Abstract
Introduction:
Breast carcinoma (BCa) is one of the most common cancers among women globally. Increased vimentin expression has been reported in various epithelial cancers.
Aim:
This study examines the expression of vimentin in BCa and its correlation with various prognostic factors such as tumor size, histological grade, lymph node status, estrogen receptor (ER), progesterone receptor (PR), HER2/neu, and Ki67 status.
Materials and Methods:
Seventy cases of BCa diagnosed between 2014 and 2015 were included in the study. A technique of manual tissue microarray was employed for the analysis of expression of immunohistochemical (IHC) markers such as vimentin, ER, PR, HER2/neu, and Ki67. Results were subjected to statistical analysis.
Results:
Vimentin was found positive in 53 (75.7%) cases of BCa, of which 18 cases (25.7%) were triple-negative BCa (TNBC). Positivity for ER, PR, Her2, and Ki67 was 32.8%, 31.4%, 60%, and 99%, respectively. Vimentin expression was significantly associated with ER negativity. All 53 cases expressing vimentin showed positive Ki67 labeling index; however, this was not statistically significant. Maximum vimentin expression was observed in the age group >50 years, postmenopausal women, BCa cases showing lymphovascular invasion (LVI), axillary lymph node metastasis, higher stage and higher grade of tumor, negative PR expression, and positive HER2/neu expression. However, this was not statistically significant. In TNBC, vimentin expression was significantly associated with histological grade and LVI.
Conclusion:
Vimentin expression was associated with well-established poor prognostic factors of BCa. Vimentin expression if routinely included in histopathology report would aid in better understanding of tumor behavior.
Keywords: Breast carcinoma, prognosis, vimentin
INTRODUCTION
Breast carcinoma (BCa) is one of the most common cancers among women globally.1 According to the population-based National Cancer Registry (India), it is one of the major cancers in Indian females.2
Conventionally, important factors such as age, menstrual status, lymph node involvement, tumor size and grade, and lymphovascular invasion (LVI) with additional factors such as histological subtype, Nottingham prognostic index, stage at presentation, hormonal receptor status, and HER2/neu receptor status influence the therapy and prognosis of BCa.3,4,5
Vimentin, a major constituent of the intermediate filament family of proteins, is ubiquitously expressed in normal mesenchymal cells and is known to maintain cellular integrity and provides resistance against stress. Aberrant vimentin expression has been reported in various epithelial cancers. Recent studies have reported that vimentin plays a major role in the epithelial–mesenchymal transition (EMT) process of BCa and its knockdown resulted in a decrease in genes linked with BCa invasion and the basal-like phenotype. In infiltrating ductal carcinoma (IDC), the expression of vimentin is associated with low ER, low PR, increased basement membrane invasiveness, and resistance to BCa chemotherapy.6
The present study was conducted to evaluate vimentin expression in BCa cases and its correlation with other clinicopathological parameters.
MATERIALS AND METHODS
This cross-sectional study was conducted in the Department of Pathology in Smt. Kashibai Navale Medical College and General Hospital, Pune, Maharashtra, India. Ethical Clearance was obtained from the Institute's Ethical Committee. Seventy cases of BCa cases diagnosed and operated from 2014 to 2015 were included in the study. The available data for all the patients as regards with age, location of tumor, grade, stage, and lymph node status were collected from the records of the histopathology section of the Department of Pathology. Cases, in which records/slides/blocks were not available, were excluded from the study.
The modified radical mastectomy specimens of the included cases received were evaluated histopathologically. All the slides were evaluated by two senior histopathologists. The Modified Bloom–Richardson system of cancer grading was used in this study. Tumor-node-metastasis classification and staging of the cases was done as per the American Joint Committee on Cancer guidelines.7
The representative tissue block of BCa cases was selected for immunohistochemical (IHC) evaluation. A technique of manual tissue microarray (TMA) was employed for the study of vimentin, estrogen receptor (ER), progesterone receptor (PR), HER2/neu, and Ki67 in all cases with one tissue core taken from each selected BCa block.8
The primary antibodies used were vimentin (clone V9, Dako), ER (Clone 6F11, Novocastra), PR (Clone PGR312, Novocastra), HER2/neu (Clone CB11, Novocastra), and Ki67 (Clone MM1, Novocastra). Negative control (without adding primary antibody) was included in all batches.
Reactive lymph node was used as a positive control for vimentin expression. Section from the endometrial tissue was used as a positive control for ER and PR. Section from BC, which previously showed unequivocal strong immunoreactivity for HER2/neu, was used as a positive control for HER2/neu. Section from the skin was used as a positive control for Ki67. Sections were examined under high-power field to observe the immunoreactivity.
Vimentin expression was considered positive if >10% of the tumor cells showed distinct granular cytoplasmic immunoreactivity.6 Allred score was used to evaluate the ER and PR, and a score of 3–8 was considered positive. Immunoreactivity for ER and PR was assessed by estimating the percentage of tumor cells showing nuclear staining. More than 10% of the tumor cells showing immunoreactivity were considered as positive.9 HER2 staining was scored according to the American Society of Clinical Oncology/College of American Pathologists guidelines. Moderate-to-strong complete membrane staining of 10% or more of the tumor cells was considered to be positive (2 + and 3+)10. Ki67 labeling index (Ki67LI) of equal to or >10% was considered to be positive.11
The Primer of Biostatistics 7.0 (manufactured by McGraw-Hill) program was used for the calculation of interrelationships between the analyzed vimentin expression and clinicopathological variables by Pearson's Chi-square test. Quantitative data was presented as mean. Qualitative data was presented as frequency and percentage table. The results were considered to be statistically significant when the value of P < 0.05 and highly statistically significant when value of P < 0.01.
RESULTS
The various clinicopathological features of BCa are presented in Table 1. In the present study, the age ranged from 34 to 72 years with a mean age of 55 years. The most common age group affected was 41–50 years with 26/70 (37%) cases. In the present study, 19/70 (27.2%) cases were triple-negative BCa (TNBC) molecular subtype. Most common age group affected was 41–70 (16/19 cases, 84.7%), with a mean age of 56.15 years. Vimentin expression and clinicopathological parameters of BCa and TNBC cases are presented in Tables 2 and 3, respectively [Figure 1].
Table 1.
Clinicopathological characteristics of breast carcinoma cases
| Clinicopathological characteristics | Number of tumors, n (%) |
|
|---|---|---|
| 70 BCa cases | 19 TNBC cases | |
| Laterality | ||
| Right | 35 (50) | 6 (31.6) |
| Left | 35 (50) | 13 (68.4) |
| Menopausal status (69) | ||
| Premenopausal | 16 (23) | 3 (15.8) |
| Postmenopausal | 53 (77) | 16 (84.2) |
| Histopathological types | ||
| IDC (NOS) | 54 (77.1) | 8 (42.1) |
| IDC + mucinous features | 1 (1.4) | 0 |
| IDC + lobular | 1 (1.4) | 1 (5.3) |
| Invasive cribriform | 2 (2.8) | 1 (5.3) |
| Medullary | 6 (8.6) | 6 (31.6) |
| Metaplastic | 3 (4.4) | 2 (10.4) |
| Mucinous | 2 (2.8) | 0 |
| Secretory | 1 (1.4) | 1 (5.3) |
| Status of lymphovascular invasion | ||
| Present | 44 (62.9) | 17 (89) |
| Absent | 26 (37.1) | 2 (11) |
| Tumor size (pT) | ||
| pT1 | 8 (11.4) | 0 |
| pT2 | 37 (52.9) | 13 (68.4) |
| pT3 | 17 (24.3) | 4 (21.1) |
| pT4 | 8 (11.4) | 2 (10.5) |
| ALN metastasis | ||
| N0 | 14 (20) | 2 (11) |
| N1 | 32 (45.7) | 12 (63) |
| N2 | 16 (22.9) | 5 (26) |
| N3 | 8 (11.4) | 0 |
| MBR Grade | ||
| Grade I | 15 (21) | 2 (10) |
| Grade II | 27 (39) | 3 (16) |
| Grade III | 28 (40) | 14 (74) |
| Stage | ||
| I | 2 (2.8) | 0 |
| II | 35 (50) | 12 (63) |
| III | 33 (47.2) | 7 (37) |
| IHC markers | ||
| Vimentin | 53 (75.7) | - |
| ER | 23 (32.8) | - |
| PR | 22 (31.4) | - |
| HER2/neu | 42 (60) | - |
| Ki67 | 69 (99) | - |
| Molecular subtype | ||
| Luminal A | 9 (12.8) | - |
| Luminal B | 14 (20) | - |
| Her2 enriched | 28 (40) | - |
| TNBC | 19 (27.2) | - |
IDC – Infiltrating duct carcinoma; NOS – Not otherwise specified; TNBC – Triple-negative breast carcinoma; MBR – Modified Bloom–Richardson; IHC – Immunohistochemistry; ALN – Axillary lymph node; ER – Estrogen receptor; PR – Progesterone receptor; BCa – Breast carcinoma
Table 2.
Vimentin expression and clinicopathological parameters of breast carcinoma cases
| Variables (n) | Vimentin expression |
P | |
|---|---|---|---|
| Positive (%) 53 (75.7) | Negative (%) 17 (24.3) | ||
| Age (years) | |||
| ≤50 (32) | 25 (35.7) | 7 (10) | 0.879 |
| >50 (38) | 28 (40) | 10 (14.3) | |
| Menopausal status (n=69) | |||
| Premenopausal (16) | 12 (17.4) | 4 (5.8) | 0.770 |
| Postmenopausal (53) | 40 (58) | 13 (18.8) | |
| Histologic tumor grade | |||
| 1 (15) | 9 (12.8) | 6 (8.6) | 0.073 |
| 2 (19) | 19 (27.2) | 8 (11.5) | |
| 3 (25) | 25 (35.7) | 3 (4.3) | |
| Lymphovascular invasion | |||
| Present (44) | 36 (51.5) | 8 (11.4) | 0.207 |
| Absent (26) | 17 (24.3) | 9 (12.8) | |
| Lymph node status | |||
| Positive (56) | 44 (62.8) | 12 (17.2) | 0.443 |
| Negative (14) | 9 (12.8) | 5 (7.2) | |
| Tumor size | |||
| T1 (4) | 3 (4.3) | 1 (1.4) | 1.000 |
| T2 (44) | 33 (47.2) | 11 (15.7) | |
| T3 (15) | 11 (15.7) | 4 (5.7) | |
| T4 (7) | 6 (8.6) | 1 (1.4) | |
| Stage | |||
| 1 (2) | 1 (1.4) | 1 (1.4) | 0.398 |
| 2 (35) | 25 (35.7) | 10 (14.3) | |
| 3 (27) | 27 (38.6) | 6 (8.6) | |
| ER | |||
| Positive (23) | 13 (18.5) | 10 (58.2) | 0.020 |
| Negative (47) | 40 (57.1) | 7 (14.4) | |
| PR | |||
| Positive (22) | 13 (18.5) | 9 (12.8) | 0.058 |
| Negative (48) | 40 (57.1) | 8 (11.5) | |
| HER2/neu | |||
| Positive (42) | 30 (42.8) | 12 (17.2) | 0.460 |
| Negative (28) | 23 (32.8) | 5 (7.2) | |
| Ki67 | |||
| Positive (69) | 53 (75.7) | 16 (22.8) | 0.243 |
| Negative (1) | 0 | 1 (1.5) | |
| Subtype | |||
| TN | 18 (25.7) | 1 (1.5) | 0.028 |
| Non-TN | 35 (50) | 16 (22.8) | |
ER – Estrogen receptor; PR – Progesterone receptor; TN – Triple negative
Table 3.
Vimentin expression and clinicopathological parameters of breast carcinoma in triple-negative breast carcinoma cases
| Variables (n) | Vimentin expression |
P | |
|---|---|---|---|
| Positive (%) 18 (94.7) | Negative (%) 1 (5.3) | ||
| Age (years) | |||
| ≤50 (8) | 7 (36.8) | 1 (5.3) | 0.870 |
| >50 (11) | 11 (57.9) | 0 | |
| Menopausal status (n=19) | |||
| Premenopausal (3) | 2 (10.5) | 1 (5.3) | 0.335 |
| Postmenopausal (16) | 16 (84.2) | 0 | |
| Histologic tumor grade | |||
| 1 | 1 (5.3) | 1 (5.3) | 0.011 |
| 2 | 3 (15.7) | 0 | |
| 3 | 14 (73.6) | 0 | |
| Lymphovascular invasion | |||
| Present (18) | 18 (94.7) | 0 | 0.040 |
| Absent (1) | 0 | 1 (5.3) | |
| Lymph node status | |||
| Positive (17) | 17 (89.4) | 0 | 0.186 |
| Negative (2) | 1 (5.3) | 1 (5.3) | |
| Tumor size | |||
| T1, T2 (13) | 13 (68.4) | 0 | 0.138 |
| T3 (4) | 3 (15.8) | 1 (5.3) | |
| T4 (2) | 2 (10.5) | 0 | |
| Stage | |||
| 1, 2 (12) | 11 (57.9) | 1 (5.3) | 0.779 |
| 3 (7) | 7 (36.8) | 0 | |
| Ki67 | |||
| Positive (19) | 18 (94.7) | 1 (5.3) | |
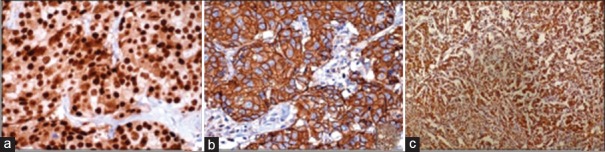
Figure 1.
Photomicrograph showing (a) strong nuclear ER (immunohistochemical, ×40), (b) uniform intense membrane HER2 (immunohistochemical, ×40) and (c) distinct granular cytoplasmic vimentin immunoreactivity in invasive ductal carcinoma (immunohistochemical, ×40)
DISCUSSION
Conventionally, vimentin has been used as a mesenchymal marker. In 1989, Raymond et al. first described the expression of vimentin in BCa.6 In this study, 75.7% of the cases of BCa showed vimentin expression, of which 25.7% were TNBC molecular subtype. Vimentin expression ranging from 7.7% to 91.3% has been documented in the literature [Table 4].6,12,13,14,15 Our patients had tumors with high stage and grade, accounting for the higher percentage of cases exhibiting vimentin expression. Our hospital is a charitable one and caters to the rural population. Due to low socioeconomic status of these patients, BCa cases are usually diagnosed in advanced stages.
Table 4.
Percentage of positive cases of vimentin in different studies and present study
| Study | Total number of cases in the study | Vimentin-positive cases in BCa | Percentage |
|---|---|---|---|
| Karihtala P et al. | 225 | 211 | 91.3 |
| Kusinska R et al. | 179 | 38 | 21.2 |
| Korsching E et al. | 272 | 21 | 7.7 |
| Hemalath A et al. | 50 | 9 | 18 |
| Yamashita et al. | 187 | 53 | 28.3 |
| Our study | 53 | 70 | 75 |
BCa – Breast carcinoma
BCa subtypes included in the present study such as invasive cribriform (2), secretory (1), medullary (6), and metaplastic (3) carcinoma expressed vimentin. However, BCa subtypes such as IDC + mucinous (1), IDC + lobular (1), and mucinous carcinoma (2) did not show vimentin expression. This is similar to studies documented in the literature.16,17,18,19,20,21
In this study, we found a significant association between vimentin expression and negative ER expression. This finding supported the observation that high vimentin expressing tumors were more frequently ER negative as documented in studies in the literature.6
Maximum vimentin expression was found in BCa cases with negative PR and HER2/neu expression. This was, however, statically insignificant. This was consistent with studies done by Yamashita et al.15 However, the study done by Seshadri et al.22 found significant negative correlation between vimentin expression and PR receptor expression.
In this study, maximum vimentin expression was observed in BCa cases showing LVI. However, this was not statistically significant. The study done by Lakhtakia et al. showed that there is a strong correlation between LVI and vimentin expression.23 In the present study, in TNBC cases, the association between vimentin expression and the status of LVI and higher tumor grade was statistically significant.
In this study, vimentin positivity was the highest in Stage 3 (38.6%) cases followed by Stage 2 (35.7%). Although the expression of vimentin increased with increasing stage of BCa, there was no statistical significance in vimentin expression and stage of tumor (P = 0.398). No correlation of vimentin expression and stage of tumor was found in the previous references.
A recent study has observed that Ki67 values above 10%–14% define a high-risk group in BCa patients making these patients successful candidates for neoadjuvant therapy.6 Considering 10% of Ki67LI as the cutoff in the present study, all BCa cases expressing vimentin were Ki67-positive though the results were not statistically significant.
18/19 (94.7%) TNBC cases showed vimentin expression. Patients with TNBC do not benefit from hormonal- or trastuzumab-based therapies. TNBC cases are biologically aggressive. Some reports suggest that they respond to chemotherapy better than other types of breast cancer. However, prognosis remains poor. This is due to the shortened disease-free interval in the adjuvant and neoadjuvant setting and a more aggressive course in the metastatic setting.24
Numerous theories have been put forward to define the role of vimentin in the pathogenesis of BCa. Vimentin expression is shown to be elevated in several aggressive breast cancer cell lines. Recent studies have reported that vimentin plays a major role in the EMT process of BCa.6
By virtue of its overexpression in many epithelial carcinomas, vimentin expression may serve as a potential target for cancer therapy. A potent breast anticancer drug Withaferin A is discovered. It acts by inducing perinuclear vimentin accumulation followed by rapid vimentin depolymerization and concomitant vimentin ser56 phosphorylation at low doses. Identifying vimentin-positive cells may have a positive impact in prolonging the life of patients with IDC (not otherwise specified).6
Limitations
TMA technique was used for ER, PR, HER2, and Ki67. Whole sections were not used for their IHC evaluation. However, utmost care was taken to sample the most representative area from the original whole section blocks for TMA. HER2 was assessed only by IHC. Evaluation by fluorescence in situ hybridization was not available, especially for the equivocal cases with HER2 expression 2+. Follow-up time for the patients was limited.
CONCLUSION
Vimentin expression was significantly associated with ER-negative BCa cases and triple-negative BCa cases with high tumor grade and LVI. Maximum vimentin expression was associated with other poor prognostic indices of BCa cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Masuda S. Breast cancer pathology: The impact of molecular taxonomy on morphological taxonomy. Pathol Int. 2012;62:295–302. doi: 10.1111/j.1440-1827.2012.02790.x. [DOI] [PubMed] [Google Scholar]
- 3.Akbar M, Akbar K, Naveed D. Frequency and correlation of molecular subtypes of breast cancer with clinicopathological features. J Ayub Med Coll Abbottabad. 2014;26:290–3. [PubMed] [Google Scholar]
- 4.Kamath R, Mahajan KS, Ashok L, Sanal TS. A study on risk factors of breast cancer among patients attending the tertiary care hospital, in Udupi district. Indian J Community Med. 2013;38:95–9. doi: 10.4103/0970-0218.112440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemalatha A, Suresh TN, Kumar ML. Expression of vimentin in breast carcinoma, its correlation with Ki67 and other histopathological parameters. Indian J Cancer. 2013;50:189–94. doi: 10.4103/0019-509X.118724. [DOI] [PubMed] [Google Scholar]
- 7.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO Classification of Tumours of the Breast. 4th ed. Lyon: IARC; 2012. pp. 8–108.pp. 152 [Google Scholar]
- 8.Pathak GS, Deshmukh SD, Ashturkar AV. Construction of tissue arrays without prefabricated recipient paraffin block experience of a novel technique in resource poor settings. Indian J Pathol Microbiol. 2011;54:654–5. doi: 10.4103/0377-4929.85139. [DOI] [PubMed] [Google Scholar]
- 9.Collins LC, Botero ML, Schnitt SJ. Bimodal frequency distribution of estrogen receptor immunohistochemical staining results in breast cancer: An analysis of 825 cases. Am J Clin Pathol. 2005;123:16–20. doi: 10.1309/hcf035n9wk40etj0. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez C, Schiff R. HER2: Biology, detection, and clinical implications. Arch Pathol Lab Med. 2011;135:55–62. doi: 10.1043/2010-0454-RAR.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tawfik K, Kimler BF, Davis MK, Fan F, Tawfik O. Ki-67 expression in axillary lymph node metastases in breast cancer is prognostically significant. Hum Pathol. 2013;44:39–46. doi: 10.1016/j.humpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Karihtala P, Auvinen P, Kauppila S, Haapasaari KM, Jukkola-Vuorinen A, Soini Y. Vimentin, zeb1 and sip1 are up-regulated in triple-negative and basal-like breast cancers: Association with an aggressive tumour phenotype. Breast Cancer Res Treat. 2013;138:81–90. doi: 10.1007/s10549-013-2442-0. [DOI] [PubMed] [Google Scholar]
- 13.Kusinska RU, Kordek R, Pluciennik E, Bednarek AK, Piekarski JH, Potemski P. Does vimentin help to delineate the so-called 'basal type breast cancer'? J Exp Clin Cancer Res. 2009;28:118. doi: 10.1186/1756-9966-28-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korsching E, Packeisen J, Liedtke C, Hungermann D, Wülfing P, van Diest PJ, et al. The origin of vimentin expression in invasive breast cancer: Epithelial-mesenchymal transition, myoepithelial histogenesis or histogenesis from progenitor cells with bilinear differentiation potential? J Pathol. 2005;206:451–7. doi: 10.1002/path.1797. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita N, Tokunaga E, Kitao H, Hisamatsu Y, Taketani K, Akiyoshi S, et al. Vimentin as a poor prognostic factor for triple-negative breast cancer. J Cancer Res Clin Oncol. 2013;139:739–46. doi: 10.1007/s00432-013-1376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Branca G, Ieni A, Barresi V, Tuccari G, Caruso RA. An updated review of cribriform carcinomas with emphasis on histopathological diagnosis and prognostic significance. Oncol Rev. 2017;11:317. doi: 10.4081/oncol.2017.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni MM, Khandeparkar SG, Joshi AR, Dhande AN. A rare case of multicentric secretory carcinoma of breast in an adult female with review of literature. Indian J Pathol Microbiol. 2016;59:209–11. doi: 10.4103/0377-4929.182021. [DOI] [PubMed] [Google Scholar]
- 18.Holck S, Pedersen L, Schiødt T, Zedeler K, Mouridsen H. Vimentin expression in 98 breast cancers with medullary features and its prognostic significance. Virchows Arch A Pathol Anat Histopathol. 1993;422:475–9. doi: 10.1007/BF01606456. [DOI] [PubMed] [Google Scholar]
- 19.Tse GM, Tan PH, Putti TC, Lui PC, Chaiwun B, Law BK. Metaplastic carcinoma of the breast: A clinicopathological review. J Clin Pathol. 2006;59:1079–83. doi: 10.1136/jcp.2005.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heatley M, Whiteside C, Maxwell P, Toner P. Vimentin expression in benign and malignant breast epithelium. J Clin Pathol. 1993;46:441–5. doi: 10.1136/jcp.46.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domagala W, Markiewski M, Kubiak R, Bartkowiak J, Osborn M. Immunohistochemical profile of invasive lobular carcinoma of the breast: Predominantly vimentin and p53 protein negative, cathepsin D and oestrogen receptor positive. Virchows Arch A Pathol Anat Histopathol. 1993;423:497–502. doi: 10.1007/BF01606541. [DOI] [PubMed] [Google Scholar]
- 22.Seshadri R, Raymond WA, Leong AS, Horsfall DJ, McCaul K. Vimentin expression is not associated with poor prognosis in breast cancer. Int J Cancer. 1996;67:353–6. doi: 10.1002/(SICI)1097-0215(19960729)67:3<353::AID-IJC8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 23.Lakhtakia R, Aljarrah A, Furrukh M, Ganguly SS. Epithelial mesenchymal transition (EMT) in metastatic breast cancer in Omani women. Cancer Microenviron. 2017;10:25–37. doi: 10.1007/s12307-017-0194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med. 2015;12:106–16. doi: 10.7497/j.issn.2095-3941.2015.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]