Abstract
STUDY QUESTION
What is the relationship between abnormal BMI and semen quality?
SUMMARY ANSWER
Underweight was significantly associated with lower sperm concentration, total sperm number and total motile sperm count, while overweight was significantly associated with lower semen volume, total sperm number and total motile sperm count.
WHAT IS KNOWN ALREADY
Abnormal BMI has been associated with lower semen quality, but the results remain somewhat controversial. In addition, most previous studies have focused on the influence of obesity or overweight on semen quality, and evidence on the association between underweight and semen quality is rare.
STUDY DESIGN, SIZE, DURATION
This research was an observational study investigating 3966 sperm donors from a large sperm bank in Wuhan city, China. These donors passed the screening for sperm donation and underwent 29 949 semen examinations between 1 January 2013 and 9 April 2018.
PARTICIPANTS/MATERIALS, SETTING, METHODS
BMI was categorized into four groups: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obese (≥30 kg/m2). Semen volume, sperm concentration, total sperm number, total motility, progressive motility and total motile sperm count were determined by trained clinical technicians. Linear mixed models were used to conduct dose–response analyses between BMI and semen quality parameters.
MAIN RESULTS AND THE ROLE OF CHANCE
Underweight was significantly associated with a 3.0% (95% CI: 0.1%, 5.8%), 6.7% (1.9%, 11.3%) and 7.4% (2.2%, 12.4%) reduction in sperm concentration, total sperm number and total motile sperm count, respectively. Overweight was significantly associated with a 4.2% (1.6%, 6.8%), 3.9% (0.9%, 6.9%) and 3.6% (0.2%, 6.9%) reduction in semen volume, total sperm number and total motile sperm count, respectively. Non-linear models including continuous BMI as a natural cubic spline function yielded similar results.
LIMITATIONS, REASONS FOR CAUTION
Our study subjects were sperm donors who are typically young and healthy, and therefore not representative of the general male population. Caution should be paid in generalizing our results to other populations. Furthermore, we did not measure the donors’ weight repeatedly along with each semen donation; instead, we only measured it once during the screening, which may cause bias due to the variations of weight across time.
WIDER IMPLICATIONS OF THE FINDINGS
Our study provides evidence that underweight and overweight are associated with lower semen quality, and highlights the importance of maintaining a normal weight for men.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by the Health and Family Planning Commission of Hubei Province (Grant number WJ2015MA027), the Hubei Provincial Committee of the Communist Youth League of China, and Center for Global and Regional Environmental Research at the University of Iowa. The authors declare that there are no conflicts of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: BMI, semen quality, underweight, overweight, obesity
Introduction
Infertility continues to be a global public health issue and a major clinical concern, affecting 15% of reproductive-age couples (Mascarenhas et al., 2012; Jiang et al., 2015). It has been estimated that 70 million couples worldwide experience subfertility or infertility, and ~40% of cases are due to male factors (Sharlip et al., 2002; Legare et al., 2014; Salas-Huetos et al., 2017). Poor semen quality is well recognized as a major condition that causes male infertility (Radwan et al., 2016; Levine et al., 2017). Recently, a rigorous and comprehensive meta-analysis of studies conducted between 1973 and 2011 reported that among men from western countries, sperm counts (measured either by sperm concentration or total sperm number) have declined by more than 50% (Levine et al., 2017). Likewise, a variety of other studies have also reported that semen quality is continuously declining (Li et al., 2016; Sengupta et al., 2017; 2018). Although previous studies have demonstrated that some risk factors, including herbicides, pesticides and environmental pollutants, might be associated with decreased semen quality, the underlying causes of decreasing semen quality are largely uncertain (Xiao et al., 2001; Sharpe, 2010; Bloom et al., 2015; Chiu et al., 2016).
Numerous studies have provided preliminary evidence that abnormal BMI is linked with various adverse health consequences, such as increased risks of metabolic and cardiovascular diseases (Pi-Sunyer, 2009). Recent studies have investigated the associations between abnormal BMI and semen quality, but the results remain inconsistent (Jensen et al., 2004; Sermondade et al., 2013; Eisenberg et al., 2014; Tsao et al., 2015). For example, Chavarro et al. suggested that the BMI ≥ 35 kg/m2 was associated with lower ejaculate volume and lower total sperm number among 483 men attending an infertility clinic (Chavarro et al., 2010). Wang et al. also reported that the increasing BMI appeared to be linked with lower total sperm number and lower sperm concentrations among 2384 subfertile men in northern China (Wang et al., 2017). In contrast, a pooled meta-analysis of 31 studies, demonstrated that there were no significant associations between increased BMI and semen volume, sperm concentration or total sperm number (MacDonald et al., 2010). On the other hand, evidence on the association between underweight and semen quality is still rare.
Taken together, the current evidence on the association between BMI and semen quality is limited and inconclusive, and further studies are warranted. In this study, we conducted a large observational study of 3966 healthy sperm donors from Wuhan city, China, and performed dose–response analyses to quantitatively assess the association between BMI and semen quality.
Materials and Methods
Study population
According to the standard protocol for human sperm banking initiated by the Chinese Ministry of Health (Ping et al., 2011), a total of 3966 men aged 22–46 years were qualified as sperm donors at a large sperm bank in Wuhan, China between 1 January 2013 and 9 April 2018. All these sperm donors were in good health, both physically and psychologically, and had no history of genetic diseases. They also underwent and passed physical examinations and laboratory tests, which helped exclude individuals at high risk for sexually transmitted infections and genetic diseases. The donors’ semen quality at screening fulfilled the following criteria: (i) fresh semen should have a liquefaction time of <60 min, sperm concentration of ≥60 × 106/ml, progressive motility of ≥32%, and normal morphology percent of ≥15%; and (ii) post-thaw semen should have a motility of ≥40%, total motile sperm count of ≥12 × 106, and a frozen–thaw survival rate of ≥60%. During the study period, the 3966 sperm donors underwent a total of 29 949 semen examinations with abstinence periods of 2–7 days.
Ethical approval
This work was approved by the Ethical Committee of Hubei Provincial Center for Disease Control and Prevention. The requirement for informed consent was waived by the committee, because the data used in this study were collected from previously routine clinical procedures and were anonymous to all research investigators.
Data collection
Structured questionnaires were used by trained physicians to collect information on demographics and lifestyle, including date of birth, ethnicity, education, smoking, marital status, season at semen collection and abstinence period. Height and weight were determined once using the same scale in the physical examination for each donor.
Semen examination
Semen sample examinations were conducted by trained clinical technicians. Semen samples were collected in a private room near the laboratory by masturbating into a sterile plastic container. The samples were then liquefied in an incubator (37°C) for 30 min and analyzed for semen volume, sperm concentration, and sperm motility within 60 min. Semen volume was measured by weighing. To assess sperm concentration and motility, 10 μl of each semen sample was placed in a Makler chamber, covered by a cover glass and then examined at ×200 magnification. Ten of the 100 squares in the microscope field were randomly scanned and the sperm number was recorded by a cytometer. Total motility was defined as the sum of progressive motility and non-progressive motility. We calculated total sperm number as semen volume multiplied by sperm concentration, and calculated total motile sperm count as total sperm number multiplied by total motility.
Statistical analysis
BMI was calculated as weight (kg) divided by the square of height (m2). According the guidelines of World Health Organization (WHO), BMI was categorized as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) or obese (≥30 kg/m2) (World Health Organization, 2000). To test the robustness of our results, we also grouped BMI based on the Chinese criteria: underweight (<18.5 kg/m2), normal weight (18.5–23.9 kg/m2), overweight (24–27.9 kg/m2) and obese (≥28 kg/m2) for sensitivity analyses (Zhou, 2002). All semen quality parameters were log-transformed before statistical analyses due to their approximate log-normal distributions, and were compared across BMI categories using repeated-measures ANOVA followed by Dunnett’s tests.
We used linear mixed models with a subject-specific random intercept to examine the dose–response associations between BMI and semen quality parameters. The linear mixed model enabled us to account for correlations between repeated semen examinations for the same donor (Verbeke and Molenberghs, 2000). All models were adjusted for age, ethnicity, education, marital status, smoking, season at semen examination and abstinence period. Compared with normal weight, percent changes with the 95% CIs were estimated as for semen quality parameters associated with underweight, overweight and obesity. Furthermore, we included a natural cubic spline function of BMI with 4 degrees of freedom in the linear mixed model to identify the non-linear dose–response relationship between continuous BMI and semen quality; percent changes were estimated for each semen quality parameter in relation to continuous BMI (minimum to maximum by 0.1 kg/m2) with the median BMI in normal weight subjects as the referent.
The robustness of our results was assessed by sensitivity analyses, including (i) using Chinese criteria for BMI category; (ii) restricting the analysis to non-smokers; and (iii) using the first semen donation sample only. All analyses were performed using R version 3.4.2 (R Core Team, 2017). All P values were two-sided, and P < 0.05 was considered as statistically significant.
Results
Characteristics of the study subjects by BMI category are summarized in Table 1. The mean age of sperm donors with normal weight was significantly higher than that of underweight subjects, and significantly lower than that of overweight subjects. About 68% of the subjects did not smoke, and the distribution of smoking did not vary significantly by BMI category. Mean BMI was 22.4 (SD: 2.8) kg/m2, with 5.6% underweight, 76.8% normal, 16.6% overweight and 1.0% obese. The mean abstinence period was 5.4 (1.3) days, and there was no significant difference across BMI categories. The average intervals between the first and the last semen sample collection were 3.7 months, and 97.5% of the sperm donors completed their entire sperm donations within 1 year.
Table I.
Characteristics of the study subjects by BMI category.
| Characteristic | All subjects | BMI categorya | |||
|---|---|---|---|---|---|
| Underweight (n = 222) | Normal (n = 3046) | Overweight (n = 660) | Obese (n = 38) | ||
| Age, years | |||||
| <25 | 1291 (32.6) | 103 (46.4) | 1047 (34.4) | 129 (19.6) | 12 (31.6) |
| 25–29 | 1356 (34.2) | 75 (33.8) | 1042 (34.2) | 228 (34.5) | 11 (28.9) |
| ≥30 | 1319 (33.2) | 44 (19.8) | 957 (31.4) | 303 (45.9) | 15 (39.5) |
| Mean (SD) | 28.5 (5.5) | 26.8 (4.9) | 28.2 (5.4) | 30.3 (5.7) | 28.7 (5.9) |
| Ethnicity | |||||
| Han | 3844 (96.9) | 216 (97.3) | 2953 (96.9) | 640 (97.0) | 35 (92.1) |
| Others | 122 (3.1) | 6 (2.7) | 93 (3.1) | 20 (3.0) | 3 (7.9) |
| Education | |||||
| High school and lower | 1122 (28.3) | 84 (37.8) | 828 (27.2) | 200 (30.3) | 10 (26.3) |
| Junior college | 1376 (34.7) | 79 (35.6) | 1067 (35.0) | 219 (33.2) | 11 (28.9) |
| Undergraduate and higher | 1468 (37.0) | 59 (26.6) | 1151 (37.8) | 241 (36.5) | 17 (44.8) |
| Marital status | |||||
| Unmarried | 2333 (58.8) | 163 (73.4) | 1853 (60.8) | 296 (44.8) | 21 (55.3) |
| Married | 1367 (34.5) | 45 (20.3) | 982 (32.2) | 323 (48.9) | 17 (44.7) |
| Divorced | 90 (2.7) | 4 (1.8) | 69 (2.3) | 17 (2.6) | 0 (0.0) |
| Living with partner | 176 (4.4) | 10 (4.5) | 142 (4.7) | 24 (3.7) | 0 (0.0) |
| Current smoking status | |||||
| Non-smoker | 2694 (67.9) | 136 (61.3) | 2100 (68.9) | 433 (65.6) | 25 (65.8) |
| Smoker | 1272 (32.1) | 86 (38.7) | 946 (31.1) | 227 (34.4) | 13 (34.2) |
| Season at semen examination | |||||
| Winter (Dec, Jan, Feb) | 5759 (19.2) | 325 (17.9) | 4446 (19.5) | 936 (18.5) | 52 (17.2) |
| Spring (Mar, Apr, May) | 7257 (24.2) | 331 (18.2) | 5623 (24.7) | 1234 (24.3) | 69 (22.9) |
| Summer (Jun, Jul, Aug) | 8309 (27.7) | 524 (28.9) | 6228 (27.4) | 1472 (29.0) | 85 (28.1) |
| Autumn (Sep, Oct, Nov) | 8624 (28.9) | 635 (35.0) | 6465 (28.4) | 1428 (28.2) | 96 (31.8) |
| Abstinence period, days | |||||
| 2–3 | 3108 (10.4) | 201 (11.1) | 2341 (10.3) | 519 (10.2) | 47 (15.6) |
| 4–5 | 12 035 (40.2) | 641 (35.3) | 8648 (38.0) | 2629 (37.9) | 117 (38.7) |
| 6–7 | 14 806 (49.4) | 973 (53.6) | 11 773 (51.7) | 1922 (51.9) | 138 (45.7) |
| Mean (SD) | 5.4 (1.3) | 5.4 (1.4) | 5.4 (1.3) | 5.4 (1.3) | 5.2 (1.4) |
| No. semen samples per subject | |||||
| 0–5 | 1537 (38.8) | 80 (36.0) | 1185 (38.9) | 257 (38.9) | 15 (39.5) |
| 6–10 | 1504 (37.9) | 77 (34.7) | 1171 (38.4) | 244 (37.0) | 12 (31.6) |
| >10 | 925 (23.3) | 65 (29.3) | 690 (22.7) | 159 (24.1) | 11 (28.9) |
Data are shown as number (percentage, %), if not specified.
aBMI was categorized using the WHO criteria: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obese (≥30 kg/m2).
Compared with subjects with normal weight, the crude analyses showed that sperm concentration, total sperm number and total motile sperm count were significantly lower in underweight subjects (Table 2, all P < 0.05), while semen volume and total sperm number were significantly lower in overweight subjects (all P < 0.05). We did not observe any significant differences for total motility or progressive motility across BMI categories (all P > 0.05).
Table II.
Distribution of semen quality parameters by BMI category.
| Characteristic | All subjects | BMI categorya | |||
|---|---|---|---|---|---|
| Underweight (n = 1815) | Normal (n = 22 762) | Overweight (n = 5070) | Obese (n = 302) | ||
| Semen volume, ml | 3.0 (1.9) | 2.6 (1.8) | 3.0 (1.9) | 2.6 (1.8)b | 2.6 (2.0) |
| Sperm concentration, 106/ml | 62.0 (7.0) | 60.0 (15.0)b | 62.0 (7.0) | 62.0 (7.0) | 63.0 (8.0) |
| Total sperm number, 106 | 168.0 (110.0) | 156.0 (95.6)b | 170.0 (110.0) | 163.2 (99.5)b | 166.5 (112.0) |
| Total motility, % | 66.0 (6.0) | 65.0 (7.0) | 66.0 (6.0) | 66.0 (7.0) | 65.0 (6.0) |
| Progressive motility, % | 61.0 (4.0) | 60.0 (8.0) | 61.0 (4.0) | 61.0 (4.0) | 61.0 (4.0) |
| Total motile sperm count, 106 | 107.2 (70.6) | 101.2 (65.3)b | 108.0 (72.5) | 105.1 (66.4) | 109.1 (75.5) |
Data are shown as median (IQR). ‘n’ refers to the number of subjects in each BMI category.
aBMI was categorized using the WHO criteria: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obese (≥30 kg/m2). The number of underweight, normal weight, overweight and obese subjects were 222, 3046, 660 and 38, respectively. Semen parameters across BMI category were compared using repeated-measures ANOVA followed by Dunnett’s tests.
bCompared with normal, P < 0.05.
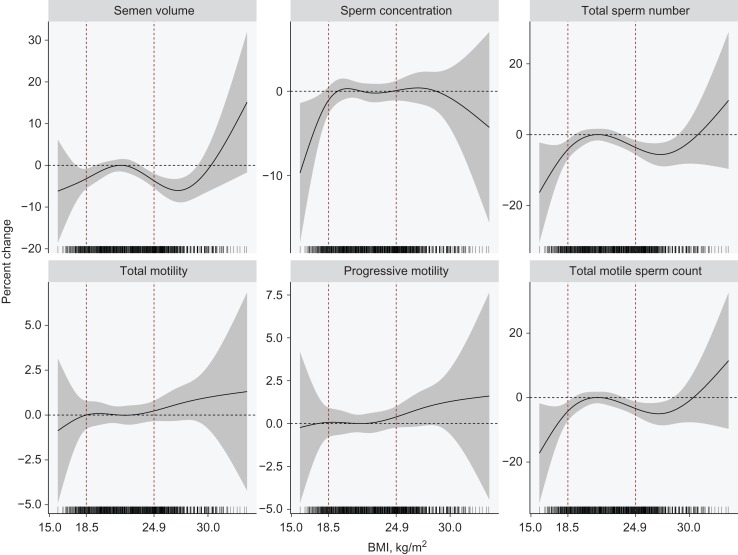
As shown in Table 3, underweight was significantly associated with a 3.0% (95% CI: 0.1%, 5.8%), 6.7% (1.9%, 11.3%) and 7.4% (2.2%, 12.4%) reduction in sperm concentration, total sperm number and total motile sperm count, respectively. Overweight was significantly associated with a 4.2% (1.6%, 6.8%), 3.9% (0.9%, 6.9%) and 3.6% (0.2%, 6.9%) reduction in semen volume, total sperm number and total motile sperm count, respectively. No significant associations were observed for sperm motility (all P > 0.05). Figure 1 shows the non-linear dose–response relationships between continuous BMI and semen quality parameters. When BMI was lower than the median BMI in normal weight subjects (21.6 kg/m2), semen volume, sperm concentration, total sperm number, total motile sperm count, but not sperm motility, appeared to decrease monotonically with decreasing BMI. For BMI from 21.6 up to 30 kg/m2, semen volume, total sperm number and total motile sperm count were inversely associated with increasing BMI. No significant relationships were observed between BMI and sperm motility.
Table III.
Estimated percent changes and 95% CIs for semen quality parameters associated with underweight, overweight and obesity compared with normal weighta.
| Characteristic | Underweight (n = 1815) | Normal (n = 22 762) | Overweight (n = 5070) | Obese (n = 302) | |||
|---|---|---|---|---|---|---|---|
| Percent change (95% CI) | P | Percent change (95% CI) | Percent change (95% CI) | P | Percent change (95% CI) | P | |
| Semen volume, ml | −3.6 (−7.7, 0.7) | 0.10 | 0 (ref.) | −4.2 (−6.8, −1.6) | 0.002 | 6.0 (−4.3, 17.5) | 0.27 |
| Sperm concentration, 106/ml | −3.0 (−5.8, −0.1) | 0.042 | 0 (ref.) | 0.3 (−1.5, 2.2) | 0.72 | 1.7 (−5.1, 9.0) | 0.63 |
| Total sperm number, 106 | −6.7 (−11.3, −1.9) | 0.007 | 0 (ref.) | −3.9 (−6.9, −0.9) | 0.012 | 7.1 (−4.6, 20.9) | 0.24 |
| Total motility, % | −0.7 (−2.1, 0.7) | 0.32 | 0 (ref.) | 0.3 (−0.6, 1.3) | 0.46 | 1.1 (−2.2, 4.6) | 0.51 |
| Progressive motility, % | −0.6 (−2.1, 1.0) | 0.47 | 0 (ref.) | 0.6 (−0.4, 1.6) | 0.25 | 1.1 (−2.6, 4.9) | 0.56 |
| Total motile sperm count, 106 | −7.4 (−12.4, −2.2) | 0.006 | 0 (ref.) | −3.6 (−6.9, −0.2) | 0.036 | 8.9 (−4.3, 24.0) | 0.20 |
N refers to the number of subjects in each BMI category.
aBMI was categorized using the WHO criteria: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obese (≥30 kg/m2). The number of underweight, normal weight, overweight and obese subjects were 222, 3046, 660 and 38, respectively.
Linear mixed models were used to estimate percent changes and 95% CIs with adjustment for age, ethnicity, education, smoking, marital status, abstinence period and season.
Figure 1.
Non-linear dose–response curves between continuous BMI and semen quality parameters. The BMI was included as a natural cubic spline function in the linear mixed model, adjusting for age, ethnicity, education, smoking, marital status, abstinence period and season. For better interpretation, the percent changes were estimated relative to the median value of normal weight (21.6 kg/m2). The vertical dotted lines gave the range of normal weight (18.5–24.9 kg/m2).
For BMI categories using Chinese criteria, the number of underweight, normal weight, overweight and obese subjects were 222 (5.6%), 2677 (67.5%), 923 (23.3%) and 144 (3.6%), respectively. Similar to results with the international BMI categories, underweight was inversely associated with sperm concentration, total sperm number and total motile sperm count, and overweight was significantly associated with lower semen volume and total sperm number (Supplementary Table S1). Among only the non-smokers, overweight was significantly associated with lower semen volume, total sperm number and total motile sperm count, which was consistent with that in all sperm donors (Supplementary Table S2, Fig. S1). In addition, we conducted analyses using the first sample for each sperm donor. The results showed that both underweight and overweight were significantly associated with lower semen volume, total sperm number and total motile sperm count (all P < 0.05), which was similar to the main analyses (Supplementary Table S3, Fig. S2).
Discussion
In this large observational study, we quantitatively investigated the association between BMI and semen quality in 3966 sperm donors from Wuhan, China, who underwent 29 949 semen examinations between 2013 and 2018. We found that underweight was inversely associated with sperm concentration, total sperm number and total motile sperm count, and overweight was significantly associated with lower semen volume, total sperm number and total motile sperm count. No significant associations were observed between obesity and semen quality in this population.
Current evidence on the relationship between abnormal BMI and semen quality remains controversial. Some studies have demonstrated that overweight or obesity is significantly linked with sperm concentration (Sekhavat and Moein, 2010; Hammiche et al., 2012; Belloc et al., 2014), total sperm number (Stewart et al., 2009; Chavarro et al., 2010; Sekhavat and Moein, 2010; Hammiche et al., 2012), sperm motility (Belloc et al., 2014) and sperm morphology (MacDonald et al., 2010; Shayeb et al., 2011). In comparison, other studies have not found significant associations (Duits et al., 2010; Eskandar et al., 2012; Eisenberg et al., 2014; Thomsen et al., 2014). Sermondade et al. (2013) conducted an updated systematic review and collaborative meta-analysis of 21 studies with 13 077 men from a general population or those attending fertility clinics, and did not observe a linear relationship between BMI and sperm concentration. However, they found that overweight and obesity were significantly associated with an increased risk of abnormal total sperm number, defined as azoospermia or oligozoospermia.
Only a few studies have examined the association between underweight and semen quality, because underweight subjects generally account for only a small proportion of study populations. Early in 2004, Jensen et al. studied 1558 Danish young men who attended a compulsory physical examination for military service between 1996 and 1998, and found that underweight (defined as BMI < 20 kg/m2) subjects had a significant reduction in sperm concentration and total sperm number, respectively (Jensen et al., 2004). Later in 2007, Qin et al. investigated 990 fertile men from a general population in 2001 and 2002, and reported that underweight was significantly associated with a lower sperm concentration and total sperm number (Qin et al., 2007). Consistent with our results, both of the two studies found that underweight was inversely associated with sperm concentration and total sperm number, and that there were no significant associations between underweight and sperm motility or semen volume. Similarly, the recent meta-analysis by Sermondade et al. observed an increased risk of abnormal sperm concentration associated with underweight, although the association between underweight and risk of abnormal total sperm number was insignificant.
Several possible reasons may account for the inconsistent associations between BMI and semen quality. First, study subjects have rarely been randomly selected in the general population due to low participation rates under 30% (Rolland et al., 2013); instead, previous studies have typically enrolled fertile men or infertile men undergoing assisted reproductive technology. We selected sperm donors who were relatively healthier in this study, which might lead to limited generalizability and yield different results (Sermondade et al., 2013). However, choosing sperm donors as the subjects has been a practical alternative to conduct semen quality studies (Sokol et al., 2006). Second, some studies have used self-reported measures of BMI, which are less accurate and may result in biased associations (Aggerholm et al., 2008; Hammoud et al., 2008). Third, most studies have used a single semen sample, which may inadequately represent a man’s testis function at any given time (Amann, 2009; Hammiche et al., 2012; Tsao et al., 2015). Finally, relatively small sample sizes, especially for underweight subjects in previous studies and obese objects in our study, may not provide sufficient statistical power to detect significant associations.
The pathophysiology behind the association between abnormal BMI and semen quality is unclear and likely complex. Overweight and obesity have been shown to affect the GnRH–FSH/LH pulse, which may impair Leydig or Sertoli cell functions and interfere with the release of sex hormones and production of mature sperm (Hammoud et al., 2008). In comparison, the relationship between underweight and lower semen quality may be due to undernutrition, which is known to have adverse effects on male reproductive hormone (Rocha-de-Melo and Guedes, 1997; Ramos et al., 2000; Santos et al., 2004). Undernutrition is associated with a decreased testosterone level (Chik et al., 1989), which may be a major reason for poor spermatogenesis through depriving the developing sperm of the signals required for normal maturation (Amory and Bremner, 1998).
Our findings have important public health implications. As the major reason for male infertility, poor semen quality has drawn much concern globally. Our study provides evidence that both underweight and overweight may adversely affect semen quality. It was estimated that the global prevalence of underweight for male adults in 2016 was 8.5%, and 39% for overweight (World Health Organization, 2016). These findings highlights the importance of maintaining a normal weight for men.
One major strength of our study is the large sample size with an average of eight repeated measures of semen quality, which allows us to account for within-subject variability and provides robust estimates. In addition, we firstly examined the non-linear dose–response relationship between BMI and semen quality. On the other hand, several limitations need to be discussed. First, our study subjects were sperm donors who were typically young and healthy, and were unable to represent the general male population. Caution should be used when generalizing our results to other populations. Second, we did not measure the donors’ weight repeatedly along with each semen quality test; instead, we only measured once during the screening, which may lead to misclassification due to the variations of weight across time. However, the BMI was unlikely to change dramatically, because BMI was measured at the time of first semen sample, and the whole duration of sperm donation (from the first semen sample to the last semen sample) for the studied sperm donors was only an average of 3.7 months. Third, although we adjusted for all available potential confounders, there is still the possibility that residual or unmeasured confounding partly contributed to the associations. Finally, BMI cannot distinguish between body fat and muscle mass. However, we did not collect information on physical activity or waist circumstance. Men who are physically active and muscular tend to be healthier and tip into overweight or obese status, which may lead to underestimation on the association between overweight/obesity and semen quality.
In conclusion, we found that underweight and overweight were both significantly associated with lower semen quality. Our findings highlight the importance of maintaining a normal weight for men.
Supplementary Material
Authors’ roles
All authors fulfill the criteria for authorship. Y.L. and T.M. designed the study, participated in data collection and analyses and revised the article. J.M. and L.W. conducted statistical analysis, summarized the results and drafted the article. Y.Z. participated in data analyses and interpretation and revised the article. C.X., H.Z. and Z.P. collected the clinical data, validated the data and revised the article. W.B. contributed significantly to the interpretation of the results and revised the article.
Funding
Health and Family Planning Commission of Hubei Province (Grant number WJ2015MA027), the Hubei Provincial Committee of the Communist Youth League of China and the Center for Global and Regional Environmental Research at the University of Iowa.
Conflict of interest
None of the authors has any conflict of interest to declare.
References
- Aggerholm AS, Thulstrup AM, Toft G, Ramlau-Hansen CH, Bonde JP. Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Fertil Steril 2008;90:619–626. [DOI] [PubMed] [Google Scholar]
- Amann RP. Considerations in evaluating human spermatogenesis on the basis of total sperm per ejaculate. J Androl 2009;30:626–641. [DOI] [PubMed] [Google Scholar]
- Amory JK, Bremner WJ. The use of testosterone as a male contraceptive. Baillieres Clin Endocrinol Metab 1998;12:471–484. [DOI] [PubMed] [Google Scholar]
- Belloc S, Cohen-Bacrie M, Amar E, Izard V, Benkhalifa M, Dalleac A, de Mouzon J. High body mass index has a deleterious effect on semen parameters except morphology: results from a large cohort study. Fertil Steril 2014;102:1268–1273. [DOI] [PubMed] [Google Scholar]
- Bloom MS, Whitcomb BW, Chen Z, Ye A, Kannan K, Buck Louis GM. Associations between urinary phthalate concentrations and semen quality parameters in a general population. Hum Reprod 2015;30:2645–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril 2010;93:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chik CL, Ho AK, Brown GM. Effect of pinealectomy on the undernutrition-induced suppression of the reproductive axis in rats. Acta Endocrinol (Copenh) 1989;120:569–573. [DOI] [PubMed] [Google Scholar]
- Chiu YH, Gaskins AJ, Williams PL, Mendiola J, Jorgensen N, Levine H, Hauser R, Swan SH, Chavarro JE. Intake of fruits and vegetables with low-to-moderate pesticide residues is positively associated with semen-quality parameters among young healthy men. J Nutr 2016;146:1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits FH, van Wely M, van der Veen F, Gianotten J. Healthy overweight male partners of subfertile couples should not worry about their semen quality. Fertil Steril 2010;94:1356–1359. [DOI] [PubMed] [Google Scholar]
- Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, Buck Louis GM. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod 2014;29:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandar M, Al-Asmari M, Babu Chaduvula S, Al-Shahrani M, Al-Sunaidi M, Almushait M, Donia O, Al-Fifi S. Impact of male obesity on semen quality and serum sex hormones. Adv Urol 2012;2012:407601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammiche F, Laven JS, Twigt JM, Boellaard WP, Steegers EA, Steegers-Theunissen RP. Body mass index and central adiposity are associated with sperm quality in men of subfertile couples. Hum Reprod 2012;27:2365–2372. [DOI] [PubMed] [Google Scholar]
- Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW. Male obesity and alteration in sperm parameters. Fertil Steril 2008;90:2222–2225. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Andersson AM, Jorgensen N, Andersen AG, Carlsen E, Petersen JH, Skakkebaek NE. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril 2004;82:863–870. [DOI] [PubMed] [Google Scholar]
- Jiang W, Sun H, Zhang J, Zhou Q, Wu Q, Li T, Zhang C, Li W, Zhang M, Xia X. Polymorphisms in Protamine 1 and Protamine 2 predict the risk of male infertility: a meta-analysis. Sci Rep 2015;5:15300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legare C, Droit A, Fournier F, Bourassa S, Force A, Cloutier F, Tremblay R, Sullivan R. Investigation of male infertility using quantitative comparative proteomics. J Proteome Res 2014;13:5403–5414. [DOI] [PubMed] [Google Scholar]
- Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 2017;23:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CJ, Tzeng CR, Chen RY, Han BC, Yeh CY, Chien LC. Decline in semen quality in men in northern Taiwan between 2001 and 2010. Chin J Physiol 2016;59:355–365. [DOI] [PubMed] [Google Scholar]
- MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update 2010;16:293–311. [DOI] [PubMed] [Google Scholar]
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012;9:e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi-Sunyer X. The medical risks of obesity. Postgrad Med 2009;121:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping P, Zhu WB, Zhang XZ, Li YS, Wang QX, Cao XR, Liu Y, Dai HL, Huang YR, Li Z. Sperm donation and its application in China: a 7-year multicenter retrospective study. Asian J Androl 2011;13:644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin DD, Yuan W, Zhou WJ, Cui YQ, Wu JQ, Gao ES. Do reproductive hormones explain the association between body mass index and semen quality? Asian J Androl 2007;9:827–834. [DOI] [PubMed] [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna, Austria, 2017. https://www.R-project.org/ (29 October 2018, date last accessed).
- Radwan M, Jurewicz J, Polanska K, Sobala W, Radwan P, Bochenek M, Hanke W. Exposure to ambient air pollution--does it affect semen quality and the level of reproductive hormones? Ann Hum Biol 2016;43:50–56. [DOI] [PubMed] [Google Scholar]
- Ramos CF, Teixeira CV, Passos MC, Pazos-Moura CC, Lisboa PC, Curty FH, de Moura EG. Low-protein diet changes thyroid function in lactating rats. Proc Soc Exp Biol Med 2000;224:256–263. [DOI] [PubMed] [Google Scholar]
- Rocha-de-Melo AP, Guedes RC. Spreading depression is facilitated in adult rats previously submitted to short episodes of malnutrition during the lactation period. Braz J Med Biol Res 1997;30:663–669. [DOI] [PubMed] [Google Scholar]
- Rolland M, Le Moal J, Wagner V, Royere D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod 2013;28:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Huetos A, Bullo M, Salas-Salvado J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update 2017;23:371–389. [DOI] [PubMed] [Google Scholar]
- Santos AM, Ferraz MR, Teixeira CV, Sampaio FJ, da Fonte Ramos C. Effects of undernutrition on serum and testicular testosterone levels and sexual function in adult rats. Horm Metab Res 2004;36:27–33. [DOI] [PubMed] [Google Scholar]
- Sekhavat L, Moein MR. The effect of male body mass index on sperm parameters. Aging Male 2010;13:155–158. [DOI] [PubMed] [Google Scholar]
- Sengupta P, Borges E Jr, Dutta S, Krajewska-Kulak E. Decline in sperm count in European men during the past 50 years. Hum Exp Toxicol 2018;37:247–255. [DOI] [PubMed] [Google Scholar]
- Sengupta P, Nwagha U, Dutta S, Krajewska-Kulak E, Izuka E. Evidence for decreasing sperm count in African population from 1965 to 2015. Afr Health Sci 2017;17:418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, Van Wely M, Cao J, Martini AC, Eskandar M et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update 2013;19:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, Schlegel PN, Howards SS, Nehra A, Damewood MD et al. Best practice policies for male infertility. Fertil Steril 2002;77:873–882. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philos Trans R Soc Lond B Biol Sci 2010;365:1697–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayeb AG, Harrild K, Mathers E, Bhattacharya S. An exploration of the association between male body mass index and semen quality. Reprod Biomed Online 2011;23:717–723. [DOI] [PubMed] [Google Scholar]
- Sokol RZ, Kraft P, Fowler IM, Mamet R, Kim E, Berhane KT. Exposure to environmental ozone alters semen quality. Environ Health Perspect 2006;114:360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart TM, Liu DY, Garrett C, Jorgensen N, Brown EH, Baker HW. Associations between andrological measures, hormones and semen quality in fertile Australian men: inverse relationship between obesity and sperm output. Hum Reprod 2009;24:1561–1568. [DOI] [PubMed] [Google Scholar]
- Thomsen L, Humaidan P, Bungum L, Bungum M. The impact of male overweight on semen quality and outcome of assisted reproduction. Asian J Androl 2014;16:749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CW, Liu CY, Chou YC, Cha TL, Chen SC, Hsu CY. Exploration of the association between obesity and semen quality in a 7630 male population. PLoS One 2015;10:e0119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. Technometrics 2000;43:375. [Google Scholar]
- Wang EY, Huang Y, Du QY, Yao GD, Sun YP. Body mass index effects sperm quality: a retrospective study in Northern China. Asian J Androl 2017;19:234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Obesity: Preventing and Managing the Global Epidemic: Report on a WHO Consultation. WHO Technical Report Series 894. 2000. http://www.who.int/iris/handle/10665/42330 (29 October 2018, date last accessed). [PubMed]
- World Health Organization Prevalence of Underweight Among Adults, BMI < 18, Crude Estimates by WHO Region 2016. http://apps.who.int/gho/data/view.main.NCDBMILT18CREGv (29 October 2018, date last accessed) .
- World Health Organization Prevalence of Obesity and Overweight 2017. http://www.who.int/gho/ncd/risk_factors/overweight/en/ (29 October 2018, date last accessed).
- Xiao G, Pan C, Cai Y, Lin H, Fu Z. Effect of benzene, toluene, xylene on the semen quality and the function of accessory gonad of exposed workers. Ind Health 2001;39:206–210. [DOI] [PubMed] [Google Scholar]
- Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 2002;15:83–96. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.