Abstract
Background
This study evaluated the outcome and survival of patients with radiologically suspected intraductal papillary mucinous neoplasms (IPMNs).
Methods
IPMN management was reviewed according to Fukuoka risk factors and IPMN localization, differentiating main‐duct (MD), mixed‐type (MT) and branch‐duct (BD) IPMNs. Perioperative results were compared with those of patients undergoing resection of pancreatic ductal adenocarcinoma (PDAC) over the same interval (2010–2014). Overall (OS) and disease‐specific (DSS) survival rates were calculated and subgroups compared.
Results
Of 142 patients with IPMNs, 26 had MD‐IPMN, eight had MT‐IPMN and 108 had BD‐IPMN. Some 74 per cent of patients with MD‐ and MT‐IPMN were managed by primary resection, whereas this was used in only 27·8 per cent of those with BD‐IPMN. The risk of secondary resection and malignant transformation for BD‐IPMNs smaller than 20 mm was 8 and 2 per cent respectively during follow‐up. Pancreatic head resection of IPMNs was associated with an increased risk of postoperative pancreatic fistula grade B/C compared with resection of PDAC (12 of 33 (36 per cent) versus 41 of 221 (18·6 per cent) respectively; P = 0·010), and greater morbidity and mortality (Clavien–Dindo grade III: 15 of 33 (45 per cent) versus 56 of 221 (25·3 per cent) respectively; grade IV: 1 (3 per cent) versus 7 (3·2 per cent); grade V: 2 (6 per cent) versus 2 (0·9 per cent); P = 0·008). Five‐year OS and DSS rates in patients with MD‐IPMN were worse than those for MT‐ and BD‐IPMN (OS: 44, 86 and 97·4 per cent respectively, P < 0·001; DSS: 60, 100 and 98·6 per cent; P < 0·001). Patients with invasive IPMN had worse OS and DSS rates than those with non‐invasive dysplasia (OS: IPMN‐carcinoma (10 patients) 33 per cent, high‐grade dysplasia 100 per cent, intermediate‐grade dysplasia 63 per cent, low grade‐dysplasia 100 per cent, P < 0·001; DSS: IPMN‐carcinoma 43 per cent, all grades of dysplasia 100 per cent, P < 0·001). Patients with high‐risk stigmata had poorer survival than those without risk factors (OS: high‐risk stigmata (35 patients) 55 per cent, worrisome features (31) 95 per cent, no risk factors (76) 100 per cent, P < 0·001; DSS: 71, 100 and 100 per cent respectively, P < 0·001).
Conclusion
The risk of malignant transformation was very low for BD‐IPMNs, but the development of high‐risk stigmata was associated with disease‐specific mortality. Patients with IPMN had greater morbidity after resection than those having resection of PDAC.
Introduction
The management and risk of progression of intraductal papillary mucinous neoplasms (IPMNs) has been stratified by the Fukuoka guidelines1, 2 into: IPMNs presenting with no risk factors, IPMNs with worrisome features and high‐risk stigmata (Table 1). IPMNs are also classified on the basis of location, distinguishing main pancreatic duct (MD‐IPMN), branch duct‐type (BD‐IPMN) and mixed‐type (MT‐IPMN) neoplasms (Fig. 1).
Table 1.
High‐risk stigmata and worrisome features according to the revised Fukuoka consensus guidelines2
| High‐risk stigmata | Worrisome features |
|---|---|
|
Obstructive jaundice in a patient with cystic lesion of the head of the pancreas Enhancing mural nodule ≥ 5 mm Main pancreatic duct ≥ 10 mm |
Pancreatitis Cyst ≥ 30 mm Thickened/enhancing cystic walls Enhancing mural nodule < 5 mm Main pancreatic duct 5–9 mm Abrupt change in calibre of pancreatic duct with distal pancreatic atrophy Lymphadenopathy Increased serum level of carbohydrate antigen 19‐9 Cyst growth rate ≥ 5 mm/2 years |
Figure 1.
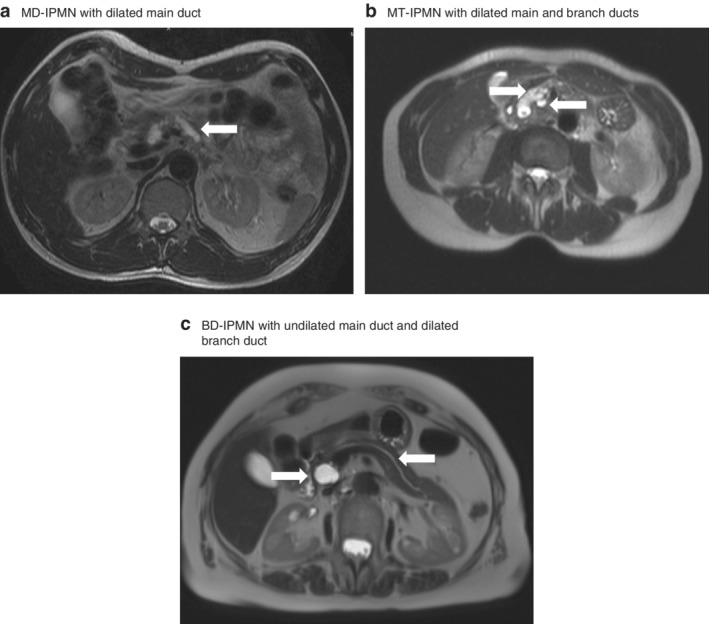
MRI scans illustrating the morphological aspects of intraductal papillary mucinous neoplasm subtypes. a Main‐duct intraductal papillary mucinous neoplasm (MD‐IPMN) showing dilated main pancreatic duct (arrow). b Mixed‐type (MT) IPMN with dilated main duct (left arrow) and branch duct (right arrow). c Branch‐duct (BD) IPMN with undilated main pancreatic duct (right arrow) and dilated branch duct (left arrow), with no involvement of main pancreatic duct
Current guidelines recommend resection of lesions with high‐risk stigmata, but in the context of BD‐IPMN, in particular, this is still a matter of debate3, 4, 5, 6, balancing the risk of progression to cancer with morbidity and mortality following pancreatic resection7, 8, in addition to risks of long‐term exocrine and endocrine insufficiency9.
The aim of this study was to evaluate the management, postoperative outcomes and survival of patients with radiologically suspected IPMNs.
Methods
Patients presenting with a cystic lesion or radiologically suspected IPMN at the Department of General and Visceral Surgery of the Medical Centre, University of Freiburg, between January 2010 and December 2014 were reviewed. Approximately 120 pancreatic resections are performed at this centre annually. The study was approved by the local ethics committee of the University of Freiburg (EK 142/17).
Cystic lesions were examined by MRI, or by CT if MRI was contraindicated. In selected patients, endoscopic ultrasonography and analysis of cyst fluid was performed. Follow‐up was performed according to Fukuoka consensus guidelines, usually every 3–6 months, with extended intervals for lesions that were stable during observation.
Cystic pancreatic tumours were categorized as IPMNs, serous cystic neoplasms (SCNs), mucinous cystic neoplasms (MCNs) and pancreatic pseudocysts.
Radiologically suspected IPMNs were classified as MD‐, MT‐ and BD‐IPMNs. Surgical resection was considered according to the Sendai or Fukuoka guidelines1, 2, and patients considered appropriate for surveillance were rescheduled for regular MRI or CT. The indication for surgical resection was reassessed throughout surveillance. Resection was also performed upon patient request for cystic tumours greater than 10 mm.
Morbidity and mortality following IPMN‐related resections were compared with rates in patients having resection of pancreatic ductal adenocarcinoma (PDAC) in the same time period. Complications were classified according to International Study Group of Pancreatic Surgery (ISGPS) definitions of postoperative pancreatic fistula (POPF)10, delayed gastric emptying (DGE)11 and postpancreatectomy haemorrhage (PPH)12, and the Clavien–Dindo general classification of postoperative complications13.
Statistical analysis
Variables are expressed as median (range) values or as numbers with percentages. Categorical variables were compared using Pearson's χ2 test. The Mann–Whitney U test was used for descriptive analysis of non‐parametric variables.
Survival data were obtained from the Comprehensive Cancer Center registry of the University of Freiburg Medical Centre. Disease‐specific mortality was defined as death related to pancreatic malignancy. Actuarial survival was calculated by univariable analysis using the Kaplan–Meier method, with log rank testing for comparison of subgroups.
Statistical analysis was performed using SPSS® version 24.0.0.1 (IBM, Armonk, New York, USA). P < 0·050 was considered statistically significant.
Results
Data from 269 patients with cystic pancreatic lesions were reviewed. Radiological findings were consistent with a possible IPMN in 142 patients (52·8 per cent); a MD‐IPMN was suspected in 26 patients, MT‐IPMN in eight and BD‐IPMN in 108. Seventy‐six of these 142 patients (53·5 per cent) were men, and the median age was 69 (range 36–88) years. Postoperative morbidity and mortality of IPMN‐related pancreatic resections in 53 patients were compared to 260 pancreatic resections undertaken for PDAC in the same period (January 2010 to December 2014).
Main‐duct intraductal papillary mucinous neoplasms
Surgery was recommended for 23 (88 per cent) of 26 patients with MD‐IPMN; three patients were deemed ineligible owing to age and co‐morbidity (Table 2).
Table 2.
Summary of the clinical course of different intraductal papillary mucinous neoplasm subtypes and postoperative histopathological examination
| BD‐IPMN | ||||||
|---|---|---|---|---|---|---|
| MD‐IPMN (n = 26) | MT‐IPMN (n = 8) | < 20 mm (n = 69) | 20–29 mm (n = 19) | ≥ 30 mm (n = 20) | ||
| Age* | 65 (52–83) | 68 (53–76) | 69 (36–82) | 70 (51–88) | 71 (47–77) | |
| Men | 16 (62) | 6 (75) | 34 (49) | 9 (47) | 11 (55) | |
| Family history of PDAC | 1 (4) | 0 (0) | 3 (4) | 0 (0) | 2 (10) | |
| Extrapancreatic tumour | 2 (8) | 2 (25) | 8 (12) | 5 (26) | 2 (10) | |
| Cyst size (mm)* | 22 (6–115) | 17 (8–70) | 11 (3–19) | 23 (20–29) | 34 (30–60) | |
| Cyst localization | Head | 16 (62) | 5 (63) | 18 (26) | 12 (63) | 13 (65) |
| Body | 2 (8) | 1 (13) | 13 (19) | 2 (11) | 0 (0) | |
| Tail | 2 (8) | 2 (25) | 14 (20) | 0 (0) | 4 (20) | |
| Multiple locations | 6 (23) | 0 (0) | 24 (35) | 5 (26) | 3 (15) | |
| Diagnostic procedure | MRI | 17 (65) | 7 (88) | 63 (91) | 16 (84) | 16 (80) |
| CT | 14 (54) | 2 (25) | 23 (33) | 7 (37) | 9 (45) | |
| EUS | 2 (8) | 0 (0) | 2 (3) | 0 (0) | 3 (15) | |
| Fukuoka classification | No risk factors | 0 (0) | 0 (0) | 57 (83) | 16 (84) | 0 (0) |
| Worrisome features | 2 (8) | 1 (13) | 8 (12) | 3 (16) | 19 (95) | |
| High‐risk stigmata | 24 (92) | 7 (88) | 4 (6) | 0 (0) | 1 (5) | |
| High‐risk stigmata | Jaundice | 2 (8) | 1 (13) | 1 (1) | 0 (0) | 0 (0) |
| Enhanced mural nodule ≥ 5 mm | 1 (4) | 0 (0) | 3 (4) | 0 (0) | 1 (5) | |
| Main duct dilatation ≥ 10 mm | 23 (88) | 6 (75) | 0 (0) | 0 (0) | 0 (0) | |
| Worrisome features | Pancreatitis | 9 (35) | 3 (38) | 6 (9) | 2 (11) | 3 (15) |
| Cyst ≥ 30 mm | 9 (35) | 0 (0) | 0 (0) | 0 (0) | 20 (100) | |
| Enhanced mural nodule < 5 mm | 1 (4) | 0 (0) | 2 (3) | 1 (5) | 2 (10) | |
| Thickened/enhanced cyst wall | 1 (4) | 0 (0) | 1 (1) | 0 (0) | 1 (5) | |
| Main duct dilatation 5–9 mm | 3 (12) | 2 (25) | 0 (0) | 0 (0) | 0 (0) | |
| Calibre change of main pancreatic duct | 3 (12) | 2 (25) | 0 (0) | 0 (0) | 0 (0) | |
| Lymphadenopathy | 5 (19) | 3 (38) | 1 (1) | 0 (0) | 0 (0) | |
| Raised serum CA19‐9 | 4 of 16 (25) | 3 (38) | 2 of 57 (4) | 0 of 16 (0) | 3 of 13 (23) | |
| Growth ≥ 5 mm/2 years | 1 of 2 (50) | 5 of 51 (10) | 1 of 12 (8) | 2 of 7 (29) | ||
| Suspicious cytology | 0 of 2 (0) | 1 of 2 (50) | 2 of 3 (67) | |||
| Symptoms | No symptoms | 4 (15) | 3 (38) | 38 (55) | 11 (58) | 11 (55) |
| Epigastric pain | 14 (54) | 4 (50) | 25 (36) | 6 (32) | 9 (45) | |
| Jaundice | 2 (8) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | |
| Weight loss | 2 (8) | 0 (0) | 6 (9) | 2 (11) | 0 (0) | |
| Other | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Pre‐existing conditions | Insulin‐dependent diabetes mellitus | 4 (15) | 2 (25) | 3 (4) | 5 (26) | 4 (20) |
| Cardiovascular | 10 (38) | 3 (38) | 24 (35) | 11 (58) | 9 (45) | |
| Pulmonary | 3 (12) | 0 (0) | 1 (1) | 0 (0) | 2 (10) | |
| Renal | 2 (8) | 1 (13) | 7 (10) | 3 (16) | 2 (10) | |
| Gastrointestinal | 15 (58) | 3 (38) | 38 (55) | 8 (42) | 12 (60) | |
| Neurological | 3 (12) | 0 (0) | 8 (12) | 3 (16) | 2 (10) | |
| Endocrine | 6 (23) | 0 (0) | 7 (10) | 0 (0) | 0 (0) | |
| Other | 6 (23) | 0 (0) | 21 (30) | 4 (21) | 4 (20) | |
| Primary resection | 19 (73) | 6 (75) | 10 (14) | 7 (37) | 13 (65) | |
| Not eligible for surgery | 3 | 2 | ||||
| Denied consent for primary resection | 4 | 1 | 1 | 1 | ||
| Surveillance | 7 (27) | 2 (25) | 59 (86) | 12 (63) | 7 (35) | |
| Secondary resection during follow‐up | 1 | 1 | 4 | 1 | 2 | |
| Denied consent for secondary resection | 3 | 1 | 1 | 1 | 2 | |
| Operation | 20 of 26 (77) | 7 of 8 (88) | 14 of 69 (20) | 8 of 19 (42) | 15 of 20 (75) | |
| PPPD | 14 of 20 (70) | 5 of 7 (71) | 5 of 14 (36) | 6 of 8 (75) | 11 of 15 (73) | |
| Whipple procedure | 1 of 20 (5) | |||||
| Distal pancreatectomy | 2 of 20 (10) | 2 of 7 (29) | 6 of 14 (43) | 2 of 8 (25) | 4 of 15 (27) | |
| Pancreatectomy | 3 of 20 (15) | |||||
| Central pancreatectomy | 2 of 14 (14) | |||||
| Enucleation | 1 of 14 (7) | |||||
| Histological examination | IPMN with low‐grade dysplasia | 5 of 20 (25) | 3 of 7 (43) | 5 of 14 (36) | 6 of 8 (75) | 4 of 15 (27) |
| IPMN with intermediate‐grade dysplasia | 4 of 20 (20) | 2 of 7 (29) | 2 of 14 (14) | 4 of 15 (27) | ||
| IPMN with high‐grade dysplasia | 1 of 7 (14) | 2 of 14 (14) | 1 of 15 (7) | |||
| IPMN‐carcinoma | 8 of 20 (40) | 1 of 7 (14) | 1 of 15 (7) | |||
| PDAC | 1 of 20 (5) | 1 (7%) | ||||
| SCN/MCN | 2 of 14 (14) | 1 of 8 (13) | 3 of 15 (20) | |||
| MCN‐associated adenocarcinoma | 1 of 14 (7) | |||||
| NET | 1 of 14 (7) | |||||
| Chronic pancreatitis | 1 of 20 (5) | 1 of 8 (13) | ||||
| Pancreatic pseudocyst | 1 of 15 (7) | |||||
| Other | 1 of 20 (5) | 1 of 14 (7) | ||||
| Malignant transformation during observation | 1 of 7 (14) | 1 of 59 (2) | 0 of 12 (0) | 2 of 7 (29) | ||
Values in parentheses are percentages unless indicated otherwise;
values are median (range). MD, main duct; IPMN, intraductal papillary mucinous neoplasm; MT, mixed type; BD, branch duct; PDAC, pancreatic ductal adenocarcinoma; EUS, endoscopic ultrasonography; CA, carbohydrate antigen; PPPD, pylorus‐preserving partial duodenopancreatectomy; SCN, serous cystic neoplasm; MCN, mucinous cystic neoplasm; NET, neuroendocrine tumour.
Surgical procedures consisted of pylorus‐preserving partial duodenopancreatectomy (PPPD) (13 patients), classic Whipple procedure (1), distal pancreatectomy (2) and total pancreatectomy (3). Histopathological examination showed five IPMNs with low‐grade dysplasia (25 per cent), four with intermediate‐grade dysplasia (20 per cent) and eight with IPMN‐associated carcinoma (40 per cent), and one each of PDAC, chronic pancreatitis and carcinosarcoma.
Four of these patients did not consent to surgery at first and decided to undergo surveillance. After an initial observation of 27 months, one of them underwent a PPPD and the histological findings were consistent with IPMN and low‐grade dysplasia. Of the six patients with suspected MD‐IPMN who did not have surgery, one died from metastatic pancreatic cancer after 2 years, one died from liver cirrhosis and four have been lost to follow‐up.
Mixed‐type intraductal papillary mucinous neoplasms
Of eight patients presenting with a radiological diagnosis of MT‐IPMN, seven underwent resection. Histological examination showed three IPMNs with low‐grade dysplasia (43 per cent), two with intermediate‐grade dysplasia (29 per cent), one high‐grade dysplasia (14 per cent) and one IPMN‐associated carcinoma (14 per cent) (Table 2).
Two patients opted not to have a resection initially. One of these patients had a resection after 24 months for low‐grade dysplasia, and the other remains under observation after 36 months with no signs of malignant transformation.
Branch‐duct intraductal papillary mucinous neoplasms
The 108 BD‐IPMNs were classified as lesions with no risk factors, those with worrisome features or those with high‐risk stigmata. The presence of risk factors together with age and co‐morbidity were all taken into account to determine further management.
Sixty‐nine (63·9 per cent) of the cystic lesions were less than 20 mm in size, 19 (17·6 per cent) were 20–29 mm, and 20 (18·5 per cent) were equal to or greater than 30 mm.
Of these 108 patients, 73 (67·6 per cent) had no risk factors for malignant transformation, 30 (27·8 per cent) had worrisome features and five (4·6 per cent) had high‐risk stigmata. Primary resection was performed in 30 patients (27·8 per cent) with BD‐IPMN (no risk factors, 6; worrisome features, 21; high‐risk stigmata, 3), and high‐grade dysplasia was detected in three patients (10 per cent). No IPMN‐carcinoma or PDAC was detected in the primary histological examination of BD‐IPMN lesions (Table 2).
Some 78 patients with BD‐IPMN underwent observation, mostly by MRI, with a median follow‐up of 47 (range 3–144) months. Of these patients, 67 (86 per cent) had no risk factors, nine (12 per cent) had worrisome features and two (3 per cent) had high‐risk stigmata.
During the study, 11 (14 per cent) of the 78 patients with BD‐IPMN who were initially considered for surveillance were referred for surgery, six owing to increasing size of the cysts, one for a newly developed neuroendocrine tumour of the pancreatic tail and four due to persisting worrisome features or high‐risk stigmata, already observed during their initial assessments. Of these patients, four had lesions smaller than 20 mm and one was found to have a pT1 pN0 cM0 MCN‐associated carcinoma (disease‐free at 36 months); the other resected patients had IPMN with low‐grade dysplasia or SCN on postoperative histopathological assessment. The malignant MCN was diagnosed owing to a rapid increase in cyst size, from 15 mm initially to 21 mm at 6 months.
In the subgroup of seven patients with BD‐IPMN of 30 mm or above (2 considered ineligible for surgery for medical reasons) who did not undergo primary resection despite worrisome features (6 patients) or high‐risk stigmata (1), surgery was recommended in four patients. Two of these patients did not consent to surgery and were lost to follow‐up, and two underwent resection. An IPMN‐associated carcinoma was found in one patient and PDAC diagnosed in the surgical specimen of the other.
The risk of malignant transformation during observation increased from 2 per cent in BD‐IPMNs smaller than 20 mm to 29 per cent in those of 30 mm or more (P = 0·005) (Table 2).
Perioperative morbidity and mortality following resection
The outcomes of 53 histologically documented IPMNs were compared with those of 260 pancreatic resections for PDAC performed over the same interval.
The rate of POPF grade B and C after pancreatic head resection for IPMN was higher than that seen after pancreatic head resection for PDAC: 12 of 33 (36 per cent) versus 41 of 221 (18·6 per cent) respectively (P = 0·010). Patients with resected IPMN had significantly more DGE grade B/C than those with resected PDAC (7 (21 per cent) versus 39 (17·6 per cent) respectively; P = 0·027) and more severe complications (Clavien–Dindo grade III: 15 (45 per cent) versus 56 (25·3 per cent); grade IV: 1 (3 per cent) versus 7 (3·2 per cent); grade V: 2 (6 per cent) versus 2 (0·9 per cent); P = 0·008) (Table 3).
Table 3.
Perioperative morbidity and mortality after pancreatic head resection
| IPMN (n = 33) | PDAC (n = 221) | P † | |
|---|---|---|---|
| Age (years)* | 68 (48–82) | 70 (31–86) | 0·375‡ |
| Men | 18 (55) | 111 (50·2) | 0·643 |
| BMI (kg/m2)* | 25·5 (16·9–37·4) | 24·6 (17·6–38·8) | 0·498‡ |
| ASA fitness grade | 0·200 | ||
| I | 3 (9) | 12 of 218 (5·5) | |
| II | 24 (73) | 126 of 218 (57·8) | |
| III | 6 (18) | 78 of 218 (35·8) | |
| IV | 0 (0) | 2 of 218 (0·9) | |
| Co‐morbidity | |||
| Coronary heart disease | 1 (3) | 36 (16·3) | 0·043 |
| Hypertension | 14 (42) | 125 (56·6) | 0·121 |
| Pulmonary disease | 4 (12) | 26 (11·8) | 0·960 |
| Kidney disease | 3 (9) | 28 (12·7) | 0·590 |
| Liver disease | 2 (6) | 21 (9·5) | 0·124 |
| Pre‐existing diabetes mellitus | 12 (36) | 55 (24·9) | 0·019 |
| Operation | 0·072 | ||
| Whipple procedure | 0 (0) | 20 (9·0) | |
| PPPD | 33 (100) | 201 (91·0) | |
| Surgical approach | 0·087 | ||
| Open surgery | 17 (52) | 159 (71·0) | |
| Laparoscopically assisted | 9 (27) | 31 (14·0) | |
| Laparoscopic | 1 (3) | 2 (0·9) | |
| Conversion rate | 6 of 16 (38) | 29 of 62 (46·8) | |
| Intraoperative blood loss (ml)* | 500 (100–2200) | 600 (0–5500) | 0·049‡ |
| Duration of surgery (min)* | 420 (170–631) | 425 (230–714) | 0·404‡ |
| Reconstruction | 0·124 | ||
| Pancreatogastrostomy | 23 (70) | 113 (51·1) | |
| Pancreatojejunostomy | 10 (30) | 105 (47·5) | |
| No reconstruction | 0 (0) | 3 (1·4) | |
| POPF grade | 0·010 | ||
| B | 8 (24) | 35 (15·8) | |
| C | 4 (12) | 6 (2·7) | |
| PPH grade B/C | 5 (15) | 19 (8·6) | 0·336 |
| DGE grade B/C | 7 (21) | 39 (17·6) | 0·027 |
| Clavien–Dindo complication grade | 0·008 | ||
| III | 15 (45) | 56 (25·3) | |
| IV | 1 (3) | 7 (3·2) | |
| V | 2 (6) | 2 (0·9) | |
| Reoperation | 10 (30) | 29 (13·1) | 0·011 |
| Intervention | 14 (42) | 52 (23·5) | 0·021 |
Values in parentheses are percentages unless indicated otherwise;
values are median (range). IPMN, intraductal papillary mucinous neoplasm; PDAC, pancreatic ductal adenocarcinoma; PPPD, pylorus‐preserving partial duodenopancreatectomy; POPF, postoperative pancreatic fistula; PPH, postpancreatectomy haemorrhage; DGE, delayed gastric emptying.
χ2 test, except
Mann–Whitney U test.
Following distal pancreatectomy for IPMN or PDAC, there were no significant differences in rates of clinically relevant POPF grade B/C (7 of 20 (35 per cent) versus 10 of 39 (26 per cent) respectively; P = 0·221), PPH grade B/C (1 (5 per cent) versus 3 (8 per cent); P = 0·697), DGE grade B/C (1 (5 per cent) versus 3 (8 per cent); P = 0·277), or morbidity and mortality (Clavien–Dindo grade III: 6 (30 per cent) versus 10 (26 per cent); grade IV: 0 (0 per cent) for both; grade V: 0 (0 per cent) versus 1 (3 per cent); P = 0·737) (Table 4).
Table 4.
Perioperative morbidity and mortality after distal pancreatectomy
| IPMN (n = 20) | PDAC (n = 39) | P † | |
|---|---|---|---|
| Age (years)* | 69 (53–83) | 66 (40–88) | 0·405‡ |
| Men | 11 (55) | 21 (54) | 0·933 |
| BMI (kg/m2)* | 24·4 (15·9–37·1) | 23·8 (17·9–32·9) | 0·798‡ |
| ASA fitness grade | 0·997 | ||
| I | 2 (10) | 4 (10) | |
| II | 12 (60) | 23 (59) | |
| III | 6 (30) | 12 (31) | |
| IV | 0 (0) | 0 (0) | |
| Co‐morbidity | |||
| Coronary heart disease | 2 (10) | 8 (21) | 0·308 |
| Hypertension | 14 (70) | 21 (54) | 0·232 |
| Pulmonary disease | 2 (10) | 4 (10) | 0·975 |
| Kidney disease | 4 (20) | 5 (13) | 0·468 |
| Liver disease | 0 (0) | 1 (3) | 0·470 |
| Pre‐existing diabetes mellitus | 5 (25) | 11 (28) | 0·880 |
| Surgical approach | 0·005 | ||
| Open surgery | 6 (30) | 28 (72) | |
| Laparoscopically assisted | 9 (45) | 4 (10) | |
| Laparoscopic | 2 (10) | 1 (3) | |
| Conversion rate | 3 of 14 (21) | 6 of 11 (55) | |
| Intraoperative blood loss (ml) | 350 (100–1000) | 550 (100–5500) | 0·181‡ |
| Duration of surgery (min) | 261 (151–391) | 314 (140–623) | 0·074‡ |
| Distal pancreatectomy with splenectomy | 9 (45) | 36 (92) | < 0·001 |
| POPF grade | 0·221 | ||
| B | 7 (35) | 8 (21) | |
| C | 0 (0) | 2 (5) | |
| PPH grade B/C | 1 (5) | 3 (8) | 0·697 |
| DGE grade B/C | 1 (5) | 3 (8) | 0·277 |
| Clavien–Dindo complication grade | 0·737 | ||
| III | 6 (30) | 10 (26) | |
| IV | 0 (0) | 0 (0) | |
| V | 0 (0) | 1 (3) | |
| Reoperation | 1 (5) | 8 (21) | 0·117 |
| Intervention | 5 (25) | 7 (18) | 0·524 |
Values in parentheses are percentages unless indicated otherwise;
values are median (range). IPMN, intraductal papillary mucinous neoplasm; PDAC, pancreatic ductal adenocarcinoma; POPF, postoperative pancreatic fistula; PPH, postpancreatectomy haemorrhage; DGE, delayed gastric emptying.
χ2 test, except
Mann–Whitney U test.
There was a higher rate of laparoscopic procedures for pancreatic head resections of IPMN than for PDAC. The outcomes of minimally invasive pancreatic head resections in these subgroups were compared; rates of POPF, postoperative interventions, reoperation, overall morbidity and mortality were significantly higher for IPMN (Table 5).
Table 5.
Perioperative morbidity and mortality after laparoscopic or laparoscopically assisted pancreatic head resection including conversion to open surgery
| IPMN (n = 16) | PDAC (n = 62) | P * | |
|---|---|---|---|
| POPF grade | 0·002 | ||
| B | 4 (25) | 12 (19) | |
| C | 2 (13) | 0 (0) | |
| PPH grade B/C | 3 (19) | 5 (8) | 0·400 |
| DGE grade B/C | 4 (25) | 8 (13) | 0·244 |
| Clavien–Dindo complication grade | 0·006 | ||
| III | 7 (44) | 13 (21) | |
| IV | 0 (0) | 1 (2) | |
| V | 2 (13) | 0 (0) | |
| Reoperation | 4 (25) | 3 (5) | 0·012 |
| Intervention | 8 (50) | 13 (21) | 0·020 |
Values in parentheses are percentages. IPMN, intraductal papillary mucinous neoplasm; PDAC, pancreatic ductal adenocarcinoma; POPF, postoperative pancreatic fistula; PPH, postpancreatectomy haemorrhage; DGE, delayed gastric emptying.
χ2 test.
Survival analysis of patients with intraductal papillary mucinous neoplasms
With a median follow‐up of 47 months, median survival was reached only for patients with MD‐IPMN (17 months).
The 5‐year OS rate was 44 per cent for the 26 patients with MD‐IPMN, 86 per cent for the eight with MT‐IPMN, and 97·4 per cent for the 108 with BD‐IPMN (P < 0·001). DSS was 60 per cent for MD‐IPMN, 100 per cent for MT‐IPMN and 98·6 per cent for BD‐IPMN (P < 0·001) (Fig. 2 a).
Figure 2.
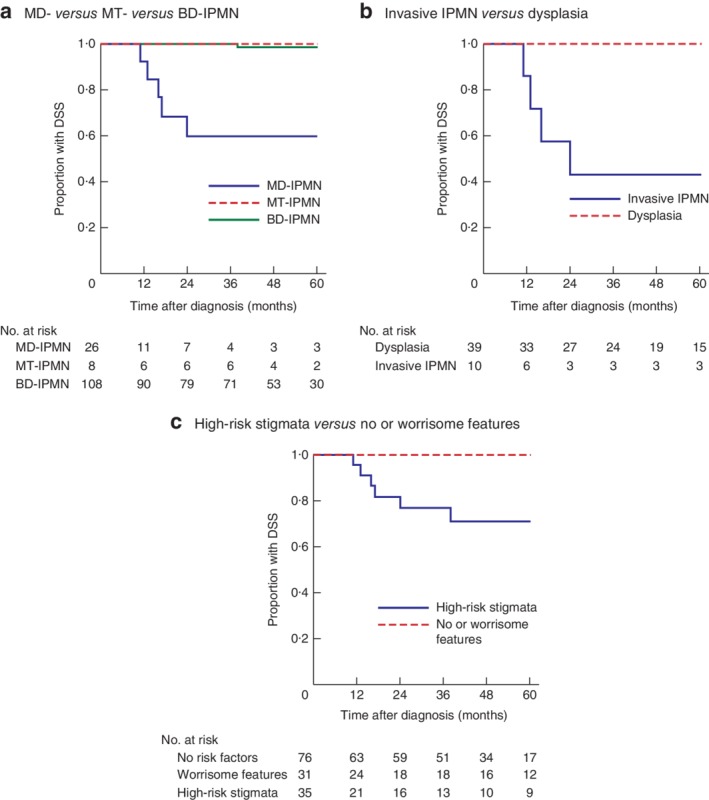
Kaplan–Meier analysis of disease‐specific survival of patients with intraductal papillary mucinous neoplasms. Comparison of disease‐specific survival (DSS) in: a patients with main‐duct (MD) intraductal papillary mucinous neoplasms (IPMNs) and those with mixed‐type (MT) and branch‐duct (BD) IPMNs; b patients with invasive IPMN‐carcinoma versus those with low‐, intermediate‐ and high‐grade dysplasia; and c patients with high‐risk stigmata versus those with worrisome features or no risk factors. a–c P < 0·001 (log rank test)
The 5‐year OS rate was also reduced in patients with invasive IPMN‐carcinoma compared with that in patients with non‐invasive dysplasia, despite oncological pancreatic resection: IPMN‐carcinoma, 33 per cent (10 patients); high‐grade dysplasia, 100 per cent (4 patients); intermediate‐grade dysplasia, 63 per cent (12 patients); low‐grade dysplasia, 100 per cent (23 patients); (P < 0·001). Five‐year DSS rates were also reduced (43 per cent for the 10 patients with IPMN‐carcinoma versus 100 per cent for the 39 with non‐invasive dysplasia; P < 0·001) (Fig. 2 b).
The 35 patients with high‐risk stigmata had poorer 5‐year survival than the 31 with worrisome features and the 76 with no risk factors (OS: 55, 95 and 100 per cent respectively; P < 0·001). Similar results were obtained for DSS (71, 100 and 100 per cent; P < 0·001) (Fig. 2 c).
Discussion
Results from this study are in line with the Sendai and Fukuoka recommendations1, 2 in terms of malignant transformation of IPMNs to pancreatic cancer. MD‐ and MT‐IPMN had high rates of high‐grade dysplasia, IPMN‐associated carcinoma and PDAC in the histopathological examination of surgical specimens. MD‐IPMN, in particular, showed malignant transformation in approximately 45 per cent of resected specimens: IPMN‐carcinoma in 40 per cent (8 of 20) and PDAC in 5 per cent (1 of 20), in accordance with previous findings14, 15.
The rate of high‐grade dysplasia and malignancy in BD‐IPMN was only 19 per cent (7 of 37), lower than in other series16, 17, although it was 29 per cent (4 of 14) in small BD‐IPMNs. Of note, in BD‐IPMNs undergoing surveillance, signs of malignant transformation rarely developed after initiating the observation. Patients with BD‐IPMNs smaller than 20 mm had an 8 per cent risk of developing factors necessitating a secondary resection, although the median follow‐up was only 47 months.
A recent study18 reported that an initial cyst size of 30 mm or above in BD‐IPMN was not associated with development of invasive IPMN or PDAC. In contrast, patients in the present study with primarily large cysts (at least 30 mm) who underwent initial surveillance had an increased risk (29 per cent) of malignant transformation during observation, compared with those with cysts smaller than 20 mm. It has also been reported19, 20, 21, 22, 23, 24 that patients with initially small cysts of less than 20 mm rarely develop signs of progression to malignancy, whereas those with larger cysts frequently develop additional risk factors.
As the indication for resecting IPMNs depends both on the risk of progression to cancer and on operative risk assessment, the consequences of surgery for IPMNs are important. An increased risk of perioperative morbidity in patients with IPMN has been suggested25, largely reflecting the risk of POPF as a result of the soft pancreatic texture that is often found in IPMN26. The present results appear to confirm this.
Survival analysis indicated that MD‐IPMN was associated with disease‐specific mortality. Patients with high‐risk stigmata also had decreased DSS, whereas worrisome features and the diagnosis of IPMN without risk factors were not associated with mortality independent of the degree of dysplasia, unless invasive IPMN occurred. This supports previous reports27, 28, 29 of the low risk of progression of IPMN with worrisome features, but higher mortality, in patients with high‐risk stigmata under surveillance.
The present study also confirms the recently reported accurate prediction rate of progression to PDAC by the Fukuoka risk stratification30.
The main limitation of this study is the median follow‐up period of 47 (range 3–144) months. The risk of progression of IPMN is not limited to 5 years, and malignant transformation can occur in the long‐term follow‐up of patients24. Therefore, the need for a longer follow‐up and large high‐quality registries must be acknowledged.
From this series, application of the Fukuoka criteria2 for managing IPMNs would seem to be safe. This includes surveillance for specific categories and resection of IPMNs with high‐risk stigmata. The histopathological degree of dysplasia did not correlate with the outcome unless IPMN‐associated carcinoma was present.
Pancreatic resection of IPMN is associated with increased perioperative risk. The decision to proceed to resection merits careful consideration in elderly patients and those with major co‐morbidities.
Disclosure
The authors declare no conflict of interest.
Funding information
No funding
References
- 1. Tanaka M, Fernández‐del Castillo C, Adsay V, Chari S, Falconi M, Jang JY et al; International Association of Pancreatology . International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012; 12: 183–197. [DOI] [PubMed] [Google Scholar]
- 2. Tanaka M, Fernández‐Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T et al Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017; 17: 738–753. [DOI] [PubMed] [Google Scholar]
- 3. Uhl W, Belyaev O, Herzog T, Mueller CA, Seelig M, Schmidt W et al [Intraductal papillary mucinous neoplasms of the pancreas: pro surgical therapy – pro surveillance.] Z Gastroenterol 2008; 46: 1290–1297. [DOI] [PubMed] [Google Scholar]
- 4. Brunner M, Weber GF, Kersting S, Grützmann R. [Branch duct intraductal papillary mucinous neoplasm – contra resection.] Chirurg 2017; 88: 918–926. [DOI] [PubMed] [Google Scholar]
- 5. Goess R, Ceyhan GO, Friess H. Pancreatic exocrine insufficiency after pancreatic surgery. Panminerva Med 2016; 58: 151–159. [PubMed] [Google Scholar]
- 6. Maker AV, Sheikh R, Bhagia V; Diabetes Control and Complications Trial (DCCT) Research Group . Perioperative management of endocrine insufficiency after total pancreatectomy for neoplasia. Langenbecks Arch Surg 2017; 402: 873–883. [DOI] [PubMed] [Google Scholar]
- 7. Hackert T, Tjaden C, Büchler MW. [Developments in pancreatic surgery during the past ten years.] Zentralbl Chir 2014; 139: 292–300. [DOI] [PubMed] [Google Scholar]
- 8. McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME et al Perioperative mortality for pancreatectomy: a national perspective. Ann Surg 2007; 246: 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weinberg BM, Spiegel BM, Tomlinson JS, Farrell JJ. Asymptomatic pancreatic cystic neoplasms: maximizing survival and quality of life using Markov‐based clinical nomograms. Gastroenterology 2010; 138: 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M et al; International Study Group on Pancreatic Surgery (ISGPS) . The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017; 161: 584–591. [DOI] [PubMed] [Google Scholar]
- 11. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR et al Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007; 142: 761–768. [DOI] [PubMed] [Google Scholar]
- 12. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ et al Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007; 142: 20–25. [DOI] [PubMed] [Google Scholar]
- 13. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marchegiani G, Mino‐Kenudson M, Sahora K, Morales‐Oyarvide V, Thayer S, Ferrone C et al IPMN involving the main pancreatic duct: biology, epidemiology, and long‐term outcomes following resection. Ann Surg 2015; 261: 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hackert T, Fritz S, Klauss M, Bergmann F, Hinz U, Strobel O et al Main‐duct intraductal papillary mucinous neoplasm: high cancer risk in duct diameter of 5 to 9 mm. Ann Surg 2015; 262: 875–881. [DOI] [PubMed] [Google Scholar]
- 16. Wong J, Weber J, Centeno BA, Vignesh S, Harris CL, Klapman JB et al High‐grade dysplasia and adenocarcinoma are frequent in side‐branch intraductal papillary mucinous neoplasm measuring less than 3 cm on endoscopic ultrasound. J Gastrointest Surg 2013; 17: 78–85. [DOI] [PubMed] [Google Scholar]
- 17. Fritz S, Klauss M, Bergmann F, Hackert T, Hartwig W, Strobel O et al Small (Sendai negative) branch‐duct IPMNs: not harmless. Ann Surg 2012; 256: 313–320. [DOI] [PubMed] [Google Scholar]
- 18. Nagata N, Kawazoe A, Mishima S, Wada T, Shimbo T, Sekine K et al Development of pancreatic cancer, disease‐specific mortality, and all‐cause mortality in patients with nonresected IPMNs: a long‐term cohort study. Radiology 2016; 278: 125–134. [DOI] [PubMed] [Google Scholar]
- 19. Kaiser J, Büchler MW, Hackert T. [Branch duct intraductal papillary mucinous neoplasm – surgical approach.] Chirurg 2017; 88: 927–933. [DOI] [PubMed] [Google Scholar]
- 20. Kim KW, Park SH, Pyo J, Yoon SH, Byun JH, Lee MG et al Imaging features to distinguish malignant and benign branch‐duct type intraductal papillary mucinous neoplasms of the pancreas: a meta‐analysis. Ann Surg 2014; 259: 72–81. [DOI] [PubMed] [Google Scholar]
- 21. Anand N, Sampath K, Wu BU. Cyst features and risk of malignancy in intraductal papillary mucinous neoplasms of the pancreas: a meta‐analysis. Clin Gastroenterol Hepatol 2013; 11: 913–921. [DOI] [PubMed] [Google Scholar]
- 22. Sadakari Y, Ienaga J, Kobayashi K, Miyasaka Y, Takahata S, Nakamura M et al Cyst size indicates malignant transformation in branch duct intraductal papillary mucinous neoplasm of the pancreas without mural nodules. Pancreas 2010; 39: 232–236. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M et al Branch‐duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology 2007; 133: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pergolini I, Sahora K, Ferrone CR, Morales‐Oyarvide V, Wolpin BM, Mucci LA et al Long‐term risk of pancreatic malignancy in patients with branch duct intraductal papillary mucinous neoplasm in a referral center. Gastroenterology 2017; 153: 1284–1294.e1. [DOI] [PubMed] [Google Scholar]
- 25. Newhook TE, LaPar DJ, Lindberg JM, Bauer TW, Adams RB, Zaydfudim VM. Morbidity and mortality of pancreaticoduodenectomy for benign and premalignant pancreatic neoplasms. J Gastrointest Surg 2015; 19: 1072–1077. [DOI] [PubMed] [Google Scholar]
- 26. Keck T, Wellner UF, Bahra M, Klein F, Sick O, Niedergethmann M et al Pancreatogastrostomy versus pancreatojejunostomy for RECOnstruction after PANCreatoduodenectomy (RECOPANC, DRKS 00000767): perioperative and long‐term results of a multicenter randomized controlled trial. Ann Surg 2016; 263: 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crippa S, Bassi C, Salvia R, Malleo G, Marchegiani G, Rebours V et al Low progression of intraductal papillary mucinous neoplasms with worrisome features and high‐risk stigmata undergoing non‐operative management: a mid‐term follow‐up analysis. Gut 2017; 66: 495–506. [DOI] [PubMed] [Google Scholar]
- 28. Del Chiaro M, Ateeb Z, Hansson MR, Rangelova E, Segersvärd R, Kartalis N et al Survival analysis and risk for progression of intraductal papillary mucinous neoplasia of the pancreas (IPMN) under surveillance: a single‐institution experience. Ann Surg Oncol 2017; 24: 1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vanella G, Crippa S, Archibugi L, Arcidiacono PG, Delle Fave G, Falconi M et al Meta‐analysis of mortality in patients with high‐risk intraductal papillary mucinous neoplasms under observation. Br J Surg 2018; 105: 328–338. [DOI] [PubMed] [Google Scholar]
- 30. Mukewar S, de Pretis N, Aryal‐Khanal A, Ahmed N, Sah R, Enders F et al Fukuoka criteria accurately predict risk for adverse outcomes during follow‐up of pancreatic cysts presumed to be intraductal papillary mucinous neoplasms. Gut 2017; 66: 1811–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]


