicillin-resistant staphylococcus aureus
- VA
Department of Veterans Affairs
REFERENCES
- 1. Mody L, Roghmann MC. Infrequent use of isolation precautions in nursing homes: implications for an evolving population. J Am Geriatr Soc. 2017;65(3):472–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shurland SM, Stine OC, Venezia RA, et al. Colonization sites of USA300 methicillin-resistant Staphylococcus aureus in residents of extended care facilities. Infect Control Hosp Epidemiol. 2009;30(4):313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roghmann MC, Johnson JK, Sorkin JD, et al. Transmission of methicillin-resistant Staphylococcus aureus (MRSA) to healthcare worker gowns and gloves during care of nursing home residents. Infect Control Hosp Epidemiol. 2015;36(9):1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pineles L, Morgan DJ, Lydecker A, et al. Transmission of methicillin-resistant Staphylococcus aureus to health care worker gowns and gloves during care of residents in Veterans Affairs nursing homes. Am J Infect Control. 2017;45(9):947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36(3):281–285. [DOI] [PubMed] [Google Scholar]
- 6. Datta R, Huang SS. Risk of infection and death due to methicillin-resistant Staphylococcus aureus in long-term carriers. Clin Infect Dis. 2008;47(2):176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albrecht JS, Croft L, Morgan DJ, et al. Perceptions of gown and glove use to prevent methicillin-resistant Staphylococcus aureus transmission in nursing homes. J Am Med Dir Assoc. 2017;18(2):158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dumyati G, Stone ND, Nace DA, et al. Challenges and strategies for prevention of multidrug-resistant organism transmission in nursing homes. Curr Infect Dis Rep. 2017;19(4):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furuno JP, McGregor JC, Harris AD, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med. 2006;166(5):580–585. [DOI] [PubMed] [Google Scholar]
- 10. Epstein L, Mu Y, Belflower R, et al. Risk factors for invasive methicillin-resistant Staphylococcus aureus infection after recent discharge from an acute-care hospitalization, 2011–2013. Clin Infect Dis. 2016;62(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mody L, Krein SL, Saint S, et al. A targeted infection prevention intervention in nursing home residents with indwelling devices: a randomized clinical trial. JAMA Intern Med. 2015;175(5):714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clinical Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA: Clinical Laboratory Standards Institute; 2006. [Google Scholar]
- 13. Centers for Medicare and Medicaid Services Long-Term Care Facility Resident Assessment Instrument User’s Manual, 2014 (Version 3.0). Baltimore, MD: Centers for Medicare and Medicaid Services, 2014.
- 14. Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York, NY: Springer-Verlag New York; 2009. [Google Scholar]
- 15. Crowson CS, Atkinson EJ, Therneau TM. Assessing calibration of prognostic risk scores. Stat Methods Med Res. 2016;25(4):1692–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ. 2016;352:i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steyerberg EW. Evaluation of model performance. (Clinical Prediction Models, chapter 15, R code and data). http://www.clinicalpredictionmodels.org/doku.php?id=rcode_and_data:chapter15. Updated January 1, 2015. Accessed July 17, 2018.
- 19. Collins GS, Reitsma JB, Altman DG, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55–63. [DOI] [PubMed] [Google Scholar]
- 20. Haley CC, Mittal D, Laviolette A, et al. Methicillin-resistant Staphylococcus aureus infection or colonization present at hospital admission: multivariable risk factor screening to increase efficiency of surveillance culturing. J Clin Microbiol. 2007;45(9):3031–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riedel S, Von Stein D, Richardson K, et al. Development of a prediction rule for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus carriage in a Veterans Affairs Medical Center population. Infect Control Hosp Epidemiol. 2008;29(10):969–971. [DOI] [PubMed] [Google Scholar]
- 22. Jinno S, Chang S, Donskey CJ. A negative nares screen in combination with absence of clinical risk factors can be used to identify patients with very low likelihood of methicillin-resistant Staphylococcus aureus infection in a Veterans Affairs hospital. Am J Infect Control. 2012;40(9):782–786. [DOI] [PubMed] [Google Scholar]
- 23. Morgan DJ, Day HR, Furuno JP, et al. Improving efficiency in active surveillance for methicillin-resistant Staphylococcus aureus or vancomycin-resistant Enterococcus at hospital admission. Infect Control Hosp Epidemiol. 2010;31(12):1230–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Medicare.gov How can I pay for nursing home care? https://www.medicare.gov/what-medicare-covers/what-part-a-covers/how-can-i-pay-for-nursing-home-care. Accessed December 8, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.