Abstract
Currently, an unprecedented number of individuals can legally access cannabis. Vaporization is increasingly popular as a method to self-administer cannabis, partly due to perception of reduced harm compared with smoking. Few controlled laboratory studies of cannabis have used vaporization as a delivery method or evaluated the acute effects of cannabis among infrequent cannabis users. This study compared the concentrations of cannabinoids in whole blood and oral fluid after administration of smoked and vaporized cannabis in healthy adults who were infrequent users of cannabis. Seventeen healthy adults, with no past-month cannabis use, self-administered smoked or vaporized cannabis containing Δ9-tetrahydrocannabinol (THC) doses of 0, 10 and 25 mg in six double-blind outpatient sessions. Whole blood and oral fluid specimens were obtained at baseline and for 8 h after cannabis administration. Cannabinoid concentrations were assessed with enzyme-linked immunosorbent assay (ELISA) and liquid chromatography–tandem mass spectrometry (LC–MS-MS) methods. Sensitivity, specificity and agreement between ELISA and LC–MS-MS results were assessed. Subjective, cognitive performance and cardiovascular effects were assessed. The highest concentrations of cannabinoids in both whole blood and oral fluid were typically observed at the first time point (+10 min) after drug administration. In blood, THC, 11-OH-THC, THCCOOH and THCCOOH-glucuronide concentrations were dose-dependent for both methods of administration, but higher following vaporization compared with smoking. THC was detected longer in oral fluid compared to blood and THCCOOH detection in oral fluid was rare and highly erratic. For whole blood, greater detection sensitivity for ELISA testing was observed in vaporized conditions. Conversely, for oral fluid, greater sensitivity was observed in smoked sessions. Blood and/or oral fluid cannabinoid concentrations were weakly to moderately correlated with pharmacodynamic outcomes. Cannabis pharmacokinetics vary by method of inhalation and biological matrix being tested. Vaporization appears to be a more efficient method of delivery compared with smoking.
The use and availability of cannabis (marijuana) has increased in recent years as its legalization has expanded in the United States (US) and elsewhere. As of this writing, cannabis is permitted for medical purposes in 33 US states and the District of Columbia and various other places throughout the developed world (e.g., the European Union, Australia). Non-medical (i.e., “recreational”) cannabis use is also permitted in nine US states, Uruguay and Canada (1). These policy reforms have coincided with decreased harm perceptions and stigma surrounding cannabis use (2) and have created a new retail cannabis marketplace in many places that permit legal use of cannabis.
The burgeoning cannabis marketplace contains a variety of cannabis-containing products and instruments to self-administer cannabis. In addition to traditional instruments used to smoke cannabis such as joints, pipes and bongs (3), cannabis vaporizers are now widely available and have become a popular method for cannabis self-administration (4, 5). Cannabis vaporizers heat either plant material (dried cannabis) or concentrated cannabis extracts/resins and produce an aerosol or “vapor” for user inhalation. Similar to electronic cigarettes (e-cigarettes, e-cigs), cannabis vaporizers do not reach temperatures associated with pyrolysis (i.e., combustion) and therefore produce fewer harmful toxicants compared with traditional smoking techniques (6, 7). Some cannabis users have reported a preference for vaporized cannabis due to lower toxicant exposure (i.e., health risk), better taste, reduced odor and stronger “high” relative to smoked cannabis (4, 5, 8).
To date, few controlled laboratory studies have directly compared the acute pharmacokinetics of smoked and vaporized cannabis in human blood and oral fluid. In the first controlled comparison of smoked and vaporized cannabis that included blood cannabinoid measurements (9), inhalation of smoked and vaporized cannabis containing 15.3, 30.6 and 61.2 mg Δ9-tetrahydrocannabinol (THC) by moderate cannabis users (3–10 administrations in past month) resulted in dose-dependent increases in plasma THC concentrations (9). THC concentrations were virtually identical across inhalation methods immediately after cannabis administration for all three doses, though at later time points (e.g., 30 and 60 min post-administration), THC concentrations tended to be greater after vaporization compared with smoked cannabis (9). In another study conducted by Huestis et al. (10, 11), the pharmacokinetics of THC and its primary metabolites were measured in whole blood and oral fluid of moderate (2–8 cannabis administrations per month) and heavy (daily or near daily) cannabis users after administration of smoked and vaporized cannabis containing ~50 mg THC. Among heavy cannabis users, maximum THC concentrations (Cmax) in whole blood were significantly higher when cannabis was smoked than when vaporized (10). There were no other significant differences in the peak magnitude or time course of cannabinoid concentrations in whole blood (e.g., 11-hydroxy-THC: 11-OH-THC; 11-nor-9-carboxy-THC: THCCOOH; THCCOOH-glucuronide) or oral fluid (e.g., THC, THCCOOH) across inhalation methods. However, cannabinoid concentrations in both whole blood and oral fluid were qualitatively higher after smoking relative to vaporization and higher among heavy cannabis users compared with moderate users (10, 11).
Several characteristics of these two studies are noteworthy and warrant additional consideration. First, it appears that frequency of cannabis use and/or demographic characteristics among study participants can impact blood THC outcomes. That is, greater THC concentrations (Cmax) were observed after smoking compared with vaporization among heavy cannabis users in the Huestis study (10), but no differences in Cmax were observed among moderate cannabis users in either study (9, 10). Interestingly, blood plasma cannabinoid concentrations were higher after vaporization at several time points in the Abrams study (9), but, conversely, blood cannabinoids tended to be higher after smoking in the Huestis study (10). A strength of the Abrams study is the use of multiple THC doses while a limitation is that THC was only examined in plasma (9). The Huestis study (10, 11) was strengthened by the inclusion of participants with varied current cannabis use frequencies and analyses of an extensive array of cannabinoids, but was limited by only administering a single dose of THC. Demographic characteristics of participants in these studies should also be considered, as factors such as sex and race can influence drug pharmacokinetics (12). Both studies enrolled predominantly male participants, the heavy cannabis users in the Huestis study were mostly African American, and the majority of the moderate cannabis users in both studies were Caucasian. Overall, the impact of cannabis use frequency, sex and race on inhaled cannabinoid pharmacokinetics needs to be better understood.
Another potentially important characteristic of these studies was the method of drug administration. Both studies used pre-rolled cannabis cigarettes for smoked cannabis administration and The Volcano Medic® (Storz and Bickel, Oakland, CA) for cannabis vaporization (9–11). Notably, THC dose standardization can be difficult to achieve with cannabis cigarettes, as users’ puff duration, puff volume, and puff number can vary substantially and alter the total volume of inhaled cannabis smoke (13). Though both studies used the same device for vaporization, Abrams and colleagues (9) set the temperature of the device to 190°C and instructed participants to inhale 2–3 “balloons” of cannabis vapor per dose while, in the Huestis study (10, 11), cannabis was vaporized at 210°C and participants only inhaled one balloon of cannabis vapor. Thus, there may have been differences between these studies in how completely cannabinoids were vaporized from the cannabis and delivered to the participants simply based on the administration procedures utilized.
Of note, all individuals included in these studies had used cannabis multiple times within the month prior to study participation. Regular cannabis users often have residual levels of THC and/or THC metabolites in their system upon study entry, which can increase the difficulty of acute cannabinoid pharmacokinetic characterization. In addition, due to the increased availability of cannabis in the growing legal cannabis market and proliferation of vaporizer products, characterization of the acute pharmacokinetics of smoked and vaporized cannabis among individuals with little to no cannabis experience is imperative. However, the vast majority of studies that have examined cannabis pharmacokinetics have enrolled daily or near daily cannabis users and administered cannabis in a smoked form. Accordingly, extant cannabinoid concentration cutoffs for biological verification of cannabis exposure/intoxication from blood and oral fluid may not be appropriate for inexperienced cannabis users or individuals who administer cannabis via vaporization or other non-smoked forms.
The current study analyzed whole blood and oral fluid specimens obtained before and after acute administration of smoked and vaporized cannabis. This study extends the Abrams and Huestis studies with the inclusion of three separate doses (0, 10 and 25 mg THC), participants who had not used cannabis in the past month, and enrollment of nearly an equal number of male and female participants. Cmax, time to maximum concentration (Tmax), and windows of detection were characterized for THC and several THC metabolites using qualitative (enzyme-linked immunosorbent assay; ELISA) and quantitative (liquid chromatography–tandem mass spectrometry; LC–MS-MS) methods. Sensitivity, specificity and agreement between ELISA and LC–MS-MS were assessed and correlations between whole blood and oral fluid cannabinoid concentrations and cannabis-induced pharmacodynamic changes (as described in Spindle et al. (14)) were performed. Importantly, these data can be used to inform standards for biological verification of recent cannabis exposure and intoxication used in roadside/workplace drug testing programs and elsewhere.
Method
Participants
Participants were recruited using media advertisements and word-of-mouth. Eligible volunteers were healthy, had experience using cannabis but had not used cannabis in the past month, and tested negative at screening and prior to each experimental session for cannabis and other illicit drugs including amphetamines, benzodiazepines, cocaine, MDMA, opioids and PCP (assessed using urine toxicology and rapid enzyme immunoassay test kits). Health status was ascertained using medical history, a 12-lead electrocardiogram (EKG), blood chemistry, hematology, and serology analysis, and a physical examination. A serum pregnancy test was conducted for female participants at screening. Participants self-reported all use of cannabis, alcohol, tobacco and illicit drugs for the three months prior to the initial screening visit, and throughout study participation, using the Time-line Follow-Back Method (15).
A total of 17 participants (nine males and eight females) completed the study. The mean (SD) age of these individuals was 27 (6) years, their mean (SD) weight was 78 (15) kg, and their mean (SD) body mass index (BMI) was 26 (3) kg/m2. Mean (SD) demographics as a function of gender were as follows: age: men: 28 (7) years vs women: 27 (5) years; weight: men: 85 (15) kg vs women: 70 (13) kg; BMI: men: 27 (2) kg vs women: 25 (4) kg/m2. No participants reported currently smoking tobacco cigarettes at study entry. Participants self-reported their race as follows: 10 Caucasian/non-Hispanic, 3 Other/Hispanic, 3 African American/Non-Hispanic and 1 Caucasian/Hispanic. At the time of randomization, an average of 398 days had passed (SD = 437; range 30–1,825) since participants last use of cannabis. Males reported their last use of cannabis was 546 (SD = 562) days prior to study entry, as opposed to 231 (SD = 124) days for females. All study participants provided written informed consent prior to participation and were compensated upon completion of study procedures. This study was approved by the Johns Hopkins Medicine Institutional Review Board and was conducted in accordance with ethical standards established in the Helsinki Declaration.
Study design and procedure
For each participant, six outpatient sessions that differed only by inhalation method (smoked vs vaporized) and THC dose (0, 10 or 25 mg) were conducted at the Johns Hopkins Behavioral Pharmacology Research Unit (BPRU). This study used a within-subjects design where participants completed all six dose conditions: smoked cannabis containing 0, 10 and 25 mg of THC and vaporized cannabis containing 0, 10 and 25 mg of THC. Sessions were clustered by inhalation method such that cannabis was either smoked for the first three sessions and then vaporized for the final three or vaporized for the initial three sessions and then smoked for the final three. Within each session cluster, THC dose order was randomized for each participant. Experimental sessions lasted ~8.5 h and were separated by at least 1 week to allow for adequate drug washout between sessions. Participants and research staff were blinded to the THC doses but not to the inhalation method.
Prior to each session, participants completed a urine drug screening and alcohol breathalyzer test to verify self-reported abstinence from illicit drugs and alcohol. Female participants also completed a urine pregnancy test before each session. All participants were fed a standard low-fat breakfast. Nursing staff inserted an intravenous catheter in a forearm vein of the participant’s non-dominant arm to allow for repeated blood sampling. During each session, participants self-administered the given THC dose by inhaling the study product ad libitum within a 10-min period. An ad-libitum inhalation protocol was used in favor of a more controlled, paced puffing procedure because in prior studies we have conducted, controlled puffing procedures can elicit significant throat irritation and discomfort in a subset of individuals. Constraining the dose administration to a 10-min period minimized variance in absorption and time course due to individual differences in inhalation patterns.
For the vaporized cannabis sessions, the Volcano Medic® (Storz and Bickel, Oakland, CA) was used to heat and aerosolize cannabis at a temperature of 204°C (400°F). These settings were used based on the manufacturer’s recommendation (personal communication, Storz and Bickel). The aerosolized cannabis was trapped in a balloon containing a one-way valve. Participants used this valve to inhale the trapped vapor until the balloon was empty. Participants inhaled a total of three balloons in the 10-min period in order to ensure that the full THC dose was delivered (in pre-testing, we verified three balloons would sufficiently vaporize the full THC doses). For each session, a new balloon was used to avoid contamination from prior THC doses. An opaque bag was placed over each balloon so that neither study participants or staff could visibly see the aerosol produced for a given dose. In pre-study testing, we found that placebo cannabis produced considerably less visible vapor than high THC cannabis.
For the smoked cannabis sessions, a small hand-held pipe pre-filled with dried cannabis was used. Participants ignited the cannabis with a lighter and inhaled the resulting smoke within the permitted 10-min period. In order to ensure that the full THC dose was administered in each session, un-blinded pharmacy staff visually inspected the contents of the pipe and verified that all of the contained plant material had turned to ash. In order to obstruct the view of the plant material, and thus more effectively blind study staff and participants, the pipe was fitted with a metal top. This metal top also served to minimize the loss of drug due to side-stream smoke. After each session, the pipe was thoroughly washed with water by pharmacy staff and left submerged overnight in ethanol for sterilization. Pharmacy staff ensured all residue was removed from the pipe (via visual inspection) prior to the next experimental session. A new pipe screen was used for each session to avoid contamination from prior THC doses.
Study drug
Cannabis used in the present study was obtained from the National Institute on Drug Abuse (NIDA) Drug Supply Program and dispensed by the Johns Hopkins BPRU Pharmacy. Participants self-administered THC doses of 0, 10 and 25 mg during smoked and vaporized conditions; plant material was weighed by pharmacy staff before being dispensed to ensure accurate dosing. Two batches of cannabis were used in this study: high THC and placebo. The high THC cannabis contained 13.4% Δ−9-THC, 0.08% Δ−8-THC, 0.03% Cannabidiol (CBD) and 0.8% Cannabinol (CBN) and the placebo cannabis contained < 0.01% Δ−9-THC and no measurable levels of Δ−8-THC, CBD or CBN. An equivalent amount of plant material (186.6 mg) was placed into the pipe or vaporizer for each experimental session. In the 0 and 25 mg conditions, 186.6 mg of placebo and high THC cannabis were used, respectively. In the 10 mg conditions, 74.6 mg of high THC and 112 mg of placebo cannabis were mixed together.
Outcome measures
Whole blood and oral fluid specimens were collected at baseline and 0.17, 0.5, 1, 1.5, 2, 3, 4, 5, 6 and 8 h after the end of the 10-min drug administration period during each experimental session. As described in further detail elsewhere (14, 16) pharmacodynamic measures including subjective drug effects, cognitive/psychomotor performance, and vital signs were also assessed. Whole blood specimens were collected from indwelling venous catheters using 10 mL “gray-top” vacutainer tubes. All specimens were mixed by inversion, aliquoted into two separate 5 mL plastic cryotubes, stored at −60°C, and shipped, frozen on dry ice, for analysis.
Blood samples were analyzed using ELISA and LC–MS-MS by the Immunalysis Corporation (Pomona, CA). The Cannabinoids Direct ELISA Kit (THC-A/C-THC) was used according to the manufacturer’s recommended procedure at a cutoff concentration for THCCOOH of 10 ng/mL. As described in the manufacturer’s brochure, cross-reactivities for this assay are as follows: THCCOOH (100%), 11-nor-9-carboxy-Δ8-THC (110%), Δ9-THC (21%), Δ8-THC (45%), 11-OH-THC (<5%), 8–11-dihydroxy-Δ9-THC (<5%), CBN (<5%) and CBD (<5%). While not listed in the manufacturer’s brochure, the cross-reactivity of THCCOOH-glucuronide was communicated by the manufacturer to be 5% cross-reactivity to THCCOOH at a 10 ng/mL cutoff concentration (personal communication, Christine Moore).
LC–MS-MS analysis (as described in depth elsewhere (17)) was used to test all blood samples for concentrations of THC, 11-OH-THC, THCCOOH and THCCOOH-glucuronide. A solid-phase extraction (SPE) technique was used to measure THC, 11-OH-THC and THCCOOH blood concentrations while THCCOOH-glucuronide was measured using a liquid/liquid extraction (LLE) technique. The limit of quantitation (LOQ) for these analyses was 1 ng/mL and the upper limit of linearity (ULOL) was 100 ng/mL. Control samples were analyzed with all whole blood specimens using two different target concentrations: 2 and 20 ng/mL (prepared from Cerillant solutions, Round Rock, TX). The ranges of percent deviation from the 2 ng/mL target concentration for control samples were: THC, (n = 16), −7.5 to 9.5%; 11-OH-THC, (n = 16), −9.5 to 8.0%; THCCOOH, (n = 16), −4.0 to 17.0%; and THCCOOH-glucuronide, (n = 10), −2.0 to 47.5%. The ranges of percent deviation from the 20 ng/mL target concentration were: THC, (n = 16), −10.2 to 15.8%; 11-OH-THC, (n = 16), −12.7 to 12.2%; THCCOOH, (n = 16), −7.3 to 15.9%; and THCCOOH-glucuronide, (n = 10), −11.1 to 23.5%.
Oral fluid samples were collected via expectoration into 8 mL glass screw culture tubes (Thermo Fisher Scientific, Waltham, MA, 16 × 100 mm, #14-959-35AA) which contained a PTFE-liner (Thermo Fisher Scientific, #4506615). Prior to collection, the inner surface of the collection tubes was silanized with Sylon-CTTM (Sigma-Aldrich, St Louis, MO, USA, #33065-U) and rinsed with ethanol and dried. Participants were given five min to produce each sample and were not allowed to eat or drink for at least 10 min prior to each collection time point. Participants did not rinse their mouth’s after cannabis inhalation, as we wanted to assess oral fluid cannabinoid concentrations under conditions that would generalize to real-world use of cannabis. After collection was complete, all samples were wrapped with para-film and stored refrigerated. All samples were shipped overnight in refrigerated containers to the Immunalysis Corporation (Pomona, CA) and analyzed using ELISA and LC–MS-MS.
The Saliva/Oral Fluids Cannabinoids Direct ELISA Kit was used according to the manufacturer’s recommended procedure at a cutoff concentration for THC of 4 ng/mL. As described in the manufacturer’s brochure, cross-reactivities for this assay are as follows: THC (100%), Δ8-THC (66.7%), CBN (4%), CBD (50%) and conjugated-THC (25%). The Ultra-Sensitive Cannabinoids Direct ELISA Kit was used according to the manufacturer’s recommended procedure at a cutoff concentration for THCCOOH of 0.05 ng/mL. As described in the manufacturer’s brochure, cross-reactivities for this assay are as follows: THCCOOH (100%), 11-nor-9-carboxy-Δ8-THC (125%), Δ9-THC (10%), 11-OH-THC (33%), CBN (<0.25%) and CBD (<0.25%).
LC–MS-MS analysis for THC and THCCOOH performed with oral fluid samples were conducted using the methods described elsewhere (17). For THC, the LOQ and ULOL for oral fluid analysis was 1 and 100 ng/mL, respectively. For THCCOOH, the LOQ and ULOL for oral fluid analysis was 0.02 and 0.1 ng/mL, respectively. Control samples were analyzed with all oral fluid specimens (prepared from Cerillant solutions; Round Rock, TX). The ranges of percent deviation from the target concentration of control samples prepared for oral fluid analyses were: THC, 5 ng/mL (n = 15), −8.0 to 14.0%; THCCOOH, 0.1 ng/mL (n = 15), −2.0 to 16%.
Data presentation and analysis
Descriptive statistics including means, standard deviations (SDs), and ranges were used to present participant demographics and/or test results for biological specimens. Sensitivity, specificity, and agreement between qualitative ELISA and quantitative LC–MS-MS results were conducted for whole blood THC test results and oral fluid THC and THCCOOH test results for the active smoked and vaporized THC doses. These analyses did not include the two placebo conditions. For whole blood THC, a THC metabolite screening cutoff of 10 ng/mL (ELISA) and confirmatory cutoff of 2 ng/mL (LC–MS-MS) were used. For oral fluid, screening cutoffs of 4 and 0.05 ng/mL were used for ELISA test results for THC and THCCOOH, respectively. Confirmatory cutoffs for oral fluid LC–MS-MS test results of 2 ng/mL for THC and 0.05 ng/mL for THCCOOH were used. Test results were categorized as either: true positive (TP; ELISA response ≥ cutoff concentration and LC–MS-MS positive), true negative (TN; ELISA response < cutoff concentration and LC–MS-MS negative), false positive (FP; ELISA response ≥ cutoff concentration and LC–MS-MS negative), or false negative (FN; ELISA response < cutoff concentration and LC–MS-MS positive). Sensitivity, specificity and agreement were calculated as follows: sensitivity (100 × [TP/(TP + FN)]), specificity (100 × [TN/(TN + FP)]) and agreement (100 × [(TP + TN)/(TP + TN + FP + FN)]).
Correlations (Pearson’s r) were conducted to examine the relationship between peak change from baseline scores for blood (i.e., THC, 11-OH-THC, THCCOOH and THCCOOH-glucuronide) and oral fluid cannabinoid concentrations (i.e., THC) and peak change from baseline scores for self-reported rating of “drug effect” (from the Drug Effect Questionnaire; DEQ; 0–100 visual analogue scale anchored from “not at all” on the left to “extremely” on the right), HR, and primary outcomes for the Digit Symbol Substitution Task (DSST; total correct), Divided Attention Task (DAT; total correct and average distance from central stimulus), and the Paced Serial Additional Task (PASAT; total correct; see Spindle et al. (14). or Vandrey et al. (16) for additional details regarding pharmacodynamic outcomes). Analyses were conducted in SPSS (version 24). A full description and results of all pharmacodynamic outcomes from this study are reported elsewhere (14).
Results
All baseline whole blood and oral fluid samples tested negative for THC and/or THC metabolites by ELISA and LC–MS-MS, further supporting compliance with pre-session cannabis abstinence requirements. Tables I and II display the full ELISA and LC–MS-MS blood and oral fluid results over time for each individual participant. Tables III and IV display the mean Cmax and time to maximum concentration (Tmax) results for blood and oral fluid, respectively, for THC and its metabolites.
Table I.
Results of ELISA and LC–MS-MS Blood Specimen Analyses Following Inhalation of Smoked and Vaporized Cannabis
| Smoked | Vaporized | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject # | Time (h) | THC dose (mg) | THC (ng/mL) | 11-OH THC (ng/mL) | THC-COOH (ng/mL) | THC-COOH GLUC (ng/mL) | ELISA (cutoff = 10 ng/mL) | THC (ng/mL) | 11-OH THC (ng/mL) | THC-COOH (ng/mL) | THC-COOH GLUC (ng/mL) | ELISA (cutoff = 10 ng/mL) |
| 15 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 15 | 0.17 | 10 | 1 | 0 | 2 | 0 | NEG | 2 | 1 | 6 | 1 | POS |
| 15 | 0.5 | 10 | 1 | 0 | 2 | 1 | NEG | 2 | 1 | 5 | 3 | POS |
| 15 | 1 | 10 | 0 | 0 | 2 | 2 | NEG | 1 | 0 | 5 | 5 | POS |
| 15 | 1.5 | 10 | 0 | 0 | 2 | 2 | NEG | 3 | 0 | 4 | 7 | POS |
| 15 | 2 | 10 | 0 | 0 | 2 | 2 | NEG | 1 | 0 | 3 | 7 | POS |
| 15 | 3 | 10 | 0 | 0 | 2 | 2 | NEG | 1 | 0 | 3 | 7 | POS |
| 15 | 4 | 10 | 0 | 0 | 1 | 2 | NEG | 0 | 0 | 3 | 6 | POS |
| 15 | 5 | 10 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 2 | 6 | POS |
| 15 | 6 | 10 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 1 | 4 | POS |
| 15 | 8 | 10 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 0 | 4 | NEG |
| 15 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 15 | 0.17 | 25 | 15 | 1 | 14 | 4 | POS | 20 | 2 | 7 | 0 | POS |
| 15 | 0.5 | 25 | 2 | 1 | 12 | 6 | POS | 2 | 1 | 6 | 3 | POS |
| 15 | 1 | 25 | 1 | 0 | 9 | 12 | POS | 3 | 0 | 5 | 5 | POS |
| 15 | 1.5 | 25 | 1 | 0 | 8 | 14 | POS | 2 | 0 | 5 | 7 | POS |
| 15 | 2 | 25 | 1 | 0 | 9 | 11 | POS | 3 | 0 | 6 | 8 | POS |
| 15 | 3 | 25 | 1 | 0 | 7 | 17 | POS | 1 | 0 | 4 | 9 | POS |
| 15 | 4 | 25 | 0 | 0 | 5 | 14 | POS | 0 | 0 | 4 | 8 | POS |
| 15 | 5 | 25 | 0 | 0 | 5 | 9 | POS | 0 | 0 | 2 | 4 | POS |
| 15 | 6 | 25 | 0 | 0 | 4 | 11 | POS | 0 | 0 | 1 | 5 | POS |
| 15 | 8 | 25 | 0 | 0 | 0 | 6 | POS | 0 | 0 | 1 | 4 | NEG |
| 20 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 20 | 0.17 | 10 | 0 | 0 | 0 | 0 | NEG | 7 | 1 | 5 | 0 | POS |
| 20 | 0.5 | 10 | 0 | 0 | 0 | 0 | NEG | 4 | 0 | 5 | 5 | POS |
| 20 | 1 | 10 | 0 | 0 | 0 | 0 | NEG | 2 | 0 | 4 | 6 | POS |
| 20 | 1.5 | 10 | 0 | 0 | 0 | 0 | NEG | 2 | 0 | 4 | 9 | POS |
| 20 | 2 | 10 | 0 | 0 | 0 | 0 | NEG | 1 | 0 | 4 | 11 | POS |
| 20 | 3 | 10 | 0 | 0 | 0 | 0 | NEG | 1 | 0 | 4 | 10 | POS |
| 20 | 4 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 3 | 11 | POS |
| 20 | 5 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 3 | 8 | POS |
| 20 | 6 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 2 | 8 | POS |
| 20 | 8 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 2 | 7 | POS |
| 20 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 20 | 0.17 | 25 | 1 | 0 | 1 | 0 | POS | 4 | 2 | 4 | 1 | POS |
| 20 | 0.5 | 25 | 3 | 0 | 2 | 1 | POS | 0 | 0 | 5 | 6 | POS |
| 20 | 1 | 25 | 1 | 0 | 2 | 3 | POS | 3 | 1 | 4 | 9 | POS |
| 20 | 1.5 | 25 | 1 | 0 | 2 | 4 | POS | 2 | 0 | 5 | 11 | POS |
| 20 | 2 | 25 | 1 | 0 | 3 | 4 | POS | 2 | 0 | 4 | 11 | POS |
| 20 | 3 | 25 | 0 | 0 | 1 | 6 | POS | 1 | 0 | 4 | 10 | POS |
| 20 | 4 | 25 | 0 | 0 | 2 | 7 | POS | 0 | 0 | 3 | 12 | POS |
| 20 | 5 | 25 | 0 | 0 | 2 | 6 | POS | 0 | 0 | 3 | 10 | POS |
| 20 | 6 | 25 | 0 | 0 | 2 | 4 | POS | 0 | 0 | 3 | 10 | POS |
| 20 | 8 | 25 | 0 | 0 | 1 | 5 | POS | 0 | 0 | 2 | 8 | POS |
| 36 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 36 | 0.17 | 10 | 0 | 0 | 9 | 0 | POS | 0 | 1 | 10 | 1 | POS |
| 36 | 0.5 | 10 | 0 | 0 | 7 | 3 | POS | 4 | 2 | 10 | 5 | POS |
| 36 | 1 | 10 | 0 | 0 | 7 | 5 | POS | 2 | 1 | 9 | 6 | POS |
| 36 | 1.5 | 10 | 0 | 0 | 7 | 7 | POS | 2 | 1 | 9 | 9 | POS |
| 36 | 2 | 10 | 0 | 0 | 6 | 8 | POS | 1 | 1 | 7 | 10 | POS |
| 36 | 3 | 10 | 0 | 0 | 6 | 5 | POS | 0 | 0 | 6 | 9 | POS |
| 36 | 4 | 10 | 0 | 0 | 4 | 6 | POS | 0 | 0 | 2 | 9 | POS |
| 36 | 5 | 10 | 0 | 0 | 5 | 5 | POS | 0 | 0 | 4 | 7 | POS |
| 36 | 6 | 10 | 0 | 0 | 5 | 4 | POS | 0 | 0 | 2 | 4 | POS |
| 36 | 8 | 10 | 0 | 0 | 2 | 3 | POS | 0 | 0 | 2 | 5 | POS |
| 36 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 36 | 0.17 | 25 | 38 | 4 | 16 | 2 | POS | 29 | 2 | 8 | 1 | POS |
| 36 | 0.5 | 25 | 9 | 2 | 18 | 9 | POS | 8 | 2 | 9 | 4 | POS |
| 36 | 1 | 25 | 8 | 2 | 14 | 13 | POS | 4 | 1 | 7 | 6 | POS |
| 36 | 1.5 | 25 | 4 | 1 | 14 | 15 | POS | 2 | 1 | 8 | 9 | POS |
| 36 | 2 | 25 | 3 | 1 | 11 | 16 | POS | 1 | 0 | 7 | 6 | POS |
| 36 | 3 | 25 | 1 | 0 | 8 | 13 | POS | 0 | 0 | 5 | 8 | POS |
| 36 | 4 | 25 | 0 | 0 | 7 | 11 | POS | 0 | 0 | 4 | 7 | POS |
| 36 | 5 | 25 | 0 | 0 | 6 | 10 | POS | 0 | 0 | 4 | 8 | POS |
| 36 | 6 | 25 | 0 | 0 | 5 | 9 | POS | 0 | 0 | 3 | 8 | POS |
| 36 | 8 | 25 | 0 | 0 | 3 | 8 | POS | 0 | 0 | 2 | 6 | POS |
| 38 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 38 | 0.17 | 10 | 3 | 1 | 4 | 0 | NEG | 8 | 2 | 7 | 1 | POS |
| 38 | 0.5 | 10 | 1 | 0 | 4 | 2 | NEG | 3 | 1 | 7 | 2 | POS |
| 38 | 1 | 10 | 1 | 0 | 2 | 2 | POS | 2 | 1 | 5 | 4 | POS |
| 38 | 1.5 | 10 | 0 | 0 | 2 | 3 | POS | 1 | 1 | 5 | 5 | POS |
| 38 | 2 | 10 | 0 | 0 | 2 | 2 | POS | 1 | 0 | 5 | 5 | POS |
| 38 | 3 | 10 | 0 | 0 | 2 | 4 | POS | 0 | 0 | 5 | 6 | POS |
| 38 | 4 | 10 | 0 | 0 | 1 | 4 | POS | 0 | 0 | 4 | 6 | POS |
| 38 | 5 | 10 | 0 | 0 | 1 | 3 | POS | 0 | 0 | 3 | 6 | POS |
| 38 | 6 | 10 | 0 | 0 | 0 | 2 | POS | 0 | 0 | 3 | 6 | POS |
| 38 | 8 | 10 | 0 | 0 | 0 | 2 | NEG | 0 | 0 | 2 | 5 | POS |
| 38 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 38 | 0.17 | 25 | 8 | 4 | 23 | 8 | POS | 6 | 2 | 11 | 1 | POS |
| 38 | 0.5 | 25 | 7 | 4 | 21 | 19 | POS | 8 | 2 | 14 | 5 | POS |
| 38 | 1 | 25 | 1 | 2 | 17 | 21 | POS | 4 | 1 | 11 | 7 | POS |
| 38 | 1.5 | 25 | 1 | 1 | 19 | 21 | POS | 2 | 1 | 10 | 9 | POS |
| 38 | 2 | 25 | 0 | 1 | 15 | 24 | POS | 1 | 0 | 7 | 11 | POS |
| 38 | 3 | 25 | 0 | 0 | 13 | 21 | POS | 1 | 0 | 8 | 12 | POS |
| 38 | 4 | 25 | 0 | 0 | 12 | 21 | POS | 0 | 0 | 7 | 10 | POS |
| 38 | 5 | 25 | 0 | 0 | 10 | 21 | POS | 0 | 0 | 6 | 7 | POS |
| 38 | 6 | 25 | 0 | 0 | 10 | 21 | POS | 0 | 0 | 5 | 9 | POS |
| 38 | 8 | 25 | 0 | 0 | 8 | 16 | POS | 0 | 0 | 5 | 8 | POS |
| 50 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 50 | 0.17 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 50 | 0.5 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 50 | 1 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 50 | 1.5 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 50 | 2 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 50 | 3 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 50 | 4 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 50 | 5 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 50 | 6 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 50 | 8 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 50 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 50 | 0.17 | 25 | 0 | 0 | 0 | 0 | NEG | 1 | 0 | 3 | 0 | NEG |
| 50 | 0.5 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 1 | NEG |
| 50 | 1 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 2 | POS |
| 50 | 1.5 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 2 | 2 | POS |
| 50 | 2 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 3 | POS |
| 50 | 3 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 2 | 4 | POS |
| 50 | 4 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 3 | POS |
| 50 | 5 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 2 | NEG |
| 50 | 6 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 2 | NEG |
| 50 | 8 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 2 | NEG |
| 55 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 55 | 0.17 | 10 | 1 | 0 | 1 | 0 | NEG | 5 | 0 | 2 | 0 | NEG |
| 55 | 0.5 | 10 | 0 | 0 | 0 | 0 | NEG | 1 | 0 | 1 | 1 | NEG |
| 55 | 1 | 10 | 0 | 0 | 0 | 0 | NEG | 1 | 0 | 1 | 1 | NEG |
| 55 | 1.5 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 1 | NEG |
| 55 | 2 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 1 | NEG |
| 55 | 3 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 1 | NEG |
| 55 | 4 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 1 | NEG |
| 55 | 5 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 1 | NEG |
| 55 | 6 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 55 | 8 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 55 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 55 | 0.17 | 25 | 0 | 0 | 1 | 0 | NEG | 9 | 0 | 4 | 0 | POS |
| 55 | 0.5 | 25 | 0 | 0 | 1 | 0 | NEG | 4 | 0 | 4 | 2 | POS |
| 55 | 1 | 25 | 0 | 0 | 1 | 1 | NEG | 3 | 0 | 3 | 3 | POS |
| 55 | 1.5 | 25 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 1 | 3 | POS |
| 55 | 2 | 25 | 0 | 0 | 1 | 1 | NEG | 1 | 0 | 3 | 3 | POS |
| 55 | 3 | 25 | 0 | 0 | 1 | 1 | NEG | 1 | 0 | 3 | 3 | POS |
| 55 | 4 | 25 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 2 | 2 | POS |
| 55 | 5 | 25 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 2 | 2 | NEG |
| 55 | 6 | 25 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 1 | 2 | NEG |
| 55 | 8 | 25 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 1 | 1 | NEG |
| 58 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 58 | 0.17 | 10 | 10 | 0 | 2 | 0 | NEG | 13 | 0 | 3 | 0 | NEG |
| 58 | 0.5 | 10 | 3 | 0 | 2 | 0 | NEG | 6 | 0 | 3 | 0 | POS |
| 58 | 1 | 10 | 2 | 0 | 1 | 0 | NEG | 4 | 0 | 3 | 1 | POS |
| 58 | 1.5 | 10 | 1 | 0 | 1 | 0 | NEG | 3 | 0 | 2 | 1 | POS |
| 58 | 2 | 10 | 0 | 0 | 1 | 0 | NEG | 2 | 0 | 2 | 1 | NEG |
| 58 | 3 | 10 | 0 | 0 | 1 | 0 | NEG | 1 | 0 | 2 | 1 | NEG |
| 58 | 4 | 10 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 2 | 0 | NEG |
| 58 | 5 | 10 | 0 | 0 | 1 | 0 | NEG | 0 | 0 | 1 | 1 | NEG |
| 58 | 6 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 1 | NEG |
| 58 | 8 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 1 | NEG |
| 58 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 58 | 0.17 | 25 | 15 | 1 | 8 | 0 | POS | 14 | 0 | 4 | 0 | NEG |
| 58 | 0.5 | 25 | 9 | 0 | 6 | 1 | POS | 6 | 0 | 3 | 0 | NEG |
| 58 | 1 | 25 | 5 | 0 | 6 | 1 | POS | 3 | 0 | 3 | 1 | POS |
| 58 | 1.5 | 25 | 4 | 0 | 5 | 2 | POS | 3 | 0 | 3 | 1 | POS |
| 58 | 2 | 25 | 2 | 0 | 5 | 2 | POS | 2 | 0 | 3 | 2 | POS |
| 58 | 3 | 25 | 1 | 0 | 5 | 2 | POS | 1 | 0 | 3 | 1 | POS |
| 58 | 4 | 25 | 1 | 0 | 5 | 3 | POS | 0 | 0 | 2 | 2 | NEG |
| 58 | 5 | 25 | 0 | 0 | 3 | 1 | NEG | 0 | 0 | 1 | 1 | NEG |
| 58 | 6 | 25 | 0 | 0 | 3 | 2 | NEG | 0 | 0 | 1 | 1 | NEG |
| 58 | 8 | 25 | 0 | 0 | 2 | 0 | NEG | 0 | 0 | 1 | 1 | NEG |
| 59 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 59 | 0.17 | 10 | 1 | 0 | 1 | 0 | NEG | 3 | 0 | 1 | 0 | NEG |
| 59 | 0.5 | 10 | 0 | 0 | 2 | 0 | POS | 1 | 0 | 1 | 0 | NEG |
| 59 | 1 | 10 | 0 | 0 | 1 | 0 | POS | 0 | 0 | 2 | 0 | NEG |
| 59 | 1.5 | 10 | 0 | 0 | 2 | 1 | NEG | 0 | 0 | 1 | 0 | NEG |
| 59 | 2 | 10 | 0 | 0 | 1 | 0 | NEG | 0 | 0 | 1 | 0 | NEG |
| 59 | 3 | 10 | 0 | 0 | 1 | 0 | NEG | 0 | 0 | 1 | 0 | NEG |
| 59 | 4 | 10 | 0 | 0 | 1 | 0 | NEG | 0 | 0 | 1 | 0 | NEG |
| 59 | 5 | 10 | 0 | 0 | 1 | 0 | NEG | 0 | 0 | 1 | 0 | NEG |
| 59 | 6 | 10 | 0 | 0 | 1 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 59 | 8 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 59 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 59 | 0.17 | 25 | 1 | 1 | 4 | 0 | POS | 16 | 3 | 17 | 0 | POS |
| 59 | 0.5 | 25 | 1 | 0 | 4 | 1 | POS | 4 | 1 | 11 | 1 | POS |
| 59 | 1 | 25 | 0 | 0 | 3 | 2 | POS | 3 | 1 | 11 | 3 | POS |
| 59 | 1.5 | 25 | 0 | 0 | 3 | 2 | POS | 2 | 0 | 7 | 4 | POS |
| 59 | 2 | 25 | 0 | 0 | 3 | 2 | POS | 1 | 0 | 6 | 6 | POS |
| 59 | 3 | 25 | 0 | 0 | 2 | 3 | POS | 0 | 0 | 6 | 5 | POS |
| 59 | 4 | 25 | 0 | 0 | 2 | 3 | POS | 0 | 0 | 4 | 2 | POS |
| 59 | 5 | 25 | 0 | 0 | 2 | 2 | POS | 0 | 0 | 3 | 2 | POS |
| 59 | 6 | 25 | 0 | 0 | 1 | 2 | POS | 0 | 0 | 3 | 2 | POS |
| 59 | 8 | 25 | 0 | 0 | 1 | 2 | NEG | 0 | 0 | 3 | 2 | POS |
| 60 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 60 | 0.17 | 10 | 0 | 0 | 0 | 0 | NEG | 5 | 0 | 4 | 1 | POS |
| 60 | 0.5 | 10 | 0 | 0 | 0 | 0 | NEG | 3 | 0 | 4 | 2 | POS |
| 60 | 1 | 10 | 0 | 0 | 0 | 0 | NEG | 2 | 0 | 4 | 3 | POS |
| 60 | 1.5 | 10 | 0 | 0 | 0 | 0 | NEG | 1 | 0 | 3 | 4 | POS |
| 60 | 2 | 10 | 0 | 0 | 0 | 0 | NEG | 1 | 0 | 4 | 3 | POS |
| 60 | 3 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 3 | 3 | POS |
| 60 | 4 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 2 | 3 | POS |
| 60 | 5 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 3 | POS |
| 60 | 6 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 3 | POS |
| 60 | 8 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 2 | NEG |
| 60 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 60 | 0.17 | 25 | 0 | 0 | 0 | 0 | NEG | 7 | 0 | 4 | 0 | POS |
| 60 | 0.5 | 25 | 0 | 0 | 0 | 0 | NEG | 2 | 0 | 3 | 2 | POS |
| 60 | 1 | 25 | 0 | 0 | 0 | 0 | NEG | 1 | 0 | 3 | 3 | POS |
| 60 | 1.5 | 25 | 0 | 0 | 0 | 0 | NEG | 1 | 0 | 2 | 4 | POS |
| 60 | 2 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 2 | 3 | POS |
| 60 | 3 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 3 | POS |
| 60 | 4 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 3 | POS |
| 60 | 5 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 2 | NEG |
| 60 | 6 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 2 | NEG |
| 60 | 8 | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 2 | NEG |
| 4 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 4 | 0.17 | 10 | 0 | 0 | 0 | 0 | NEG | 10 | 6 | 17 | 1 | POS |
| 4 | 0.5 | 10 | 0 | 0 | 0 | 0 | NEG | 5 | 5 | 18 | 4 | POS |
| 4 | 1 | 10 | 0 | 0 | 0 | 0 | NEG | 3 | 3 | 16 | 0 | POS |
| 4 | 1.5 | 10 | 0 | 0 | 0 | 0 | NEG | 1 | 2 | 12 | 0 | POS |
| 4 | 2 | 10 | 0 | 0 | 0 | 0 | NEG | 1 | 1 | 11 | 14 | POS |
| 4 | 3 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 10 | 14 | POS |
| 4 | 4 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 8 | 9 | POS |
| 4 | 5 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 6 | 8 | POS |
| 4 | 6 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 5 | 6 | POS |
| 4 | 8 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 4 | 6 | POS |
| 4 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 4 | 0.17 | 25 | 4 | 1 | 4 | 0 | POS | 13 | 2 | 7 | 0 | POS |
| 4 | 0.5 | 25 | 2 | 0 | 2 | 1 | NEG | 5 | 2 | 6 | 2 | POS |
| 4 | 1 | 25 | 1 | 0 | 2 | 1 | NEG | 3 | 1 | 5 | 3 | POS |
| 4 | 1.5 | 25 | 1 | 0 | 3 | 2 | NEG | 2 | 1 | 4 | 4 | POS |
| 4 | 2 | 25 | 1 | 0 | 3 | 1 | NEG | 1 | 0 | 4 | 3 | POS |
| 4 | 3 | 25 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 3 | 3 | POS |
| 4 | 4 | 25 | 0 | 0 | 1 | 2 | NEG | 0 | 0 | 2 | 4 | NEG |
| 4 | 5 | 25 | 0 | 0 | 1 | 2 | NEG | 0 | 0 | 2 | 3 | NEG |
| 4 | 6 | 25 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 2 | 2 | NEG |
| 4 | 8 | 25 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 1 | 3 | NEG |
| 25 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 25 | 0.17 | 10 | 0 | 0 | 0 | 0 | NEG | 17 | 2 | 10 | 1 | POS |
| 25 | 0.5 | 10 | 0 | 0 | 0 | 1 | NEG | 5 | 1 | 8 | 6 | POS |
| 25 | 1 | 10 | 0 | 0 | 0 | 1 | NEG | 4 | 1 | 9 | 4 | POS |
| 25 | 1.5 | 10 | 0 | 0 | 0 | 1 | NEG | 2 | 1 | 8 | 9 | POS |
| 25 | 2 | 10 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 10 | 4 | POS |
| 25 | 3 | 10 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 5 | 6 | POS |
| 25 | 4 | 10 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 3 | 6 | POS |
| 25 | 5 | 10 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 4 | 6 | POS |
| 25 | 6 | 10 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 3 | 4 | POS |
| 25 | 8 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 2 | 4 | POS |
| 25 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 25 | 0.17 | 25 | 0 | 0 | 0 | 0 | NEG | 15 | 2 | 7 | 1 | POS |
| 25 | 0.5 | 25 | 0 | 0 | 0 | 0 | NEG | 7 | 2 | 7 | 6 | POS |
| 25 | 1 | 25 | 0 | 0 | 0 | 1 | NEG | 4 | 1 | 6 | 9 | POS |
| 25 | 1.5 | 25 | 0 | 0 | 0 | 1 | NEG | 2 | 1 | 5 | 8 | POS |
| 25 | 2 | 25 | 0 | 0 | 0 | 1 | NEG | 2 | 1 | 5 | 9 | POS |
| 25 | 3 | 25 | 0 | 0 | 0 | 1 | NEG | 1 | 0 | 4 | 9 | POS |
| 25 | 4 | 25 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 3 | 7 | POS |
| 25 | 5 | 25 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 3 | 6 | POS |
| 25 | 6 | 25 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 3 | 6 | POS |
| 25 | 8 | 25 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 2 | 6 | POS |
| 29 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 29 | 0.17 | 10 | 2 | 0 | 2 | 3 | POS | 7 | 2 | 8 | 1 | POS |
| 29 | 0.5 | 10 | 2 | 0 | 3 | 4 | POS | 2 | 1 | 7 | 3 | POS |
| 29 | 1 | 10 | 1 | 0 | 3 | 5 | POS | 1 | 1 | 6 | 4 | POS |
| 29 | 1.5 | 10 | 0 | 0 | 2 | 5 | POS | 1 | 0 | 6 | 5 | POS |
| 29 | 2 | 10 | 0 | 0 | 2 | 5 | POS | 0 | 0 | 6 | 6 | POS |
| 29 | 3 | 10 | 0 | 0 | 1 | 5 | POS | 0 | 0 | 5 | 5 | POS |
| 29 | 4 | 10 | 0 | 0 | 1 | 4 | POS | 0 | 0 | 4 | 6 | POS |
| 29 | 5 | 10 | 0 | 0 | 0 | 3 | POS | 0 | 0 | 3 | 5 | POS |
| 29 | 6 | 10 | 0 | 0 | 1 | 3 | POS | 0 | 0 | 3 | 6 | POS |
| 29 | 8 | 10 | 0 | 0 | 1 | 3 | NEG | 0 | 0 | 2 | 6 | NEG |
| 29 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 29 | 0.17 | 25 | 2 | 0 | 1 | 0 | NEG | 13 | 6 | 14 | 2 | POS |
| 29 | 0.5 | 25 | 1 | 0 | 1 | 1 | NEG | 6 | 2 | 12 | 9 | POS |
| 29 | 1 | 25 | 0 | 0 | 1 | 2 | NEG | 4 | 2 | 14 | 7 | POS |
| 29 | 1.5 | 25 | 0 | 0 | 1 | 2 | NEG | 2 | 1 | 10 | 10 | POS |
| 29 | 2 | 25 | 0 | 0 | 1 | 2 | NEG | 1 | 1 | 9 | 11 | POS |
| 29 | 3 | 25 | 0 | 0 | 1 | 2 | NEG | 0 | 0 | 8 | 10 | POS |
| 29 | 4 | 25 | 0 | 0 | 1 | 2 | NEG | 0 | 0 | 7 | 17 | POS |
| 29 | 5 | 25 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 5 | 15 | POS |
| 29 | 6 | 25 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 4 | 9 | POS |
| 29 | 8 | 25 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 4 | 6 | POS |
| 53 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 53 | 0.17 | 10 | 1 | 0 | 2 | 0 | NEG | 5 | 2 | 10 | 1 | POS |
| 53 | 0.5 | 10 | 0 | 0 | 1 | 1 | NEG | 2 | 2 | 7 | 3 | POS |
| 53 | 1 | 10 | 0 | 0 | 1 | 1 | NEG | 1 | 1 | 7 | 4 | POS |
| 53 | 1.5 | 10 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 5 | 4 | POS |
| 53 | 2 | 10 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 5 | 4 | POS |
| 53 | 3 | 10 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 4 | 3 | POS |
| 53 | 4 | 10 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 3 | 3 | POS |
| 53 | 5 | 10 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 3 | 3 | POS |
| 53 | 6 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 2 | 2 | POS |
| 53 | 8 | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 1 | 2 | NEG |
| 53 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 53 | 0.17 | 25 | 3 | 1 | 3 | 1 | NEG | 2 | 5 | 12 | 1 | POS |
| 53 | 0.5 | 25 | 1 | 0 | 2 | 2 | NEG | 2 | 5 | 13 | 6 | POS |
| 53 | 1 | 25 | 1 | 0 | 2 | 3 | NEG | 0 | 2 | 11 | 8 | POS |
| 53 | 1.5 | 25 | 0 | 0 | 2 | 6 | NEG | 0 | 2 | 9 | 10 | POS |
| 53 | 2 | 25 | 0 | 0 | 2 | 3 | NEG | 0 | 1 | 8 | 10 | POS |
| 53 | 3 | 25 | 0 | 0 | 1 | 3 | NEG | 0 | 1 | 6 | 10 | POS |
| 53 | 4 | 25 | 0 | 0 | 1 | 3 | NEG | 0 | 0 | 5 | 7 | POS |
| 53 | 5 | 25 | 0 | 0 | 1 | 3 | NEG | 0 | 0 | 4 | 7 | POS |
| 53 | 6 | 25 | 0 | 0 | 1 | 2 | NEG | 0 | 0 | 3 | 6 | POS |
| 53 | 8 | 25 | 0 | 0 | 1 | 1 | NEG | 0 | 0 | 4 | 4 | POS |
| 54 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 54 | 0.17 | 10 | 5 | 1 | 4 | 0 | POS | 7 | 1 | 7 | 0 | POS |
| 54 | 0.5 | 10 | 2 | 0 | 3 | 1 | POS | 3 | 1 | 6 | 1 | POS |
| 54 | 1 | 10 | 1 | 0 | 3 | 2 | POS | 2 | 0 | 5 | 2 | POS |
| 54 | 1.5 | 10 | 0 | 0 | 2 | 2 | POS | 0 | 0 | 4 | 4 | POS |
| 54 | 2 | 10 | 0 | 0 | 2 | 2 | POS | 0 | 0 | 4 | 4 | POS |
| 54 | 3 | 10 | 0 | 0 | 2 | 3 | POS | 0 | 0 | 3 | 5 | POS |
| 54 | 4 | 10 | 0 | 0 | 2 | 2 | POS | 0 | 0 | 3 | 6 | POS |
| 54 | 5 | 10 | 0 | 0 | 2 | 3 | POS | 0 | 0 | 3 | 6 | POS |
| 54 | 6 | 10 | 0 | 0 | 1 | 3 | POS | 0 | 0 | 3 | 5 | POS |
| 54 | 8 | 10 | 0 | 0 | 1 | 3 | NEG | 0 | 0 | 3 | 6 | POS |
| 54 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 54 | 0.17 | 25 | 11 | 1 | 6 | 0 | POS | 29 | 3 | 6 | 1 | POS |
| 54 | 0.5 | 25 | 5 | 1 | 5 | 2 | POS | 5 | 1 | 7 | 3 | POS |
| 54 | 1 | 25 | 2 | 0 | 5 | 4 | POS | 2 | 1 | 6 | 4 | POS |
| 54 | 1.5 | 25 | 1 | 0 | 6 | 5 | POS | 2 | 0 | 6 | 6 | POS |
| 54 | 2 | 25 | 1 | 0 | 4 | 5 | POS | 1 | 0 | 5 | 7 | POS |
| 54 | 3 | 25 | 0 | 0 | 4 | 5 | POS | 0 | 0 | 5 | 6 | POS |
| 54 | 4 | 25 | 0 | 0 | 3 | 6 | POS | 0 | 0 | 4 | 7 | POS |
| 54 | 5 | 25 | 0 | 0 | 3 | 3 | POS | 0 | 0 | 4 | 8 | POS |
| 54 | 6 | 25 | 0 | 0 | 3 | 6 | POS | 0 | 0 | 4 | 7 | POS |
| 54 | 8 | 25 | 0 | 0 | 3 | 5 | POS | 0 | 0 | 3 | 7 | POS |
| 63 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 63 | 0.17 | 10 | 21 | 1 | 1 | 0 | NEG | 15 | 1 | 2 | 0 | NEG |
| 63 | 0.5 | 10 | 6 | 1 | 2 | 3 | POS | 5 | 1 | 3 | 5 | POS |
| 63 | 1 | 10 | 4 | 0 | 2 | 5 | POS | 3 | 0 | 3 | 4 | POS |
| 63 | 1.5 | 10 | 2 | 0 | 2 | 5 | POS | 1 | 0 | 2 | 4 | POS |
| 63 | 2 | 10 | 1 | 0 | 2 | 5 | POS | 0 | 0 | 2 | 7 | POS |
| 63 | 3 | 10 | 0 | 0 | 1 | 7 | POS | 0 | 0 | 2 | 9 | POS |
| 63 | 4 | 10 | 0 | 0 | 1 | 5 | POS | 0 | 0 | 1 | 4 | NEG |
| 63 | 5 | 10 | 0 | 0 | 1 | 4 | POS | 0 | 0 | 1 | 2 | NEG |
| 63 | 6 | 10 | 0 | 0 | 1 | 3 | POS | 0 | 0 | 0 | 2 | NEG |
| 63 | 8 | 10 | 0 | 0 | 0 | 3 | NEG | 0 | 0 | 0 | 1 | NEG |
| 63 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 63 | 0.17 | 25 | 27 | 2 | 5 | 1 | POS | 33 | 2 | 2 | 0 | NEG |
| 63 | 0.5 | 25 | 14 | 3 | 7 | 3 | POS | 14 | 3 | 7 | 4 | POS |
| 63 | 1 | 25 | 7 | 2 | 7 | 5 | POS | 5 | 2 | 8 | 10 | POS |
| 63 | 1.5 | 25 | 4 | 1 | 6 | 7 | POS | 4 | 2 | 8 | 7 | POS |
| 63 | 2 | 25 | 2 | 1 | 5 | 7 | POS | 2 | 1 | 6 | 8 | POS |
| 63 | 3 | 25 | 1 | 0 | 4 | 6 | POS | 1 | 1 | 6 | 14 | POS |
| 63 | 4 | 25 | 0 | 0 | 3 | 6 | POS | 0 | 0 | 5 | 9 | POS |
| 63 | 5 | 25 | 0 | 0 | 2 | 5 | POS | 0 | 0 | 3 | 7 | POS |
| 63 | 6 | 25 | 0 | 0 | 1 | 6 | POS | 0 | 0 | 3 | 6 | POS |
| 63 | 8 | 25 | 0 | 0 | 1 | 6 | NEG | 0 | 0 | 1 | 7 | POS |
| 64 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 64 | 0.17 | 10 | 14 | 0 | 1 | 0 | NEG | 18 | 2 | 9 | 1 | POS |
| 64 | 0.5 | 10 | 4 | 0 | 1 | 2 | POS | 8 | 1 | 8 | 5 | POS |
| 64 | 1 | 10 | 2 | 0 | 1 | 4 | POS | 5 | 1 | 8 | 9 | POS |
| 64 | 1.5 | 10 | 1 | 0 | 1 | 4 | POS | 4 | 1 | 8 | 10 | POS |
| 64 | 2 | 10 | 1 | 0 | 1 | 5 | POS | 2 | 0 | 6 | 11 | POS |
| 64 | 3 | 10 | 0 | 0 | 1 | 4 | NEG | 1 | 0 | 6 | 11 | POS |
| 64 | 4 | 10 | 0 | 0 | 1 | 4 | NEG | 0 | 0 | 5 | 11 | POS |
| 64 | 5 | 10 | 0 | 0 | 1 | 3 | NEG | 0 | 0 | 4 | 10 | POS |
| 64 | 6 | 10 | 0 | 0 | 1 | 3 | NEG | 0 | 0 | 4 | 9 | POS |
| 64 | 8 | 10 | 0 | 0 | 1 | 3 | NEG | 0 | 0 | 3 | 9 | POS |
| 64 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 64 | 0.17 | 25 | 15 | 0 | 4 | 1 | POS | 18 | 2 | 4 | 0 | POS |
| 64 | 0.5 | 25 | 7 | 1 | 7 | 4 | POS | 5 | 1 | 5 | 3 | POS |
| 64 | 1 | 25 | 5 | 0 | 5 | 6 | POS | 3 | 0 | 4 | 5 | POS |
| 64 | 1.5 | 25 | 3 | 0 | 5 | 7 | POS | 2 | 0 | 4 | 6 | POS |
| 64 | 2 | 25 | 2 | 0 | 4 | 8 | POS | 1 | 0 | 4 | 6 | POS |
| 64 | 3 | 25 | 1 | 0 | 4 | 8 | POS | 0 | 0 | 3 | 6 | POS |
| 64 | 4 | 25 | 0 | 0 | 3 | 9 | POS | 0 | 0 | 3 | 7 | POS |
| 64 | 5 | 25 | 0 | 0 | 2 | 7 | POS | 0 | 0 | 2 | 6 | POS |
| 64 | 6 | 25 | 0 | 0 | 2 | 5 | POS | 0 | 0 | 2 | 5 | POS |
| 64 | 8 | 25 | 0 | 0 | 1 | 5 | POS | 0 | 0 | 2 | 5 | POS |
| 65 | BL | 10 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 65 | 0.17 | 10 | 5 | 0 | 2 | 0 | NEG | 6 | 0 | 1 | 0 | NEG |
| 65 | 0.5 | 10 | 2 | 0 | 2 | 1 | NEG | 2 | 0 | 2 | 1 | NEG |
| 65 | 1 | 10 | 1 | 0 | 1 | 2 | NEG | 1 | 0 | 2 | 3 | NEG |
| 65 | 1.5 | 10 | 1 | 0 | 1 | 3 | NEG | 1 | 0 | 2 | 3 | NEG |
| 65 | 2 | 10 | 0 | 0 | 1 | 3 | NEG | 0 | 0 | 1 | 3 | NEG |
| 65 | 3 | 10 | 0 | 0 | 1 | 3 | NEG | 0 | 0 | 1 | 4 | NEG |
| 65 | 4 | 10 | 0 | 0 | 1 | 3 | NEG | 0 | 0 | 1 | 3 | NEG |
| 65 | 5 | 10 | 0 | 0 | 1 | 2 | NEG | 0 | 0 | 1 | 3 | NEG |
| 65 | 6 | 10 | 0 | 0 | 0 | 2 | NEG | 0 | 0 | 1 | 3 | NEG |
| 65 | 8 | 10 | 0 | 0 | 0 | 1 | NEG | 0 | 0 | 0 | 3 | NEG |
| 65 | BL | 25 | 0 | 0 | 0 | 0 | NEG | 0 | 0 | 0 | 0 | NEG |
| 65 | 0.17 | 25 | 34 | 3 | 11 | 3 | POS | 15 | 2 | 5 | 1 | POS |
| 65 | 0.5 | 25 | 7 | 2 | 9 | 11 | POS | 6 | 1 | 5 | 5 | POS |
| 65 | 1 | 25 | 6 | 1 | 8 | 19 | POS | 4 | 1 | 4 | 8 | POS |
| 65 | 1.5 | 25 | 5 | 1 | 8 | 20 | POS | 3 | 1 | 4 | 13 | POS |
| 65 | 2 | 25 | 3 | 1 | 7 | 25 | POS | 1 | 0 | 4 | 13 | POS |
| 65 | 3 | 25 | 1 | 0 | 6 | 25 | POS | 1 | 0 | 4 | 12 | POS |
| 65 | 4 | 25 | 1 | 0 | 4 | 24 | POS | 0 | 0 | 3 | 11 | POS |
| 65 | 5 | 25 | 0 | 0 | 3 | 22 | POS | 0 | 0 | 3 | 11 | POS |
| 65 | 6 | 25 | 0 | 0 | 3 | 17 | POS | 0 | 0 | 2 | 11 | POS |
| 65 | 8 | 25 | 0 | 0 | 2 | 11 | POS | 0 | 0 | 1 | 8 | POS |
Note: NS = No sample; POS = positive sample; NEG = negative sample. The first nine participants (i.e., #15–60) in this table were male while the last eight (i.e., #4–65) were females.
Table II.
ELISA and LC–MS-MS Analyses of Oral Fluid Samples Following Inhalation of Smoked and Vaporized Cannabis
| Smoked | Vaporized | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject # | Time (h) | THC dose (mg) | ELISA THC (cutoff = 4 ng/mL) | ELISA THC-COOH (cutoff = 0.05 ng/mL) | THC (ng/mL) | THC-COOH (ng/mL) | ELISA THC (cutoff = 4 ng/mL) | ELISA THC-COOH (cutoff = 0.05 ng/mL) | THC (ng/mL) | THC-COOH (ng/mL) |
| 15 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 15 | 0.17 | 10 | POS | POS | 23 | 0 | NS | NS | NS | NS |
| 15 | 0.5 | 10 | POS | POS | 35 | 0 | POS | POS | 21 | 0 |
| 15 | 1 | 10 | POS | POS | 10 | 0 | POS | POS | 22 | 0 |
| 15 | 1.5 | 10 | NEG | NEG | 0 | 0 | POS | POS | 6 | 0 |
| 15 | 2 | 10 | NEG | NEG | 2 | 0 | POS | NEG | 3 | 0 |
| 15 | 3 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 1 | 0 |
| 15 | 4 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 15 | 5 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 2 | 0 |
| 15 | 6 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 15 | 8 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 15 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 15 | 0.17 | 25 | NS | NS | NS | NS | NS | NS | 179 | 0 |
| 15 | 0.5 | 25 | NS | NS | NS | NS | POS | POS | 37 | 0 |
| 15 | 1 | 25 | NS | NS | NS | NS | POS | POS | 52 | 0 |
| 15 | 1.5 | 25 | NS | NS | NS | NS | POS | POS | 30 | 0 |
| 15 | 2 | 25 | NS | NS | NS | NS | POS | POS | 23 | 0 |
| 15 | 3 | 25 | NS | NS | 66 | 0 | NEG | NEG | 3 | 0 |
| 15 | 4 | 25 | POS | POS | 13 | 0 | NEG | POS | 1 | 0 |
| 15 | 5 | 25 | POS | POS | 51 | 0 | POS | POS | 3 | 0 |
| 15 | 6 | 25 | POS | POS | 11 | 0 | NEG | NEG | 2 | 0 |
| 15 | 8 | 25 | POS | POS | 12 | 0 | NEG | POS | 2 | 0 |
| 20 | BL | 0 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 20 | 0.17 | 0 | POS | POS | 49 | 0 | NEG | NEG | 0 | 0 |
| 20 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 20 | 0.17 | 10 | POS | POS | 27 | 0 | POS | POS | 36 | 0 |
| 20 | 0.5 | 10 | POS | POS | 11 | 0 | POS | POS | 18 | 0 |
| 20 | 1 | 10 | POS | POS | 7 | 0 | POS | NEG | 3 | 0 |
| 20 | 1.5 | 10 | POS | POS | 11 | 0 | NEG | NEG | 1 | 0 |
| 20 | 2 | 10 | POS | POS | 7 | 0 | NEG | NEG | 1 | 0 |
| 20 | 3 | 10 | POS | POS | 8 | 0 | NEG | NEG | 1 | 0 |
| 20 | 4 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 20 | 5 | 10 | POS | NEG | 5 | 0 | NEG | NEG | 1 | 0 |
| 20 | 6 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 20 | 8 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 20 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 20 | 0.17 | 25 | POS | POS | 280 | 0 | POS | POS | 594 | 0 |
| 20 | 0.5 | 25 | POS | POS | 125 | 0 | POS | POS | 196 | 0 |
| 20 | 1 | 25 | POS | POS | 61 | 0 | POS | POS | 57 | 0 |
| 20 | 1.5 | 25 | POS | POS | 61 | 0 | POS | POS | 88 | 0 |
| 20 | 2 | 25 | POS | POS | 17 | 0 | POS | POS | 7 | 0 |
| 20 | 3 | 25 | POS | POS | 7 | 0 | POS | POS | 4 | 0 |
| 20 | 4 | 25 | POS | POS | 2 | 0 | POS | POS | 7 | 0 |
| 20 | 5 | 25 | POS | NEG | 3 | 0 | POS | NEG | 2 | 0 |
| 20 | 6 | 25 | NEG | NEG | 1 | 0 | POS | NEG | 2 | 0 |
| 20 | 8 | 25 | NEG | NEG | 1 | 0 | POS | POS | 3 | 0 |
| 36 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 36 | 0.17 | 10 | POS | POS | 279 | 0 | NS | NS | NS | NS |
| 36 | 0.5 | 10 | POS | POS | 43 | 1.023 | NS | NS | NS | NS |
| 36 | 1 | 10 | POS | NEG | 6 | 0 | NS | NS | NS | NS |
| 36 | 1.5 | 10 | POS | POS | 8 | 0 | POS | NEG | 5 | 0.043 |
| 36 | 2 | 10 | POS | POS | 5 | 1.037 | POS | NEG | 2 | 0.059 |
| 36 | 3 | 10 | POS | POS | 4 | 0 | NEG | NEG | 2 | 0.072 |
| 36 | 4 | 10 | NEG | POS | 0 | 1.095 | POS | NEG | 1 | 0.034 |
| 36 | 5 | 10 | NEG | NEG | 1 | 0.131 | NS | NS | NS | NS |
| 36 | 6 | 10 | NEG | NEG | 0 | 0 | NS | NS | NS | NS |
| 36 | 8 | 10 | NEG | NEG | 0 | 0 | NS | NS | NS | NS |
| 36 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 36 | 0.17 | 25 | NS | NS | 694 | 0 | POS | POS | 523 | 0 |
| 36 | 0.5 | 25 | POS | POS | 517 | 0 | POS | POS | 162 | 0 |
| 36 | 1 | 25 | POS | POS | 93 | 0 | POS | NEG | 10 | 0 |
| 36 | 1.5 | 25 | POS | POS | 29 | 0 | POS | NEG | 6 | 0 |
| 36 | 2 | 25 | POS | POS | 9 | 0 | POS | NEG | 2 | 0 |
| 36 | 3 | 25 | POS | POS | 9 | 0 | POS | NEG | 1 | 0 |
| 36 | 4 | 25 | NEG | NEG | 0 | 0 | POS | NEG | 2 | 0 |
| 36 | 5 | 25 | POS | POS | 0 | 0 | NEG | NEG | 0 | 0 |
| 36 | 6 | 25 | POS | POS | 0 | 0 | NEG | NEG | 3 | 0 |
| 36 | 8 | 25 | POS | POS | 4 | 0 | NEG | NEG | 0 | 0 |
| 38 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 38 | 0.17 | 10 | NS | NS | 51 | 0 | NS | NS | 383 | 0 |
| 38 | 0.5 | 10 | POS | POS | 25 | 0 | POS | POS | 49 | 0 |
| 38 | 1 | 10 | POS | POS | 1 | 0 | POS | POS | 36 | 0 |
| 38 | 1.5 | 10 | NEG | POS | 0 | 0 | POS | POS | 27 | 0 |
| 38 | 2 | 10 | POS | POS | 1 | 0 | POS | POS | 18 | 0 |
| 38 | 3 | 10 | NEG | NEG | 0 | 0 | POS | POS | 11 | 0 |
| 38 | 4 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 2 | 0 |
| 38 | 5 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 2 | 0 |
| 38 | 6 | 10 | NEG | NEG | 0 | 0 | POS | NEG | 2 | 0 |
| 38 | 8 | 10 | NEG | NEG | 0 | 0 | POS | NEG | 5 | 0 |
| 38 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 38 | 0.17 | 25 | NS | NS | NS | NS | NS | NS | NS | NS |
| 38 | 0.5 | 25 | NS | NS | NS | NS | NS | NS | 218 | 0 |
| 38 | 1 | 25 | NS | NS | NS | NS | POS | POS | 122 | 0 |
| 38 | 1.5 | 25 | NS | NS | NS | NS | POS | POS | 58 | 0 |
| 38 | 2 | 25 | NS | NS | NS | NS | POS | POS | 60 | 0 |
| 38 | 3 | 25 | POS | POS | 27 | 0.034 | POS | POS | 30 | 0 |
| 38 | 4 | 25 | POS | POS | 36 | 0.071 | POS | POS | 8 | 0 |
| 38 | 5 | 25 | NS | NS | NS | NS | POS | POS | 3 | 0 |
| 38 | 6 | 25 | POS | NEG | 6 | 0.027 | NEG | NEG | 5 | 0 |
| 38 | 8 | 25 | POS | NEG | 14 | 0.065 | POS | NEG | 1 | 0 |
| 50 | BL | 0 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 50 | 0.17 | 0 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 50 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 50 | 0.17 | 10 | POS | POS | 230 | 0 | POS | POS | 108 | 0 |
| 50 | 0.5 | 10 | POS | POS | 39 | 0 | POS | POS | 36 | 0 |
| 50 | 1 | 10 | POS | POS | 41 | 0 | POS | POS | 21 | 0 |
| 50 | 1.5 | 10 | POS | POS | 63 | 0 | POS | POS | 7 | 0 |
| 50 | 2 | 10 | POS | POS | 25 | 0 | POS | POS | 29 | 0 |
| 50 | 3 | 10 | POS | POS | 23 | 0 | POS | POS | 9 | 0 |
| 50 | 4 | 10 | POS | POS | 10 | 0 | POS | NEG | 5 | 0 |
| 50 | 5 | 10 | POS | POS | 12 | 0 | POS | NEG | 7 | 0 |
| 50 | 6 | 10 | POS | POS | 9 | 0 | NEG | NEG | 1 | 0 |
| 50 | 8 | 10 | POS | NEG | 2 | 0 | NEG | NEG | 3 | 0 |
| 50 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 50 | 0.17 | 25 | POS | POS | 2368 | 0 | NS | NS | 1374 | 0 |
| 50 | 0.5 | 25 | POS | POS | 358 | 0 | POS | POS | 118 | 0 |
| 50 | 1 | 25 | POS | POS | 69 | 0 | POS | NEG | 5 | 0 |
| 50 | 1.5 | 25 | POS | POS | 104 | 0 | POS | POS | 9 | 0 |
| 50 | 2 | 25 | POS | POS | 63 | 0 | POS | POS | 13 | 0 |
| 50 | 3 | 25 | POS | POS | 61 | 0 | POS | POS | 11 | 0 |
| 50 | 4 | 25 | POS | POS | 38 | 0 | POS | NEG | 19 | 0 |
| 50 | 5 | 25 | POS | POS | 20 | 0 | POS | POS | 10 | 0 |
| 50 | 6 | 25 | POS | NEG | 19 | 0 | NEG | NEG | 4 | 0 |
| 50 | 8 | 25 | POS | NEG | 16 | 0 | POS | NEG | 3 | 0 |
| 55 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 55 | 0.17 | 10 | POS | POS | 56 | 0 | POS | POS | 74 | 0 |
| 55 | 0.5 | 10 | POS | POS | 13 | 0 | NEG | NEG | 1 | 0 |
| 55 | 1 | 10 | POS | POS | 8 | 0 | NEG | NEG | 1 | 0 |
| 55 | 1.5 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 55 | 2 | 10 | NEG | NEG | 2 | 0 | NEG | NEG | 0 | 0 |
| 55 | 3 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 55 | 4 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 55 | 5 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 55 | 6 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 55 | 8 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 55 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 55 | 0.17 | 25 | POS | POS | 133 | 0 | POS | POS | 60 | 0 |
| 55 | 0.5 | 25 | POS | POS | 30 | 0 | POS | NEG | 14 | 0 |
| 55 | 1 | 25 | POS | POS | 41 | 0 | NEG | NEG | 4 | 0 |
| 55 | 1.5 | 25 | POS | NEG | 15 | 0 | NEG | NEG | 3 | 0 |
| 55 | 2 | 25 | POS | POS | 25 | 0 | NEG | NEG | 2 | 0 |
| 55 | 3 | 25 | POS | POS | 21 | 0 | NEG | NEG | 2 | 0 |
| 55 | 4 | 25 | NEG | NEG | 2 | 0 | NEG | NEG | 1 | 0 |
| 55 | 5 | 25 | POS | POS | 5 | 0 | NEG | NEG | 1 | 0 |
| 55 | 6 | 25 | POS | NEG | 3 | 0 | NEG | NEG | 0 | 0 |
| 55 | 8 | 25 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 58 | BL | 0 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 58 | 0.17 | 0 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 58 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 58 | 0.17 | 10 | POS | POS | 1063 | 0 | NS | NS | 8 | 0 |
| 58 | 0.5 | 10 | POS | POS | 65 | 0 | POS | POS | 92 | 0.086 |
| 58 | 1 | 10 | POS | POS | 46 | 0 | POS | POS | 40 | 0.09 |
| 58 | 1.5 | 10 | POS | POS | 14 | 0 | POS | POS | 38 | 0.098 |
| 58 | 2 | 10 | POS | POS | 4 | 0 | POS | POS | 16 | 0.081 |
| 58 | 3 | 10 | POS | NEG | 3 | 0 | POS | POS | 2 | 0.02 |
| 58 | 4 | 10 | NEG | NEG | 2 | 0 | NEG | NEG | 2 | 0.02 |
| 58 | 5 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 2 | 0.04 |
| 58 | 6 | 10 | POS | NEG | 2 | 0 | NEG | NEG | 0 | 0 |
| 58 | 8 | 10 | POS | NEG | 2 | 0 | NEG | NEG | 0 | 0 |
| 58 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 58 | 0.17 | 25 | NS | NS | NS | NS | NS | NS | 1646 | 0 |
| 58 | 0.5 | 25 | POS | POS | 266 | 0.063 | POS | POS | 899 | 0.128 |
| 58 | 1 | 25 | POS | POS | 97 | 0.081 | POS | POS | 47 | 0.039 |
| 58 | 1.5 | 25 | POS | POS | 89 | 0.101 | POS | POS | NS | NS |
| 58 | 2 | 25 | POS | POS | 68 | 0.101 | NEG | NEG | 5 | 0.021 |
| 58 | 3 | 25 | POS | POS | 25 | 0.148 | POS | POS | 7 | 0.052 |
| 58 | 4 | 25 | POS | POS | 11 | 0.114 | POS | POS | 5 | 0.057 |
| 58 | 5 | 25 | POS | POS | 2 | 0.109 | POS | POS | 10 | 0.069 |
| 58 | 6 | 25 | POS | POS | 5 | 0.131 | NEG | POS | 5 | 0.084 |
| 58 | 8 | 25 | POS | POS | 17 | 0.175 | NEG | POS | 0 | 0.06 |
| 59 | BL | 0 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 59 | 0.17 | 0 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 59 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 59 | 0.17 | 10 | POS | POS | 435 | 0 | POS | POS | 7 | 0 |
| 59 | 0.5 | 10 | POS | POS | 36 | 0 | POS | POS | 9 | 0 |
| 59 | 1 | 10 | POS | POS | 52 | 0 | POS | NEG | 8 | 0 |
| 59 | 1.5 | 10 | POS | POS | 37 | 0 | NEG | NEG | 3 | 0 |
| 59 | 2 | 10 | POS | POS | 21 | 0 | NEG | NEG | 3 | 0 |
| 59 | 3 | 10 | POS | POS | 41 | 0 | NEG | NEG | 1 | 0 |
| 59 | 4 | 10 | POS | NEG | 6 | 0 | NEG | NEG | 0 | 0 |
| 59 | 5 | 10 | POS | NEG | 5 | 0 | NEG | NEG | 0 | 0 |
| 59 | 6 | 10 | POS | NEG | 3 | 0 | NEG | NEG | 0 | 0 |
| 59 | 8 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 59 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 59 | 0.17 | 25 | POS | POS | 825 | 0 | POS | POS | 398 | 0 |
| 59 | 0.5 | 25 | POS | POS | 173 | 0 | NS | NS | NS | NS |
| 59 | 1 | 25 | POS | POS | 16 | 0 | NS | NS | NS | NS |
| 59 | 1.5 | 25 | POS | POS | 34 | 0 | POS | POS | 46 | 0.074 |
| 59 | 2 | 25 | POS | NEG | 4 | 0 | NS | NS | NS | NS |
| 59 | 3 | 25 | POS | POS | 16 | 0 | POS | POS | 36 | 0.1 |
| 59 | 4 | 25 | NEG | POS | 3 | 0 | POS | POS | 9 | 0.07 |
| 59 | 5 | 25 | NEG | NEG | 5 | 0 | NEG | NEG | 0 | 0 |
| 59 | 6 | 25 | POS | NEG | 3 | 0 | NEG | NEG | 1 | 0 |
| 59 | 8 | 25 | NEG | NEG | 3 | 0 | NEG | NEG | 1 | 0 |
| 60 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 60 | 0.17 | 10 | POS | POS | 30 | 0 | NS | NS | 187 | 0 |
| 60 | 0.5 | 10 | POS | POS | 29 | 0 | NEG | NEG | 6 | 0 |
| 60 | 1 | 10 | POS | POS | 10 | 0 | POS | POS | 26 | 0 |
| 60 | 1.5 | 10 | POS | NEG | 4 | 0 | POS | NEG | 11 | 0 |
| 60 | 2 | 10 | NEG | NEG | 4 | 0 | POS | NEG | 3 | 0 |
| 60 | 3 | 10 | NEG | NEG | 3 | 0 | NEG | NEG | 3 | 0 |
| 60 | 4 | 10 | NEG | NEG | 1 | 0 | POS | NEG | 4 | 0 |
| 60 | 5 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 60 | 6 | 10 | NEG | NEG | 3 | 0 | NEG | NEG | 1 | 0 |
| 60 | 8 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 60 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 60 | 0.17 | 25 | POS | POS | 99 | 0 | POS | POS | 267 | 0 |
| 60 | 0.5 | 25 | POS | POS | 33 | 0 | POS | POS | 65 | 0 |
| 60 | 1 | 25 | POS | POS | 15 | 0 | POS | NEG | 26 | 0 |
| 60 | 1.5 | 25 | POS | POS | 10 | 0 | POS | NEG | 10 | 0 |
| 60 | 2 | 25 | POS | POS | 5 | 0 | POS | NEG | 12 | 0 |
| 60 | 3 | 25 | POS | POS | 14 | 0 | NEG | NEG | 5 | 0 |
| 60 | 4 | 25 | POS | NEG | 6 | 0 | NEG | NEG | 4 | 0 |
| 60 | 5 | 25 | NEG | NEG | 2 | 0 | NEG | NEG | 2 | 0 |
| 60 | 6 | 25 | NEG | NEG | 1 | 0 | NEG | NEG | 3 | 0 |
| 60 | 8 | 25 | NEG | NEG | 1 | 0 | NEG | NEG | 1 | 0 |
| 4 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 4 | 0.17 | 10 | POS | POS | 88 | 0 | NS | NS | NS | NS |
| 4 | 0.5 | 10 | POS | POS | 22 | 0 | POS | POS | 93 | 0 |
| 4 | 1 | 10 | POS | POS | 17 | 0 | POS | POS | 27 | 0 |
| 4 | 1.5 | 10 | POS | POS | 21 | 0 | POS | POS | 17 | 0 |
| 4 | 2 | 10 | POS | POS | 6 | 0 | POS | POS | 19 | 0 |
| 4 | 3 | 10 | POS | POS | 5 | 0 | POS | NEG | 5 | 0 |
| 4 | 4 | 10 | NEG | NEG | 1 | 0 | POS | NEG | 6 | 0 |
| 4 | 5 | 10 | NEG | NEG | 2 | 0 | NEG | NEG | 3 | 0 |
| 4 | 6 | 10 | NEG | NEG | 2 | 0 | NEG | NEG | 3 | 0 |
| 4 | 8 | 10 | NEG | NEG | 2 | 0 | NEG | NEG | 2 | 0 |
| 4 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 4 | 0.17 | 25 | POS | POS | 707 | 0 | POS | POS | 689 | 0 |
| 4 | 0.5 | 25 | POS | POS | 125 | 0 | POS | POS | 134 | 0 |
| 4 | 1 | 25 | POS | POS | 40 | 0 | POS | POS | 51 | 0 |
| 4 | 1.5 | 25 | POS | POS | 19 | 0 | POS | POS | 17 | 0 |
| 4 | 2 | 25 | POS | POS | 17 | 0 | POS | POS | 14 | 0 |
| 4 | 3 | 25 | POS | POS | 8 | 0 | POS | NEG | 6 | 0 |
| 4 | 4 | 25 | POS | NEG | 5 | 0 | POS | NEG | 2 | 0 |
| 4 | 5 | 25 | NEG | NEG | 5 | 0 | POS | NEG | 3 | 0 |
| 4 | 6 | 25 | POS | POS | 3 | 0 | POS | NEG | 2 | 0 |
| 4 | 8 | 25 | NEG | POS | 2 | 0 | NEG | NEG | 4 | 0 |
| 25 | BL | 0 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 25 | 0.17 | 0 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 25 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 25 | 0.17 | 10 | NEG | NEG | 0 | 0 | NS | NS | NS | NS |
| 25 | 0.5 | 10 | POS | NEG | 29 | 0 | NS | NS | NS | NS |
| 25 | 1 | 10 | POS | NEG | 25 | 0 | POS | POS | 63 | 0 |
| 25 | 1.5 | 10 | NEG | NEG | 4 | 0 | POS | POS | 27 | 0 |
| 25 | 2 | 10 | POS | NEG | 4 | 0 | POS | POS | 12 | 0 |
| 25 | 3 | 10 | POS | NEG | 5 | 0 | POS | POS | 6 | 0.064 |
| 25 | 4 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 2 | 0 |
| 25 | 5 | 10 | NEG | NEG | 1 | 0 | POS | NEG | 3 | 0 |
| 25 | 6 | 10 | NEG | NEG | 1 | 0 | POS | NEG | 2 | 0 |
| 25 | 8 | 10 | NEG | NEG | 1 | 0 | POS | POS | 3 | 0 |
| 25 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 25 | 0.17 | 25 | POS | POS | 112 | 0 | NS | NS | NS | NS |
| 25 | 0.5 | 25 | POS | POS | 37 | 0 | POS | POS | 27 | 0 |
| 25 | 1 | 25 | POS | POS | 70 | 0 | POS | POS | 26 | 0 |
| 25 | 1.5 | 25 | POS | POS | 21 | 0 | POS | NEG | 6 | 0 |
| 25 | 2 | 25 | POS | POS | 14 | 0 | POS | POS | 30 | 0 |
| 25 | 3 | 25 | POS | POS | 12 | 0 | NEG | NEG | 1 | 0 |
| 25 | 4 | 25 | POS | NEG | 5 | 0 | NEG | NEG | 2 | 0 |
| 25 | 5 | 25 | NEG | NEG | 4 | 0 | NEG | NEG | 1 | 0 |
| 25 | 6 | 25 | POS | POS | 3 | 0 | NEG | NEG | 2 | 0 |
| 25 | 8 | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 2 | 0 |
| 29 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 29 | 0.17 | 10 | POS | POS | 41 | 0 | POS | POS | 150 | 0 |
| 29 | 0.5 | 10 | POS | NEG | 15 | 0 | POS | NEG | 10 | 0 |
| 29 | 1 | 10 | POS | NEG | 12 | 0 | POS | NEG | 3 | 0 |
| 29 | 1.5 | 10 | POS | NEG | 4 | 0 | POS | NEG | 8 | 0 |
| 29 | 2 | 10 | POS | NEG | 4 | 0 | NEG | NEG | 0 | 0 |
| 29 | 3 | 10 | POS | NEG | 8 | 0 | NEG | NEG | 1 | 0 |
| 29 | 4 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 29 | 5 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 29 | 6 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 29 | 8 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 29 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 29 | 0.17 | 25 | POS | POS | 383 | 0 | POS | POS | 588 | 0 |
| 29 | 0.5 | 25 | POS | POS | 87 | 0 | POS | POS | 113 | 0 |
| 29 | 1 | 25 | POS | POS | 19 | 0 | POS | NEG | 13 | 0 |
| 29 | 1.5 | 25 | POS | POS | 12 | 0 | POS | NEG | 15 | 0 |
| 29 | 2 | 25 | POS | POS | 16 | 0 | NEG | NEG | 3 | 0 |
| 29 | 3 | 25 | POS | POS | 5 | 0 | POS | POS | 12 | 0 |
| 29 | 4 | 25 | POS | NEG | 2 | 0 | NEG | NEG | 1 | 0 |
| 29 | 5 | 25 | POS | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 29 | 6 | 25 | POS | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 29 | 8 | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 53 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 53 | 0.17 | 10 | POS | POS | 96 | 0 | POS | POS | 80 | 0 |
| 53 | 0.5 | 10 | POS | POS | 15 | 0 | POS | POS | 15 | 0 |
| 53 | 1 | 10 | POS | NEG | 9 | 0 | POS | POS | 17 | 0 |
| 53 | 1.5 | 10 | NEG | NEG | 2 | 0 | NEG | NEG | 4 | 0 |
| 53 | 2 | 10 | POS | NEG | 4 | 0 | POS | NEG | 4 | 0 |
| 53 | 3 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 2 | 0 |
| 53 | 4 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 53 | 5 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 1 | 0 |
| 53 | 6 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 53 | 8 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 53 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 53 | 0.17 | 25 | POS | POS | 274 | 0 | NS | NS | 150 | 0 |
| 53 | 0.5 | 25 | POS | POS | 45 | 0 | NS | NS | 10 | 0 |
| 53 | 1 | 25 | POS | POS | 31 | 0 | POS | POS | 43 | 0 |
| 53 | 1.5 | 25 | POS | NEG | 2 | 0 | POS | POS | 15 | 0 |
| 53 | 2 | 25 | POS | POS | 4 | 0 | POS | POS | 13 | 0 |
| 53 | 3 | 25 | POS | POS | 2 | 0 | POS | NEG | 3 | 0 |
| 53 | 4 | 25 | POS | POS | 4 | 0 | NEG | NEG | 1 | 0 |
| 53 | 5 | 25 | POS | POS | 3 | 0 | NEG | NEG | 1 | 0 |
| 53 | 6 | 25 | POS | NEG | 2 | 0 | NEG | NEG | 0 | 0 |
| 53 | 8 | 25 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 54 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 54 | 0.17 | 10 | POS | POS | 101 | 0 | POS | POS | 41 | 0 |
| 54 | 0.5 | 10 | NEG | NEG | 2 | 0 | POS | POS | 13 | 0 |
| 54 | 1 | 10 | POS | NEG | 5 | 0 | NEG | NEG | 2 | 0 |
| 54 | 1.5 | 10 | NEG | NEG | 3 | 0 | NEG | NEG | 1 | 0 |
| 54 | 2 | 10 | POS | NEG | 3 | 0 | NEG | NEG | 1 | 0 |
| 54 | 3 | 10 | NEG | NEG | 3 | 0 | NEG | NEG | 0 | 0 |
| 54 | 4 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 54 | 5 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 54 | 6 | 10 | NEG | NEG | 0 | 0.028 | NEG | NEG | 0 | 0 |
| 54 | 8 | 10 | NEG | NEG | 1 | 0.034 | NEG | NEG | 0 | 0 |
| 54 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 54 | 0.17 | 25 | POS | POS | 89 | 0 | NS | NS | 138 | 0 |
| 54 | 0.5 | 25 | POS | POS | 24 | 0 | POS | POS | 39 | 0 |
| 54 | 1 | 25 | POS | POS | 5 | 0 | NEG | NEG | 8 | 0 |
| 54 | 1.5 | 25 | NEG | NEG | 1 | 0 | NEG | NEG | 2 | 0 |
| 54 | 2 | 25 | NEG | NEG | 2 | 0 | NEG | NEG | 1 | 0 |
| 54 | 3 | 25 | NEG | NEG | 1 | 0 | NEG | NEG | 1 | 0 |
| 54 | 4 | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 54 | 5 | 25 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 54 | 6 | 25 | NEG | NEG | 0 | 0.023 | NEG | POS | 0 | 0.063 |
| 54 | 8 | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 63 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 63 | 0.17 | 10 | POS | POS | 290 | 0 | POS | POS | 14 | 0 |
| 63 | 0.5 | 10 | POS | POS | 13 | 0 | POS | NEG | 5 | 0 |
| 63 | 1 | 10 | POS | POS | 5 | 0 | NEG | NEG | 1 | 0 |
| 63 | 1.5 | 10 | POS | NEG | 4 | 0 | NEG | NEG | 0 | 0 |
| 63 | 2 | 10 | POS | NEG | 9 | 0 | NEG | NEG | 0 | 0 |
| 63 | 3 | 10 | POS | NEG | 5 | 0 | NEG | NEG | 0 | 0 |
| 63 | 4 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 63 | 5 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 63 | 6 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 63 | 8 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 63 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 63 | 0.17 | 25 | POS | POS | 429 | 0 | POS | POS | 168 | 0 |
| 63 | 0.5 | 25 | POS | POS | 46 | 0 | POS | POS | 69 | 0 |
| 63 | 1 | 25 | POS | NEG | 10 | 0 | POS | POS | 36 | 0 |
| 63 | 1.5 | 25 | NEG | NEG | 3 | 0 | POS | POS | 10 | 0 |
| 63 | 2 | 25 | POS | NEG | 3 | 0 | POS | POS | 8 | 0 |
| 63 | 3 | 25 | POS | NEG | 3 | 0 | POS | POS | 6 | 0 |
| 63 | 4 | 25 | NEG | NEG | 2 | 0 | NEG | NEG | 1 | 0 |
| 63 | 5 | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 2 | 0 |
| 63 | 6 | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 63 | 8 | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 64 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 64 | 0.17 | 10 | POS | POS | 24 | 0 | POS | POS | 68 | 0 |
| 64 | 0.5 | 10 | POS | POS | 8 | 0 | NS | NS | NS | NS |
| 64 | 1 | 10 | POS | NEG | 3 | 0 | POS | NEG | 6 | 0 |
| 64 | 1.5 | 10 | POS | NEG | 2 | 0 | POS | NEG | 6 | 0.025 |
| 64 | 2 | 10 | POS | NEG | 3 | 0 | POS | NEG | 4 | 0 |
| 64 | 3 | 10 | NEG | NEG | 2 | 0 | NEG | NEG | 0 | 0 |
| 64 | 4 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 0 | 0 |
| 64 | 5 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 1 | 0 |
| 64 | 6 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 1 | 0 |
| 64 | 8 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 64 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 64 | 0.17 | 25 | POS | POS | 58 | 0 | POS | POS | 103 | 0 |
| 64 | 0.5 | 25 | POS | POS | 24 | 0 | POS | POS | 12 | 0 |
| 64 | 1 | 25 | POS | POS | 16 | 0 | POS | POS | 17 | 0 |
| 64 | 1.5 | 25 | POS | POS | 9 | 0 | POS | NEG | 6 | 0 |
| 64 | 2 | 25 | POS | POS | 5 | 0 | POS | POS | 4 | 0 |
| 64 | 3 | 25 | POS | NEG | 8 | 0 | NEG | NEG | 2 | 0 |
| 64 | 4 | 25 | POS | NEG | 3 | 0 | NEG | NEG | 2 | 0 |
| 64 | 5 | 25 | POS | NEG | 1 | 0 | NEG | NEG | 1 | 0 |
| 64 | 6 | 25 | POS | NEG | 2 | 0 | NEG | NEG | 6 | 0 |
| 64 | 8 | 25 | NEG | NEG | 2 | 0 | NEG | NEG | 1 | 0 |
| 65 | BL | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 65 | 0.17 | 10 | POS | POS | 11 | 0 | POS | POS | 31 | 0 |
| 65 | 0.5 | 10 | NEG | NEG | 2 | 0 | POS | POS | 14 | 0 |
| 65 | 1 | 10 | NEG | NEG | 2 | 0 | POS | POS | 8 | 0 |
| 65 | 1.5 | 10 | NEG | NEG | 2 | 0 | POS | NEG | 3 | 0 |
| 65 | 2 | 10 | NEG | NEG | 1 | 0 | NEG | NEG | 3 | 0 |
| 65 | 3 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 65 | 4 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 65 | 5 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 65 | 6 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 65 | 8 | 10 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 65 | BL | 25 | NEG | NEG | 0 | 0 | NEG | NEG | 0 | 0 |
| 65 | 0.17 | 25 | NS | NS | NS | NS | POS | POS | 707 | 0 |
| 65 | 0.5 | 25 | NS | NS | NS | NS | POS | POS | 124 | 0 |
| 65 | 1 | 25 | NS | NS | NS | NS | POS | POS | 119 | 0 |
| 65 | 1.5 | 25 | POS | POS | 16 | 0 | POS | POS | 98 | 0 |
| 65 | 2 | 25 | POS | NEG | 6 | 0 | POS | POS | 23 | 0 |
| 65 | 3 | 25 | POS | NEG | 2 | 0 | POS | POS | 12 | 0 |
| 65 | 4 | 25 | NEG | NEG | 1 | 0 | POS | POS | 8 | 0 |
| 65 | 5 | 25 | NEG | NEG | 0 | 0 | POS | NEG | 2 | 0 |
| 65 | 6 | 25 | NEG | NEG | 0 | 0 | POS | NEG | 1 | 0 |
| 65 | 8 | 25 | NEG | NEG | 0 | 0 | POS | NEG | 1 | 0 |
Note: NS = no sample; POS = positive sample; NEG = negative sample. The first nine participants (i.e., #15–60) in this table were male while the last eight (i.e., #4–65) were females.
Table III.
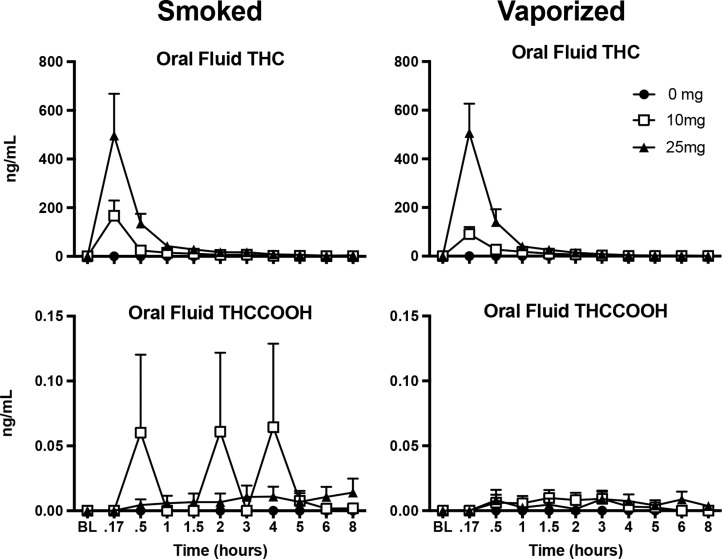
Mean THC, 11-OH-THC, THCCOOH, and THCCOOH-Glucuronide Blood Maximum Concentration (Cmax), Time to Maximum Concentration (Tmax), and Individual Ranges by THC Dose (10, 25 mg) and Inhalation Method (Smoked and Vaporized)
| Dose (mg) | THC Cmax (ng/mL; range) | THC Tmax (h; range) | 11-OH-THC Cmax (ng/mL; range) | 11-OH-THC Tmax (h; range) | THCCOOH Cmax (ng/mL; range) | THCCOOH Tmax (h; range) | THCCOOH-GLUC Cmax (ng/mL; range) | THCCOOH- GLUC Tmax (h; range) |
|---|---|---|---|---|---|---|---|---|
| Smoked | ||||||||
| 10 | 3.76 (0–21) | 0.11 (0.0–0.17) | 0.18 (0–1) | 0.06 (0.0–0.17) | 1.82 (0–9) | 0.17 (0.0–0.5) | 2.06 (0–8) | 1.06 (0.0–3.0) |
| 25 | 10.24 (0–38) | 0.13 (0.0–0.17) | 1.12 (0–4) | 0.15 (0.0–0.5) | 5.94 (0–23) | 0.21 (0.0–0.5) | 6.71 (0–25) | 1.79 (0.0–4.0) |
| Vaporized | ||||||||
| 10 | 7.53 (0–18) | 0.18 (0.0–0.5) | 1.24 (0–6) | 0.14 (0.0–0.5) | 6.0 (0–18) | 0.25 (0.0–1.0) | 5.53 (0–14) | 1.70 (0.0–4.0) |
| 25 | 14.36 (1–33) | 0.19 (0.17–0.5) | 2.06 (0–6) | 0.15 (0.0–0.5) | 7.0 (2–17) | 0.34 (0.17–1.0) | 7.35 (1–17) | 2.41 (1.0–4.0) |
Note: All LC–MS-MS analyses were performed with solid phase extraction (SPE) with the exception of THCCOOH-glucuronide which was performed using liquid/liquid extraction (LLE).
Table IV.
Mean THC and THCCOOH Oral Fluid Maximum Concentration (Cmax), Time to Maximum Concentration (Tmax), and Individual Ranges by THC Dose (10, 25 mg) and Inhalation Method (Smoked and Vaporized)
| Dose (mg) | THC Cmax (ng/mL) | THC Tmax (h) | THCCOOH Cmax (ng/mL) | THCCOOH Tmax(h) |
|---|---|---|---|---|
| Smoked | ||||
| 10 | 167 (0–1063) | 0.19 (0.17–0.5) | 0.064 (0–1.095) | 0.71 (0.0–8.0) |
| 25 | 496 (58–2368) | 0.17 (0.17-0.17) | 0.014 (0-0.175) | 0.88 (0.0–8.0) |
| Vaporized | ||||
| 10 | 91 (7–383) | 0.22 (0.17–0.5) | 0.0097 (0–0.098) | 0.53 (0.0–3.0) |
| 25 | 506 (60–1646) | 0.17 (0.17–0.0.17) | 0.0089 (0–0.10) | 0.41 (0.0–6.0) |
Whole blood results
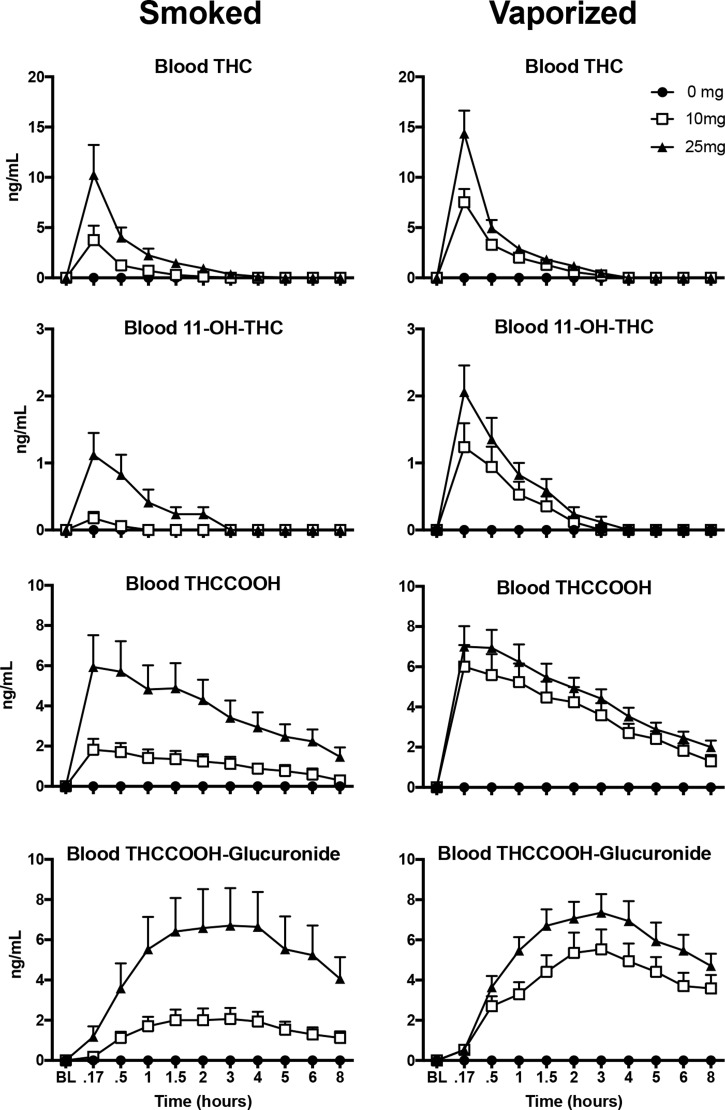
Figure 1 presents mean concentrations of THC, 11-OH-THC, THCCOOH and THCCOOH-glucuronide in whole blood before and after inhalation of smoked and vaporized cannabis. Mean detection time windows and individual ranges to first and last positive for THC and THC metabolites in whole blood are displayed in Table V. Whole blood THC, 11-OH-THC and THCCOOH concentrations peaked within the first 10–30 min after the end of the smoked and vaporized cannabis administration periods. THC and 11-OH-THC levels declined rapidly and were not detected after the 4 h time point; THCCOOH was eliminated at a much slower rate and was still detectable after 8 h following 16 of 34 smoked cannabis administrations and 25 of 34 vaporized cannabis administrations. The time course of THCCOOH-glucuronide in blood was discordant from the other cannabinoids, with a slower onset to peak concentration and decay compared with THC and the other metabolites. Inhalation of vaporized cannabis resulted in higher mean concentrations of THC, 11-OH-THC and THCCOOH in whole blood than the same dose of smoked cannabis. At the 10 mg THC dose, THCCOOH-glucuronide concentrations were also higher for vaporized, as opposed to smoked cannabis, but concentrations of this metabolite were similar across inhalation methods at the 25 mg THC dose. Neither THC or any of its metabolites were detected at any time point in the 0 mg THC smoked and vaporized conditions.
Figure 1.
Quantitative whole blood THC, 11-OH-THC, THCCOOH and THCCOOH-glucuronide mean (+SEM) concentrations.
Table V.
Mean Detection Times and Ranges of THC and THC Metabolites in Whole Blood by THC Dose (10, 25 mg) and Inhalation Method (Smoked and Vaporized)
| Dose (mg) | THCCOOH ELISA (cutoff = 10 ng/mL) | THC LC–MS-MS (LOQ = 1 ng/mL) | 11-OH-THC, LC–MS-MS (LOQ = 1 ng/mL) | THCCOOH LC–MS-MS (LOQ = 1 ng/mL) | THCCOOH-GLUC LC–MS-MS (LOQ = 1 ng/mL) |
|---|---|---|---|---|---|
| Smoked: Detection time (h) to first positive | |||||
| 10 | 0.43 (0.17–1.0) | 0.17 (0.17–0.17) | 0.17 (0.17–0.17) | 0.17 (0.17–0.17) | 0.82 (0.17–4.0) |
| 25 | 0.17 (0.17–0.17) | 0.17 (0.17–0.17) | 0.20 (0.17–0.5) | 0.17 (0.17–0.17) | 0.36 (0.17–1.0) |
| Smoked: Detection time (h) to last positive | |||||
| 10 | 5.29 (1.0–8.0) | 1.0 (0.17–2.0) | 0.28 (0.17–0.5) | 5.51 (0.17–8.0) | 6.71 (1.50–8.0) |
| 25 | 6.56 (0.17–8.0) | 2.27 (0.5–4.0) | 1.64 (0.17–2.0) | 7.71 (6.0–8.0) | 7.87 (6.0–8.0) |
| Vaporized: Detection time (h) to first positive | |||||
| 10 | 0.24 (0.17–0.5) | 0.19 (0.17–0.5) | 0.17 (0.17–0.17) | 0.17 (0.17–0.17) | 0.29 (0.17–1.0) |
| 25 | 0.27 (0.17–1.0) | 0.17 (0.17–0.17) | 0.17 (0.17–0.17) | 0.17 (0.17–0.17) | 0.34 (0.17–1.0) |
| Vaporized: Detection time (h) to last positive | |||||
| 10 | 6.75 (1.5–8.0) | 1.84 (0.5–3.0) | 1.11 (0.17–2.0) | 6.94 (5.0–8.0) | 7.67 (5.0–8.0) |
| 25 | 6.59 (3.0–8.0) | 2.27 (0.17–3.0) | 1.58 (0.5–3.0) | 7.89 (6.0–8.0) | 8.0 (8.0–8.0) |
Oral fluid results
Figure 2 displays mean concentrations of THC and THCCOOH in oral fluid before and after smoked and vaporized cannabis administration. Similar to whole blood, oral fluid THC concentrations peaked within 10 min after the end of the cannabis administration period for both inhalation methods and declined rapidly thereafter. THCCOOH detection was highly erratic in oral fluid. At the 10 mg dose, mean Cmax THC and THCCOOH concentrations were marginally higher for smoked relative to vaporized cannabis although concentrations of THC and THCCOOH were comparable across inhalation methods at the 25 mg dose. Overall, oral fluid THC concentrations were considerably higher than THCCOOH concentrations for each active dose of smoked and vaporized cannabis. THCCOOH was rarely detected in participant’s oral fluid. Indeed, for several participants (i.e., participants #15, #20, #50, #55, #60, #4, #29, #53, #63, #65), THCCOOH was not detected in any experimental session and it was only detected sporadically for the remaining participants. For participant #36, THCCOOH concentrations at several time points (i.e., 30-min, 2 h and 4 h collection points) in the 10 mg smoked condition were ~10 times greater than the next highest THCCOOH concentrations observed for all other participants across all conditions and time points (Table II). However, for several intermediate time points (e.g., 1 and 3 h collection points) in the same experimental session, THCCOOH concentrations were below the LOQ for participant #36, further highlighting the erratic nature of THCCOOH detection in oral fluid. Interestingly, at the 0 mg THC dose, THC was detected in five participant’s oral fluid specimens immediately after cannabis administration (three vaporized and two smoked cannabis sessions), but THC concentrations subsequently fell below the LOQ 30 min post-cannabis administration in each of these instances (Table II). This may have resulted from trace amounts of THC remaining in the placebo cannabis following its production via ethanol cannabinoid extraction.
Figure 2.
Quantitative oral fluid THC and THCCOOH mean (+SEM) concentrations.
Analogous to ELISA testing for whole blood THCCOOH, time to first positive ELISA test for THC (cutoff = 4 ng/mL) and THCCOOH (cutoff = 0.05 ng/mL) in oral fluid was observed at the 10 or 30-min collection points for all participants and the time to last detection varied considerably (Table VI). For LC–MS-MS analyses, THC was detected immediately after cannabis use for both inhalation methods. Overall, THC was detected for longer in oral fluid than in whole blood. In fact, there were several instances in which THC persisted in oral fluid for all collection time points (i.e., up to 8 h). Unlike whole blood, THCCOOH in oral fluid was often not detected for several hours after cannabis exposure.
Table VI.
Mean Detection Times and Ranges of THC and THCCOOH in Oral Fluid by THC Dose (10, 25 mg) and Inhalation Method (Smoked and Vaporized)
| Dose (mg) | THC ELISA (cutoff = 4 ng/mL) | THCCOOH ELISA (cutoff = 0.05 ng/mL) | THC LC–MS-MS (LOQ = 0.5 mg/mL) | THCCOOH LC–MS-MS (LOQ = 0.02 ng/mL) |
|---|---|---|---|---|
| Smoked: Detection time (h) to first positive | ||||
| 10 | 0.19 (0.17–0.5) | 0.17 (0.17–0.17) | 0.19 (0.17–0.5) | 3.25 (0.5–6.0) |
| 25 | 0.17 (0.17–0.17) | 0.17 (0.17–0.17) | 0.17 (0.17–0.17) | 6.0 (6.0–6.0) |
| Smoked: Detection time (h) to last positive | ||||
| 10 | 3.16 (0.17–8.0) | 1.87 (0.17–6.0) | 5.89 (2.0–8.0) | 6.5 (5.0–8.0) |
| 25 | 5.76 (1.0–8.0) | 4.35 (0.5–8) | 7.06 (4.0–8.0) | 7.3 (6.0–8.0) |
| Vaporized: Detection time (h) to first positive | ||||
| 10 | 0.17 (0.17–0.17) | 0.17 (0.17–0.17) | 0.17 (0.17–0.17) | 1.67 (0.5–3.0) |
| 25 | 0.17 (0.17–0.17) | 0.17 (0.17–0.17) | 0.17 (0.17–0.17) | 3.25 (0.5–6.0) |
| Vaporized: Detection time (h) to last positive | ||||
| 10 | 2.72 (0.17–8.0) | 1.61 (0.17–8.0) | 5.15 (1.0–8.0) | 3.17 (1.5–5.0) |
| 25 | 4.23 (0.5–8.0) | 3.72 (0.17–8.0) | 7.06 (4.0–8.0) | 6.0 (4.0–8.0) |
Note: Sessions in which participants could not produce a saliva sample immediately after self-administration were not included in time to first detection analyses (Table II).
Sensitivity/specificity and agreement between ELISA and LC–MS-MS
The specificity between ELISA and LC–MS-MS results for THCCOOH in whole blood were similar for smoked and vaporized cannabis, but higher sensitivity was observed following vaporized compared with smoked cannabis administration (Table VII). Agreement between ELISA and LC–MS/MS results was slightly higher in vaporized cannabis sessions compared to smoked. Conversely, for oral fluid, higher sensitivity was observed in smoked, compared with vaporized, cannabis sessions for THC and THCCOOH. Specificity and agreement for THC in oral fluid were comparable across inhalation methods. Specificity and agreement for THCCOOH in oral fluid were poor, but were better after vaporized compared with smoked cannabis administration.
Table VII.
Comparisons of Immunoassay responses (ELISA) to confirmation analyses (LC–MS-MS) in Blood and Oral Fluid by Cannabis Administration Method (Smoked vs Vaporized) and THC dose (10 and 25 mg)
| Blood THCCOOH ELISA (cutoff = 10 ng/mL) vs THCCOOH LC–MS-MS (confirmation = 2 ng/mL) | Oral Fluid THC ELISA (cutoff = 4 ng/mL) vs THC LC–MS-MS (confirmation = 2 ng/mL) | Oral Fluid THCCOOH ELISA (cutoff = 0.05 ng/mL) vs THCCOOH LC–MS-MS (confirmation = 0.05 ng/mL) | |
|---|---|---|---|
| Smoked | |||
| #True Positive (%) | 35.9 | 53.8 | 4.0 |
| #True Negative (%) | 51.3 | 31.8 | 52.3 |
| #False Positive (%) | 6.1 | 2.0 | 38.6 |
| #False Negative (%) | 6.8 | 7.8 | 0.5 |
| N | 396 | 378 | 378 |
| % Sensitivity | 84.0 | 87.3 | 88.9 |
| % Specificity | 89.4 | 94.0 | 58.0 |
| % Agreement | 87.1 | 89.7 | 59.0 |
| Vaporized | |||
| #True Positive (%) | 65.7 | 42.4 | 3.8 |
| #True Negative (%) | 23.5 | 34.8 | 58.8 |
| #False Positive (%) | 3.0 | 1.3 | 27.0 |
| #False Negative (%) | 5.1 | 11.9 | 0.8 |
| N | 385 | 358 | 358 |
| % Sensitivity | 92.9 | 78.1 | 83.3 |
| % Specificity | 88.6 | 96.5 | 68.5 |
| % Agreement | 91.7 | 85.5 | 69.3 |
Note: 0 mg smoked and vaped conditions were not included in sensitivity and specificity analyses.
Correlations between blood and oral fluid concentrations and pharmacodynamics
Statistically significant positive correlations were observed between self-reported drug effect severity (i.e., ratings of “Drug Effect” on DEQ) and whole blood THC, 11-OH-THC and THCCOOH, and oral fluid THC after administration of all active cannabis doses. Correlations were larger for blood cannabinoids compared with oral fluid THC. Interestingly, in blood, correlations were largest for THCCOOH rather than for THC and 11-OH-THC, which are the compounds that pharmacologically drive the acute intoxicating effects of cannabis. Correlations between THCCOOH-glucuronide and self-reported drug effect were generally low except for smoked cannabis at the 10 mg THC dose (r = 0.70). HR change from baseline was positively correlated with blood THC and 11-OH-THC at both active doses for each inhalation method. THCCOOH-glucuronide was modestly inversely correlated with changes in HR (Table VIII).
Table VIII.
Correlations (Pearson’s r) Between Individual Change From Baseline Values for THC and Metabolite Concentrations in Blood and Oral Fluid and Pharmacodynamic Measures
| Blood THC | Blood 11-OH-THC | Blood THCCOOH | Blood THCCOOH-GLUC | OF THC | |
|---|---|---|---|---|---|
| Smoked 10 mg THC dose | |||||
| Drug effect (DEQ) | 0.38* | 0.24* | 0.70* | 0.46* | 0.21* |
| Heart rate | 0.25* | 0.28* | 0.07 | −0.25* | 0.17* |
| DSST (total correct) | −0.06 | −0.02 | 0.16 | 0.18* | −0.20* |
| DAT (Dist. from stimulus) | 0.08 | 0.04 | 0.02 | −0.24* | 0.08 |
| DAT (total correct) | 0.07 | 0.01 | 0.01 | 0.01 | 0.08 |
| PASAT (total correct) | 0.11 | 0.12 | −0.09 | 0.05 | −0.01 |
| Smoked 25 mg THC dose | |||||
| Drug effect (DEQ) | 0.51* | 0.47* | 0.57* | 0.19* | 0.21* |
| Heart rate | 0.37* | 0.51* | 0.43* | 0.01 | 0.14 |
| DSST (total correct) | −0.18* | −0.13 | −0.16 | 0.05 | −0.16 |
| DAT (Dist. from stimulus) | 0.01 | −0.03 | 0.004 | −0.08 | 0.10 |
| DAT (total correct) | −0.05 | 0.004 | −0.09 | 0.07 | −0.04 |
| PASAT (total correct) | −0.07 | −0.02 | −0.03 | 0.17* | −0.04 |
| Vaporized 10 mg THC dose | |||||
| Drug effect (DEQ) | 0.54* | 0.48* | 0.54* | −0.03 | 0.28* |
| Heart rate | 0.40* | 0.36* | 0.12 | −0.31* | 0.32* |
| DSST (total correct) | −0.23* | −0.11 | −0.03 | 0.13 | −0.18* |
| DAT (Dist. from stimulus) | 0.05 | 0.06 | −0.05 | −0.23* | 0.11 |
| DAT (total correct) | 0.01 | −0.08 | −0.06 | −0.03 | −0.07 |
| PASAT (total correct) | −0.07 | −0.07 | −0.01 | 0.03 | −0.03 |
| Vaporized 25 mg THC dose | |||||
| Drug effect (DEQ) | 0.52* | 0.52* | 0.56* | −0.19* | 0.26* |
| Heart rate | 0.37* | 0.57* | 0.53* | −0.25* | 0.30* |
| DSST (total correct) | −0.32* | −0.24* | −0.40* | 0.17* | −0.16 |
| DAT (Dist. from stimulus) | 0.14 | 0.14 | 0.25* | −0.03 | −0.004 |
| DAT (total correct) | −0.14 | −0.15 | −0.26* | 0.09 | −0.12 |
| PASAT (total correct) | −0.19* | −0.20* | −0.38* | 0.01 | −0.02 |
Note: Asterisks (*) denote statistical significance (P < 0.05).
In contrast to subjective drug effect ratings, cognitive/psychomotor performance was not strongly correlated with blood/oral fluid cannabinoid concentrations, despite the fact that significant cognitive/psychomotor deficits were observed after acute administration of cannabis (14). Indeed, for both the 10 and 25 mg doses of smoked cannabis and the 10 mg vaporized dose, few statistically significant inverse correlations were observed between blood/oral fluid cannabinoid concentrations and cognitive/psychomotor performance (Table VIII), and even those were modest (r’s ≤ 0.23). However, at the 25 mg vaporized dose, several significant small to moderate inverse correlations were observed between blood THC, 11-OH-THC, and THCCOOH and cognitive/psychomotor performance: correct responses on the DSST and PASAT were inversely correlated with THC, 11-OH-THC and THCCOOH concentrations. In addition, blood THCCOOH concentrations were correlated with performance on the DAT (average distance from central stimulus and total correct responses) and blood THCCOOH-glucuronide concentrations were positively correlated with correct responses on the DSST at the 25 mg vaporized dose. Small inverse correlations were also observed between oral fluid THC concentrations and total correct responses on the DSST at the 10 mg dose for both inhalation methods though no correlations were observed between these outcomes at either of the 25 mg doses. Notably, oral fluid THC concentrations were not correlated with performance on the DAT or the PASAT for either THC dose or method of inhalation (Table III).
Sex differences
Table IX displays Cmax values for whole blood THC and THC metabolites divided by sex. On average, whole blood THC and 11-OH-THC Cmax values were qualitatively higher for females than males for both methods of inhalation within a given dose. Females also exhibited qualitatively higher THCCOOH Cmax concentrations after vaporized cannabis at both doses. Conversely, males displayed qualitatively higher average THCCOOH concentrations after inhalation of smoked cannabis, particularly at the 25 mg dose. Average THCCOOH-glucuronide concentrations in whole blood were qualitatively higher for females relative to males, except at the 25 mg smoked cannabis dose where THCCOOH-glucuronide concentrations were comparable. Notably, on average, males weighed more than females (: 85 vs 70 kg) which could account for the higher observed blood cannabinoid concentrations in females. For oral fluid, unlike with blood, males had qualitatively higher mean Cmax concentrations of THC compared with females for both doses and inhalation methods. These seemingly contradictory findings are likely because a subset of male participants (participants #20, #50 and #59) exhibited extremely high levels of THC in oral fluid despite little to no THC in whole blood (Tables I and II).
Table IX.
Mean Maximum Blood Concentrations (Cmax) of THC and THC metabolites, and Individual Ranges, by THC Dose (10, 25 mg), Inhalation Method (Smoked and Vaporized) and Gender (Nine Males and Eight Females)
| Dose (mg) | Male THC Cmax (ng/mL) | Female THC Cmax (ng/mL) | Male 11-OH-THC Cmax (ng/mL) | Female 11-OH-THC Cmax (ng/mL) | Male THCCOOH Cmax (ng/mL) | Female THCCOOH Cmax (ng/mL) | Male THCCOOH-GLUC Cmax (ng/mL) | Female THCCOOH-GLUC Cmax (ng/mL) | Male THC Cmax (ng/mL) Oral Fluid | Female THC Cmax (ng/mL) Oral fluid |
|---|---|---|---|---|---|---|---|---|---|---|
| Smoked | ||||||||||
| 10 | 1.78 (0–10) | 6.0 (0–21) | 0.11 (0–1) | 0.25 (0–1) | 2.11 (0–9) | 1.5 (0–4) | 1.44 (0–8) | 3.0 (0.4–7) | 244 (23–1063) | 81 (0–290) |
| 25 | 8.67 (0–38) | 12 (0–34) | 1.22 (0–4) | 1.0 (0–3) | 7.44 (0–23) | 4.25 (0–11) | 7.0 (0–21) | 6.63 (0.8–25) | 733 (99–2,368) | 293 (58–707) |
| Vaporized | ||||||||||
| 10 | 4.78 (0–13) | 10.63 (5–18) | 0.55 (0–2) | 2.0 (0–6) | 4.22 (0–10) | 8.0 (1–18) | 4.22 (0–11) | 7.13 (0–14) | 115 (7–383) | 64 (14–150) |
| 25 | 11.78 (1–29) | 17.25 (2–33) | 1.22 (0–3) | 3.0 (2–6) | 6.89 (3–17) | 7.75 (2–14) | 6.11 (1–12) | 8.75 (0–17) | 630 (60–1,646) | 363 (103–707) |
Description of adverse events
Three adverse events occurred after inhalation of cannabis in this study. One participant vomited 13 min after the 25 mg THC vaporized cannabis administration period and another vomited 15 min after the 25 mg THC smoked cannabis administration period. In both cases, emesis was short lived and immediately resolved all related nausea. In addition, one participant experienced an adverse event that included both auditory and visual hallucinations after vaporization of the 25 mg THC cannabis dose. This specific adverse event is described in further detail elsewhere (18).
Discussion
A comprehensive understanding of cannabis pharmacokinetics is necessary to refine procedures for detection of cannabis use and impairment related to acute intoxication. These procedures are widely utilized and are relevant for toxicological drug testing in workplace, roadside, research (e.g., randomized clinical trials), criminal justice (e.g., criminal probation) and substance abuse treatment settings. Further understanding of the comparative pharmacokinetics of smoked and vaporized cannabis in infrequent cannabis users is particularly important given that vaporizers have become a popular method for cannabis self-administration (4, 5), and because most prior research has enrolled individuals who use cannabis at a weekly or greater frequency. The present study examined the pharmacokinetics of various doses of smoked and vaporized cannabis, using LC–MS-MS and ELISA analyses, in blood and oral fluid of healthy adults who had not used cannabis for at least one month prior to participation.
Higher concentrations of THC and THC metabolites were detected in whole blood after inhalation of vaporized cannabis compared with the same doses of smoked cannabis. For example, for the 25 mg THC dose, mean Cmax concentrations in whole blood after inhalation of smoked and vaporized cannabis were 10.24 and 14.36 ng/mL, respectively. Thus, cannabis vaporizers can deliver cannabinoids more efficiently to the user than smoked implements, likely because less drug is lost during vaporization than during pyrolysis (i.e., combustion) (7). These results are in contrast to prior studies which detected either comparable maximum THC concentrations in plasma after smoked and vaporized cannabis inhalation (9), or higher concentrations of THC and its metabolites in blood after smoking compared with vaporization (10).
Methodological differences likely account for the divergent findings between the present study and the two former direct comparisons of smoked and vaporized cannabis. In the Newmeyer et al. study (10), participants inhaled a single “balloon” of cannabis vapor at each dose, compared with 2–3 balloons in the Abrams et al. study (9) and 3 balloons in the present study. Both prior studies allowed for variance in cannabinoid delivery by using a paced puffing procedure for inhalation and/or using cannabis cigarettes in smoked conditions rather than an independently measured amount of plant material for smoked administration. Paced puffing procedures control for the number of puffs and the duration of exposure to cannabis, but do not control for the depth or intensity of inhalation across puffs, which can vary considerably across participants and within individual smoking bouts (13). Variability in the amount and distribution of plant material within a cannabis cigarette may also contribute to variability in drug delivery. In the present study, participants were able to self-administer cannabis ad-libitum, but within a constrained time period and using methods that ensured complete delivery of a fixed dose of cannabis (exact amount of cannabis placed into a contained hand-held pipe). Of note, though no differences between smoked and vaporized cannabis were observed at peak plasma concentrations (immediately post-cannabis administration) in the Abrams et al. study (9), plasma THC levels were higher after vaporized cannabis administration compared with smoked for most subsequent time points, which is consistent with the present study.
Overall, cannabinoid concentrations in this study were substantially lower than those observed in prior cannabis administration studies which enrolled moderate or heavy cannabis users (including the two other direct comparisons of smoked and vaporized cannabis(9, 10)). For example, when chronic cannabis users inhale smoked or vaporized cannabis containing comparable THC doses (e.g., ~15–30 mg) to the present study (i.e., 10 and 25 mg), mean Cmax concentrations for blood THC can range from 50 to over 100 ng/mL (9, 19, 20). Conversely, mean Cmax concentrations in the present study did not exceed 15 ng/mL.
Several factors may account for the lower blood cannabinoid concentrations observed in this study. First, in the present study, the first blood sampling occurred 10 min after drug administration while, in prior studies, blood sampling generally occurred much earlier. For instance, in the Abrams study (9), the first blood sampling occurred 2 min after cannabis administration and in the Newmeyer study (10), blood sampling occurred at 2 min increments during the cannabis administration bouts. Given that THC is highly lipophilic, these differences in blood sampling time points likely contributed to the lower cannabinoid concentrations observed here. The use of plasma, as opposed to whole blood, in the Abrams study may have also contributed to higher cannabinoid concentrations compared to this study. Finally, the moderate/heavy cannabis users who participated in former studies may have differed from the infrequent cannabis users in this study in ways which further contributed to pharmacokinetic differences. In some studies, select participants exhibited elevated cannabinoid concentrations at baseline (prior to cannabis administration) (10). Differences in puffing behaviors (e.g., puff duration, puff volume, inhalation depth) between infrequent and regular cannabis users could also conceivably contribute to differential cannabinoid delivery, though this has never been demonstrated empirically.
The time course for detection of THC and its metabolites in whole blood were similar across inhalation methods and also consistent with prior controlled laboratory examinations of smoked and vaporized cannabis (10, 21, 22). That is, smoked and vaporized cannabis inhalation resulted in rapid increases of THC and 11-OH-THC in the blood, which peaked within 10–30 min and returned to baseline within 1–4 h. THCCOOH generally exhibited a similar onset to detection and THCCOOH-glucuronide detection was typically delayed, but both of these metabolites often persisted in the blood throughout the experimental sessions (i.e., up to 8 h).
Despite differences observed for whole blood across inhalation methods, oral fluid THC and THCCOOH mean Cmax concentrations were similar for the 25 mg conditions (smoked THC: 496 ng/mL; vaporized THC: 506 ng/mL), and slightly higher for smoked cannabis at the 10 mg THC dose (THC: 167 vs 91 ng/mL). THC was often detected in oral fluid well after it was last detected in whole blood, and oral fluid THCCOOH detection was erratic, often delayed relative to whole blood, and in several instances, it was not detected at all. Thus, after cannabis inhalation, cannabinoid concentrations detected in oral fluid often do not mirror those found in whole blood, suggesting oral fluid concentrations may reflect cannabinoid deposition in the oral cavity rather than systemic cannabinoid bioavailability. These results could suggest that cannabis users’ puff topography can influence cannabinoid deposition in the oral cavity and that, in some instances, smoked cannabis may be retained in the oral cavity to a greater extent than vaporized cannabis. This assertion may be supported by the fact that blood ELISA testing was more sensitive after vaporized cannabis administration (93%) relative to smoked (84%), while oral fluid ELISA testing was more sensitive during smoked (THC: 87%; THCCOOH: 89%) relative to vaporized sessions (THC: 78%; THCCOOH: 83%). Interestingly, Swortwood et al. (11), in the same study as Newmeyer et al. (10), similarly detected higher THC concentrations in oral fluid after inhalation of smoked cannabis compared with vaporized though, as mentioned above, participants only inhaled 1 “balloon” of cannabis aerosol in that study which could have resulted in incomplete delivery of the vaporized THC dose.
In some instances, whole blood concentrations of THC, 11-OH-THC, THCCOOH and THCCOOH-glucuronide and oral fluid concentrations of THC were significantly correlated with cannabis-induced changes on pharmacodynamic outcomes including self-reported drug effect, HR, and primary end points on a battery of cognitive tests (DSST, DAT and the PASAT). The strength of correlation between cannabinoid concentrations and pharmacodynamic outcomes was generally greater during the 25 mg THC sessions compared with the 10 mg sessions, particularly for subjective drug effect ratings and HR. In addition, whole blood THC concentrations were more strongly correlated with pharmacodynamic outcomes than oral fluid THC concentrations, suggesting THC in blood may be a more accurate predictor of cannabis impairment. Importantly, however, blood and oral fluid cannabinoid concentrations and cognitive/psychomotor performance were only moderately correlated at best, and often not correlated at all. Indeed, as described elsewhere (14), self-reported drug effects and cognitive/psychomotor impairment in this study often persisted for several hours after cannabinoid concentrations in blood and oral fluid fell below the LOQ. Interestingly, no single cannabinoid measured, either in blood or oral fluid, was universally associated with cannabis-related drug effects, physiological changes and cognitive performance, highlighting the need to combine analytical blood/oral fluid drug testing with behavioral assessments to accurately determine instances of cannabis intoxication/impairment.
Several limitations of the present study are noteworthy. First, a limited range of doses, one type of cannabis (high THC, low CBD), and one type of vaporizer held at a constant temperature were used. Additional studies should explore the generality of the effects found here to other types of cannabis (e.g., cannabis extracts, those with varied THC:CBD ratios, or with different terpenoid profiles), other more popular vapor delivery devices (e.g., hand-held devices), and with variations in temperature settings. The small sample size of the present study is also a limitation, as this precluded the evaluation of participant characteristics such as genetic factors that could influence acute cannabis pharmacokinetics. Lastly, inclusion of only infrequent users could be considered a limitation. Future studies should explore whether regular or intermittent cannabis users with a preference for smoked or vaporized cannabis exhibit different pharmacokinetic profiles to those observed in this study.
Conclusion
The present study determined that the acute pharmacokinetics of smoked and vaporized cannabis can differ, as vaporized cannabis generally delivered more THC and THC metabolites to whole blood of study participants. Blood cannabinoid concentrations tended to return to zero prior to the offset of subjective drug effects or impairment measured on a battery of performance tests. Though correlations between individual analytes and select pharmacodynamic outcomes were statistically significant in several cases, no single biomarker appeared to serve as a reliable predictor of impairment or subjective intoxication. Oral fluid cannabinoid concentrations did not follow the same time course as whole blood, suggesting residual deposition of THC in the oral cavity may have influenced the assay’s results. THCCOOH detection in oral fluid was also highly erratic. Sensitivity between ELISA and LC–MS-MS results differed across inhalation method. For whole blood, greater sensitivity was observed in vaporized conditions while, conversely, for oral fluid, greater sensitivity was observed when cannabis was smoked. Future studies are need to further characterize the pharmacokinetics of cannabis across different populations and methods for cannabis administration. This work will become increasingly important as legal access to cannabis for both medicinal and non-medicinal use becomes more accepted and the diversity of product formulations continues to expand.
Acknowledgments
We thank the support staff of the Johns Hopkins University Behavioral Pharmacology Research Unit for outstanding contributions to the implementation of this study. We also thank Dr Christine Moore and Ms Cynthia Coulter at Immunalysis Inc., support staff at RTI International, and the NIDA Drug Supply Program.
Funding
This research was funded by the Substance Abuse and Mental Health Services Administration (SAMHSA).
References
- 1. Cyrenne P., Shanahan M. (2018) Toward a regulatory framework for the legalization of cannabis: how do we get to there from here? Canadian Public Policy, 44, 54–76. [Google Scholar]
- 2. Berg C.J., Stratton E., Schauer G.L., Lewis M., Wang Y., Windle M., et al. (2015) Perceived harm, addictiveness, and social acceptability of tobacco products and marijuana among young adults: marijuana, hookah, and electronic cigarettes win. Substance Use & Misuse, 50, 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Russell C., Rueda S., Room R., Tyndall M., Fischer B. (2018) Routes of administration for cannabis use–basic prevalence and related health outcomes: a scoping review and synthesis. International Journal of Drug Policy, 52, 87–96. [DOI] [PubMed] [Google Scholar]
- 4. Lee D.C., Crosier B.S., Borodovsky J.T., Sargent J.D., Budney A.J. (2016) Online survey characterizing vaporizer use among cannabis users. Drug and Alcohol Dependence, 159, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morean M.E., Kong G., Camenga D.R., Cavallo D.A., Krishnan-Sarin S. (2015) High school students’ use of electronic cigarettes to vaporize cannabis. Pediatrics, 136, 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goniewicz M.L., Knysak J., Gawron M., Kosmider L., Sobczak A., Kurek J., et al. (2014) Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco Control, 23, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pomahacova B., Van der Kooy F., Verpoorte R. (2009) Cannabis smoke condensate III: the cannabinoid content of vaporised Cannabis sativa. Inhalation Toxicology, 21, 1108–1112. [DOI] [PubMed] [Google Scholar]
- 8. Etter J.F. (2015) Electronic cigarettes and cannabis: an exploratory study. European Addiction Research, 21, 124–130. [DOI] [PubMed] [Google Scholar]
- 9. Abrams D.I., Vizoso H.P., Shade S.B., Jay C., Kelly M.E., Benowitz N.L. (2007) Vaporization as a smokeless cannabis delivery system: a pilot study. Clinical Pharmacology & Therapeutics, 82, 572–578. [DOI] [PubMed] [Google Scholar]
- 10. Newmeyer M.N., Swortwood M.J., Barnes A.J., Abulseoud O.A., Scheidweiler K.B., Huestis M.A. (2016) Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: identification of recent cannabis intake. Clinical Chemistry, 62, 1579–1592. [DOI] [PubMed] [Google Scholar]
- 11. Swortwood M.J., Newmeyer M.N., Andersson M., Abulseoud O.A., Scheidweiler K.B., Huestis M.A. (2017) Cannabinoid disposition in oral fluid after controlled smoked, vaporized, and oral cannabis administration. Drug Testing and Analysis, 9, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris R.Z., Benet L.Z., Schwartz J.B. (1995) Gender effects in pharmacokinetics and pharmacodynamics. Drugs, 50, 222–239. [DOI] [PubMed] [Google Scholar]
- 13. McClure E.A., Stitzer M.L., Vandrey R. (2012) Characterizing smoking topography of cannabis in heavy users. Psychopharmacology, 220, 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spindle T.R., Cone E.J., Schlienz N.J., Mitchell J.M., Bigelow G.E., Flegel R., et al. (2018). Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: a crossover trial. JAMA Network Open, 1, e184841–e184841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sobell L.C., Sobell M.B. (1992) Timeline follow-back. In Raye Z.L., John P.A. (eds). Measuring Alcohol Consumption, Humana Press, Totowa, NJ, pp. 41–72. [Google Scholar]
- 16. Vandrey R., Herrmann E.S., Mitchell J.M., Bigelow G.E., Flegel R., LoDico C., et al. (2017) Pharmacokinetic profile of oral cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. Journal of Analytical Toxicology, 41, 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coulter C., Miller E., Crompton K., Moore C. (2008) Tetrahydrocannabinol and two of its metabolites in whole blood using liquid chromatography-tandem mass spectrometry. Journal of Analytical Toxicology, 32, 653–658. [DOI] [PubMed] [Google Scholar]
- 18. Barrett F.S., Schlienz N.J., Lembeck N., Waqas M., Vandrey R. (2018) “Hallucinations” following acute cannabis dosing: a case report and comparison to other hallucinogenic drugs. Cannabis and Cannabinoid Research, 3, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cooper Z.D., Haney M. (2009) Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug and Alcohol Dependence, 103, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papafotiou K., Carter J.D., Stough C. (2005) The relationship between performance on the standardised field sobriety tests, driving performance and the level of Δ9-tetrahydrocannabinol (THC) in blood. Forensic Science International, 155, 172–178. [DOI] [PubMed] [Google Scholar]
- 21. Fabritius M., Chtioui H., Battistella G., Annoni J.M., Dao K., Favrat B., et al. (2013) Comparison of cannabinoid concentrations in oral fluid and whole blood between occasional and regular cannabis smokers prior to and after smoking a cannabis joint. Analytical and Bioanalytical Chemistry, 405, 9791–9803. [DOI] [PubMed] [Google Scholar]
- 22. Schwope D.M., Bosker W.M., Ramaekers J.G., Gorelick D.A., Huestis M.A. (2012) Psychomotor performance, subjective and physiological effects and whole blood Δ9-tetrahydrocannabinol concentrations in heavy, chronic cannabis smokers following acute smoked cannabis. Journal of Analytical Toxicology, 36, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]