Abstract
An interneuron alteration has been proposed as a source for the modified balance of excitation / inhibition in the cerebral cortex in autism. We previously demonstrated a decreased number of parvalbumin (PV)-expressing interneurons in prefrontal cortex in autism. PV-expressing interneurons include chandelier (Ch) and basket (Bsk) cells. We asked whether the decreased PV+ interneurons affected both Ch cells and Bsk cells in autism. The lack of single markers to specifically label Ch cells or Bsk cells presented an obstacle for addressing this question. We devised a method to discern between PV-Ch and PV-Bsk cells based on the differential expression of Vicia villosa lectin (VVA). VVA binds to N-acetylgalactosamine, that is present in the perineuronal net surrounding some cell types where it plays a role in intercellular communication. N-acetylgalactosamine is present in the perineuronal net surrounding Bsk but not Ch cells. We found that the number of Ch cells is consistently decreased in the prefrontal cortex of autistic (n = 10) when compared with control (n = 10) cases, while the number of Bsk cells is not as severely affected. This finding expand our understanding of GABAergic system functioning in the human cerebral cortex in autism, which will impact translational research directed towards providing better treatment paradigms for individuals with autism.
Keywords: autism, basket cells, chandelier cell, parvalbumin, prefrontal cortex
Introduction
A modification in the function of cortical interneurons has been proposed as a potential source for the altered balance of excitation/inhibition in the cerebral cortex in autism. Our recent finding that the number of parvalbumin (PV)-expressing interneurons is decreased in several areas of the prefrontal cortex in autism (Hashemi et al. 2016), provides a potential explanation for altered excitation/inhibition in the cerebral cortex. PV+ interneurons modulate the output of pyramidal neurons by directly innervating the soma and the axon initial segment. PV-expressing interneurons include 2 distinct subtypes: chandelier cells (Ch) and basket (Bsk) cells (Somogyi 1977; DeFelipe and Gonzalez-Albo 1998). Bsk cells are more numerous than Ch cells and together Ch and Bsk cells account for up to 50% of total interneurons in some areas of the cortex. Both Ch and Bsk cells are fast-spiking interneurons that innervate a large number of pyramidal neurons. However, Ch cells and Bsk cells exhibit substantially different connectional properties. Ch cells innervate the axon initial segment of pyramidal neurons, while Bsk cells innervate the soma and proximal dendrites of pyramidal neurons. A single Ch or Bsk cell can innervate up to 200 pyramidal cells. Therefore, the loss of even a small number of PV+ cells could potentially change and impair pyramidal neuron output and regional cortical function (DeFelipe 1999).
Here, we asked whether the number of PV+ Ch and PV+ Bsk cells was equally or differentially affected in the prefrontal cortex in autism. However, the lack of single markers to specifically label Ch cells or Bsk cells presented an obstacle for addressing this question. We therefore devised a method to discern between these 2 PV-expressing cell types based on the differential expression of Vicia villosa lectin (VVA) by Ch and Bsk cells. VVA lectin binds to the carbohydrate N-acetylgalactosamine, which is present in the perineuronal net surrounding some cell types. The perineuronal net has a net-like appearance that results from the perisynaptic arrangement of astrocytic processes that surround cells as well as proteoglycans and glycoproteins present in the extracellular matrix surrounding these cells (Bruckner et al. 1993; Murakami and Ohtsuka 2003). VVA is not a constituent of the cells themselves, but accumulates within the perineural net, along the membranes and extracellular space surrounding these cells. Functions associated with the perineural net include intercellular communication, cell polarity, and synapse formation and stabilization. In the visual cortex, the perineural net inhibits experience dependent plasticity (Pizzorusso et al. 2002; Ye and Miao 2013), and VVA in particular, inhibits the invasion of the tectum by retinal axons (Inoue and Sanes 1997). The perineural net has also been shown altered in traumatic stages, such as in traumatic brain injury (Bhattacharyya et al. 2015).
Based in intracellular electrophysiological recording and dye labeling experiments, it was determined that N-acetylgalactosamine is present in the perineuronal net surrounding Bsk cells and other cell types, but it is not associated with Ch cell bodies or cellular processes, including cartridges (Mulligan et al. 1989; Naegele and Katz 1990; Schweizer et al. 1993). In one of these experiments performed in cats, electrophysiological recording were performed in cells intracellularly injected with Lucifer yellow that after fixation were immunostained with VVA-Texas red. Double-labeled and single-labeled cells were reconstructed with camera lucida. Based on morphology and electrophysiological properties they were able to unambiguously determine that Ch cells are not surrounded by VVA. Among the Bsk cells the exception are the small Bsk cells that account for 2% of the total number of BSk cells and which soma and dendritic arbor are confined to layer IV (Naegele and Katz 1990). We double stained human prefrontal cortex with PV antibodies and VVA lectin to distinguish Ch cells from Bsk cells and quantify their numbers. Our data demonstrate that the number of PV+ Ch cells is consistently decreased in the areas of the autistic prefrontal cortex included in this study, while the number of PV+ Bsk cells is not as severely affected in autistic prefrontal cortex. These data provide evidence that deficits in a specific neuronal population may contribute to the altered cognitive functioning in some cases of autism.
Material and Methods
Samples
We collected prefrontal areas BA9, BA46, and BA47 from 10 autism and 10 control age-matched cases (these cases were included in Hashemi et al. 2016; see Table 1). The tissue was obtained from the Autism Tissue Program, currently known as Autism BrainNet, and the UC Davis Medical Center. The autistic cases were all diagnosed as classical autism. The diagnosis of autism was confirmed by standard postmortem use of the autism diagnostic interview-revised (ADI-R) in all cases. ADI-R data included sociability, verbal/nonverbal communication, and repetition scores. The presence of mental retardation and seizures was also reported. The control cases were determined to be free of neurological disorders, including autism, based on medical records and information gathered at the time of death from next of kin. Cases were all males, except for one control case. Age, hemisphere, brain weight, severity of symptoms, and postmortem interval varied from case to case; details are listed in Table 1. The 10 control cases averaged 45.0 years old with an age range of 6–56 years old. The 10 autism cases averaged 37.8 with a range from 7 to 48 years old. One of the subjects with autism also suffered from seizures, and 3 from mental retardation (IQ < 70), (see Table 1). Control patients did not have a history of seizures, mental retardation, or dementia.
Table 1.
Information for the autism and control cases included in this study. Information includes sex, age, diagnosis, PMI (postmortem interval), brain mass, hemisphere, cause of death, the presence of seizures and/or mental retardation, and the scores for repetition, verbal or nonverbal, and social abilities
| Case ID | Sex | Age | Diagnosis | PMI (Hours) | Brain mass (g) | Hemisphere | Cause of death | Seizure | Mental retardation | Rept. score | Verbal score | Nonverbal score | Social score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UCD-13AP86 | M | 6 | Control | NK | NK | NK | NK | NA | NA | NA | NA | NA | NA |
| AN07444 (B7387) | M | 17 | Control | 30.75 | 1460 | Right | Asphyxia | NA | NA | NA | NA | NA | NA |
| AN00544 (B6951) | M | 17 | Control | 28.92 | 1250 | Left | NK | NA | NA | NA | NA | NA | NA |
| AN19760 (B5873) | M | 28 | Control | 23.25 | 1580 | Right | NK | NA | NA | NA | NA | NA | NA |
| AN12137 (B5352) | M | 31 | Control | 32.92 | 1810 | Right | Asphyxia | NA | NA | NA | NA | NA | NA |
| AN15566 (B6316) | F | 32 | Control | 28.92 | 1360 | Right | NK | NA | NA | NA | NA | NA | NA |
| AN05475 (B7561) | M | 39 | Control | NK | 1350 | Right | Cardiac arrest | NA | NA | NA | NA | NA | NA |
| AN17868 (B5812) | M | 46 | Control | 18.78 | 1588 | Right | Cardiac arrest | NA | NA | NA | NA | NA | NA |
| AN19442 (B6259) | M | 50 | Control | 20.4 | 1740 | Right | NK | NA | NA | NA | NA | NA | NA |
| AN13295 (B6860) | M | 56 | Control | 22.12 | 1370 | Left | NK | NA | NA | NA | NA | NA | NA |
| AN03221 (B6242) | M | 7 | Autism | 11.42 | 1560 | NK | Drowning | No | No | 8 | 16 | NA | 27 |
| AN01293 (B6349) | M | 9 | Autism | 4.41 | 1690 | Left | Cardiac arrest | No | Yes | 5 | NA | 12 | 26 |
| AN00394 (B4323) | M | 14 | Autism | 10.3 | 1615 | NK | Cardiac arrest | No | No | NK | NK | NK | NK |
| AN00764 (B5144) | M | 20 | Autism | 23.66 | 1144 | Right | Accident | No | No | NK | NK | NK | NK |
| AN00493 (B5000) | M | 27 | Autism | 8.3 | 1575 | Right | Drowning | No | Yes | NK | NK | NK | NK |
| AN18892 (B4871) | M | 31 | Autism | 99 | 1600 | Left | Gun shot | No | No | NK | NK | NK | NK |
| AN09901 (B5764) | M | 32 | Autism | 28.65 | 1694 | Right | Heat shock | No | No | NK | NK | NK | NK |
| AN06746 (B4541) | M | 44 | Autism | 30.8 | 1530 | NK | Cardiac arrest | No | No | NK | NK | NK | NK |
| AN19534 (B6143) | M | 45 | Autism | 40.16 | 1360 | Right | Aspiration | Yes | No | 4 | NA | 14 | 27 |
| AN18838 (B6202) | M | 48 | Autism | NK | 1260 | Right | Asphyxia | No | Yes | 8 | NA | 14 | 29 |
Tissue Processing
After removal of the brain from the skull, brain tissue was immersed in 10% buffered formalin for at least 8 weeks. A 3 cm block of each of the prefrontal areas (BA46, BA47, BA9) was cut based on their anatomical location in the cortex following Broadmann cortical classification. The block was placed into a cryoprotectant solution (10% glycerol in 0.1 M phosphate buffer for 2 days and 20% glycerol for 5 days) in preparation for freezing. Tissue blocks were frozen with 2-methyl butane (isopentane) and serially sectioned on a cryostat. We obtained between 80 and 130 sections per block. One section from each block was stained with cresyl violet (Nissl). Remaining sections adjacent to the Nissl-stained section were used for immunoschemistry.
Anatomical and Cytoarchitectural Considerations
We used Brodmann cortical neuroanatomy to isolate a block containing area BA9 in the superior prefrontal cortex, BA46 in the middle prefrontal cortex, and BA47 in the inferior prefrontal cortex, from each case. Summarizing, BA9 (magnocellular “granular frontal area”, or FDm of von Economo) consists of a strip-like zone whose medial boundary is the callosomarginal sulcus and its ventral boundary is the inferior frontal sulcus. BA46 (“middle granular frontal area”, or FD∆ of von Economo) includes the middle third of the middle and the most anterior part of the inferior frontal gyri at the transition to the orbital surface. BA47 (“orbital area”, or FFα of von Economo) surrounds the posterior branches of the orbital sulcus, and laterally crosses the orbital part of the inferior frontal gyrus).
We cut 14 µm coronal sections on a cryostat, stained one section with Nissl, and based on von Economo cytoarchitecture selected the region in each section that exactly matched the von Economo description for BA9, BA46, and BA47. Briefly, in BA46 cortical layers are more differentiated than those in BA9. Layers III and V exhibit a clear sub-lamination in BA9, but not in BA46. Neurons in BA46 are more homogeneous in size than those in BA9. BA9 is characterized by the presence of large pyramidal cells in sub-layers IIIc and Va1, a pale violet-stained sub-layer Vb, and a narrow and indistinct layer IV. BA46 is characterized by relatively low cell-packing density and a violet-pale sub-layer Vb. BA45 and BA11 surround BA47. BA45 contains a violet-pale sub-layer Vb, while BA47 layer Vb has a higher cell density. BA45 has large and packed pyramidal cells in layer IIIc, which is replaced by small and medium cells in BA47. BA11 lacks layer IV, has small size neuronal cell bodies in all layers, a non-differentiated layers I–II, and lacks a clear border between layers II and III and between layer VI and white matter (von Economo 1929). For more cytoarchitectural considerations see (Hashemi et al. 2016). Within selected regions of interest we chose a 3 mm wide bin extending perpendicularly from the pia through the thickness of the cortical gray matter to include all cortical layers. On adjacent sections we performed double immunostaining for PV and VVA.
Histochemistry
We performed enzymatic immunohistochemistry to label specific cell types and histochemistry to label VVA+ cells. For primary antibodies we used monoclonal mouse anti-PV (1:500, Swant 235), mouse anti-NeuN (1:500, Chemicon, MAB377) mouse anti-calbindin (CB-D28k; 1:500, Swant 300), mouse anti-calretinin (CR; 1:500, Swant 7697). We used donkey anti-mouse secondary antibodies that were conjugated with biotin (1:200, Abcam), amplified with avidin-biotin complex (ABC), and developed with DAB or ImmPACT SG substrates (Vector). We used biotinilated-VVA (1:100, Vector B-1235), amplified with ABC, and developed with ImmPACT SG substrates (Vector). All tissue sections were then co-stained with cresyl violet, dehydrated and defatted, mounted on glass slides, and coverslipped with DPX (Martinez-Cerdeno and Clasca 2002; Martinez-Cerdeno et al. 2002; Martinez-Cerdeno et al. 2003).
Quantification
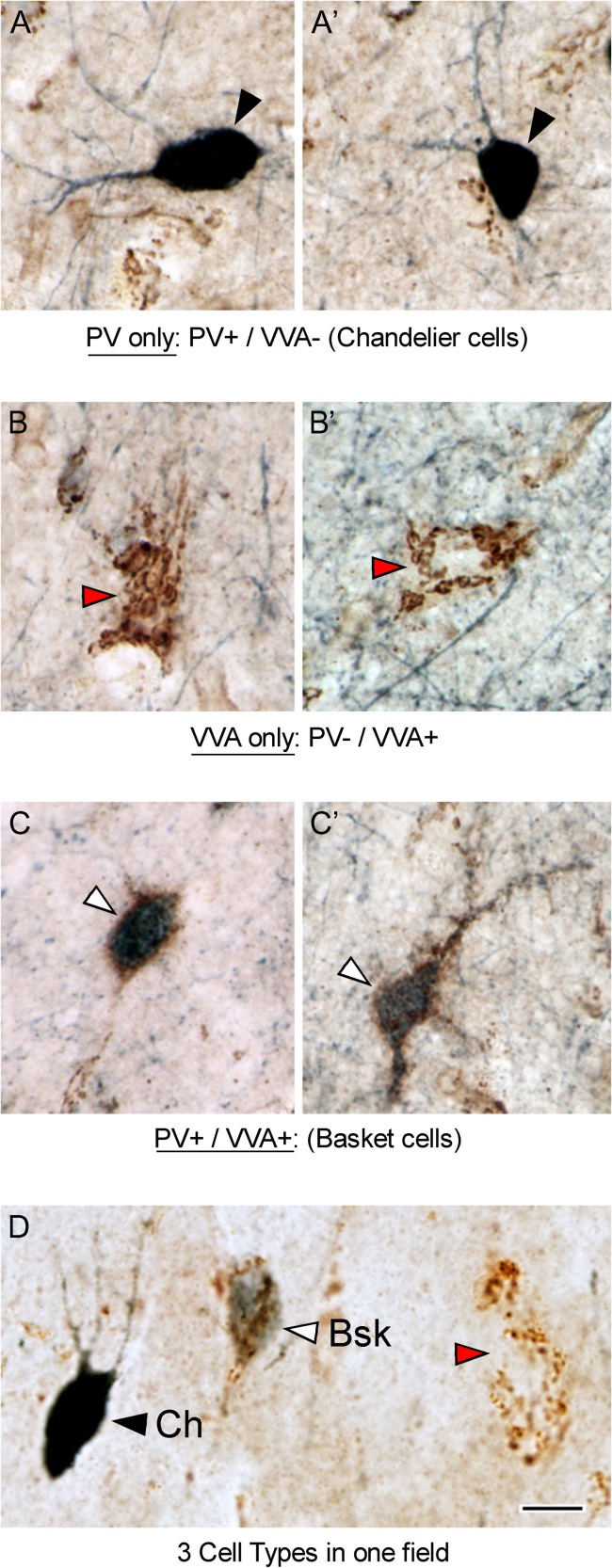
Since we only had access to small blocks of tissue from each case that included a portion of BA9, BA46, and BA47, but did not encompass the entire Brodmann area, we could not apply stereological methods. We therefore employed an approach based on quantifying total cell number in 3 mm wide bins within select regions encompassing the entire thickness of the cortical gray matter within representative samples of each BA. We combined PV immunohistochemical and VVA histochemical staining to distinguish PV+ Bsk interneurons from PV+ Ch interneurons. We double stained sections of prefrontal cortex with anti-PV antibodies and biotinylated VVA. PV-expressing cells were identified with a dark blue reaction product that fills the soma and proximal dendrites of interneurons in the cerebral cortex (Fig. 1A, black arrowheads). VVA+ cells were labeled with a brown reaction product that labels the perineural net that intimately envelopes the entire soma and proximal processes of cells. The VVA+ cells were identified by numerous brown puncta that provide an outline of labeled cells in the cerebral cortex (Fig. 1B, red arrowheads), as described in previous publications (Blazquez-Llorca et al. 2010). In some cases the plane of section bisected VVA+ cell bodies, and the location of the soma was apparent due to the lack of cytoplasmic VVA-labeling (Fig. 1B’).
Figure 1.
Prefrontal cortical tissue from BA9 stained with anti-PV antibodies (dark blue) and VVA lectin (brown). PV antibodies label the soma and proximal processes of PV-expressing interneurons. VVA binds to the carbohydrate N-acetylgalactosamine, which is present in the perineuronal net surrounding Bsk cells, but not Ch cells. (A, A’) Ch cells are PV+ / VVA−. Ch cells exhibit a dark blue PV-reaction product that fills the soma and proximal dendrites (black arrowheads). (B, B’) PV− / VVA+ cells present with a brown mesh-like puncta over the soma and proximal dendrites (red arrowheads). (C, C’) Bsk cells are PV+ / VVA+ and present with a dark blue PV-reaction product in the cytoplasm and proximal dendrites, and with a brown mesh-like puncta of VVA staining over the soma and proximal dendrites (white arrowheads). (D) All 3 cell types were present in the prefrontal cortical areas we analyzed. Scale bar: 5 µm.
Statistics
The goal of the statistical analysis was to compare percentage of each type of interneuron between autistic and control cases, and to assess the relationship between anatomical parameters and other patient/sample characteristics (such as age, hemisphere, presence of mental retardation or seizures, and severity of autism). Each variable was compared between autistic and control cases using t-tests. The joint influence of autism and patient/sample characteristics on variables was assessed using multiple regression modeling.
Results
PV/VVA Double Labeling Differentiates Ch Cells from Bsk Cells
In order to differentiate Ch from Bsk cells, we performed PV/VVA double histochemistry and identified 3 subsets of cells: one subset that stained positive for PV only—these cells were PV+ and VVA-negative (Fig. 1A, black arrowheads); a second subset that stained positive for VVA only—these cells were PV-negative and VVA+ (Fig. 1B, red arrowheads); and a third subset of cells that stained positive for both PV and VVA (Fig. 1C, white arrowheads). The 3 cell types were present in the prefrontal cortex. Among the population of PV-expressing cells, the VVA+ and VVA-negative subtypes were easily distinguished using the PV/VVA double labeling protocol. PV+/VVA+ cells displayed the mesh-like VVA+ puncta over the soma and proximal dendrites, while the PV+/VVA-negative cells clearly lacked the VVA staining (Fig. 1D). Therefore, we concluded that this approach would reliably distinguish Ch cells from Bsk cells, since only Bsk cells are associated with VVA lectin staining (Mulligan et al. 1989; Naegele and Katz 1990; Schweizer et al. 1993).
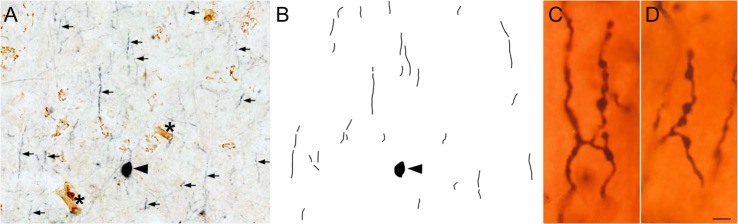
To further test the idea that PV+/VVA-negative cells were Ch cells, we examined a notable feature possessed only by Ch cells: axonal processes called cartridges. Ch cell axons form vertical rows of synaptic boutons that resemble a candlestick and are called cartridges (Szentagothai and Arbib 1974; DeFelipe et al. 1985), and are labeled with anti-PV antibodies. We therefore asked whether PV+ cartridges were associated exclusively with the PV+/VVA-negative cells—the presumed Ch cells. We found that PV+ cartridges, although could not be linked to a specific cell soma, were surrounding the PV+/VVA-negative cells, and that the vertically oriented PV+ cartridges were not labeled with VVA (Fig. 2A,B). Together this data supports the concept that PV+/VVA-negative cells are Ch cells. In contrast, the PV+/VVA+ cells were not associated with PV+ cartridges, and we defined these as Bsk cells.
Figure 2.
PV-expressing cartridges were associated with the PV+/VVA-negative cells. Vertically oriented PV+ cartridges were not labeled with VVA. (A) Ch cell soma (black arrowhead) and cartridges (small arrows) were stained with dark blue anti-PV reaction product, but not with VVA lectin. VVA+ cells (brown) are visible, as well as VVA+ capillaries (asterisks), but VVA does not label the Ch cell body or cartridges. (B) Neurolucida reconstruction of PV staining in the image shown in panel A. (C, D) Golgi stained tissue showing the structure of boutons that comprise each PV-expressing Ch cell cartridge. Scale bar: A,B. 15 µm., C,D: 1 µm.
Using the PV/VVA double labeling protocol we found that approximately 75% of PV+ cells were labeled with VVA lectin in prefrontal cortex (BA46:79.23 ± 1.8; BA47:74.77 ± 3.0; BA9:75.27 ± 4.0). Thus, in the human prefrontal areas we analyzed, approximately 75% of the PV+ cells were Bsk cells, and the remaining 25% were Ch cells. Our data on the proportions of PV+ subtypes in human prefrontal cortex are in line with previous studies of human non-frontal cortical areas (Kobayashi et al. 1989; Blazquez-Llorca et al. 2010). In summary, and most pertinent for this investigation, among the population of cortical interneurons that express PV in 3 areas of prefrontal cortex, we found that approximately 25% of the PV+ cells were Ch cells, while the remaining 75% were Bsk cells.
VVA Expression in the Human Prefrontal Cortex: Methodological Considerations
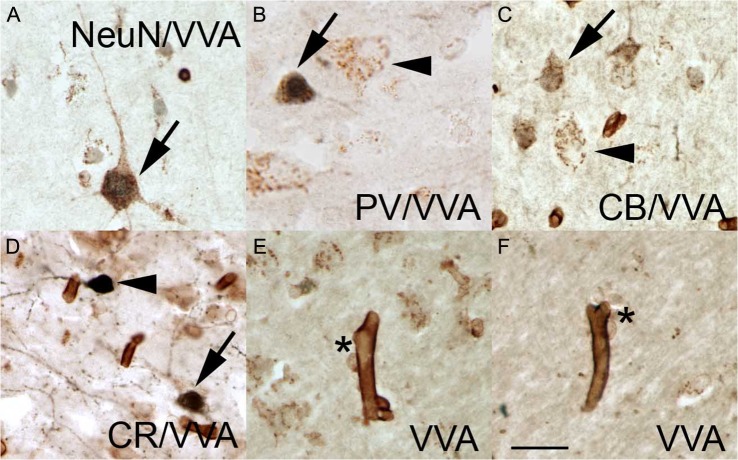
Due to differences in cerebro-cortical VVA staining across species, and due to the lack of comprehensive information on VVA staining in the human prefrontal cortex, we characterized VVA+ cells in the 3 prefrontal BAs that were included in this study. We phenotyped the VVA+ cells in prefrontal cortex of 5 control cases by co-staining with the NeuN pan-neuronal marker, and with 3 interneuron-specific markers. We performed double staining for VVA and NeuN to determine the percentage of cortical neurons labeled by VVA, and double-stained tissue with VVA and one of 3 interneuron markers: VVA and PV; VVA and CB; or VVA and CR.
In the human prefrontal cortex VVA staining labeled cells with a mesh-like pattern of puncta over the soma and proximal dendrites, as previously described for macaque cerebral cortex (Mulligan et al. 1989), and as shown in Figure 1. We found that the distribution of VVA-expressing cells in the human prefrontal cortex was widespread in comparison with data that has been reported in the cortex for other species (Mulligan et al. 1992; Ojima et al. 1995; Wintergerst et al. 1996; Hitomi et al. 1997; Ohyama and Ojima 1997). We quantified the total number of single and double stained cells in the 3 mm wide bins that were selected for analysis in each BA area from each case. The number of VVA-expressing subtypes in each BA was homogeneous across the control cases. We found that approximately 95% of NeuN+ cells in BA9, BA46, and BA47 were also VVA+ (Fig. 3A), indicating that most cortical neurons have a VVA+ perineural net, as previously shown in several species (Mulligan et al. 1992; Ohyama and Ojima 1997; Ojima et al. 1998). We also found that around 45% of cortical VVA-expressing neurons labeled with antibodies against PV, CR, or CB (Fig. 3B–D; see Supplementary Table 1). Some non-neuronal cell types were also labeled with VVA. In addition, we noted that 35% did not express NeuN. We counter stained sections with cresyl-violet, which labels the soma and nuclei of neurons but only the nucleus of glial cells, and determined that the VVA+/NeuN-negative cells were glia. These VVA+ glial cells were always associated with capillaries, suggesting an astrocytic phenotype (Fig. 3E,F). Our data match that by Murakami et al., who described VVA+ glial cells in human visual cortex (Murakami et al. 1995). In addition, we found that capillaries were intensely stained with VVA (Fig. 3E,F), as previously reported (Jones et al. 2015).
Figure 3.
VVA lectin labeled pyramidal neurons, interneurons, and astrocytes. (A) NeuN+ pyramidal cell (dark blue nucleus) with a VVA+ perineural net outlining the soma and proximal dendrites (brown puncta). (B) Some VVA+ cells express PV (arrow) while other VVA+ cells do not (arrowhead). (C) Some VVA+ cells express CB (arrow), while other VVA+ cells do not (arrowhead). (D) CR+ cells that express VVA (arrow) and that do not express VVA (arrowhead). (E, F) VVA+ cells (asterisk) associated with VVA+ capillaries. Scale bars: 15 µm.
Previous studies in mouse have described that VVA labels PV+ cells, and our data support that conclusion. However, it should be noted that a broad range of cortical cell types beyond PV+ interneurons, were labeled with VVA in human. Summarizing, most neurons but not all—95%—are VVA+. Ch cells can be included among the NeuN+ neurons that are not stained with VVA. In total, approximately 45% of the VVA+ neurons express interneuronal makers. While the total VVA+ cell population comprised about 65% neurons, the remaining 35% appeared to be astrocytes.
Ch Cells Are Decreased in the Prefrontal Cortex (BA46, BA47, and BA9) in Autism
We quantified and compared the number of PV+/VVA+ and PV+/VVA− cells between autism and control groups. We used light microscopy with a 100× lens to quantify the total number of each interneuron subtype in the defined bins from each case. We calculated the proportion of interneuron subtypes: PV+/VVA− (Ch cells), PV+/VVA+ (Bsk cells), as a percentage of the total number of cells quantified in each bin. The total number of labeled cells was the sum of all cells that were stained with PV, VVA, or both markers. The proportion of PV+/VVA+ double positive cells (Bsk cells), and of PV+/VVA-negative cells (Ch cells) among all labeled cells in the prefrontal cortical areas was then quantified and compared between control and autism cases. Quantifying the number of PV-expressing interneuron subtypes as a percentage avoids the introduction of error due to differential tissue shrinkage that can occur in each case, and which can impact cell density measurements.
Using this quantification method on the entire set of prefrontal tissue from 10 control cases, we found that Bsk cells outnumbered Ch cells (Supplementary Table 2). In BA46 Bsk cells comprised 12.38 ± 1.2% of all labeled cells, while Ch cells were 5.91 ± 2.0%. In BA47 Bsk cells made up 14.05 ± 3.1% of all cells, while Ch cells were 4.05 ± 0.5%. And in BA9 Bsk cells were 8.87 ± 0.5% of all cells, while Ch cells were 2.93 ± 0.4%. These results match previously published data on the relative number of Ch cells and Bsk cells in cerebral cortex (DeFelipe et al. 1985).
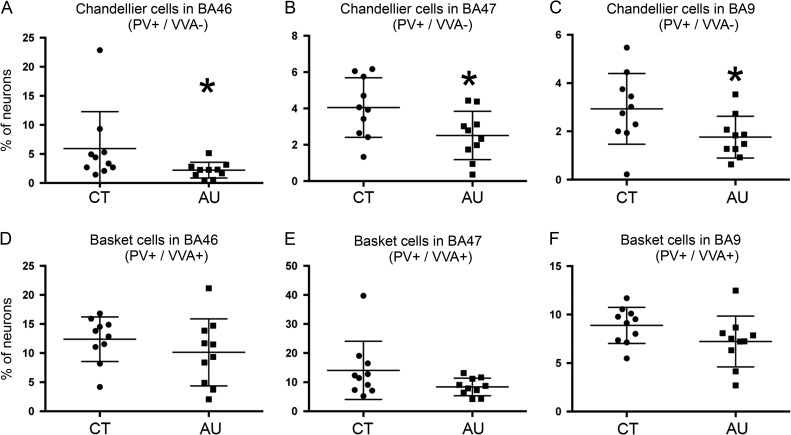
We next quantified cell numbers in the prefrontal cortex of the autistic cases (Fig. 4, Supplementary Table 2). The total number of VVA+ cells did not decreased in any of the BA areas. In BA46 Bsk cells comprised 10.13 ± 1.9% of all labeled cells, while Ch cells were 2.12 ± 0.4%. In BA47 Bsk cells made up 8.37 ± 0.9% of all cells, while Ch cells were 2.51 ± 0.4%. And in BA9 Bsk cells were 7.22 ± 0.6% and Ch cells were 1.76 ± 0.6%. Thus, we found a decrease in the percentage of Ch cells in autistic cases in the 3 areas of prefrontal cortex analyzed. In BA46 the proportion of Ch cells was significantly decreased by 65.1% (P = 0.05). In BA47 Ch cells were decreased by 39.1%, but this difference was marginally significant (P = 0.07). In BA9 Ch cells were significantly decreased by 40.0% (P = 0.04, see Fig. 4). There was also a slight, but not significant, decrease in the percentage of Bsk cells in these BAs (P = 0.1, 0.3, and 0.1, respectively, see Supplementary Table 2). We did not find any correlation or trend between the number of each cell type with the variables of age (Supplementary Fig. 1), PMI, or hemisphere. There were not enough cases to correlate data by gender, presence of seizures, mental retardation, or ADI-R scores.
Figure 4.
(A–C) The number of Ch cells in Brodmann Area (BA)46, BA47, and BA9 of prefrontal cortex is significantly lower in the autism cases (AU) than in control cases (CT). (D–F) The number of Bsk cells in BA46, BA47, and BA9 did not statistically differ between groups.
We next performed a replication study to test our quantification approach. We analyzed a second 3 mm wide bin within each BA in all control and autism cases as previously performed (Hashemi et al. 2016). Once again, we found a decrease in the percentage of Ch cells that was significant in all 3 BAs tested (BA46: P = 0.03; BA47: P = 0.01; BA9: P = 0.01; see Supplementary Table 2). These data support our finding that the number of Ch cells is decreased in prefrontal cortex in autism. In the replication study, there was not a significant change in Bsk cells in BA46 or BA9, but the number of Bsk cells in BA47 was significantly decreased at the P = 0.05 level (BA46: P = 0.1; BA47: P = 0.05; BA9: P = 0.55). The data indicate that some areas of the autistic prefrontal cortex may be also characterized by a mild decrease in PV-expressing Bsk cells.
In summary, our data support the hypothesis that a subpopulation of PV+ interneurons, Ch cells, is decreased in number in the prefrontal cortex in some cases of autism. We also found the number of PV+ Bsk cells may also be slightly decreased in some areas of the prefrontal cortex in autism.
Discussion
We previously reported a decrease in the number of PV+ interneurons in the prefrontal cortex in autism (Hashemi et al. 2016). PV is expressed by 2 subtypes of interneurons, Bsk and Ch cells. These 2 interneuron subtypes synthesize and release GABA as do most cortical interneurons. However, only PV+ interneurons generate fast-spiking action potentials that synchronize the activity of numerous pyramidal cells through rhythmic inhibition. These cells also participate in the generation of gamma oscillations and in executive functions associated with prefrontal brain regions (Kawaguchi et al. 1987; Kawaguchi and Kubota 1997; Goldman-Rakic 1999; Salinas and Sejnowski 2001; Markram et al. 2004; Woodruff et al. 2009). Ch and Bsk cells account for 20–50% of interneurons depending of the cortical area. We recently showed that PV+ interneurons approximately account for 50% of interneurons in BA46, 25% in BA47, and 28% in BA9 in control brain tissue obtained from children, adolescents, and adults (Hashemi et al. 2016).
Ch cells have unique morphological characteristics: The terminal portions of Ch cell axons form vertical rows of synaptic boutons, called cartridges that resemble candlesticks (Szentagothai and Arbib 1974; DeFelipe et al. 1985). Each Ch cell has multiple cartridges (see Fig. 2A,B), and each cartridge innervates a single pyramidal axonal initial segment (AIS). Each Ch cell can therefore regulate the final output of many pyramidal cells. While GABAergic input on dendrites originates from many types of interneurons, the AIS only receives input from one or at most 2 Ch cells (DeFelipe 1999). Therefore, the loss of small numbers of Ch cells may critically alter pyramidal neuron output and impair cerebral cortex function (DeFelipe 1999). Bsk cells are multipolar PV+ interneurons located throughout all cortical layers that establish multiple synaptic connections with the soma and proximal dendrites of pyramidal neurons in a manner that outlines the pyramidal cell body, producing a basket-like appearance (Kawaguchi and Kubota 1997; Wang et al. 2002; Goldberg et al. 2008). As in the case of Ch cells, Bsk cells regulate the output of many pyramidal cells.
As discussed in our previous publication investigating PV+ interneurons in the autistic neocortex (Hashemi et al. 2016), the decreased number of PV+ Ch cells we detected in the present study in autism cases may represent an actual decrease in cell number, or an apparent decrease in cell number resulting from reduced PV protein levels in Ch cells. A decrease in the number of detected PV+ Ch cells could therefore result from different factors including 1) decreased production of Ch cells by precursor cells during prenatal development, 2) increased cell death among Ch cells during development, or 3) a lack of PV expression by Ch cells. However, in a mouse model, maternal immune activation produced deficits in the function of PV+ interneurons was reported despite normal levels of PV-expression (Canetta et al. 2016), suggesting that the deficit detect results from reduced cell number, not protein expression. The decreased number of Ch cells we detected in the autism cases could also potentially result from altered expression of the N-acetylgalactosamine that VVA lectin labels. However, we did not detect a decrease in the number of VVA+ cells between control and autistic groups. In addition, examination of cortical tissue in a PV-knockout mouse found no change in the number of VVA+ cells despite a loss of PV protein (Vreugdenhil et al. 2003), demonstrating that expression of the N-acetylgalactosamine component of the perineural net is not influenced by some variables, including PV expression. Nonetheless, future studies should more closely investigate whether changes in the extracellular matrix, such as the perineural net, occur in autism.
Early onset autism becomes apparent when affected children do not develop expected social and communication skills. Regressive forms of autism become apparent later when children who initially develop along a normal trajectory lose acquired milestones and abilities. Consequently, it is possible that early onset autism results from prenatal insult(s), while regressive forms result from adverse postnatal event(s). Critically sensitive periods of development include cell production, cell maturation, and synaptic development. Cortical neuron production in humans begins during the first trimester, and interneuron production continues through the third trimester until birth (Arshad et al. 2016). The precise timing of generation for interneuron subtypes has been mapped out in rodents (Batista-Brito and Fishell 2009). For example, Ch cells are generated in embryonic day (E)15.5 in mice, while Bsk cells are generated in E13.5 (Inan et al. 2012; Taniguchi et al. 2013). However, the timing of generation for specific interneuron subtypes has not yet been determined for the human brain. Nonetheless, if the basic timeline of cell generation in rodents can be extrapolated to human development, we would expect critical periods of development when specific interneuron subtypes, such as Ch cells, generate, mature, and develop intercellular connections. Thus, intrinsic or extrinsic insults that potentially impact Ch cell development and viability could occur at any point during an extended period including much of gestation and several years of early childhood.
Changes in morphology and/or function of Ch and Bsk cells have previously been reported in neurological diseases, such as schizophrenia and epilepsy (Woo et al. 1998; Defelipe et al. 1999; Pierri et al. 1999; Volk and Lewis 2002; Volk et al. 2002; Lewis et al. 2012). For example, Glausier et al. quantified PV+ Bsk cell inputs in BA9 of schizophrenic and matched control subjects and found that the level of PV protein expression was lower in PV+ Bsk cell boutons in schizophrenia (Glausier et al. 2014). Woo et al. found that the density of Ch cartridges was decreased by 40% in the prefrontal cortex of schizophrenic subjects compared with matched groups of normal control and non-schizophrenic psychiatric subjects (Woo et al. 1998). The changes in Ch and Bsk cells reported in these cases may be associated with altered information processing within the prefrontal cortex and could contribute to the cognitive impairments seen in these disorders (Woo et al. 1998). Our data showing that Ch cells, and to some degree Bsk cells, are numerically reduced in the autistic cerebral cortex suggests a possible deficit of inhibition on pyramidal neurons.
As a consequence of the decrease in PV+ cells, the pyramidal AIS experiences an alteration in the input that receives from interneurons. The AIS is the thin unmyelinated initial region of the pyramidal axon as it exits the cell body—between the axon hillock and the beginning of myelinated portion of the axon—and it is approximately 10–60 μm in length. It is in the AIS where action potentials are initiated, and can be identified by the dense expression of ion channels and associated proteins (Kole and Stuart 2012). Since small changes in membrane potential at the AIS can generate profound changes in excitability, a change in quantity or quality of inhibitory or excitatory inputs at the AIS may contribute to the cognitive features of neurological disorders. Decreased number of Ch cell projections to the AIS of pyramidal cells in autism would likely have functional implications. Other neurodevelopmental diseases have previously been linked to abnormalities in the AIS. For example, pyramidal neurons in CA1 in a mouse model of Angelman Syndrome present with an altered resting membrane potential, threshold potential, and action potential amplitude in the AIS (Kaphzan et al. 2011). An alteration in the AIS has also been associated with epilepsy and schizophrenia (Ribak 1985; Lewis 2011). The focal loss of Ch cells in temporal lobe epilepsy has led to the hypothesis that decreased inhibition at the AIS contributes to the etiology of epilepsy (DeFelipe 1999). Based on the data we presented here, we cannot positively correlate an alteration in the AIS in autism with the presence of epilepsy, but we can hypothesize that an alteration of the AIS could be related with the presence of epilepsy in the 30–40% of the cases of autism that develop epilepsy.
More studies on the functional characteristics of Ch and Bsk cells are needed to achieve a fuller understanding of their potential contributions to the cellular basis of autism. Establishing the underlying mechanisms at play in autism will be essential for the development of new therapeutic interventions. These data will greatly expand our understanding of GABAergic system functioning in the human cerebral cortex in autism, which will impact translational research directed towards providing better treatment paradigms for individuals with autism.
Supplementary Material
Supplementary Material
Supplementary material is available at Cerebral Cortex online.
Funding
National Institute for Mental Health (MH094681 and MH101188); the MIND Institute (IDDRC; U54 HD079125), the Shriners Hospitals, and the Pathology Department at UCD.
Notes
Conflict of Interest: None declared.
References
- Arshad A, Vose LR, Vinukonda G, Hu F, Yoshikawa K, Csiszar A, Brumberg JC, Ballabh P. 2016. Extended production of cortical interneurons into the third trimester of human gestation. Cereb Cortex. 26:2242–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Fishell G. 2009. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol. 87:81–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Zhang X, Feferman L, Johnson D, Tortella FC, Guizzetti M, Tobacman JK. 2015. Decline in arylsulfatase B and Increase in chondroitin 4-sulfotransferase combine to increase chondroitin 4-sulfate in traumatic brain injury. J Neurochem. 134:728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez-Llorca L, Garcia-Marin V, DeFelipe J. 2010. GABAergic complex basket formations in the human neocortex. J Comp Neurol. 518:4917–4937. [DOI] [PubMed] [Google Scholar]
- Bruckner G, Brauer K, Hartig W, Wolff JR, Rickmann MJ, Derouiche A, Delpech B, Girard N, Oertel WH, Reichenbach A. 1993. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 8:183–200. [DOI] [PubMed] [Google Scholar]
- Canetta S, Bolkan S, Padilla-Coreano N, Song LJ, Sahn R, Harrison NL, Gordon JA, Brown A, Kellendonk C. 2016. Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol Psychiatry. 21:956–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. 1999. Chandelier cells and epilepsy. Brain. 122 (Pt 10):1807–1822. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Gonzalez-Albo MC. 1998. Chandelier cell axons are immunoreactive for GAT-1 in the human neocortex. Neuroreport. 9:467–470. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Gonzalez-Albo MC, Del Rio MR, Elston GN. 1999. Distribution and patterns of connectivity of interneurons containing calbindin, calretinin, and parvalbumin in visual areas of the occipital and temporal lobes of the macaque monkey. J Comp Neurol. 412:515–526. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Jones EG, Schmechel D. 1985. Variability in the terminations of GABAergic chandelier cell axons on initial segments of pyramidal cell axons in the monkey sensory-motor cortex. J Comp Neurol. 231:364–384. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Fish KN, Lewis DA. 2014. Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry. 19:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. 2008. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 58:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. 1999. The “psychic” neuron of the cerebral cortex. Ann N Y Acad Sci. 868:13–26. [DOI] [PubMed] [Google Scholar]
- Hashemi E, Ariza J, Rogers H, Noctor SC, Martinez-Cerdeno V. 2016. The number of parvalbumin-expressing interneurons is decreased in the medial prefrontal cortex in autism. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi S, Su WD, Hong LJ, Ohtsuka A, Murakami T. 1997. Perineuronal sulfated proteoglycans and cell surface glycoproteins in the visual cortex of adult and newborn cats. Acta Med Okayama. 51:295–299. [DOI] [PubMed] [Google Scholar]
- Inan M, Welagen J, Anderson SA. 2012. Spatial and temporal bias in the mitotic origins of somatostatin- and parvalbumin-expressing interneuron subgroups and the chandelier subtype in the medial ganglionic eminence. Cereb Cortex. 22:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Sanes JR. 1997. Lamina-specific connectivity in the brain: regulation by N-cadherin, neurotrophins, and glycoconjugates. Science. 276:1428–1431. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Wilsher SA, Wooding FB, Benirschke K, Allen WR. 2015. The binucleate cell of okapi and giraffe placenta shows distinctive glycosylation compared with other ruminants: a lectin histochemical study. Mol Phylogenet Evol. 83:184–190. [DOI] [PubMed] [Google Scholar]
- Kaphzan H, Buffington SA, Jung JI, Rasband MN, Klann E. 2011. Alterations in intrinsic membrane properties and the axon initial segment in a mouse model of Angelman syndrome. J Neurosci. 31:17637–17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Katsumaru H, Kosaka T, Heizmann CW, Hama K. 1987. Fast spiking cells in rat hippocampus (CA1 region) contain the calcium-binding protein parvalbumin. Brain Res. 416:369–374. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. 1997. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 7:476–486. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Emson PC, Mountjoy CQ. 1989. Vicia villosa lectin-positive neurones in human cerebral cortex. Loss in Alzheimer-type dementia. Brain Res. 498:170–174. [DOI] [PubMed] [Google Scholar]
- Kole MH, Stuart GJ. 2012. Signal processing in the axon initial segment. Neuron. 73:235–247. [DOI] [PubMed] [Google Scholar]
- Lewis DA. 2011. The chandelier neuron in schizophrenia. Dev Neurobiol. 71:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. 2012. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 35:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. 2004. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 5:793–807. [DOI] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Clasca F. 2002. Reelin immunoreactivity in the adult neocortex: a comparative study in rodents, carnivores, and non-human primates. Brain Res Bull. 57:485–488. [DOI] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Galazo MJ, Cavada C, Clasca F. 2002. Reelin immunoreactivity in the adult primate brain: intracellular localization in projecting and local circuit neurons of the cerebral cortex, hippocampus and subcortical regions. Cereb Cortex. 12:1298–1311. [DOI] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Galazo MJ, Clasca F. 2003. Reelin-immunoreactive neurons, axons, and neuropil in the adult ferret brain: evidence for axonal secretion of reelin in long axonal pathways. J Comp Neurol. 463:92–116. [DOI] [PubMed] [Google Scholar]
- Mulligan KA, van Brederode JF, Hendrickson AE. 1989. The lectin Vicia villosa labels a distinct subset of GABAergic cells in macaque visual cortex. Vis Neurosci. 2:63–72. [DOI] [PubMed] [Google Scholar]
- Mulligan KA, Van Brederode JF, Mehra R, Hendrickson AE. 1992. VVA-labelled cells in monkey visual cortex are double-labelled by a polyclonal antibody to a cell surface epitope. J Neurocytol. 21:244–259. [DOI] [PubMed] [Google Scholar]
- Murakami T, Hitomi S, Ohtsuka A, Taguchi T. 1995. Neurons with perineuronal sulfated proteoglycans in the human visual cortex, with special reference to their reactions to lectins. Arch Histol Cytol. 58:357–364. [DOI] [PubMed] [Google Scholar]
- Murakami T, Ohtsuka A. 2003. Perisynaptic barrier of proteoglycans in the mature brain and spinal cord. Arch Histol Cytol. 66:195–207. [DOI] [PubMed] [Google Scholar]
- Naegele JR, Katz LC. 1990. Cell surface molecules containing N-acetylgalactosamine are associated with basket cells and neurogliaform cells in cat visual cortex. J Neurosci. 10:540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama J, Ojima H. 1997. Labeling of pyramidal and nonpyramidal neurons with lectin Vicia villosa during postnatal development of the guinea pig. J Comp Neurol. 389:453–468. [PubMed] [Google Scholar]
- Ojima H, Kuroda M, Ohyama J, Kishi K. 1995. Two classes of cortical neurones labelled with Vicia villosa lectin in the guinea-pig. Neuroreport. 6:617–620. [DOI] [PubMed] [Google Scholar]
- Ojima H, Sakai M, Ohyama J. 1998. Molecular heterogeneity of Vicia villosa-recognized perineuronal nets surrounding pyramidal and nonpyramidal neurons in the guinea pig cerebral cortex. Brain Res. 786:274–280. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo TU, Lewis DA. 1999. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 156:1709–1719. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. 2002. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 298:1248–1251. [DOI] [PubMed] [Google Scholar]
- Ribak CE. 1985. Axon terminals of GABAergic chandelier cells are lost at epileptic foci. Brain Res. 326:251–260. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. 2001. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M, Streit WJ, Muller CM. 1993. Postnatal development and localization of an N-acetylgalactosamine containing glycoconjugate associated with nonpyramidal neurons in cat visual cortex. J Comp Neurol. 329:313–327. [DOI] [PubMed] [Google Scholar]
- Somogyi P. 1977. A specific ‘axo-axonal’ interneuron in the visual cortex of the rat. Brain Res. 136:345–350. [DOI] [PubMed] [Google Scholar]
- Szentagothai J, Arbib MA. 1974. Conceptual models of neural organization. Neurosci Res Program Bull. 12:305–510. [PubMed] [Google Scholar]
- Taniguchi H, Lu J, Huang ZJ. 2013. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 339:70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. 2002. Impaired prefrontal inhibition in schizophrenia: relevance for cognitive dysfunction. Physiol Behav. 77:501–505. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. 2002. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 12:1063–1070. [DOI] [PubMed] [Google Scholar]
- von Economo C. 1929. The cytoarchitectonics of the human cerebral cortex. London: Humphrey Milford - Oxford University. [Google Scholar]
- Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B. 2003. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 89:1414–1422. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gupta A, Toledo-Rodriguez M, Wu CZ, Markram H. 2002. Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cereb Cortex. 12:395–410. [DOI] [PubMed] [Google Scholar]
- Wintergerst ES, Vogt Weisenhorn DM, Rathjen FG, Riederer BM, Lambert S, Celio MR. 1996. Temporal and spatial appearance of the membrane cytoskeleton and perineuronal nets in the rat neocortex. Neurosci Lett. 209:173–176. [DOI] [PubMed] [Google Scholar]
- Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. 1998. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA. 95:5341–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff A, Xu Q, Anderson SA, Yuste R. 2009. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits. 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Miao QL. 2013. Experience-dependent development of perineuronal nets and chondroitin sulfate proteoglycan receptors in mouse visual cortex. Matrix Biol. 32:352–363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.