Abstract
Background
Early treatment is the only potential cure for periampullary cancer. The pathway to surgery is complex and involves multiple procedures across local and specialist hospitals. The aim of this study was to analyse variability within this pathway, and its impact on cost and outcomes.
Methods
Patients undergoing surgery for periampullary cancer (2011–2016) were identified retrospectively and their pathway to surgery was analysed. Patients who had early surgery (shortest quartile, Q1) were compared with those having late surgery (longest quartile, Q4).
Results
A total of 483 patients were included in the study, with 121 and 124 patients in Q1 and Q4 respectively. The median time from initial CT to surgery was 21 days for Q1 versus 112 days for Q4 (P < 0·001). Diagnostic delays were common in Q4; these patients required significantly more investigations than those in Q1 (endoscopic ultrasonography (EUS): 74·2 versus 18·2 per cent respectively, P < 0·001; MRI: 33·6 versus 20·6 per cent, P = 0·036). The median time to diagnostic EUS was 13 days in Q1 versus 59 days in Q4 (P < 0·001). Some 42·1 per cent of jaundiced patients in Q1 underwent preoperative biliary drainage, compared with all patients in Q4. There were significantly more unplanned admissions and associated longer duration of hospital stay per patient and costs in Q4 than in Q1 (median: 8 versus 3 days respectively; €5652 versus €2088; both P < 0·001). There was a higher likelihood of potentially curative surgery in Q1 (82·6 per cent versus 66·9 per cent in Q4; P = 0·005).
Conclusion
There is wide variation across the entire pathway, suggesting that multiple strategies are required to enable early surgery. Defining an effective pathway by anticipating the need for investigations and avoiding biliary drainage reduces unplanned admissions and costs and increases resection rates.
Introduction
The prognosis for patients with periampullary cancer is poor1, 2. However, for selected patients outcomes have improved; surgical resection with adjuvant chemotherapy offers a 5‐year survival rate of 28 or 40 per cent in patients with pancreatic or periampullary cancer respectively3, 4. Increasing time to surgery has been shown to relate to unresectability at the time of surgery5, as a proportion of patients are found to be unresectable due to occult local or distant spread. Consequently, early surgery is crucially important6.
The pathway from presentation, through diagnosis and to treatment for these patients is more complex than that for patients with other tumour types. Centralization has improved surgical outcomes, but made the pathway to surgery complex, requiring clear and rapid communication between local and specialist centres. When cross‐sectional imaging is not diagnostic, obtaining tissue for diagnosis is challenging: brushings from endoscopic retrograde cholangiopancreatography (ERCP) have a low sensitivity for detection of cancer, and, although endoscopic ultrasonography (EUS) has a higher accuracy, it also is often non‐diagnostic and requires repeating7, 8. The National Institute for Health and Care Excellence (NICE) in the UK has recently recommended PET for patients with potentially resectable pancreatic cancer9. In the absence of a coordinated pathway, the need for multiple and/or specialist investigations, as well as multidisciplinary reviews, inevitably prolongs the time from presentation to surgery. Preoperative interventions to correct malnutrition and jaundice, both common problems in patients with periampullary cancer, may also serve to complicate the pathway further9, 10, 11, 12. Despite evidence that preoperative biliary drainage is harmful, patients with potentially resectable disease frequently undergo ERCP before referral to a specialist centre13.
Thus, the pathway to surgery is complex; although early surgery is clearly desirable, it often may not be achieved. To the authors' knowledge, there are no studies in the literature analysing the pathway and its economic implications for patients with periampullary cancer within the UK. The aim of this study was therefore to define variability within the pathway from presentation to treatment among patients with potentially resectable periampullary cancer, to understand the reasons for this variability, and to compare outcomes between patients undergoing early or late treatment. The hypothesis was that a delay to surgery has a negative impact for patients and healthcare providers.
Methods
The study was conducted in line with the STROBE guidelines14, and was approved by the Institutional Review Board.
All consecutive patients who underwent surgical exploration for potentially resectable periampullary malignancy (pancreatic ductal adenocarcinoma, cholangiocarcinoma and ampullary carcinoma) at University Hospitals Birmingham from January 2011 to December 2016 were reviewed retrospectively. This is a tertiary referral hospital that treats 2·2 million patients per year. Consecutive patients were identified prospectively by a dedicated data manager on a daily basis. Patients who received neoadjuvant chemotherapy were excluded. The primary endpoint was time to surgery, defined as the number of days from the date of initial diagnostic CT to the date of surgery.
The dates of all investigations pertinent to diagnosis and treatment were recorded up to the point of surgery. Signs of biliary and pancreatic duct dilatation with a hypodense mass in the head of the pancreas on CT were considered sufficient for diagnosis. EUS, biopsy, MRI of the liver, staging laparoscopy and PET were used selectively. The lymph node ratio (LNR) was calculated from the ratio of positive lymph nodes from the total lymph node yield. Data related to investigations, interventions, outpatient appointments, clinical tests and operative details were obtained from electronic hospital records. All readmissions, complications and length of stay from hospital episodes, regardless of location (as long as the healthcare provider was a National Health Service (NHS) organization) between initial CT and surgery were identified by review of national data sets including Hospital Episodes Statistics by a dedicated informatics data manager. Costs of treatments/hospital admissions, based on national tariffs, were obtained from the NHS informatics team. The cost of each admission (excluding the admission for the operation) and costs of treatment were totalled for each patient, to provide the cost before surgery.
Statistical analysis
The time to surgery was summarized using median (i.q.r.) values, and compared across the referring hospitals using the Kruskal–Wallis test. A Jonckheere–Terpstra test was then used, with the hospitals ordered by number of referrals, to assess whether the time to surgery correlated with the number of referrals from a hospital.
The cohort was divided into two groups, based on the quartiles of times from diagnostic imaging to surgery (Q1 and Q4), in order to compare patients with the shortest and longest times to surgery. Patient demographics were then compared between these groups using independent‐samples t tests for normally distributed continuous variables and Mann–Whitney U tests for non‐normally distributed continuous variables or ordinal variables. Dichotomous variables were assessed with Fisher's exact test. The χ2 test was used for other categorical variables. The times between milestones of the patient pathway, and factors relating to treatment and imaging, were then assessed using a similar approach.
A multivariable analysis was performed using a binary logistic regression model, to identify independent predictors of a patient being in Q4 (predictors of longer time to surgery). A backwards stepwise approach was used for variable selection. Continuous variables were divided into categories, before the analysis, to improve model fit. For age and LNR these were based on tertiles of the distribution, whereas for BMI categories of less than 25, 25–29 and 30 kg/m2 or above were used, as is conventional.
All analyses were performed using IBM SPSS® version 22 (IBM, Armonk, New York, USA). Two‐sided P values were used in all analyses, with P < 0·050 deemed to be indicative of statistical significance throughout.
Results
Time to surgery
Between 1 January 2011 and 31 December 2016, a total of 483 patients with periampullary cancer had initial diagnostic imaging. The median time from imaging to surgery was 61 (i.q.r. 37–90) days (Fig. 1). Comparisons across the referring hospitals are shown in Fig. 2. A total of 12 hospitals had at least five referrals during the study period, with a range of 8–75 referrals. Times to surgery were similar across these 12 hospitals (P = 0·203), with medians ranging from 44 (i.q.r. 24–78) to 80 (22–138) days. No significant correlation between the number of referrals and time to surgery was detected (P = 0·696).
Figure 1.
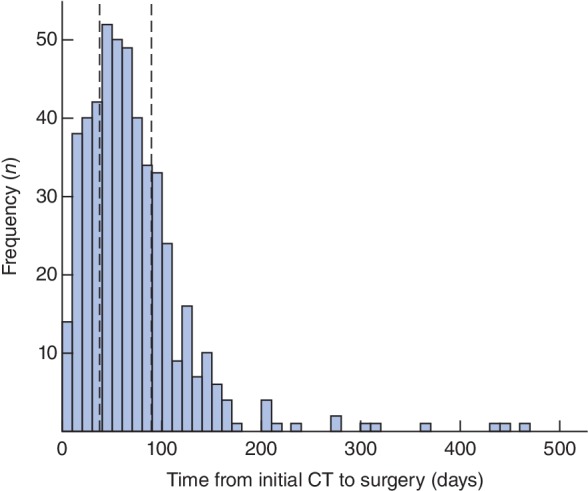
Histogram of the time from diagnostic imaging to surgery. The plot includes all 483 patients who underwent surgery during the study interval. The bars have an interval width of 10 days. The dashed lines represent the lower and upper quartiles (37 and 90 days respectively)
Figure 2.
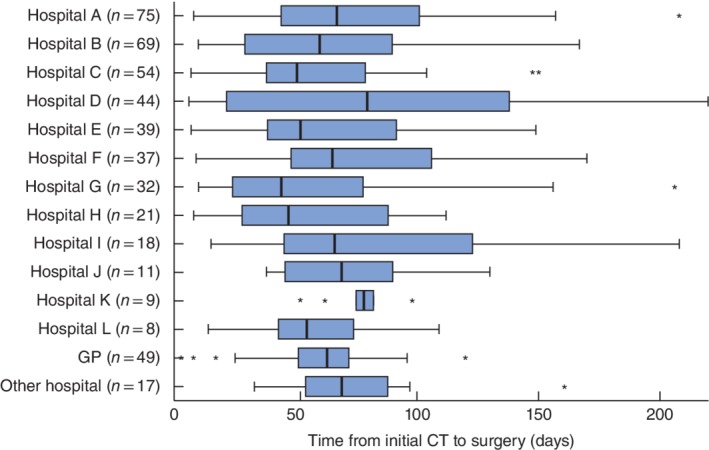
Comparison of time from diagnostic imaging to surgery by the referral hospital. The numbers reported on the y‐axis represent the total number of referrals during the period; ‘other hospital’ groups hospitals with fewer than five referrals. Median values, interquartile ranges and ranges are denoted by thick bars, boxes and error bars respectively; asterisks represent outliers, defined as values more than 1·5 times the length of the box. The x‐axis was truncated at 220 days as 99 per cent of patients fell within this range. GP, general practitioner. P = 0·203 for comparisons across hospitals A–L (Kruskal–Wallis test); P = 0·696 when the hospital was treated as an ordinal factor based on total number of referrals (Jonckheere–Terpstra test)
Patient demographics
Patients in the lower (Q1, 37 days or less) and upper (Q4, at least 90 days) quartiles with regard to time to surgery were identified and interrogated further, giving sample sizes for analysis of 121 and 124 respectively. Patient demographics were generally similar in the two groups for sex, BMI, smoking status and Charlson Co‐morbidity Index (Table 1).
Table 1.
Comparison of patient and diagnostic factors
| Quartile of time from CT to surgery | |||
|---|---|---|---|
| Q1 (n = 121) | Q4 (n = 124) | P † | |
| Age at surgery (years)* | 66·2(9·5) | 68·5(9·2) | 0·056‡ |
| Sex ratio (M : F) | 63 : 58 | 70 : 54 | 0·523 |
| BMI (kg/m2)* | 26·2(4·9) | 26·0(5·1) | 0·786‡ |
| Smoking status | 1·000 | ||
| Non‐smoker | 95 (78·5) | 97 (78·2) | |
| Smoker | 11 (9·1) | 11 (8·9) | |
| Ex‐smoker | 15 (12·4) | 16 (12·9) | |
| Charlson Co‐morbidity Index > 2 | 41 of 119 (34·5) | 52 (41·9) | 0·238 |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.).
Fisher's exact test, except
t test.
Patient pathway
The median time to surgery was 112 days in Q4 and 21 days in Q1. Breaking this pathway down into individual components showed that all stages were significantly longer in Q4 than in Q1 (P < 0·001) (Table 2). The largest difference was in the time from the specialist clinic to surgery: median 75 days for Q4 versus 7 days for Q1.
Table 2.
Components of the patient pathway
| Quartile of time from CT to surgery | ||||
|---|---|---|---|---|
| n | Q1 (n = 121) | Q4 (n = 124) | P ‡ | |
| Time (days)* | ||||
| CT to surgery | 245 | 21 (14–29) | 112 (99–142) | < 0·001 |
| CT to referral | 222 | 3 (1–6) | 20 (7–41) | < 0·001 |
| CT to final PBD† | 157 | 3 (0–8) | 14 (2–46) | < 0·001 |
| Referral to specialist MDT | 218 | 3 (1–7) | 4 (2–15) | < 0·001 |
| Specialist clinic to surgery | 241 | 7 (1–14) | 75 (45–103) | < 0·001 |
Values are median (i.q.r.).
Where pathway components were reversed (for example, where patients were referred before imaging) a time of 0 days was assigned.
Includes only those patients with jaundice who had preoperative biliary drainage (PBD); see Table 3 for further details. MDT, multidisciplinary team.
Mann–Whitney U test.
Diagnostic tests
All patients had at least one diagnostic CT scan, but the total number of scans required was significantly higher in the Q4 group (mean per patient: 1·8 versus 1·0 in Q1; P < 0·001) (Table 3). Patients in Q4 were significantly more likely to require EUS than those in Q1 (74·2 versus 18·2 per cent respectively; P < 0·001). In addition, where EUS was performed, patients in Q4 had a greater number of tests (mean 1·4 versus 1·1 per patient; P = 0·024) and had significantly longer time from initial CT to both their first (median 38 versus 13 days; P < 0·001) and diagnostic (59 versus 13 days; P < 0·001) EUS. Patients in Q4 were also significantly more likely to require MRI than those in Q1 (33·6 versus 20·6 per cent respectively; P = 0·036), and tended to have a higher rate of PET, although this was not significantly different (5·9 versus 0·9 per cent; P = 0·069).
Table 3.
Diagnostic tests and management of jaundice
| Quartile of time from CT to surgery | ||||
|---|---|---|---|---|
| n | Q1 (n = 121) | Q4 (n = 124) | P § | |
| Diagnostic tests | ||||
| CT | 245 | 121 (100) | 124 (100) | – |
| Total no. of scans | 245 | 122 (1·0 per patient) | 229 (1·8 per patient) | < 0·001¶ |
| EUS | 245 | 22 (18·2) | 92 (74·2) | < 0·001 |
| Total no. of EUS scans | 114† | 24 (1·1 per patient) | 133 (1·4 per patient) | 0·024¶ |
| Time from CT to first EUS (days)* | 114† | 13 (7–19) | 38 (19–61) | < 0·001¶ |
| Time from CT to diagnostic EUS (days)* | 105† | 13 (7–19) | 59 (39–80) | < 0·001¶ |
| MRI | 226‡ | 22 of 107 (20·6) | 40 of 119 (33·6) | 0·036 |
| PET | 226‡ | 1 of 107 (0·9) | 7 of 119 (5·9) | 0·069 |
| Management of jaundice | ||||
| No. of patients with jaundice | 245 | 107 (88·4) | 112 (90·3) | 0·682 |
| ERCP | 219 | 42 of 107 (39·3) | 112 of 112 (100) | < 0·001 |
| No. of patients having ERCP | 219 | 29 of 107 (27·1) | 84 of 112 (75·0) | < 0·001 |
| Total no. of procedures | 154† | 46 (1·1 per patient) | 142 (1·3 per patient) | 0·103¶ |
| PTC | 219 | 6 of 107 (5·6) | 23 of 112 (20·5) | 0·001 |
| Total no. of procedures | 29† | 6 (1·0 per patient) | 39 (1·7 per patient) | 0·058¶ |
| PBD | 219 | 45 of 107 (42·1) | 112 of 112 (100) | < 0·001 |
| No. of patients having PBD before referral | 216‡ | 37 of 106 (34·9) | 97 of 110 (88·2) | < 0·001 |
| No. of patients with > 1 attempt at PBD | 219 | 6 of 107 (5·6) | 41 of 112 (36·6) | < 0·001 |
| Total no. of procedures | 157† | 51 (1·1 per patient) | 181 (1·6 per patient) | 0·002¶ |
| Time from CT to final PBD (days)* | 157† | 3 (0–8) | 14 (2–46) | < 0·001¶ |
Values in parentheses are percentages unless indicated otherwise;
values are median (i.q.r.).
In the subgroup of patients for whom at least one procedure was performed.
Excludes patients for whom details of additional imaging or timing of the earliest preoperative biliary drainage (PBD) were not available. EUS, endoscopic ultrasonography; ERCP, endoscopic retrograde cholangiopancreatography; PTC, percutaneous transhepatic cholangiography.
Fisher's exact test, except
Mann–Whitney U test.
Treatment of jaundice
Rates of jaundice were similar in the two groups: 88·4 per cent in Q1 and 90·3 per cent in Q4 (P = 0·682) (Table 3). In those with jaundice, patients in Q4 were significantly more likely to undergo preoperative biliary drainage than those in Q1 (100 versus 42·1 per cent; P < 0·001). Where biliary drainage was performed, patients in Q4 required significantly more procedures to achieve successful drainage than those in Q1: mean 1·6 versus 1·1 procedures per patient (P = 0·002). Successful biliary drainage was achieved a median of 3 and 14 days after diagnostic CT in Q1 and Q4 respectively (P < 0·001).
Preoperative admissions and costs of treatment
Patients in Q4 required significantly more hospital admissions, including for performance of initial CT and surgery, than those in Q1 (median 3 versus 1 respectively; P < 0·001) (Table 4). Consequently, the Q4 group also had a significantly longer cumulative length of stay (median 8 versus 3 days; P < 0·001). In light of this, and the fact that the Q4 group required a greater number of scans and procedures, these patients had a median cost before surgery of €5652, which was significantly greater than the €2088 in Q1 (P < 0·001).
Table 4.
Comparisons of admissions, tumour and operative factors
| Quartile of time from imaging to surgery | ||||
|---|---|---|---|---|
| n | Q1 (n = 121) | Q4 (n = 124) | P § | |
| Preoperative admissions | ||||
| No. of hospital episodes* | 218 | 1 (1–1) | 3 (2–5) | < 0·001¶ |
| Cumulative length of stay (days)* | 218 | 3 (0–8) | 8 (1–20) | < 0·001¶ |
| Costs before surgery (€)* | 218 | 2088 (0–3666) | 5652 (3299–8450) | < 0·001¶ |
| Tumour factors | ||||
| Diagnosis | 245 | < 0·001 | ||
| Ductal adenocarcinoma | 95 (78·5) | 66 (53·2) | ||
| Cholangiocarcinoma | 9 (7·4) | 25 (20·2) | ||
| Ampullary cancer | 17 (14·0) | 33 (26·6) | ||
| T category† | 182 | 0·185¶ | ||
| T0 | 0 of 100 (0) | 3 of 82 (4) | ||
| T1 | 5 of 100 (5·0) | 5 of 82 (6) | ||
| T2 | 6 of 100 (6·0) | 10 of 82 (12) | ||
| T3 | 86 of 100 (86·0) | 59 of 82 (72) | ||
| T4 | 3 of 100 (3·0) | 5 of 82 (6) | ||
| N category† | 182 | 0·031¶ | ||
| N0 | 20 of 100 (20·0) | 28 of 82 (34) | ||
| N1 | 78 of 100 (78·0) | 53 of 82 (65) | ||
| N2 | 2 of 100 (2·0) | 1 of 82 (1) | ||
| Lymph node ratio*, † | 183 | 0·18 (0·06–0·34) | 0·13 (0·00–0·29) | 0·062¶ |
| Operative factors | ||||
| Operation | 245 | 0·005 | ||
| Resection | 100 (82·6) | 83 (66·9) | ||
| Bypass | 21 (17·4) | 41 (33·1) | ||
| Vascular reconstruction† | 182 | 20 of 99 (20) | 10 of 83 (12) | 0·163 |
| R1 status† | 183 | 33 of 100 (33·0) | 13 of 83 (16) | 0·010 |
Values in parentheses are percentages unless indicated otherwise;
values are median (i.q.r.). T and N categories were assessed pathologically for resections and radiologically for bypasses.
Includes only patients who had a resection.
Fisher's exact test, except
Mann–Whitney U test.
Tumour and operative factors
Patients with the longest time from imaging to surgery (Q4) had a significantly lower proportion of ductal adenocarcinoma (53·2 per cent versus 78·5 per cent for Q1; P < 0·001) (Table 4). There was no significant difference in T category or LNR between the groups, although N category was significantly higher in the Q1 group (P < 0·031). Resection rates were significantly lower in Q4: 66·9 per cent versus 82·6 per cent in Q1 (P = 0·005). Patients in Q1 had a higher rate of vascular reconstruction (20 versus 12 per cent for Q4; P = 0·163), and those treated by resection had a significantly higher rate of positive resection margins (33·0 versus 16 per cent respectively; P = 0·010).
Predictors of extended times to surgery
Multivariable analysis was performed to identify factors independently associated with patients in Q4 (Table 5). The number of CT scans was not included in this analysis because repeat scans were performed to check for progression of disease in those with long times to surgery. Hence, the requirement for multiple CT scans was a result of an extended time to surgery rather than a causal factor.
Table 5.
Predictors of extended times from CT to surgery
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Odds ratio | P | Odds ratio | P | |
| Age at surgery (years) | 0·019 | 0·088 | ||
| < 65 | 1·00 (reference) | 1·00 (reference) | ||
| 65–74 | 1·00 (reference) | 0·574 | 2·47 (0·94, 6·50) | 0·066 |
| ≥ 75 | 1·00 (reference) | 0·007 | 3·26 (1·02, 10·36) | 0·046 |
| Female sex | 0·84 (0·51, 1·39) | 0·491 | n.s. | |
| BMI (kg/m2) | 0·536 | n.s. | ||
| < 25 | 1·00 (reference) | |||
| 25–29 | 0·80 (0·46, 1·39) | 0·424 | ||
| ≥ 30 | 1·17 (0·58, 2·39) | 0·656 | ||
| Smoking status | 0·992 | n.s. | ||
| Non‐smoker | 1·00 (reference) | |||
| Smoker | 0·98 (0·41, 2·37) | 0·963 | ||
| Ex‐smoker | 1·04 (0·49, 2·23) | 0·910 | ||
| Charlson Co‐morbidity Index > 2 | 1·37 (0·82, 2·31) | 0·231 | n.s. | |
| Diagnosis | < 0·001 | n.s. | ||
| Ductal adenocarcinoma | 1·00 (reference) | |||
| Cholangiocarcinoma | 4·00 (1·75, 9·12) | < 0·001 | ||
| Ampullary cancer | 2·79 (1·44, 5·43) | 0·002 | ||
| Jaundice | 1·22 (0·54, 2·76) | 0·631 | n.s. | |
| T3–4 category | 0·32 (0·18, 0·56) | < 0·001 | n.s. | |
| N1–2 category | 0·34 (0·20, 0·59) | < 0·001 | n.s. | |
| Lymph node ratio | < 0·001 | 0·033 | ||
| 0 | 1·00 (reference) | 1·00 (reference) | ||
| 0·01–0·20 | 0·31 (0·16, 0·60) | < 0·001 | 0·50 (0·19, 1·30) | 0·154 |
| > 0·20 | 0·31 (0·16, 0·58) | < 0·001 | 0·23 (0·08, 0·70) | 0·010 |
| No. of EUS scans | < 0·001 | < 0·001 | ||
| 0 | 1·00 (reference) | 1·00 (reference) | ||
| 1 | 9·6 (5·0, 18·2) | < 0·001 | 11·9 (4·7, 30·7) | < 0·001 |
| > 1 | 46·4 (10·5, 205·1) | < 0·001 | 67·2 (7·1, 635·8) | < 0·001 |
| MRI | 1·96 (1·07, 3·58) | 0·029 | n.s. | |
| PET | 6·6 (0·8, 54·8) | 0·079 | 9·4 (0·6, 142·6) | 0·108 |
| No. of PBD procedures | < 0·001 | < 0·001 | ||
| 0 | 1·00 (reference) | 1·00 (reference) | ||
| 1 | 11·5 (5·6, 23·8) | < 0·001 | 10·8 (4·2, 27·8) | < 0·001 |
| > 1 | 43·3 (15·1, 123·8) | < 0·001 | 29·9 (7·6, 118·6) | < 0·001 |
Values in parentheses are 95 per cent confidence intervals. Results are from binary logistic regression analysis, with Q4 versus Q1 as the dependent variable; hence odds ratios greater than 1 represent a greater chance of extended time to surgery. The multivariable model used a backwards stepwise approach to variable selection, with all factors analysed in the univariable analysis considered for inclusion. The final model was based on 221 patients, after exclusions owing to missing data. n.s., Not selected for inclusion by the stepwise procedure; EUS, endoscopic ultrasonography; PBD, preoperative biliary drainage.
As in the univariable analysis, none of the demographic factors was found to be significant in the multivariable model. Of the tumour factors considered, increasing LNR was independently associated with a lower probability of being in Q4 (P = 0·033). However, the strongest predictors of a longer time to surgery were the number of times EUS was performed (P < 0·001) and preoperative biliary drainage was required (P < 0·001).
Discussion
This retrospective review of the pathway to surgery taken by patients with potentially resectable periampullary cancer identified a broad range in time to surgery for those who undergo early or late surgery (21 versus 112 days respectively). Related findings were the particularly long time to establish a tissue diagnosis, where required, for patients in Q4 (13 versus 59 days), as well as increased time for every measured stage of the pathway and increased use of every observed investigation. Furthermore, the majority of patients with jaundice in Q1 had surgery without preoperative biliary drainage, whereas draining was required by every jaundiced patient in Q4. Ultimately, patients in Q1 had lower rates of readmission, shorter length of stay, lower treatment costs and a higher resection rate, highlighting the importance of rapid progression from presentation to surgery among these patients.
The start of this pathway was the date of initial CT, which is considered the single best investigation required for diagnosing, or at least raising suspicion of, periampullary cancer; this has been used elsewhere when reviewing outcomes of patients treated within these pathways10. The sensitivity and specificity of CT in identifying pancreatic cancer is 70–100 per cent11, and its ability to evaluate resectability based on locoregional, distant and vascular involvement is 81–99 per cent12, 13, 15. For some patients, CT can be the single best imaging required to determine definitive management, as CT has been shown to be superior to MRI and ultrasound imaging12.
When there is diagnostic uncertainty, ERCP has previously been relied on to provide tissue for diagnosis16. However, the role of ERCP in the pathway has devolved from diagnostic to therapeutic, certainly within the era of improved CT accuracy and EUS. However, when EUS is required, this study suggests it is associated with significant delay among patients who proceed slowly to surgery and is, therefore, a particular area of the pathway that is prone to causing delay. Anticipating the need for EUS, rapid reporting of pathology and, when needed, repeating EUS urgently could all reduce time to diagnosis. Same‐day reporting of histological material from suspected cancer is possible17, 18, 19, and is something this study suggests could have a major impact on the cancer pathway.
It is common for patients (42–79 per cent) to have had preoperative biliary drainage before referral to specialist surgical review20, 21, 22, 23. However, this is associated with significant harm24, including a 4 per cent risk of pancreatitis, haemorrhage and bowel perforation25, 26, 27, 28, 29, 30, 31, 32, 33, 34. Biliary colonization following ERCP has been reported to be as high as 27 per cent35, 36, with a resultant impact on surgical‐site infections after pylorus‐preserving pancreaticoduodenectomy (PPPD)37.
A direct‐to‐surgery approach among jaundiced patients, rather than preoperative biliary drainage, is associated with a marked reduction in complications and readmissions38. After preoperative biliary drainage, the need for further procedures and stent changes is common due to cholangitis39.
In August 2015, a pathway to offer surgery without preoperative biliary drainage was implemented at the authors' centre; before this, surgery without preoperative biliary drainage was performed on an ad hoc basis40. It is interesting to observe that this reduced the time from CT to referral, suggesting that changes in specialist care pathways influence the behaviour of referring colleagues in non‐specialist centres. Patients within this ‘fast track’ pathway were within the shortest quartile in time to surgery where diagnostic and staging investigations were prioritized and organized in parallel, rather than in series, reducing times to diagnosis and treatment40. Patients in the longest quartile to surgery were treated within pre‐existing pathways provided within NHS standard treatment times for cancer. These state that treatment for cancer should be provided within 62 days of referral. The experience of these patients shows that provision of early treatment is a challenge within a nationally funded healthcare system of limited resource without some form of support over and above those provided within existing pathways.
Owing to the multiple benefits, avoidance of preoperative biliary drainage among jaundiced patients is recommended in best practice guidelines41 and by NICE9, where possible.
The recently published NICE guidelines on pancreatic cancer9 aim to ensure quicker and more accurate staging. These guidelines recommend PET for all patients to avoid overtreatment of those with occult metastatic disease. This could potentially delay treatment further, and thus it is important that, where implemented, PET is done with minimal impact to the patient pathway. PET–CT showed low accuracy in differentiating benign from malignant periampullary lesions in 57 per cent of patients42, although it had a relatively good ability to detect lymph node metastasis43. The recent PET‐PANC study44 showed similar sensitivity and specificity between multidetector CT and PET–CT. PET–CT upstaged disease, preventing resection in 11 per cent of patients.
The present study, and others5, 45, 46, however, highlight that treatment is time critical. Consequently, concerns over understaging must be balanced against introducing undue delays to surgery. Indeed, overuse of preoperative investigations prolongs time to surgery and results in complications incurred as a result of them39, 47.
Patients undergoing resection in both quartiles had similar pathological profiles and yet there was a higher rate of resection, despite more borderline cases requiring vascular reconstruction and involved margins, in the faster quartile.
There are clear limitations to this study. The data were derived from a single hospital and its network of referring teams, and thus may not be generalizable. However, the median time to surgery (61 days) is representative for patients in the UK40. There is currently a prospective national audit of pancreatic cancer in the UK, the RICOCHET study, which will map the patient pathway in detail. Aspects of the pathway, such as local preference for use of various diagnostic tests and the surgical approach to borderline resectable and locally advanced cancers, may not be generalizable. The strength of this study is that it originates from a centre that currently performs roughly 10 per cent of the UK pancreatic cancer resections per year (based on hospital resection volume and data on cancer resections available at the UK NHS Hospital Episodes Statistics database)48, and it is possible that the data presented here may actually underestimate diagnostic delays in other units, particularly if surgery without preoperative biliary drainage is not routine.
Focusing on establishing rapid diagnosis and staging (including efficient and effective use of EUS) and avoiding preoperative biliary drainage appear to be strategies that can effectively reduce time to surgery. The need for multiple staging investigations must be balanced against risks of cancer progression if they cause delays to treatment. Cost saving from providing early surgery and avoiding preoperative biliary drainage could be redirected into supporting these pathways.
Disclosure
The authors declare no conflict of interest.
Funding information
No funding
References
- 1. Parasher JGL. The role of ERCP in pancreatobiliary malignancies. Adv Dig Endosc 2006; 6: 120–141. [Google Scholar]
- 2. Cancer Research UK . Pancreatic Cancer Statistics . https://www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/pancreatic‐cancer?_ga=2.51142689.902754631.1552057165‐1585745111.1461691245 [accessed 1 November 2018].
- 3. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM et al; European Study Group for Pancreatic Cancer . Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC‐4): a multicentre, open‐label, randomised, phase 3 trial. Lancet 2017; 389: 1011–1024. [DOI] [PubMed] [Google Scholar]
- 4. Acharya A, Markar SR, Sodergren MH, Malietzis G, Darzi A, Athanasiou T et al Meta‐analysis of adjuvant therapy following curative surgery for periampullary adenocarcinoma. Br J Surg 2017; 104: 814–822. [DOI] [PubMed] [Google Scholar]
- 5. Sanjeevi S, Ivanics T, Lundell L, Kartalis N, Andrén‐Sandberg Å, Blomberg J et al Impact of delay between imaging and treatment in patients with potentially curable pancreatic cancer. Br J Surg 2016; 103: 267–275. [DOI] [PubMed] [Google Scholar]
- 6. Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg 2007; 246: 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jailwala J, Fogel EL, Sherman S, Gottlieb K, Flueckiger J, Bucksot LG et al Triple‐tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc 2000; 51: 383–390. [DOI] [PubMed] [Google Scholar]
- 8. Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR et al Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology 2006; 131: 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Institute of Health and Care Excellence (NICE) . Pancreatic Cancer in Adults: Diagnosis and Management. NICE Guideline; 2018. https://www.nice.org.uk/guidance/ng85/resources/pancreatic‐cancer‐in‐adults‐diagnosis‐and‐management‐pdf‐1837696373701 [accessed 1 November 2018]. [PubMed]
- 10. Marchegiani G, Andrianello S, Perri G, Secchettin E, Maggino L, Malleo G et al Does the surgical waiting list affect pathological and survival outcome in resectable pancreatic ductal adenocarcinoma? HPB (Oxford) 2018; 20: 411–417. [DOI] [PubMed] [Google Scholar]
- 11. Shrikhande SV, Barreto SG, Goel M, Arya S. Multimodality imaging of pancreatic ductal adenocarcinoma: a review of the literature. HPB (Oxford) 2012; 14: 658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bipat S, Phoa SS, van Delden OM, Bossuyt PM, Gouma DJ, Laméris JS et al Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: a meta‐analysis. J Comput Assist Tomogr 2005; 29: 438–445. [DOI] [PubMed] [Google Scholar]
- 13. Squillaci E, Fanucci E, Sciuto F, Masala S, Sodani G, Carlani M et al Vascular involvement in pancreatic neoplasm: a comparison between spiral CT and DSA. Dig Dis Sci 2003; 48: 449–458. [DOI] [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 15. Valls C, Andía E, Sanchez A, Fabregat J, Pozuelo O, Quintero JC et al Dual‐phase helical CT of pancreatic adenocarcinoma: assessment of resectability before surgery. AJR Am J Roentgenol 2002; 178: 821–826. [DOI] [PubMed] [Google Scholar]
- 16. Niederau C, Grendell JH. Diagnosis of pancreatic carcinoma. Imaging techniques and tumor markers. Pancreas 1992; 7: 66–86. [DOI] [PubMed] [Google Scholar]
- 17. Lodewijk L, Vriens MR, Vorselaars WM, van der Meij NT, Kist JW, Barentsz MW et al Same‐day fine‐needle aspiration cytology diagnosis for thyroid nodules achieves rapid anxiety decrease and high diagnostic accuracy. Endocr Pract 2016; 22: 561–566. [DOI] [PubMed] [Google Scholar]
- 18. Khurana KK, Graber B, Wang D, Roy A. Telecytopathology for on‐site adequacy evaluation decreases the nondiagnostic rate in endoscopic ultrasound‐guided fine‐needle aspiration of pancreatic lesions. Telemed J E Health 2014; 20: 822–827. [DOI] [PubMed] [Google Scholar]
- 19. Collins BT, Murad FM, Wang JF, Bernadt CT. Rapid on‐site evaluation for endoscopic ultrasound‐guided fine‐needle biopsy of the pancreas decreases the incidence of repeat biopsy procedures. Cancer Cytopathol 2013; 121: 518–524. [DOI] [PubMed] [Google Scholar]
- 20. Isla AM, Griniatsos J, Riaz A, Karvounis E, Williamson RC. Pancreaticoduodenectomy for periampullary malignancies: the effect of bile colonization on the postoperative outcome. Langenbecks Arch Surg 2007; 392: 67–73. [DOI] [PubMed] [Google Scholar]
- 21. Morris‐Stiff G, Tamijmarane A, Tan YM, Shapey I, Bhati C, Mayer AD et al Pre‐operative stenting is associated with a higher prevalence of post‐operative complications following pancreatoduodenectomy. Int J Surg 2011; 9: 145–149. [DOI] [PubMed] [Google Scholar]
- 22. Cortes A, Sauvanet A, Bert F, Janny S, Sockeel P, Kianmanesh R et al Effect of bile contamination on immediate outcomes after pancreaticoduodenectomy for tumor. J Am Coll Surg 2006; 202: 93–99. [DOI] [PubMed] [Google Scholar]
- 23. Coates JM, Beal SH, Russo JE, Vanderveen KA, Chen SL, Bold RJ et al Negligible effect of selective preoperative biliary drainage on perioperative resuscitation, morbidity, and mortality in patients undergoing pancreaticoduodenectomy. Arch Surg 2009; 144: 841–847. [DOI] [PubMed] [Google Scholar]
- 24. Cotton PB, Garrow DA, Gallagher J, Romagnuolo J. Risk factors for complications after ERCP: a multivariate analysis of 11 497 procedures over 12 years. Gastrointest Endosc 2009; 70: 80–88. [DOI] [PubMed] [Google Scholar]
- 25. Eloubeidi MA, Tamhane A, Varadarajulu S, Wilcox CM. Frequency of major complications after EUS‐guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc 2006; 63: 622–629. [DOI] [PubMed] [Google Scholar]
- 26. Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F et al Incidence rates of post‐ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007; 102: 1781–1788. [DOI] [PubMed] [Google Scholar]
- 27. Paquin SC, Gariépy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS‐guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc 2005; 61: 610–611. [DOI] [PubMed] [Google Scholar]
- 28. Shah JN, Fraker D, Guerry D, Feldman M, Kochman ML. Melanoma seeding of an EUS‐guided fine needle track. Gastrointest Endosc 2004; 59: 923–924. [DOI] [PubMed] [Google Scholar]
- 29. Fisher L, Segarajasingam DS, Stewart C, Deboer WB, Yusoff IF. Endoscopic ultrasound guided fine needle aspiration of solid pancreatic lesions: performance and outcomes. J Gastroenterol Hepatol 2009; 24: 90–96. [DOI] [PubMed] [Google Scholar]
- 30. Al‐Haddad M, Wallace MB, Woodward TA, Gross SA, Hodgens CM, Toton RD et al The safety of fine‐needle aspiration guided by endoscopic ultrasound: a prospective study. Endoscopy 2008; 40: 204–208. [DOI] [PubMed] [Google Scholar]
- 31. Jagannath P, Dhir V, Shrikhande S, Shah RC, Mullerpatan P, Mohandas KM. Effect of preoperative biliary stenting on immediate outcome after pancreaticoduodenectomy. Br J Surg 2005; 92: 356–361. [DOI] [PubMed] [Google Scholar]
- 32. Sewnath ME, Birjmohun RS, Rauws EA, Huibregtse K, Obertop H, Gouma DJ. The effect of preoperative biliary drainage on postoperative complications after pancreaticoduodenectomy. J Am Coll Surg 2001; 192: 726–734. [DOI] [PubMed] [Google Scholar]
- 33. Garcea G, Chee W, Ong SL, Maddern GJ. Preoperative biliary drainage for distal obstruction: the case against revisited. Pancreas 2010; 39: 119–126. [DOI] [PubMed] [Google Scholar]
- 34. Qiu YD, Bai JL, Xu FG, Ding YT. Effect of preoperative biliary drainage on malignant obstructive jaundice: a meta‐analysis. World J Gastroenterol 2011; 17: 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thosani N, Zubarik RS, Kochar R, Kothari S, Sardana N, Nguyen T et al Prospective evaluation of bacteremia rates and infectious complications among patients undergoing single‐operator choledochoscopy during ERCP. Endoscopy 2016; 48: 424–431. [DOI] [PubMed] [Google Scholar]
- 36. Du M, Xing Y, Suo J, Liu B, Jia N, Huo R et al Real‐time automatic hospital‐wide surveillance of nosocomial infections and outbreaks in a large Chinese tertiary hospital. BMC Med Inform Decis Mak 2014; 14: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barreto SG, Singh MK, Sharma S, Chaudhary A. Determinants of surgical site infections following pancreatoduodenectomy. World J Surg 2015; 39: 2557–2563. [DOI] [PubMed] [Google Scholar]
- 38. van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ et al Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med 2010; 362: 129–137. [DOI] [PubMed] [Google Scholar]
- 39. Jinkins LJ, Parmar AD, Han Y, Duncan CB, Sheffield KM, Brown KM et al Current trends in preoperative biliary stenting in patients with pancreatic cancer. Surgery 2013; 154: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roberts KJ, Prasad P, Steele Y, Marcon F, Faulkner T, Cilliers H et al A reduced time to surgery within a ‘fast track’ pathway for periampullary malignancy is associated with an increased rate of pancreatoduodenectomy. HPB (Oxford) 2017; 19: 713–720. [DOI] [PubMed] [Google Scholar]
- 41. Fang Y, Gurusamy KS, Wang Q, Davidson BR, Lin H, Xie X et al Pre‐operative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev 2012; (9)CD005444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng MF, Wang HP, Tien YW, Liu KL, Yen RF, Tzen KY et al Usefulness of PET/CT for the differentiation and characterization of periampullary lesions. Clin Nucl Med 2013; 38: 703–708. [DOI] [PubMed] [Google Scholar]
- 43. Raj P, Kaman L, Singh R, Dahyia D, Bhattacharya A, Bal A. Sensitivity and specificity of FDG PET–CT scan in detecting lymph node metastasis in operable periampullary tumours in correlation with the final histopathology after curative surgery. Updates Surg 2013; 65: 103–107. [DOI] [PubMed] [Google Scholar]
- 44. Ghaneh P, Hanson R, Titman A, Lancaster G, Plumpton C, Lloyd‐Williams H et al PET‐PANC: multicentre prospective diagnostic accuracy and health economic analysis study of the impact of combined modality 18fluorine‐2‐fluoro‐2‐deoxy‐d‐glucose positron emission tomography with computed tomography scanning in the diagnosis and management of pancreatic cancer. Health Technol Assess 2018; 22: 1–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Glant JA, Waters JA, House MG, Zyromski NJ, Nakeeb A, Pitt HA et al Does the interval from imaging to operation affect the rate of unanticipated metastasis encountered during operation for pancreatic adenocarcinoma? Surgery 2011; 150: 607–616. [DOI] [PubMed] [Google Scholar]
- 46. Raman SP, Reddy S, Weiss MJ, Manos LL, Cameron JL, Zheng L et al Impact of the time interval between MDCT imaging and surgery on the accuracy of identifying metastatic disease in patients with pancreatic cancer. AJR Am J Roentgenol 2015; 204: W37–W42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cooper M, Newman NA, Ibrahim AM, Lam E, Herman JM, Singh VK et al Unnecessary tests and procedures in patients presenting with solid tumors of the pancreas. J Gastrointest Surg 2013; 17: 1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. NHS Digital . Hospital Episode Statistics (HES). https://digital.nhs.uk/data‐and‐information/data‐tools‐and‐services/data‐services/hospital‐episode‐statistics [accessed 1 November 2018].


