Abstract
Simple limbal epithelial transplantation (SLET) is an innovative limbal stem cell transplantation technique that has gained increasing popularity over the last few years. Different groups from across the world have published the clinical results of SLET in large case series with varying types and severities of limbal stem cell deficiency (LSCD). This review attempts to place all the available knowledge on SLET together in one place for the benefit of not only cornea specialists and trainees but also for residents and general ophthalmologists. It follows a balanced approach of blending evidence with experience by providing an objective analysis of published results along with helpful insights from subject experts, starting from preoperative considerations including the role of newer imaging modalities to the technical aspects of the surgery itself and the management of possible complications. Original data and novel insights on allogeneic SLET for bilateral LSCD are included in the review to address the few remaining lacunae in the existing literature on this topic. This review intends to inform, educate, and empower all aspiring and practicing SLET surgeons to optimize their clinical outcomes and to have maximal positive impact on the lives of the individuals affected by unilateral or bilateral chronic LSCD.
Keywords: Limbal stem cell transplantation, limbal stem cells, limbal transplantation, simple limbal epithelial transplantation, SLET
A delicate layer of transparent nonkeratinized, stratified squamous epithelium covers the corneal surface. These epithelial cells have high turnover and are constantly exposed to the environment. Therefore, they need to be replaced continuously throughout life by stem cells present at the limbus.[1] Severe trauma or inflammation of the limbus may lead to corneal epithelial dysfunction due to limbal stem cell deficiency (LSCD), clinically characterized by progressive vascularization, conjunctivalization, and scarring of the corneal surface. In severe and chronic cases, LSCD can lead to visual impairment and even blindness.[2] Fortunately, limbal stem cell transplantation (LSCT) can reverse this potentially blinding condition by transferring healthy limbal tissue containing the stem cells from a normal donor eye. Depending on the source of the donor tissue, LSCT can either be autologous (from the unaffected fellow eye of the same person) or allogeneic (from another person).
Several different surgical techniques of LSCT have emerged with time. The conventional approach, first described by Kenyon and Tseng in 1989 for autologous transplants has since come to be known as conjunctival-limbal autografting (CLAU).[3] In this technique, two large conjunctival-limbal lenticules are harvested from a healthy eye and directly transplanted to the affected eye. Unfortunately, CLAU is known to be associated with complications including the risk of developing iatrogenic LSCD in the donor eye.[4,5,6,7,8] A significant advancement in LSCT was made by Pellegrini et al. in 1997, who developed the technique of cultivated limbal epithelial transplantation (CLET). In this approach, a tiny limbal biopsy from the healthy eye was used to create a multilayered sheet of corneal epithelium ready for transplantation.[9] Although CLET minimized the problems of CLAU, cell expansion necessitated a clinical-grade laboratory with regulatory approvals which was and still is extremely expensive to build and maintain. In 2012, Sangwan et al. described an innovative technique of LSCT called simple limbal epithelial transplantation (SLET), which combined the advantages of CLAU and CLET while avoiding the limitations of both approaches.[10] Since then, SLET has become the preferred technique of LSCT.[11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29] In this review, the authors enumerate the indications, surgical technique, mechanism of action, outcomes, limitations, and impact of SLET on patients with blinding LSCD.
Indications
Unilateral LSCD is the primary indication for autologous SLET. The most frequent cause of unilateral LSCD is ocular burns and, therefore, it is not surprising that almost all of the published literature on autologous SLET pertains to this indication.[2,11,12,13] However, autologous SLET has also been described in few cases with LSCD secondary to ocular surface squamous neoplasia excision (OSSN) and multiple surgical interventions.[15,16] Both primary and recurrent pterygia and failed prior LSCT are other reported indications of autologous SLET.[17,18,19,20]
Allogeneic SLET for bilateral LSCD was first described in two separate case reports of a patient with severe chemical burns[21] and in a patient with dry eyes.[22] In both cases, the allogeneic donor tissue used was cadaveric in origin. It is important to note that unlike autologous SLET, patients with bilateral LSCD undergoing allogeneic SLET, either cadaveric or live-related, need long-term systemic immunosuppression for graft survival.[21] This review provides detailed information on the management and outcomes of allogeneic SLET in cases of chronic bilateral LSCD due to Stevens–Johnson syndrome (SJS), mucous membrane pemphigoid (MMP), chemical burns, and ocular allergy. Although cadaveric allogeneic SLET has also been used in the treatment of severe cases of acute chemical burns to achieve faster ocular surface epithelialization, the authors of this review strongly recommend the use of SLET as a reconstructive procedure only for chronic, established LSCD and not for acute injury or inflammation.[23]
Preoperative Considerations
Donor: Analogous to any organ or tissue transplantation, the main factor in determining the outcomes of SLET is the health of the donor limbus. Therefore, a careful preoperative inspection of the donor site is critical to ensure its viability. Typically, the superior limbus is preferred as the limbal palisades are more in number at this location. In case of cadaveric SLET, the tissue should : (i) be fresh (<48 hrs from the time of harvesting), (ii) have visibly intact limbal palisades, (iii) have no epithelial sloughing, and should be from a donor aged 60-years of age or less.[30] These criteria are recommended to ensure that cadaveric tissue have a proliferative potential similar to live-tissue.[31,32]
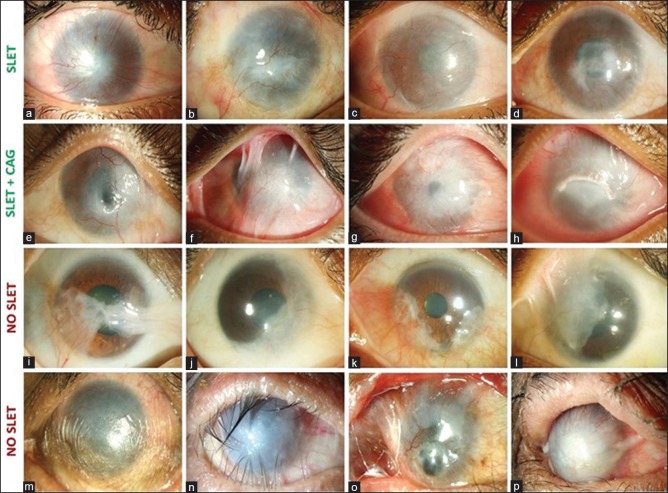
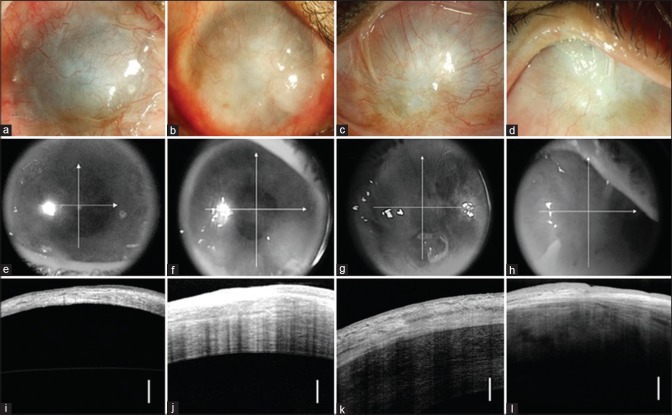
Recipient: The classification of cases for SLET into different prognostic categories based on presenting features in the recipient eye is summarized in Table 1. The presence of any of the following attributes in the affected eye should be considered as absolute contraindications for SLET: (i) dry ocular surface (defined as repeated Schirmer's I score with anesthesia of less than 10 mm or presence of corneal or bulbar conjunctival keratinization), (ii) blind eye with no visual potential; (iii) disorganized anterior segment (adherent leukoma, anterior staphyloma, or extensive peripheral anterior synechiae), and (iv) presence of uncorrected adnexal pathologies like lagophthalmos, ectropion, entropion, trichiasis, and dacryocystitis [Fig. 1]. Since SLET is an epithelial regenerative procedure, it has limited impact on corneal stromal opacification. Thus, cases with severe stromal opacification (leukoma) will additionally require corneal transplantation in the form of either anterior lamellar or penetrating keratoplasty (PK). A preoperative high-resolution anterior segment optical coherence tomography (AS-OCT) of the diseased eye is extremely useful in this context. It not only reveals the underlying stromal thickness (thereby alerting for likely perforation of extremely thin areas during dissection of the conjunctivalized pannus), but the infrared photograph of the cornea also shows the degree of opacification of the underlying stroma [Fig. 2]. The ideal cases for autologous SLET, particularly for beginners, are those with no history of any trauma, inflammation, or surgery in the donor eye; Similarly, the ideal case criteria for the affected eye are: (i) wet ocular surface without adnexal pathologies; (ii) minimal or no symblepharon; and (iii) clear to translucent underlying corneal stroma. Those cases which have severe symblepharon will also require additional conjunctival autografting (CAG), either during or after SLET [Fig. 1]. The authors also recommend that (i) primary or secondary pterygium/pseudopterygium (partial LSCD) be treated with CAG alone and (ii) cases with severe stromal opacification or disorganized anterior segment, which would also need a corneal grafting, be cosmetically rehabilitated if unilateral or undergo keratoprosthesis if the pathology is bilateral. In addition to the absolute and relative contraindications listed above, allogeneic SLET should be performed with caution in children because of the problems associated with long-term systemic immunosuppression and should be managed with the help of a rheumatologist experienced in pediatric immunosuppression.
Table 1.
Prognostic categorization for simple limbal epithelial transplantation (SLET) based on presenting features in the affected eye
| PRESENTING FEATURE | EXCELLENT | GOOD | FAIR/MODERATE | POOR |
|---|---|---|---|---|
| HISTORY | ||||
| Prior AMG | Yes | Yes | No | No |
| Prior LK/PK | No | No | Yes | Yes |
| Prior SLET/LSCT | No | Yes | Yes | Yes |
| Prior corneal melting or perforation | No | No | Yes | Yes |
| Prior multiple surgeries | No | No | Yes | Yes |
| Prior glaucoma | No | No | No | Yes |
| CLINICAL FEATURES | ||||
| Eye Lids | ||||
| Entropion/ectropion | No | No | No | Yes |
| Irregular margin | No | No | No | Yes |
| Tarsal papillae | No | No | Yes | Yes |
| Lagophthalmos | No | No | Yes (good Bell’s) | Yes (poor Bell’s) |
| Blink | Complete | Complete | Incomplete | Poor blink rate |
| Conjunctiva | ||||
| Inflammation | Minimal | Mild | Moderate | Severe |
| Symblepharon[11] | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
| Keratinization | Absent | Absent | Absent | Present |
| Dryness | Absent | Absent | Absent | Present |
| Cornea | ||||
| Stromal thickness | Normal (400-500µ) | Adequate (300-400µ) | Thin (200-300µ) | Thin/Edema (<200/>600µ) |
| Opacification | Minimal | Mild | Moderate | Severe |
| Integrity | Intact | Intact | Intact | Distorted |
| Other features | ||||
| Anterior segment | Organized | Organized | Disorganized | Disorganized |
| Digital IOP | Normal | Normal | Normal | Hard/Soft |
AMG=amniotic membrane grafting; LK=lamellar keratoplasty; PK=penetrating keratoplasty; LSCT=limbal stem cell transplantation; IOP=Intraocular pressure; Cases with excellent prognosis will usually regain good vision and cosmesis with SLET alone and can be performed by any ophthalmologist; Cases with good prognosis will regain good cosmesis but may not regain optimal vision with SLET alone and are recommended to be handled by cornea specialists; Cases with fair to moderate prognosis will require additional procedures for optimal cosmetic and visual recovery and are recommended to be handled by cornea specialists with ocular surface experience; cases with poor prognosis are those where SLET is best avoided
Figure 1.
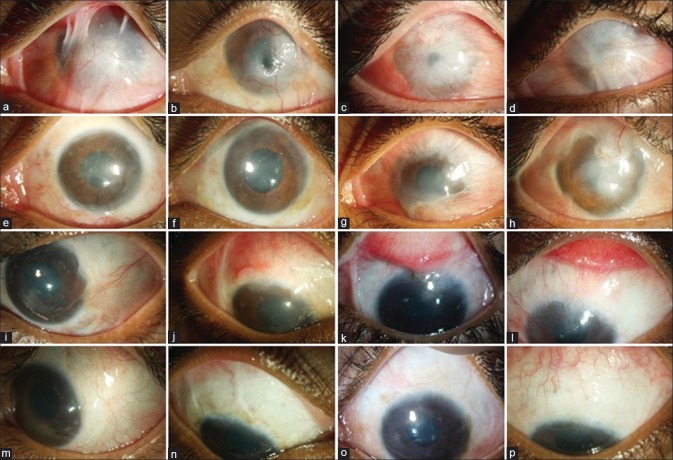
Case selection for simple limbal epithelial transplantation (SLET). Ideal cases for SLET are those of unilateral total limbal stem cell deficiency (LSCD) with wet ocular surface, without eyelid pathologies, with minimal symblepharon, and with relatively clear underlying corneal stroma (a to d). Cases satisfying all the above criteria but with advanced symblephara will need both SLET and conjunctival autografting (CAG) from the healthy eye (e to h). Cases of pterygium, partial LSCD, or pseudopterygium are best treated with ipsilateral or contralateral CAG without SLET (i to l). Cases of total LSCD associated with dry ocular surface, keratinization, entropion, adherent leukoma, and anterior staphyloma are not amenable to SLET or CAG and will need more complex procedures like keratoprosthesis (m to p)
Figure 2.
Utility of anterior-segment optical coherence tomography (AS-OCT) before simple limbal epithelial transplantation (SLET). The top row shows four different cases of total limbal stem cell deficiency (LSCD), where clinically the fibrovascular pannus is too thick to estimate underlying corneal stromal clarity or thickness (a to d). The middle row shows the infrared photographs of the same eyes captured by the AS-OCT, revealing increasing grades of underlying stromal opacification (from left to right) obscuring the discernibility of the pupil (e to h). The bottom row shows the linear scans of the AS-OCT imaging, revealing the huge variation in the underlying corneal stromal thickness (i to l). The vertical white bar in the bottom row indicates 250 microns of corneal thickness. The second case, summarized in the images of the second column (b, f, and j) is ideal for SLET. The first case (a, e, and i) would require very careful dissection and there is a serious risk of intraoperative corneal perforation; it may be preferable to do an anterior lamellar keratoplasty with SLET. The third (c, g, and k) and fourth cases (d, h, and i) show significant underlying corneal damage and SLET alone is not recommended as it will not improve corneal clarity (vision) or appearance of the eye (cosmesis)
Preoperative Counseling
Like all ocular surface procedures, patients and their attendants and/or guardians need to be counseled patiently. One common concern the parents particularly have is about the effect of the biopsy on the healthy eye in unilateral cases. Surgeons must not interpret this query as an aspersion being cast on their intent or doubts being raised about their skilfulness or competence. Since the patient is dependent on the only seeing eye, this concern is justified and must be addressed with gentle reassurance. Patients should also be explained about the possible need for additional surgeries and examinations under general anesthesia for children. Finally, the goals of surgery must be clearly explained and the patients must realize that (i) the appearance of the eye will improve drastically but will never become equal to the normal healthy eye, so the benchmark of evaluating cosmetic success should be the preoperative appearance of the affected eye and not a normal eye; (ii) in long-standing cases, certain attributes like the degree of ptosis, squint, or poor vision due to amblyopia may not improve after surgery. For medico-legal purposes, surgical advice and informed consent for autologous SLET should always be taken for both eyes, clearly indicating that two procedures, limbal graft harvesting and SLET, will be done in the normal and the affected eye, respectively. It is best to avoid the term “biopsy” in the consent or surgical advice because Indian medical insurance companies will often not reimburse for a “biopsy” until a pathology report is furnished for the same.
Surgical Technique
Anesthesia: For children, general anesthesia is mandatory. For adults, limbal biopsy can be harvested from the donor eye under topical anesthesia, but beginners may prefer peribulbar or subtenons anesthesia. The affected eye in adults requires a peribulbar block.
Preoperative vasoconstriction: It is recommended to use two to three applications of brimonidine tartrate 0.15% and phenylephrine 5% eye drops alternatively for 5–10 mins before shifting the patient to the operating room. This practice significantly reduces intraoperative bleeding in both the donor and recipient eyes.
Donor eye: In live-donors (autologous or allogeneic), the donor limbus is not marked directly with a skin-marking pen, as the alcohol in the ink can damage the delicate limbal stem cells (Video). One-clock hour or roughly 3.5 to 4 mm is measured with a caliper and marking is done slightly behind the limbus on the conjunctiva. A conjunctival bleb is created with fluid just behind the selected area of the biopsy and a limbus-based conjunctival flap is lifted until the insertion of the Tenon's capsule at the limbus. This area is lined by blood vessels and marks the posterior boundary of the limbus. Dissection with a no. 15 blade held as flat as possible is then carried forward in the same plane until the grey clear cornea is visible. The flap is reposed, and the conjunctival part is excised off. The limbal tissue is excised separately using a pair of Vannas or Wescott scissors and preserved in balanced salt solution (BSS). This tissue should not be left to dry.
In cadaveric donors, a conjunctival flap is not possible, because there is hardly any remnant conjunctiva in corneo-scleral rims. The authors’ preferred technique is a snip biopsy in which the limbal tissue is pinched using a Lim's forceps and one-clock hour is cut out using a Vannas or Wescott's scissors (Video). This tissue is usually thicker and has more stromal component than a live-biopsy and may require further stromal trimming before transplantation. It is important not to take a lengthier biopsy because the risk of immunological rejection increases with the increase in the amount of transplanted allogeneic tissue.
Recipient eye: Any symblepharon preventing the insertion of the speculum needs to be excised first. A peritomy is performed 360 degrees around the cornea about 2–3 mm beyond the estimated limbus. Dissection is then carried forward using a pair of Vannas scissors to release the conjunctivalized pannus covering the cornea from the limbus 360 degrees before proceeding centripetally. The pannus is then removed from the corneal surface using a combination of sharp and blunt dissection. Any attempt to manually debulk the corneal stroma to reach a clearer plane is strongly discouraged. A recession of the surrounding conjunctiva is performed by blunt dissection using tenotomy scissors. The human amniotic membrane (hAM; basement membrane side up) is then placed and secured over the recipient cornea with the help of fibrin sealant. It is critical to ensure that the hAM is tucked under the conjunctival edge in all quadrants. The hAM is also smoothened out over the cornea using a blunt spatula to ensure that there are no folds. The limbal tissue is then removed from the BSS and approximately cut into 6–10 pieces with the help of Vannas scissors. These pieces are placed (epithelial side up) in the mid-periphery of the cornea in a concentric pattern over the hAM [Fig. 3]. The correct orientation of the small pieces can be identified from the pigmentation and/or smooth surface of the epithelial side and the white fibrous strands on the stromal side. Care is taken to ensure that the pieces of limbal tissue are not placed over the pupillary area or on the limbus. A drop of fibrin sealant is placed over each piece to ensure that they adhere to the hAM. After waiting for at least one minute for the fibrin glue to polymerize over the limbal pieces, a soft therapeutic bandage contact lens (BCL) is placed on the eye. Care is taken to cut away the excess glue that may be sticking to the speculum as it is being removed and not to pull at the strands which may dislodge the film of glue on the surface that is holding the transplants in place. In very young children, a suture tarsorrhaphy is recommended for the first couple of weeks to prevent early loss of the BCL or transplants, because of the risk that children may inadvertently rub their own eyes.
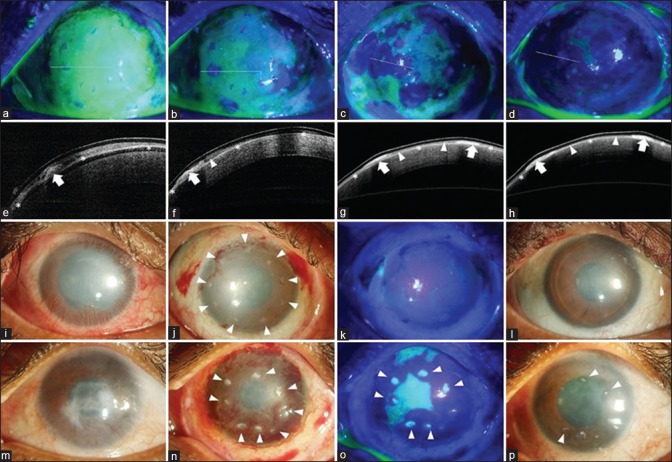
Figure 3.
Mechanism of corneal healing after simple limbal epithelial transplantation (SLET). The top row shows cobalt blue–illuminated fluorescein-stained images of the ocular surface immediately after SLET (a to d) with corresponding anterior segment optical coherence tomography (AS-OCT) images in the second row (e to h). On postoperative day (POD) 1, the cornea is covered with fibrin glue with the epithelium-up limbal transplant pieces visible as tiny islands of negative staining (a); the white line denotes the location of the AS-OCT section which in the corresponding image below shows the hyperreflective limbal piece (bold white arrow, e) while the white asterisks denote the high-reflective human amniotic membrane graft. On POD 5, areas of negative staining denoting epithelial outgrowth are seen around several of the individual transplants (b); which corresponds to the hyporeflective mass (white arrowhead) extending from the edge of the transplant (bold white arrow, f). Subsequent images on POD 7 and 10 show coalescing of the neighboring epithelial sheets to form a stratified epithelial sheet (c and g; d and h). The third row shows the typical postoperative course in a case of total limbal stem cell deficiency (LSCD, i) when the transplants are correctly oriented epithelial side up (white arrowheads, j); complete epithelization is usually seen by POD 14 (k) and the transplants are barely visible at 3 months with significant reduction in surface inflammation and improvement in corneal clarity (l) as compared to baseline (i). In a similar case of total LSCD (m), where the transplants were inadvertently placed with the epithelial side down (white arrowheads, n), epithelial healing is delayed at POD 14 and each individual transplant stains positively with fluorescein dye (o) and the stromal side of the transplants are still visible at 3 months as white opacities (white arrowheads, p)
Post-Operative Regimen
Autologous transplants: Postoperatively topical prednisolone acetate 1% eye drops are administered six times a day for 1 week and then tapered every week over the next 6 weeks in both the recipient and the donor eyes while topical moxifloxacin 0.5% eye drops are administered four times a day in both eyes and continued till the epithelial defect heals. The BCL is removed at the 1-week postoperative visit. The cornea is stained with fluorescein, and if complete epithelial healing has not occurred, then a BCL is replaced. It is also imperative to check the epithelial healing at the donor site simultaneously. The condition of the recipient eye is monitored until the epithelial defect completely heals. After the first 6 weeks, the donor eye is off medications completely and the recipient eye is kept on lubricants if needed. If there is superficial corneal haze, topical cyclosporine 0.05% eye drops are prescribed for several months. Long-term topical corticosteroid use is unnecessary and should be avoided in autologous transplants. If a temporary suture tarsorrhaphy is in place in the recipient eye, oral steroids (to reduce peri-ocular tissue edema) and topical antibiotic-steroid ointments (instead of eye drops – preferably chloramphenicol-dexamethasone-polymyxin-B combination) are prescribed, until the tarsorrhaphy is released, typically during the 1- to 2-week visit.
Allogeneic transplants: Since the transplanted allogeneic limbal tissue is placed on the mid-peripheral avascular corneal stroma, the risk of immunological rejection is theoretically less than that of solid-organ transplants or even conjunctival/kerato-limbal allografting. Even when used in inflamed eyes with acute burns, allogeneic SLET can sustain the corneal surface for several weeks to months.[23] However, eventually without some immunosuppression the transplanted tissue does reject, and the LSCD can recur if not immediately treated.[21] The authors recommend using a pulse intravenous (IV) immunosuppression regimen. The advantage of pulse IV therapy is that it is administered under direct supervision, without being dependent on the patient's compliance. The patients are given 500 mg of intravenous methylprednisolone (IVMP), on the day of transplantation, and postoperatively at one week, 6 weeks, every 6 weeks thereafter until 6 months, every 2 months thereafter until 1-year; every 3 months thereafter until 2 years, and every 6 months thereafter. Alternatively, oral regimens as described for allogeneic CLET can also be used.[33] Oral cyclosporine is started in a dosage of 5 to 7 mg/kg, 48-hours before surgery, along with methylprednisolone, 1g intravenously, for the first 3 consecutive postoperative days. During the postoperative period, cyclosporine is tapered to the maintenance dosage of 1.5 to 2 mg/kg over 4 to 8 weeks, with diltiazem hydrochloride, 90 mg, added as an adjunct to cyclosporine to reduce the cost and increase the serum levels of cyclosporine. patients are also given oral prednisolone, 1 mg/kg, which is tapered on a weekly basis to the maintenance dosage of 5 mg/day. Irrespective of the regimen, hematological investigations and hepatic and renal parameters need to be reassessed every 4–6 weeks.
Topical steroids are never tapered off completely and continued at a maintenance dose of 1 or 2 times/day along with lubricants. Patients can be prescribed scleral contact lenses for optimal visual recovery after 6–8 weeks of the procedure. Episodes of allograft rejection usually present with a sudden drop in vision associated with epithelial haze, positive fluorescein staining, and engorged superficial blood vessels encroaching toward the transplants. Each rejection episode is treated aggressively by administering a top-up dose of IVMP and stepping up topical steroids.
Mechanism of Action
Mittal et al. serially imaged eyes that had undergone SLET using fluorescein staining to elegantly demonstrate the multidirectional growth of epithelial cells from each transplant until the individual epithelial islands merged to form a confluent continental sheet of epithelium on the corneal surface.[27] In all eyes, complete ocular surface epithelialization occurred within the first 14 days of surgery. They also observed that each transplant did not assume activity simultaneously or at the same rate and inactive transplants were overrun by the epithelial sheet expanding from adjoining transplants [Fig. 3]. This study clearly demonstrated that hAM acts as a substrate for secure attachment of the epithelial cells and supports their proliferation and migration in eyes in which SLET has been performed. Amescua et al. used AS-OCT to also show the persistence of hAM under the proliferating epithelial cells.[14] In another series, excised corneal buttons from eyes undergoing PK after SLET were subjected to both histopathology and immunohistochemistry and cytokeratin (CK) expression, to confirm that not only was the epithelium of corneal phenotype (CK3+, CK12+, CK19-, MUC5AC-) but also that there was focal retention of stem cells (ABCG2+, ΔP63α+) in the basal epithelial layer of the newly regenerated epithelium.[11] This study confirmed that SLET is a true regenerative procedure that rejuvenates the corneal surface with limbal stem cell–derived corneal epithelia and has the potential to sustain it in the future.
Clinical Efficacy
Autologous SLET: The details of published studies reporting the outcomes of autologous SLET are summarized in Table 2. All were noncomparative case series except for one study that compared CLAU and SLET in 10 eyes each and found both surgical techniques to be equally effective in achieving a stable epithelized ocular surface and for regression of corneal vascularization.[24] The four major studies included a total of 253 cases of unilateral LSCD.[11,12,13,20] The indication in 96% (243/253) of the eyes was unilateral LSCD secondary to ocular burns. At a mean follow-up period of 1.48 years, a successful outcome (defined as a stable, epithelized and avascular corneal surface) was achieved in 78% (197/253) of eyes. A two-line improvement in best corrected visual acuity (BCVA) (mentioned in 223 eyes across three studies) was seen in 69% (153/223) of eyes at a mean follow-up period of 1.2 years.[11,12,13]
Table 2.
Summary of published studies on outcomes of simple limbal epithelial transplantation (SLET) for the treatment of various ocular surface pathologies
| Most common indication (number of eyes) | Author | Year | Country | Study design | Total number of eyes | Amount of limbal tissue harvested | Success rate in percentage (Number of eyes) | Percentage of eyes with 2-line improvement in BCVA (Number of eyes) | Percentage of eyes which underwent simultaneous/ subsequent LK/PK | Mean follow-up in years (range) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chemical burn (5) | Sangwan et al.[10] | 2012 | India | R | 6 | 2×2 mm/1 clock h | 100 (6/6) | 100 (6/6) | None | 0.8 (0.6-1) |
| Chemical burn (2) | Amescua et al.[14] | 2014 | U.S.A | R | 4 | 2×2 mm/1 clock h | 100 (4/4) | NA | None | 0.6 (0.5-0.75) |
| Simultaneously after primary pterygium excision (9) | Hernadez- Bogantes et al.[17] | 2015 | Mexico | R | 9 | 2×2 mm from ipsilateral eye | 100 (9/9) | NA | NA | 0.67 |
| Chemical burn (125) | Basu et al.[11] | 2016 | India | P | 125 | 2×2 mm/1 clock h | 76 (95/125) | 75 (94/125) | 8 (10/125) | 1.5 (1-4) |
| Chemical burn (62) | Vazirani et al.[12] | 2016 | Multi-centre | R | 68 | 1-2 clock hours | 84 (57/68) | 65 (44/68) | 7 (5/68) | 1 (0.5-4.9) |
| Chemical burn (4) | Quieroz et al.[29] | 2016 | Brazil | R | 4 | 4×2 mm | 50 (2/4) | 24 (1/4) | None | 0.5 |
| Chemical burn (26) | Gupta et al.[13] | 2018 | India | P | 30 | 1-2 clock h | 70 (21/30) | 50 (15/30) | 10 (3/30) | 1.1 (0.5-3.4) |
| Simultaneously after OSSN excision (8) | Kaliki et al.[15] | 2017 | India | R | 8 | 1 clock hour from ipsilateral or contralateral eye | 100 (8/8) | NA | NA | 1 |
| Simultaneously after recurrent pterygium excision (4) | Mednick et al.[18] | 2018 | Canada | R | 4 | 4×2mm | 100 (4/4) | NA | NA | 1.2 (0.7-2.5) |
| Chemical burn-eyes with previous failed CLET (30) | Basu et al.[20] | 2018 | India | R | 30 | 1 clock h | 80 (24/30) | NA | 13 (4/30) | 2.3 (0.8-3.8) |
OSSN=ocular surface squamous neoplasia; R=retrospective; P=prospective; SLET=simple limbal epithelial transplantation; BCVA=best-corrected visual acuity; LK=lamellar keratoplasty; PK=penetrating keratoplasty; NA=not applicable; CLET=Cultivated limbal epithelial transplantation
The outcomes of sequential secondary surgeries such as PK and deep anterior lamellar keratoplasty (DALK) after SLET were also reported.[25,26] In seven eyes of seven patients who underwent PK 9.5 ± 11.9 months after SLET, six eyes maintained a clear graft at 15.1 ± 5.4 months after PK.[25] Another report of 11 eyes of children who underwent DALK following SLET for unilateral severe chemical injury with LSCD with follow-up of 13 ± 4.6 months following DALK reported anatomical success in 8 (72%) eyes and visual acuity improvement in six (54%) eyes.[26]
Allogeneic SLET: Since there is little published information available on the outcomes of allogeneic SLET in chronic bilateral LSCD, the authors report their experience in 30 eyes of 29 patients undergoing either live-related or cadaveric allogeneic SLET with an identical postoperative immunosuppression regimen, as mentioned above. Of the 30 eyes, 16 eyes of 16 patients underwent live-related (but without HLA or ABO matching), while 14 eyes of 13 patients underwent cadaveric allogeneic SLET. The two groups were comparable at baseline in terms of demographics and clinical features [Supplementary Table 1]. The median follow-up was 28 months (range 13–66 months) with no significant difference between the live-related and cadaveric groups (P = 0.23). One year after operation, statistically significant improvement was seen in the median grades of corneal conjunctivalization (2 to 0, P=<0.0001), vascularization (2 to 0, P = 0.0001), and opacification (2 to1, P = 0.002) in all treated eyes. Although clinically the median grade of symblepharon improved from 1 to 0, this change was not found to be statistically significant (P = 0.34). The median BCVA improved from hand-motions to 20/80 (P < 0.0001) and more than 60% of eyes had a visual recovery of 20/60 or better irrespective of the source of donor tissue. Overall allogeneic SLET was successful in 25/30 (83.3%) eyes at final follow-up [Fig. 4]. Successful outcomes were maintained in 14/16 (87.5%) eyes in the live-related group and 11/14 (78.6%) eyes in the cadaveric group at one-year postoperatively and at final follow-up (P = 0.51). Kaplan-Meier survival analysis showed a 5-year cumulative survival probability of 90+/−4% in the live-related and 82+/− 7% in the cadaveric donor group (P = 0.12).
Supplementary Table 1.
Baseline characteristics of cases undergoing allogeneic simple limbal epithelial transplantation (SLET) for bilateral limbal stem cell deficiency (LSCD)
| Characteristics | Live-related | Cadaveric | P |
|---|---|---|---|
| Gender | |||
| Male | 10 | 8 | 0.47 |
| Female | 6 | 5 | |
| Laterality | |||
| Right eye | 7 | 7 | 0.14 |
| Left eye | 9 | 7 | |
| Etiology of Limbal Stem Cell Deficiency | |||
| SJS | 9 | 7 | 0.85 |
| MMP | 3 | 2 | |
| Chemical Burns | 2 | 2 | |
| Allergic Conjunctivitis | 2 | 2 | |
| OSSN excision | 0 | 1 | |
| Preoperative BCVA | |||
| 20/20-20/80 | 0 | 0 | 0.87 |
| >20/80-<20/400 | 0 | 0 | |
| >20.400 or worse | 16 | 14 | |
| Prior Surgical Procedures | |||
| Lid-MMG | 9 | 8 | 0.29 |
| Bulbar MMG | 3 | 2 | |
| AMG | 5 | 4 | |
| DALK/PK | 0 | 0 | |
| Preoperative Grade of Symblepharon | |||
| Grade 0 | 3 | 3 | 0.35 |
| Grade 1 | 8 | 7 | |
| Grade 2 | 5 | 4 | |
| Grade 3 | 0 | 0 | |
| Preoperative Grade of Vascularization | |||
| Grade 0 | 0 | 0 | 0.67 |
| Grade 1 | 0 | 0 | |
| Grade 2 | 7 | 7 | |
| Grade 3 | 9 | 7 | |
| Preoperative Grade of Corneal Opacity | |||
| Grade 0 | 0 | 0 | 0.33 |
| Grade 1 | 3 | 2 | |
| Grade 2 | 10 | 7 | |
| Grade 3 | 3 | 5 | |
| Preoperative Grade of Conjunctivalization | |||
| Grade 0 | 0 | 0 | 0.47 |
| Grade 1 | 0 | 0 | |
| Grade 2 | 6 | 5 | |
| Grade 3 | 10 | 9 |
SJS=Stevens-Johnson syndrome; MMP=mucous membrane pemphigoid; OSSN=ocular surface squamous neoplasia; BCVA=best corrected visual acuity; MMG=mucous membrane grafting; AMG=amniotic membrane grafting; DALK=deep anterior lamellar keratoplasty; PK=penetrating keratoplasty
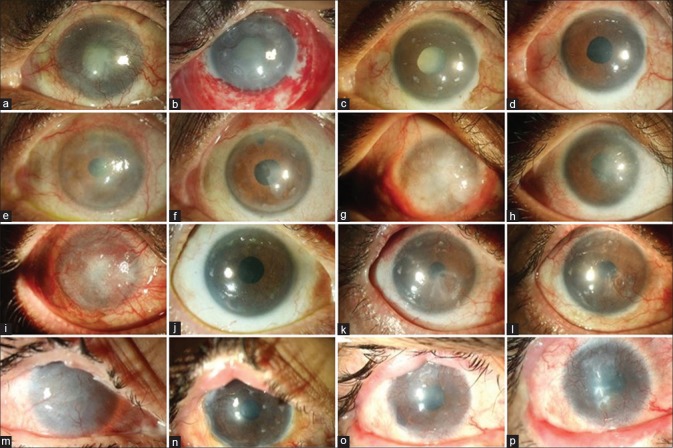
Figure 4.
Clinical outcomes of allogenic simple limbal epithelial transplantation (SLET). The top row shows the 1-year progressive outcomes in a case of mucous membrane pemphigoid (MMP) with advanced senile cataract (a to d). Preoperative image showing total limbal stem cell deficiency (LSCD) with mature senile cataract (a); postoperative day (POD) 1 image showing intact transplants on the cornea with multiple hemorrhages under the human amniotic membrane graft (b); POD 90 image showing a epithelized avascular cornea, at this visit the patient was planned for cataract surgery (c); 12 and 9 months after allogeneic SLET and cataract surgery, respectively, the aided visual acuity is 20/20 for distance and n6 for near (d). Pre- (e) and postoperative (f) 1-year images of a one-eyed patient with OSSN excision–induced LSCD. Pre- (g) and postoperative (h) 1.5-year images of a case of bilateral LSCD due to severe chronic ocular allergy. The third row from top summarizes the 2-year timeline of another case of MMP with total LSCD (i) where a successful outcome was maintained until 1.5 years, following which there was an episode of immunological rejection (k) which was reversed but the patient developed partial LSCD (l). The bottom row shows the 4-year timeline of a case of bilateral LSCD (m) due to Stevens–Johnson syndrome (SJS) who first underwent lid-margin mucous membrane grafting followed by allogeneic SLET and maintained a stable surface (n) for 2.5 years following which he gradually developed recurrence of LSCD (o-p)
There were no intraoperative complications noted during allogeneic SLET in the donor or recipient eyes. The most common postoperative complication in recipient eyes was the recurrence of LSCD which occurred in five (16.7%) eyes [Fig. 4]. The indication in four of the five eyes with failure was SJS while the remaining one was a case of MMP. In three of the five eyes with failure, Boston type 1 keratoprosthesis was implanted for visual rehabilitation. Hemorrhage beneath the amniotic membrane was seen in nine (30%) eyes, which resolved spontaneously. Progression or development of visually significant cataract was noted in seven (23.3%) eyes, all of which underwent uneventful phacoemulsification with implantation of posterior chamber intraocular lens. Allograft rejection was noted in two eyes which were treated with stepping-up of topical steroids and pulse dose of IVMP. The only postoperative complication noted in four (25%) eyes of live-related donors was subconjunctival hemorrhage which resolved spontaneously. There was no LSCD or ocular surface disease noted in any of the eyes of live-related donors. No serious systemic complications were noted in the follow-up period. The blood sugar, HbA1c, blood pressure, liver function tests, and blood counts remained within normal levels for all patients.
Complications
No studies have reported serious adverse outcomes of the donor eye. Localized, nonprogressive focal LSCD of donor site after SLET was reported in two cases which did not affect visual acuity.[12,28] Pyogenic granuloma was also reported in two donor eyes at the site of limbal tissue excision.[11] The most common complication of the recipient eye after SLET was focal recurrence of LSCD which has been reported in 18% to 31% eyes.[11,12,13] Most clinical failures after SLET occurred in the first six months after surgery.[12,28] Early complications included hemorrhage under the hAM which usually resolved without any consequence.[11] Early loss of the SLET transplants and detachment of the hAM can also occur rarely and usually lead to failure.[11,29] Pre-existing symblepharon, if not addressed at the time of surgery with a conjunctival autograft, was noted to be a risk factor for recurrent conjunctivalization and failure of the primary procedure.[11,12,13] PK done simultaneously with SLET was also a risk factor for early failure.[11,12,13] Indications such as acid injury was noted to have a higher rate of failure than SLET performed for other forms of chemical burns such as alkali injury.[11,12,13] Other rare complications that have been reported are sterile keratitis, microbial keratitis, persistent epithelial defect which could lead to thinning and perforation if not addressed in time, and recurrence of corneal neovascularization. Strategies to prevent and manage the commonly encountered immediate postoperative complications are summarized in Table 3, while the prevention and management of symblepharon-associated progressive conjunctivalization/recurrence of LSCD after SLET is discussed in [Fig. 5].
Table 3.
Management of immediate postoperative complications known to be associated with simple limbal epithelial transplantation
| Possible Causes | Mechanism | How to Prevent These from Happening |
|---|---|---|
| Post-operative Loss of Limbal Transplants with Intact hAM | ||
| Excess Glue | Fibrin glue holds transplants in place for the first 48-72 hours after which the epithelial cells growing out of each piece anchors the pieces to the hAM. Excess glue forms a mound that can dislodge abruptly taking the piece with it before the epithelial cells have even started to grow out. |
Use optimal but not excess glue. TISSEEL® from Baxter is highly recommended above other products. Use separate applicators (1mL Insulin syringe) for each component. DUPLOJECT injectors are best avoided. |
| Reverse Orientation of Limbal Transplants | If the transplants are placed epithelium down, due to the reversed polarity of epithelial cells, they take more time to grow out. Hence, the transplants don’t get properly anchored to the hAM by the time the glue disintegrates and can fall off. | Attach a 26g needle to the syringe containing the fibrin sealant and a 29/30g needle to the one with the thrombin solution, to have better control. Less than one drop of each component is enough for each transplant piece. |
| Excess Glue with Reverse Orientation of Limbal Transplants | Excess glue either dislodges abruptly or prevents cells from growing on the hAM. While cells from transplants placed upside down grow very slowly. Instead of cancelling each other out, these factors have an additive effect. | Thrombin solution tends to spurt, so squirt a little outside the surgical field to avoid excess application. Place transplants epithelium up, stroma down. Examine transplants under high magnification: epithelial side is shiny and may be pigmented, while stromal side is fibrous, uneven and whiter. Wait 1 minute before applying the BCL to ensure that the glue has gelled in place. Do not use a skin-marking pen to demarcate the epithelial surface (alcohol in ink damages the epithelial cells). |
| Bulky Transplants | Deeper dissection makes the limbal biopsy too thick. Chopped pieces tend to lie on one side rather than flat on the hAM and have higher risk of getting dislodged. | Keep the dissection superficial, just deep enough to avoid button-holing. Don’t dissect deeper into the stroma or anteriorly into clear cornea. |
| Early Loss of BCL | BCL protects the transplants from the impact of the blinking lid during this critical early period. The lid-wiper action can dislodge transplants particularly those which are bulky or are covered with excess glue. |
Choose the correct size of BCL: the BCL should fit neatly within the edges of the recessed conjunctiva. 14mm is ideal, larger BCLs tend to fold over the conjunctival edge. Additional tarsorrhaphy may help in very young children to avoid inadvertent displacement. |
| Post-operative Loss of Both Limbal Transplants and hAM | ||
| Freeze-dried or Lyophilized hAM | These types of hAMs do not stick well with fibrin glue. The entire membrane may come off in a few days. | It is recommended to always use fresh-frozen hAM. If fresh-frozen hAM is not available, suture the hAM to the peripheral cornea using long 10-0 nylon circumferential (parallel to limbus) sutures. |
| Free Floating Peripheral Edge of hAM | If the peripheral margin of hAM is not buried/tucked under recessed edge of conjunctiva, the free edge can get rolled up. As tears percolate under the hAM and dissolve the glue, the hAM can peel off and dislodge while the transplants are still stuck on. | Do Tenotomy under the conjunctival rim using blunt dissection to make sure there is enough space to tuck-in the hAM. Tuck and bury the peripheral free edge of the hAM under the recessed cut-margin of the conjunctiva. |
| Reverse orientation of hAM | hAM stuck BM down doesn’t stick well, especially if there are some viable epithelial cells on it. The hAM will float, tears can collect below and dislodge it. | Always use hAM in the BM-up or stromal side-down orientation. Check for the correct orientation by touching the AM with a dry sponge. The stromal side is sticky. |
| Trauma | Inadvertent blunt trauma to the operated eye can dislodge the hAM. | Prescribe eye protection: shields or glasses. In very young children do a temporary suture tarsorrhaphy and open it after 2 weeks. |
| Hematoma under hAM | Haemorrhage is normal and self-limiting, but if a hematoma does form, it may lift off the hAM from its edges. Hematomas also inevitably dislodge the BCL and further compound the problem. | Use vaso-constrictive eyedrops pre-operatively and cauterize bleeding vessels, particularly at the limbus. |
| Wound Leak | If SLET is combined with PK or an intraoperative perforation has occurred, aqueous can collect under the hAM and dislodge it. There is bullous elevation of the hAM with a wide separation from the corneal stroma. | Don’t forget to check for water-tight closure after the PK before doing the SLET. Small perforations during pannus excision may be patched with a Tenons graft, but always check for water-tightness before proceeding with SLET. |
hAM=Human amniotic membrane; BCL=Bandage contact lens; BM=Basement membrane; SLET=Simple limbal epithelial transplantation; PK=Penetrating keratoplasty
Figure 5.
Prevention and management of symblepharon-associated progressive recurrence of limbal stem cell deficiency (LSCD) after simple limbal epithelial transplantation (SLET). The top row shows similar cases of total unilateral limbal stem cell deficiency (LSCD) with severe symblepharon extending from the lids to the cornea (a to d). In the first two instances (a, b), excellent recurrence-free long-term outcomes are seen when SLET is combined with conjunctival autografting (CAG, e,f). However, in cases of the next two cases (c, d), recurrence of LSCD along with the symblepharon are seen when SLET alone is performed (g, h). in such cases, CAG should always be combined with SLET to prevent recurrence of LSCD. The third row shows four cases of early recurrence of symblepharon after 3–6 months of SLET (i to l), which were treated successfully with CAG, shown in corresponding images of the fourth row (m to p). Since the late failure of SLET is almost always due to recurrence of previously unaddressed symblepharon, it is important to look for early recurrence and treat it using CAG and not by repeating SLET
Summary: Limitations and Impact
In unilateral LSCD, the clinical efficacy of autologous SLET has been validated in large studies with extensive follow-up;[11,12,13] the persistence and viability of stem cells on the corneal surface have been demonstrated;[11] and the mechanism of corneal regeneration after SLET is now well understood.[11,14,27] Although no head-to-head randomized controlled trial has so far been published comparing the efficacy of SLET, CLAU, or CLET, the advantages of SLET over the other techniques are quite obvious. In resource-limited settings, which would include most of the developing world, the option of CLET is largely theoretical, and the choice for the corneal surgeon is essentially between CLAU and SLET. CLET also has other limitations, such as the possibility of contamination of the cells during transport, and safety issues secondary to the use of xenogenic material.[34] Also, in a resource-limited setting, doing staged surgeries with an interval of 2 weeks (first surgery where the limbal biopsy is harvested, second surgery when the sheet with expansion of cells is transplanted) might not be feasible for patients. The outcomes of CLET in pediatric patients with chemical burns have not been favorable as opposed to SLET.[11,35] The potential benefit of CLET is that one can edit a defective gene while the cells are grown in culture so this could be beneficial in indications like epidermolysis bullosa or other single gene defect–induced LSCD.
Between CLAU and SLET, the scales seem easily tilted in favor of SLET since it can achieve with one-clock-hour of limbus what CLAU does with three to six. In bilateral LSCD too, SLET can be quite effective with systemic immunosuppression, as described in this review. The challenge in bilateral LSCD is that it is rare to find cases which have normal eyelids, wet surfaces free of symblepharon, and a relatively clear underlying corneal stroma, except in the instance of chronic ocular allergy. Unfortunately, most cases of bilateral LSCD in our country are secondary to SJS, MMP, and severe chemical burns,[2] which either have extensive cicatrization or have dryness or both. This challenge is exemplified by the fact that despite having performed more than 500 cases of autologous SLET in the last several years, the authors found only 30 cases suitable for allogeneic SLET, while having performed 164 keratoprosthesis procedures for patients with bilateral LSCD.[36,37,38]
It is also important to reiterate that even in unilateral LSCD, SLET alone is not effective in cases with severe symblepharon, and needs both limbal and conjunctival grafting. Therefore, while in cases of total LSCD with extensive symblepharon, SLET needs to be combined with CAG, in cases of partial LSCD with pseudopterygium, CAG alone may be adequate. In bilateral LSCD with symblepharon, there is no scope for CAG and therefore allogeneic SLET should be avoided or combined with an oral mucous membrane graft. An exceptional scenario where CLAU may hold advantage over SLET is in cases of complex reconstruction requiring conjunctival, limbal, and corneal grafting. In these cases, the corneal graft remains at high-risk of immunological rejection and may need to be replaced in the future, therefore placing the limbal graft in its anatomical location beyond the cornea as in CLAU may be advantageous over SLET. This is because the limbal transplants placed on the corneal graft will be lost when the corneal graft is replaced. It is recommended, however, to use the modified technique of mini-CLAU in such instances to protect the donor eye.[39]
One may think of SLET as in vivo CLET, where the cell expansion takes place on the surface of the eye instead of a petri dish in a laboratory, using the natural environment, growth factors, and tears as tissue-culture reagents. The central hypothesis of CLET that one-clock hour of limbal tissue is enough to regenerate the entire corneal surface is reaffirmed by SLET and it should be considered as a corollary or natural extension of the same concept. In fact, the developers of SLET transitioned to it only after a decade of working on CLET.[40] This concept of in vivo cultivation has at least two parallels in dermatology, where epidermis grafts are divided into tiny pieces and spread across a large surface area to epithelialize or restore pigmentation to the bare areas in between.[41,42]
The advent of SLET has made life significantly easier for corneal surgeons, particularly those in the developing world dealing with a huge burden of unilateral LSCD due to chemical burns.[2] The technique has a relatively short learning curve and surgeons otherwise inexperienced in ocular surface surgery are quickly able to replicate the same results as experienced ones.[11] This easy replicability has also allowed SLET to be adapted by other specialties like oculoplastics, where it has been successfully adapted to both treating and preventing LSCD after extensive OSSN excision by surgeons who were otherwise naïve to LSCT.[15,16] The reliability and replicability of SLET is also demonstrated in the consistent outcomes reported across large studies by different surgeons from all over the world.[11,12,13] This review aimed to help cornea specialists and trainees, general ophthalmologists, and ophthalmology residents to understand the indications, surgical technique, mechanism, outcomes, and also limitations of this technique based on the experience of hundreds of procedures performed during the first decade of the SLET-era. Although SLET does not require any specialized instrumentation, beginners are advised to pursue short clinical rotations with experienced SLET surgeons, follow the recommendations of this review, and attend educational instructional courses in regional and national conferences to ensure best results for their patients.
Financial support and sponsorship
Hyderabad Eye Research Foundation.
Conflicts of interest
There are no conflicts of interest.
Video Available on: www.ijo.in
References
- 1.Kinoshita S, Kiorpes TC, Friend J, Thoft RA. Limbal epithelium in ocular surface wound healing. Invest Ophthalmol Vis Sci. 1982;23:73–80. [PubMed] [Google Scholar]
- 2.Vazirani J, Nair D, Shanbhag S, Wurity S, Ranjan A, Sangwan V. Limbal stem cell deficiency-demography and underlying causes. Am J Ophthalmol. 2018;188:99–103. doi: 10.1016/j.ajo.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–22. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 4.Baradaran-Rafii A, Eslani M, Jamali H, Karimian F, Tailor UA, Djalilian AR. Postoperative complications of conjunctival limbal autograft surgery. Cornea. 2012;31:893–9. doi: 10.1097/ICO.0b013e31823f095d. [DOI] [PubMed] [Google Scholar]
- 5.Miri A, Said DG, Dua HS. Donor site complications in autolimbal and living related allolimbal transplantation. Ophthalmology. 2011;118:1265–71. doi: 10.1016/j.ophtha.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Basti S, Mathur U. Unusual intermediate-term outcome in three cases oflimbal autograft transplantation. Ophthalmology. 1999;106:958–63. doi: 10.1016/S0161-6420(99)00516-3. [DOI] [PubMed] [Google Scholar]
- 7.Tan DT, Ficker LA, Buckley RJ. Limbal transplantation. Ophthalmology. 1996;103:29–36. doi: 10.1016/s0161-6420(96)30737-9. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins C, Tuft S, Liu C, Buckley R. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye. 1993;7:629–33. doi: 10.1038/eye.1993.145. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–3. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 10.Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:931–4. doi: 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 11.Basu S, Sureka SP, Shanbhag SS, Kethiri AR, Singh V, Sangwan VS. Simple limbal epithelial transplantation: Long-term clinical outcomes in 125 cases of unilateral chronic ocular surface burns. Ophthalmology. 2016;123:1000–10. doi: 10.1016/j.ophtha.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Vazirani J, Ali MH, Sharma N, Gupta N, Mittal V, Atallah M, et al. Autologous simple limbal epithelial transplantation for unilateral limbal stem cell deficiency: Multicentre results. Br J Ophthalmol. 2016;100:1416–20. doi: 10.1136/bjophthalmol-2015-307348. [DOI] [PubMed] [Google Scholar]
- 13.Gupta N, Joshi J, Farooqui JH, Mathur U. Results of simple limbal epithelial transplantation in unilateral ocular surface burn. Indian JOphthalmol. 2018;66:45–52. doi: 10.4103/ijo.IJO_602_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amescua G, Atallah M, Nikpoor N, Galor A, Perez VL. Modified simple limbal epithelial transplantation using cryopreserved amniotic membrane for unilateral limbal stem cell deficiency. Am J Ophthalmol. 2014;158:469–75.e2. doi: 10.1016/j.ajo.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Kaliki S, Mohammad FA, Tahiliani P, Sangwan VS. Concomitant simple limbal epithelial transplantation after surgical excision of ocular surface squamous neoplasia. Am J Ophthalmol. 2017;174:68–75. doi: 10.1016/j.ajo.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Mittal V, Narang P, Menon V, Mittal R, Honavar S. Primary simple limbal epithelial transplantation along with excisional biopsy in the management of extensive Ocular Surface Squamous Neoplasia. Cornea. 2016;35:1650–2. doi: 10.1097/ICO.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Bogantes E, Amescua G, Navas A, Garfias Y, Ramirez-Miranda A, Lichtinger A, et al. Minor ipsilateral simple limbal epithelial transplantation (mini-SLET) for pterygium treatment. Br J Ophthalmol. 2015;99:1598–600. doi: 10.1136/bjophthalmol-2015-306857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mednick Z, Boutin T, Einan-Lifshitz A, Sorkin N, Slomovic A. Simple limbal epithelial transplantation for recurrent pterygium: A case series. Am JOphthalmol Case Rep. 2018;12:5–8. doi: 10.1016/j.ajoc.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhalekar S, Basu S, Lal I, Sangwan VS. Successful autologous simple limbal epithelial transplantation (SLET) in previously failed paediatric limbal transplantation for ocular surface burns. BMJ Case Rep 2013. 2013 doi: 10.1136/bcr-2013-009888. 10.1136/bcr-2013-009888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basu S, Mohan S, Bhalekar S, Singh V, Sangwan V. Simple limbal epithelial transplantation (SLET) in failed cultivated limbal epithelial transplantation (CLET) for unilateral chronic ocular burns. Br J Ophthalmol. 2018;102:1640–5. doi: 10.1136/bjophthalmol-2017-311506. [DOI] [PubMed] [Google Scholar]
- 21.Bhalekar S, Basu S, Sangwan VS. Successful management of immunological rejection following allogeneic simple limbal epithelial transplantation (SLET) for bilateral ocular burns. BMJ Case Rep 2013. 2013 doi: 10.1136/bcr-2013-009051. doi: 10.1136/bcr-2013-009051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arya SK, Bhatti A, Raj A, Bamotra RK. Simple limbal epithelial transplantation in acid injury and severe dry eye. J Clin Diagn Res. 2016;10:Nd06–7. doi: 10.7860/JCDR/2016/19306.7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyer G, Srinivasan B, Agarwal S, Tarigopula A. Outcome of allo simple limbal epithelial transplantation (alloSLET) in the early stage of ocular chemical injury. Br J Ophthalmol. 2017;101:828–33. doi: 10.1136/bjophthalmol-2016-309045. [DOI] [PubMed] [Google Scholar]
- 24.Arora R, Dokania P, Manudhane A, Goyal JL. Preliminary results from the comparison of simple limbal epithelial transplantation with conjunctival limbal autologous transplantation in severe unilateral chronic ocular burns. Indian J Ophthalmol. 2017;65:35–40. doi: 10.4103/0301-4738.202312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta N, Farooqui JH, Patel N, Mathur U. Early results of penetrating keratoplasty in patients with unilateral chemical injury after simple limbal epithelial transplantation. Cornea. 2018;37:1249–54. doi: 10.1097/ICO.0000000000001681. [DOI] [PubMed] [Google Scholar]
- 26.Singh D, Vanathi M, Gupta C, Gupta N, Tandon R. Outcomes of deep anterior lamellar keratoplasty following autologous simple limbal epithelial transplant in pediatric unilateral severe chemical injury. Indian JOphthalmol. 2017;65:217–22. doi: 10.4103/ijo.IJO_880_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittal V, Jain R, Mittal R. Ocular surface epithelialization pattern after simple limbal epithelial transplantation: An in vivo observational study. Cornea. 2015;34:1227–32. doi: 10.1097/ICO.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 28.Mittal V, Jain R, Mittal R, Vashist U, Narang P. Successful management of severe unilateral chemical burns in children using simple limbal epithelial transplantation (SLET) Br J Ophthalmol. 2016;100:1102–8. doi: 10.1136/bjophthalmol-2015-307179. [DOI] [PubMed] [Google Scholar]
- 29.Queiroz AG, Barbosa MM, Santos MS, Barreiro TP, Gomes JA. Assessment of surgical outcomes of limbal transplantation using simple limbal epithelial transplantation technique in patients with total unilateral limbal deficiency. Arq Bras Oftalmol. 2016;79:116–8. doi: 10.5935/0004-2749.20160034. [DOI] [PubMed] [Google Scholar]
- 30.Notara M, Shortt AJ, O’Callaghan AR, Daniels JT. The impact of age on the physical and cellular properties of the human limbal stem cell niche. Age (Dordr) 2013;35:289–300. doi: 10.1007/s11357-011-9359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vemuganti GK, Kashyap S, Sangwan VS, Singh S. Ex-vivo potential of cadaveric and fresh limbal tissues to regenerate cultured epithelium. Indian J Ophthalmol. 2004;52:113–120. [PubMed] [Google Scholar]
- 32.Kethiri AR, Basu S, Shukla S, Sangwan VS, Singh V. Optimizing the role oflimbal explant size and source in determining the outcomes of limbaltransplantation: An in vitro study. PLoS One. 2017;12:e0185623. doi: 10.1371/journal.pone.0185623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu S, Fernandez MM, Das S, Gaddipati S, Vemuganti GK, Sangwan VS. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:1504–9. doi: 10.1136/bjophthalmol-2012-301869. [DOI] [PubMed] [Google Scholar]
- 34.Schwab IR, Johnson NT, Harkin DG. Inherent risks associated with manufacture of bioengineered ocular surface tissue. Arch Ophthalmol. 2006;124:1734–40. doi: 10.1001/archopht.124.12.1734. [DOI] [PubMed] [Google Scholar]
- 35.Sejpal K, Ali MH, Maddileti S, Basu S, Ramappa M, Kekunnaya R, et al. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol. 2013;131:731–6. doi: 10.1001/jamaophthalmol.2013.2308. [DOI] [PubMed] [Google Scholar]
- 36.Vazirani J, Mariappan I, Ramamurthy S, Fatima S, Basu S, Sangwan VS. Surgical management of bilateral limbal stem cell deficiency. Ocul Surf. 2016;14:350–64. doi: 10.1016/j.jtos.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Basu S, Pillai VS, Sangwan VS. Mucosal complications of modified osteo-odonto keratoprosthesis in chronic Stevens-Johnson syndrome. Am J Ophthalmol. 2013;156:867–73.e2. doi: 10.1016/j.ajo.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Basu S, Serna-Ojeda JC, Senthil S, Pappuru RR, Bagga B, Sangwan VS. The auroKPro versus the Boston type I Keratoprosthesis: 5-year clinical outcomes in 134 cases of bilateral corneal blindness. Am J Ophthalmol. 2018 doi: 10.1016/j.ajo.2019.03.016. doi: 10.1016/j.ajo.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Kheirkhah A, Raju VK, Tseng SC. Minimal conjunctival limbal autograft for total limbal stem cell deficiency. Cornea. 2008;27:730–3. doi: 10.1097/QAI.0b013e31815cea8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sangwan VS, Basu S, Vemuganti GK, Sejpal K, Subramaniam SV, Bandyopadhyay S, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: A 10-year study. Br J Ophthalmol. 2011;95:1525–9. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 41.Lari AR, Gang RK. Expansion technique for skin grafts (Meek technique) in the treatment of severely burned patients. Burns. 2001;27:61–6. doi: 10.1016/s0305-4179(00)00066-8. [DOI] [PubMed] [Google Scholar]
- 42.Gupta S, Sandhu K, Kanwar A, Kumar B. Melanocyte transfer via epidermal grafts for vitiligo of labial mucosa. Dermatol Surg. 2004;30:45–8. doi: 10.1111/j.1524-4725.2004.29380.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.