Abstract
Purpose:
To compare the lipid layer thickness (LLT) using the LipiView® ocular surface interferometer (TearScience® Inc, Morrisville, NC) between the eye treated with glaucoma medication and untreated normal eye in the unilateral glaucoma patients, and evaluate the effect of topical glaucoma medication on the LLT parameters in glaucoma eyes.
Methods:
The participants in this cross-sectional comparative study were unilateral glaucoma patients treated with topical glaucoma medications for more than 12 months. Three LLT parameters (average, minimum, and maximum) obtained by the LipiView® were compared between the glaucomatous eye and normal eye. The factors associated with LLT parameters in the eyes treated with glaucoma medication were investigated with multiple regression analysis.
Results:
Thirty patients with unilateral normal tension glaucoma were enrolled in the present study. Lipid layer average, minimum, and maximum were 64.83 ± 16.50, 51.63 ± 16.73, and 82.53 ± 20.62 in glaucomatous eyes, 77.26 ± 17.81, 62.83 ± 20.99, and 86.13 ± 15.42 in normal eyes. Lipid layer average and minimum were significantly thinner than those in normal eyes (P < 0.001, P < 0.001, respectively). Longer duration of glaucoma eye drops and a greater number of glaucoma medications were associated with the lower LLT average (β = −0.456, P < 0.001, β = −8.517, P = 0.003, respectively), and increasing glaucoma medications have a significant correlation with lower LLT minimum in glaucoma eyes (β = −8.814, P = 0.026).
Conclusion:
The present study highlights that patients with long-term glaucoma medications need to be assessed for LLT parameters objectively evaluate their ocular surface health.
Keywords: Glaucoma, LipiView interferometer, ocular surface disease, tear lipid layer
Because intraocular pressure (IOP) is the only modifiable factor in patients with glaucoma, treatment with IOP-lowering topical medication has been essential to prevent the worsening of glaucomatous damage.[1,2,3] However, previous studies reported that long-term use of glaucoma medication has led to ocular surface disease (OSD) in some patients with glaucoma, and OSD would make a negative effect on the adherence to the glaucoma treatment regimen in these patients, subsequently.[4,5,6,7] Therefore, evaluating and prompt management OSD in patients treated with topical glaucoma medication might be a critical issue for improving the glaucoma treatment compliance.
OSD is characterized by an inadequate quantity of tears, an unstable tear film, and disruption of the integrity of the ocular surface.[2,4,8] Both the instability of the lipid layer of the tear film and meibomian gland dysfunction are reported to contribute to the emergence of OSD.[9,10,11] Recently, LipiView interferometer (TearScience Inc., Morrisville, NC, USA), a new device that can measure tear film lipid layer thickness (LLT) quantitatively using interferometry, has been reported to be a beneficial tool to evaluate the relationships between OSD and meibomian gland dysfunction in other ocular disorders.[9,10,12] Meanwhile, glaucoma medication can lead to several adverse events on the ocular surface, we suggest that LipiView interferometer may provide meaningful information about the relationship between the changes in the lipid layer and topical glaucoma medication.
In the present study, we compared the effect of topical glaucoma medication a on LLT using the LipiView interferometer between the eye treated with glaucoma medication a and the untreated normal eye in the same patient with unilateral glaucoma.
Methods
This cross-sectional study included patients with unilaterally diagnosed glaucoma who had been using topical medications for more than 12 months. The study protocol was approved by the Pusan National University Yangsan Hospital Review Board, and the study was performed in accordance with the tenets set forth in the Declaration of Helsinki. The patients provided informed consent for participating in the study.
All enrolled patients underwent a complete ophthalmologic examination including measurement of best-corrected visual acuity, slit lamp examination, axial length, gonioscopy, and fundoscopy. Automated visual field examination was performed on all subjects using the 30-2 SITA standard program on the Humphrey 740i Visual Field Analyzer (Carl Zeiss Meditec, Dublin, CA, USA). All patients had red-free photographs, optic disc stereo photographs, and Fourier domain optical coherence tomography (OCT) (Cirrus, Carl Zeiss Meditec, Dublin, CA, USA). The diagnosis of glaucoma was according to the presence of glaucomatous optic nerve appearance, corresponding with typical visual field loss. Subjects were excluded, if they have used any topical anti-inflammatory, antibiotic, or other medication. Subjects with a history of using artificial tear drops within 3 months before participating in the study were also excluded. Eyes with previous trauma, ocular surgery or laser treatment, systemic treatments that are known to affect tear secretion, autoimmune disease, current use of contact lenses, and any history or slit-lamp evidence of eye surface disorders were excluded.
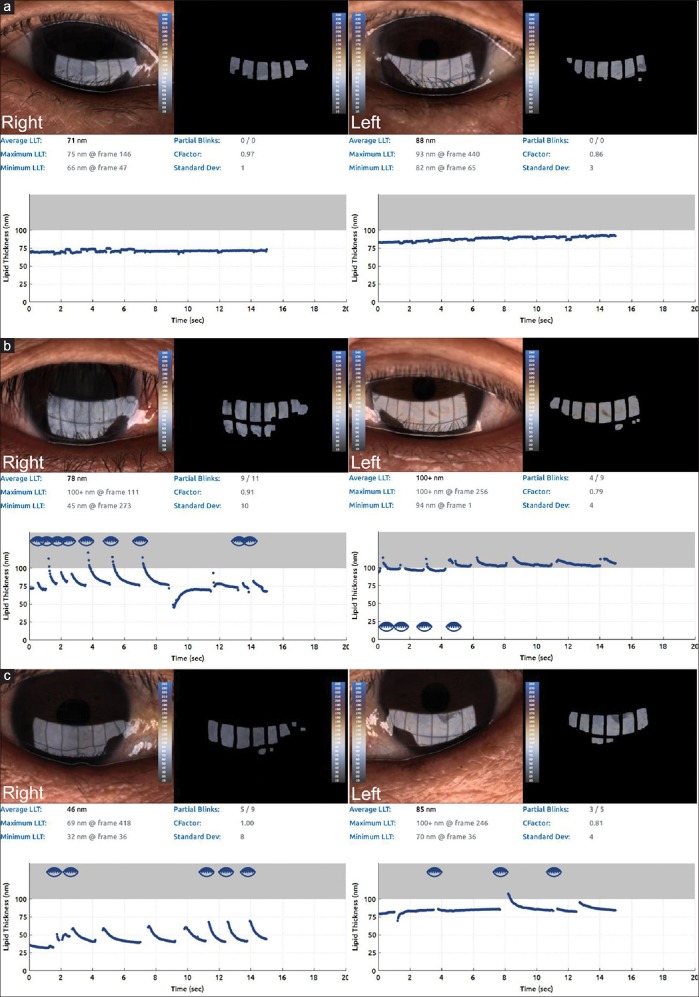
Tear LLT was measured with the LipiView® ocular surface interferometer (TearScience® Inc, Morrisville, NC, USA) analyzing more than one billion data points of the interferometric image of the tear film. Interferometric color units (ICUs) were used to measure tear film LLT with the interferometer, with 1 ICU equal to 1 nm of LLT. The following measurements were recorded for each study subject: the average tear film LLT from all frame averages, and the maximum and minimum LLT. Patients with interferometer results showing a quality of less than 0.8 were excluded from the present study. The LipiView® had an upper cut-off of 100 ICU [Fig. 1].
Figure 1.
(a) A 75-year-old woman using fixed combination brinzolamide 1%/timolol 0.5% two times per day in right eye displays lower average, maximum, and minimum lipid layer thickness (LLT) in the right eye (71 nm, 75 nm, 66 nm) than those in the left untreated eye (88 nm, 93 nm, 82 nm). (b) A 50-year-old man treated with both combination dorzolamide 2%/timolol 0.5% two times and tafluprost 0.0015% once per day in right eye shows lower average and minimum LLT (78 nm, 45 nm) than those in the left untreated eye 100nm, 94nm), and the same readings of maximum LLT in both eyes. (c) A 55-year-old man treated with combination dorzolamide 2%/timolol 0.5%, brimonidine tartrate 0.15% two times, and latanoprost 0.005% once per a day in right eye displays significantly decreased thickness in average, maximum, and minimum LLT (46 nm, 69 nm, 32 nm) compared with all LLT parameters in left eye (85 nm, 100 nm, 70 nm)
Statistical analysis
All statistical analyses were performed with SPSS for Windows 21.0 (SPSS Inc, Chicago, IL, USA). The data were presented as mean standard deviation. The assumption of data normality was checked with the Kolmogorov–Smirnov test. A paired t test was used for comparison of variables between the glaucomatous eye and the untreated normal eye in each patient. A multiple linear regression analysis with stepwise variable selection was used to identify the ophthalmic factors associated with LLT measurements. Each variable was first analyzed using a univariate model; all significant variables (P < 0.10) were then evaluated using a multivariate model. The relationship between LLT parameters and associated factors was additionally examined using scatter plots and linear regression. The coefficient of determination (R2) in the linear regression was reported and P values < 0.05 were considered statistically significant.
Results
The present study enrolled 30 patients with unilateral normal-tension glaucoma who fulfilled the eligibility criteria. The mean age of the subjects was 61.33 ± 11.75 years (range: 34–79 years; 13 men and 17 women). Demographic and clinical data of the subjects are summarized in Table 1. There was no difference in refractive error and IOP between eyes with glaucomatous and untreated normal eyes. Treated eyes showed significantly lower mean deviation (MD), visual field index, and average retinal nerve fiber layer on OCT, and higher pattern standard deviation than normal eyes. In the LipiView interferometer, LLT average, minimum, and maximum were 64.83 ± 16.50, 51.63 ± 16.73, and 82.53 ± 20.62, respectively, in glaucomatous eyes; and 77.26 ± 17.81, 62.83 ± 20.99, and 86.13 ± 15.42, respectively, in normal eyes, and then the average and minimum LLT were significantly lower in glaucomatous eyes than in normal eyes (P < 0.001 and P < 0.001, respectively). As the grading system of meibomian gland dysfunction by Arita et al., in glaucomatous eyes, the prevalence of the grade from 0 to 3 have shown as 3.3%, 23.3%, 26.7%, 46.7%, and in normal eyes, 20%, 40%, 40%, 0%, respectively.
Table 1.
Demographic and ophthalmic data for the unilateral glaucoma patients
| Glaucoma eye | Normal eye | P* | |
|---|---|---|---|
| Age ( mean±SD, year) | 61.33±11.75 | ||
| Male/female | 13/17 | ||
| Refractive error (mean±SD, Diopter) | -1.44±3.15 | -1.22±2.82 | 0.987 |
| Intraocular pressure (mean±SD, mmHg) | 14.43±2.59 | 14.53±3.10 | 0.851 |
| Automated perimetry | |||
| MD ( mean±SD, dB) | -6.93±8.51 | -0.77±1.70 | 0.033 |
| PSD ( mean±SD, dB) | 5.83±4.57 | 2.51±1.64 | 0.025 |
| VFI ( mean±SD, %) | 84.11±19.36 | 98.18±2.44 | 0.008 |
| RNFL average thickness (mean±SD, µm) | 75.36±13.09 | 86.96±9.47 | 0.005 |
| Glaucoma medications | |||
| No. medications (mean±SD, bottles) | 1.40±0.77 | - | |
| Total daily drops (mean±SD, drops) | 2.43±1.16 | - | |
| Current medication duration (mean±SD, months) | 35.67±21.05 | - | |
| LipiView® Interferometer | |||
| Lipid layer average (mean±SD, nm) | 64.83±16.50 | 77.26±17.81 | <0.001 |
| Lipid layer minimum (mean±SD, nm) | 51.63±16.73 | 62.83±20.99 | <0.001 |
| Lipid layer maximum (mean±SD, nm) | 82.53±20.62 | 86.13±15.42 | 0.261 |
* Paired t-test, P values in bold letters indicate statistical significance (P < 0.05)
Correlation coefficients were calculated to evaluate the effects of age, spherical equivalent, IOP, MD in the glaucomatous eyes, total number of glaucoma medications, total number of daily drops, the duration of topical glaucoma medication, and three LLT parameters (average, minimum, and maximum) in normal eyes on the LipiView interferometer measurements with LLT average and minimum in glaucomatous eyes [Table 2]. Both the LLT average and minimum in glaucomatous eyes were negatively correlated with total glaucoma medications, total number of daily drops, and the duration of topical glaucoma medication, and positively correlated with MD in the glaucomatous eyes and three LLT parameters in the normal eyes.
Table 2.
Pearson correlation coefficients between lipid layer thickness (LLT) parameters in the treated eyes and various demographic, ocular factors, and LLT parameters in the untreated eyes
| LLT average in glaucoma eye | LLT minimum in glaucoma eye | |
|---|---|---|
| Age | r=0.276 | r=0.352 |
| P=0.140 | P=0.067 | |
| SE | r=0.379 | r=0.292 |
| P=0.092 | P=0.117 | |
| IOP | r=−0.016 | r=0.090 |
| P=0.941 | P=0.670 | |
| Mean deviation | r=0.585** | r=0.443* |
| P=0.005 | P=0.044 | |
| total glaucoma medications | r=−0.554** | r=−0.406* |
| P=0.002 | P=0.026 | |
| total number of daily drops | r=−0.543** | r=−0.384* |
| P=0.002 | P=0.036 | |
| the duration of topical glaucoma medication | r=−0.689** | r=−0.351* |
| P<0.001 | P=0.045 | |
| LLT average in normal eye | r=0.778** | r=0.585** |
| P<0.001 | P<0.001 | |
| LLT minimum in normal eye | r=0.663** | r=0.680** |
| P<0.001 | P<0.001 | |
| LLT maximum in normal eye | r=0.645** | r=0.511** |
| P<0.001 | P<0.001 |
**P<0.01, *P<0.05, LLT: Lipid layer thickness; SE: Spherical equivalent; IOP: Intraocular pressure
When age, spherical equivalent, MD, total glaucoma medications, total number of eye drops, duration of topical glaucoma medication, and LLT parameters in normal eyes were entered into linear regression analysis as independent variables, the lower average LLT in the normal eye, and the longer duration of topical glaucoma medication were significantly associated with lower LLT average in glaucomatous eyes, and the LLT minimum in normal eyes showed positive association with that in glaucomatous eyes (P < 0.001, adjusted R2 = 0.734; P < 0.001, adjusted R2 = 0.443, respectively). Stepwise multiple regression analyses excluding LLT parameters in normal eyes as independent variables revealed that longer duration of glaucoma eye drops and higher number of glaucoma medications were associated with the lower LLT average, and an increasing number of glaucoma medications have significant correlation with lower LLT minimum in glaucomatous eyes (P < 0.001, adjusted R2 = 0.593; P < 0.001, adjusted R2 = 0.135, respectively). On multiple regression analysis, there were no significant variables associated with LLT maximum in glaucomatous eyes [Table 3].
Table 3.
Results of multiple regression analysis with lipid layer thickness average and minimum in glaucoma eyes as dependent variables
| LLT average in glaucoma eye | LLT minimum in glaucoma eye | |||
|---|---|---|---|---|
| β (95% CI) | P* | β (95% CI) | P* | |
| Model 1 | ||||
| LLT average in normal eye | 0.545 (0.341-0.748) | <0.001 | ||
| The duration of topical glaucoma medication | -0.335 (-0.507--0.163) | <0.001 | ||
| LLT minimum in normal eye | 0.542 (0.316-0.768) | <0.001 | ||
| Model 2 | ||||
| The duration of topical glaucoma medication | -0.456 (-0.654--0.259) | <0.001 | ||
| Total glaucoma medications | -8.517 (-13.921--3.113) | 0.003 | -8.814 (-16.50--1.121) | 0.026 |
*by stepwise method, LLT, lipid layer thickness, Model 1 : including lipid layer thickness (LLT) parameters in normal eyes as the independent factors in the multiple regression analysis, Model 2 : excluding LLT parameters in normal eyes as the independent factors
Discussion
The purpose of the present study was to evaluate the change of the tear LLT in eyes with or without topical glaucoma medication in the same subjects and to investigate the factors that have an effect on decreased LLT in glaucomatous eyes. To measure LLT quantitatively, we used an interferometer that measured three LLT parameters: LLT average, minimum, and maximum. We found that the LLT average and minimum in the eyes treated with glaucoma medications were significantly lower than in untreated normal eyes (P < 0.001). When we analyzed the glaucoma medication-related factors affecting the decreased LLT in glaucomatous eyes, the present study revealed that a longer duration of topical glaucoma medication was associated with both lower average and minimum LLT, and increasing number of medications were also correlated with a lower LLT average.
Although previous studies have shown a relationship between topical glaucoma medication and OSD, they analyzed the impact of topical glaucoma medication on OSD according to subjective assessments including a graded questionnaire.[13] Recently, Lee et al. investigated the effects of topical medications on the OSD and LLT using LipiView interferometer and revealed that LLT average in the glaucoma group was significantly lower than that in normal subjects and negatively correlated with total medication duration.[9] Unlikely the present study, they demonstrated that LLT average has not been associated with the number of eyedrops reflecting short-term changes on the cornea, but a duration of glaucoma medications and insisted that the LipiView interferometer could be helpful to detect chronic damages in the ocular surface. However, because they have not compared the change of other LLT parameters between glaucoma and normal subjects and the parameters of both eyes in the same subjects, individual non-ocular factors might cause the bias on the results. Further, on the basis of the results in the present study that have shown the significant association between LLT minimum and the number of glaucoma medications, lipiView interferometer could provide useful information in long-term as well as short-term changes in ocular surfaces.
In accordance with the previous study,[4,9,13] we found that a longer duration of topical glaucoma medication and more glaucoma medications were associated with lower LLT in glaucomatous eyes. Arita et al. demonstrated that long-term use of topical glaucoma medication was associated with alterations in meibomian gland morphology and function and supported the idea that abnormalities in meibomian glands induced by long-term exposure of topical glaucoma medication contribute to OSD.[13] Ramli et al. showed that the prevalence of OSD varied from 37% to 91% in the glaucoma group regardless of preservative content in the eye drops, and OSD was associated with increasing numbers of topical glaucoma medications.[4] On the basis of their results, the authors suggested that long-term use and greater numbers of topical glaucoma medications resulted in the subclinical conjunctival inflammation related with abnormal changes of the meibomian glands. Accordingly, because the meibomian glands secrete lipids into tear film for increasing tear stability, these previous studies support our findings that lipid layer abnormalities are associated with longer duration of topical glaucoma medication and more glaucoma medications.
The present study showed that the LLT parameters in normal eyes have an association with those in glaucomatous eyes. These results indicated that risk factors other than glaucoma have an important role in the instability of the LLT. In fact, previous studies reported that the stability of the lipid layer was affected by various factors such as age, sex, contact lens wear, ocular surgical history, and environment.[10,14,15,16] Kottaiyan et al. developed a chamber in which controlled environmental circumstances influencing the OSD, such as high temperature, low humidity, and increased airflow could be used to precisely assess their effect on tear parameters.[15] Because there are likely various physical or environmental factors that influence tear evaporation rate and tear production quality, the present study including only unilateral eyes treated with topical glaucoma medication is meaningful in that it can exclude various factors that may influence LLT. To our knowledge, this is the first study to compare LLT between the treated eye and the untreated eye in the same subject and analyze factors that influence changes in LLT.
Lipid layer instability in OSD was associated with meibomian gland dysfunction, which is defined as a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal obstruction and/or qualitative/quantitative changes in the glandular secretion.[17,18,19] Lee et al. reported that malfunction of the meibomian glands could be an important clinical finding associated with compliance in using glaucoma medication and recommended careful inspection of the meibomian gland function in patients treated with glaucoma medication.[20]
In the current study, because longer the duration and the greater number of the glaucoma medication were associated with lower LLT parameters in glaucomatous eyes, there may be a tendency for these patients to have lower adherence to prescribed treatment. Therefore, assessment and continued monitoring of the change of LLT related to OSD may have a positive effect on treatment adherence in patients with glaucoma and ultimately may contribute to prevent the worsening of glaucoma.
The findings of this study are subject to the following limitations. First, the sample size of patients with unilateral glaucoma was relatively small because the prevalence of unilateral glaucoma treated with topical glaucoma medication in the affected eye only is much less than the prevalence of bilateral glaucoma. Second, we could not consider the effect of preservatives in topical glaucoma medications on the LLT. Third, because we did not restrict the types of glaucoma medications used in the present study, there may be bias caused by the use of different types of medications. Finally, the present study did not compare the parameters in the LipiView interferometer with other measurements including tear break-up time, ocular surface disease index, or tear osmolarity for OSDI. Then, the further study will be needed for evaluating the correlations between conventional measurements in OSDI and LipiView interferometers.
Conclusion
In summary, the average and minimum LLT measured using a lipid interferometer in glaucomatous eyes were lower than those in normal eyes. In glaucomatous eyes, a longer duration of treatment and increased number of topical glaucoma medications were associated with lower LLT.
OSD (defined as instability and reduction of LLT) is strongly related to treatment compliance of topical glaucoma medications. Patients undergoing long-term glaucoma treatment or using many glaucoma eye drops should be carefully followed with respect to LLT parameters, which are useful for OSD assessment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Arici MK, Arici DS, Topalkara A, Güler C. Adverse effects of topical antiglaucoma drugs on the ocular surface. Clin Exp Ophthalmol. 2000;28:113–7. doi: 10.1046/j.1442-9071.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh S, O’Hare F, Lamoureux E, Vajpayee RB, Crowston JG. Prevalence of signs and symptoms of ocular surface disease in individuals treated and not treated with glaucoma medication. Clin Exp Ophthalmol. 2012;40:675–81. doi: 10.1111/j.1442-9071.2012.02781.x. [DOI] [PubMed] [Google Scholar]
- 3.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 4.Ramli N, Supramaniam G, Samsudin A, Juana A, Zahari M, Choo MM. Ocular surface disease in glaucoma. Optom Vis Sci. 2015;92:222–6. doi: 10.1097/OPX.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 5.Alessandro GA, Teresa R. Ocular surface alterations and topical antiglaucomatous therapy: A review. Open Ophthalmol J. 2014;8:67–72. doi: 10.2174/1874364101408010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kastelan S, Tomic M, Metez Soldo K, Salopek-Rabatic J. How ocular surface disease impacts the glaucoma treatment outcome. Biomed Res Int. 2013;2013:696328. doi: 10.1155/2013/696328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with laucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29:618–21. doi: 10.1097/ICO.0b013e3181c325b2. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Tatham AJ, Zangwill LM, Weinreb RN, Zhang C, Medeiros FA. Diagnostic ability of retinal nerve fiber layer imaging by swept-source optical coherence tomography in glaucoma. Am J Ophthalmol. 2015;159:193–201. doi: 10.1016/j.ajo.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SY, Lee H, Bae HW, Kim TI, Kim CY. Tear lipid layer thickness change and topical anti-glaucoma medication use. Optom Vis Sci. 2016;93:1210–7. doi: 10.1097/OPX.0000000000000943. [DOI] [PubMed] [Google Scholar]
- 10.Jung JW, Park SY, Kim JS, Kim EK, Seo KY, Kim TI. Analysis of factors associated with the tear film lipid layer thickness in normal eyes and patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2016;57:4076–83. doi: 10.1167/iovs.16-19251. [DOI] [PubMed] [Google Scholar]
- 11.Hwang H, Jeon HJ, Yow KC, Hwang HS, Chung EH. Image-based quantitative analysis of tear film lipid layer thickness for meibomian gland evaluation. Biomed Eng Online. 2017;16:1–15. doi: 10.1186/s12938-017-0426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markoulli M, Duong TB, Lin M, Papas E. Imaging the tear film: A comparison between the subjective keeler tearscope-plus™ and the objective oculus® keratograph 5M and LipiView® interferometer. Curr Eye Res. 2018;43:155–62. doi: 10.1080/02713683.2017.1393092. [DOI] [PubMed] [Google Scholar]
- 13.Arita R, Itoh K, Maeda S, Maeda K, Furuta A, Tomidokoro A, et al. Comparison of the long-term effects of various topical antiglaucoma medications on meibomian glands. Cornea. 2012;31:1229–34. doi: 10.1097/ICO.0b013e31823f8e7d. [DOI] [PubMed] [Google Scholar]
- 14.Dartt DA, Willcox MD. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Exp Eye Res. 2013;117:1–3. doi: 10.1016/j.exer.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kottaiyan R, Yoon G, Wang Q, Yadav R, Zavislan JM, Aquavella JV. Integrated multimodal metrology for objective and noninvasive tear evaluation. Ocul Surf. 2012;10:43–50. doi: 10.1016/j.jtos.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Sweeney DF, Millar TJ, Raju SR. Tear film stability: A review. Exp Eye Res. 2013;117:28–38. doi: 10.1016/j.exer.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 17.McCulley JP, Shine WE. Meibomian gland function and the tear lipid layer. Ocul Surf. 2003;1:97–106. doi: 10.1016/s1542-0124(12)70138-6. [DOI] [PubMed] [Google Scholar]
- 18.Green-Church KB, Butovich I, Willcox M, Borchman D, Paulsen F, Barabino S, et al. The international workshop on meibomian gland dysfunction: Report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52:1979–93. doi: 10.1167/iovs.10-6997d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniel Nelson J, Shimazaki J, Benitez-del-Castillo JM, Craig JP, McCulley JP, Den S, et al. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52:1930–7. doi: 10.1167/iovs.10-6997b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee TH, Sung MS, Heo H, Park SW. Association between meibomian gland dysfunction and compliance of topical prostaglandin analogs in patients with normal tension glaucoma. PLoS One. 2018;13:1–12. doi: 10.1371/journal.pone.0191398. [DOI] [PMC free article] [PubMed] [Google Scholar]