Abstract
Background
The aim of this network meta‐analysis was to compare the performance of blue dye alone or in combination with radioisotope (technetium‐99m, Tc) with three novel techniques for sentinel lymph node detection in breast cancer: indocyanine green fluorescence (ICG), superparamagnetic iron oxide (SPIO) nanoparticles and contrast‐enhanced ultrasound imaging (CEUS).
Methods
PubMed, Embase, the Cochrane Library, China Knowledge Research Integrated Database, ClinicalTrials.gov and OpenGrey databases were searched up to 31 November 2017, without language restriction. Studies that compared the detection performance of at least one of the novel methods (ICG, SPIO and CEUS) with that of traditional methods (blue dye and/or radioisotope) were included in network meta‐analysis.
Results
Thirty‐five studies were included. Pooled risk ratios (RRs) for Tc (1·09, 95 per cent c.i. 1·04 to 1·15), ICG (1·12, 1·07 to 1·16) and SPIO (1·09, 1·01 to 1·18) showed statistically better performance in detecting sentinel lymph nodes than blue dye alone. ICG had the lowest false‐negative rate, with a RR of 0·29 (0·16 to 0·54), followed by Tc (RR 0·44, 0·20 to 0·96) and SPIO (RR 0·45, 0·14 to 1·45), with blue dye alone as the reference group.
Conclusion
SPIO or ICG alone are superior to blue dye alone and comparable to the standard dual‐modality technique of blue dye with Tc.
Introduction
Lymphatic staging of breast cancer was initially established by axillary lymph node dissection followed by sentinel lymph node biopsy1. There may be more than one sentinel node, depending on the location of the tumour and the network of surrounding lymph vessels2. For patients with early breast cancer and clinical and radiological absence of axillary lymph node metastases, sentinel lymph node biopsy is currently considered the standard of care3.
The most widely used technique for sentinel lymph node identification is the dual‐modality method involving the injection of technetium‐99m (Tc)‐labelled nanocolloid and blue dye (BD) into the peritumoral or periareolar region4. ‘Hot’ nodes detected using a handheld scintillation counter (γ‐probe) and ‘blue’ nodes identified during surgery are both recognized as sentinel nodes and removed, followed by histopathological confirmation of metastases. An identification rate of 96 per cent was reported in a large meta‐analysis5 of 8000 patients. In another meta‐analysis6 of more than 9000 patients, the false‐negative rate was 5·9 per cent with the dual‐modality technique, in comparison with 8·6 and 7·4 per cent for dye‐only and tracer‐only techniques respectively6. The dual technique, nevertheless, has shortcomings, including radiation exposure to healthcare professionals and patients, issues with radiotracer availability, dependency on availability of nuclear medicine units and allergic reactions to BD.
New techniques have been developed to improve the clinical value of sentinel lymph node biopsy with similar accuracy, but avoiding irradiation and risks of allergy. Novel techniques studied in recent years include those using indocyanine green (ICG) fluorescence, superparamagnetic iron oxide (SPIO) nanoparticles, and contrast‐enhanced ultrasound imaging using microbubbles (CEUS). A systematic review by Ahmed and colleagues7 in 2014 reported that these techniques showed clinical potential but had high false‐negative rates. The review was based on pairwise meta‐analysis and was unable to compare relative performance between techniques not previously studied in a direct comparison. The aim of the present network meta‐analysis was to compare these techniques directly and indirectly in terms of detection and false‐negative rates.
Methods
Literature search and eligibility criteria
A literature search was performed in electronic databases, including PubMed, Embase, the Cochrane Library, China Knowledge Research Integrated Database, ClinicalTrials.gov and OpenGrey, up to 31 November 2017. The specific concepts used in the search strategy were ‘Breast Neoplasms’, ‘Sentinel Lymph Node Biopsy’, ‘Indocyanine Green’, ‘Superparamagnetic Iron Oxide’ and ‘Contrast‐enhanced Ultrasound’. Medical Subject Headings (MeSH) or Emtree, and free‐text terms were both searched (Table S1, supporting information). There were no restrictions on language. All bibliographies listed in relevant review papers and included publications were also checked.
Two reviewers independently searched for eligible studies based on predefined eligibility criteria. Studies that compared the detection performance of at least one of the novel methods (ICG, SPIO and CEUS) with that of traditional methods (BD and/or Tc) were included. Single‐arm studies that assessed only one of the methods, reviews, case reports, animal studies, commentaries and letters to editors were excluded. Discrepancies were discussed between reviewers until consensus was reached.
Data extraction and quality assessment
The following data were extracted from the included studies: study characteristics (publication year, number of subjects); baseline characteristics (mean age and recruitment method); and outcome events (number of detections and number of false‐negative reports). Relevant data were also extracted for quality assessment.
A network geometry was constructed based on the included studies8. Each node represented various techniques, and the size was weighted by the number of subjects from whom data on each technique were derived. The connecting line between two nodes represented the presence of a direct comparison, and the number of studies included determined the thickness of each line. Hence, the thicker the line, the more studies comparing the two methods were included.
The quality of each study was evaluated by two independent investigators, using the Risk Of Bias in Non‐randomized Studies of Interventions (ROBINS‐I) tool9. Seven domains (confounding, selection of participants into the study, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes and selection of the reported result) were evaluated to assess the risk of bias. For each of the seven domains in the ROBINS‐I tool, responses to the signalling questions were taken together to arrive at the judgement of low, moderate, serious or critical risk of bias, and judgements within each domain were summarized to provide an overall risk‐of‐bias judgement for the outcome being assessed. Any disagreement in quality assessment was resolved by discussion and consensus.
Statistical analysis
A network meta‐analysis, comparing the detection methods for the proposed outcomes, was performed using a multivariable meta‐regression model with random effects, adopting a frequentist approach. The model allowed the inclusion of potential co‐variables and accounted for correlations from multiarm trials. Risk ratios (RRs) with 95 per cent confidence intervals were estimated for detection rate and false‐negative rate.
To rank the effect size of all the methods, surface under the cumulative ranking (SUCRA) value, indicating the probability of each method being the best, was used10. A higher SUCRA value indicated a higher rank of performance for the method.
To check for the assumption of consistency, the design‐by‐treatment approach was used. This approach tested whether the difference between RRs from direct comparison (either by 2‐arm or multiarm design) and those from network meta‐analysis was statistically significant. The χ2 test was used to test the difference throughout the entire network, and P < 0·050 indicated the need for further investigation to identify the sources of inconsistency11.
The network meta‐analyses were generated using Stata/MP® version 13 with network and network graphs packages12, 13, 14.
Results
Study characteristics and network geometry
Of 567 studies identified from the initial search, 35 cohort studies15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 (4244 subjects) satisfied the inclusion and exclusion criteria, and were included in this meta‐analysis (Fig. 1, Table 1). Among the studies included, 2215,16,20–23,26,27,30–32,34,35,38–41,43–45,47,49 reported on the performance of ICG, eight17, 19, 24, 25, 29, 33, 37, 42 on SPIO and five18, 28, 36, 46, 48 on CEUS. The mean age of included subjects was 57 years.
Figure 1.
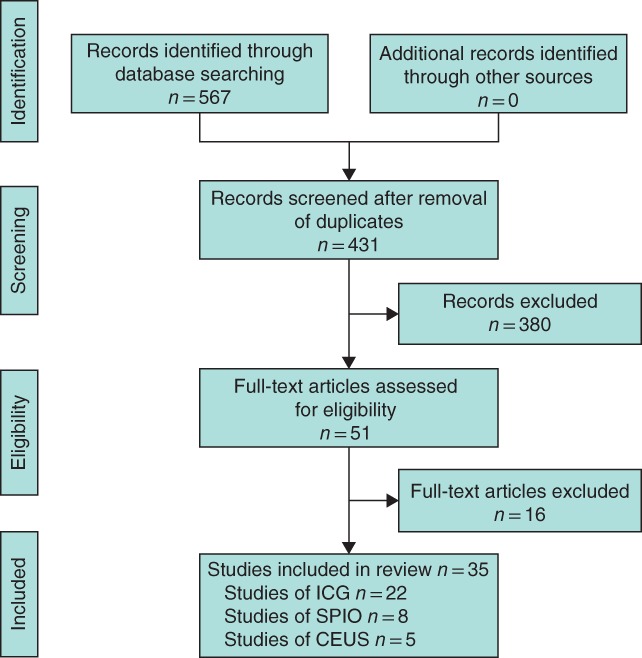
PRISMA flow chart showing selection of studies for review. ICG, indocyanine green; SPIO, superparamagnetic iron oxide; CEUS, contrast‐enhanced ultrasound imaging
Table 1.
Baseline characteristics of included studies
| Reference | Country | Methods compared | No. of subjects | Mean age (years) | Recruitment method |
|---|---|---|---|---|---|
| Abe et al.15 | Japan | BD, ICG | 128 | 52·6 | Non‐consecutive |
| Ballardini et al.16 | Italy | Tc, ICG | 134 | 56 | Non‐consecutive |
| Douek et al.17 | UK, Netherlands | Tc/BD, SPIO | 160 | n.a. | Non‐consecutive |
| Esfehani et al.18 | Iran | Tc/BD, CEUS | 50 | 53 | Non‐consecutive |
| Ghilli et al.19 | Italy | Tc, SPIO | 193 | 61 | Consecutive |
| Grischke et al.20 | Germany | Tc, ICG | 105 | 57·5 | Non‐consecutive |
| Guo et al.21 | China | BD, ICG | 86 | 52·3 | Consecutive |
| Hirano et al.22 | Japan | BD, ICG | 108 | 60·5 | Non‐consecutive |
| Hojo T et al.23 | Japan | BD, ICG | 131 | 57·6 | Non‐consecutive |
| Houpeau et al.24 | France | Tc/BD, SPIO | 108 | 58 | Non‐consecutive |
| Karakatsanis et al.25 | Sweden | Tc, SPIO | 206 | 61·7 | Non‐consecutive |
| Mieog et al.26 | USA | BD, Tc, ICG | 24 | 59·5 | Consecutive |
| Murawa et al.27 | Japan | Tc, ICG | 20 | 56 | Non‐consecutive |
| Omoto et al.28 | Japan | BD, Tc, CEUS | 20 | 50·9 | Non‐consecutive |
| Piñero‐Madrona et al.29 | Spain | Tc/BD, SPIO | 181 | 56 | Non‐consecutive |
| Pitsinis et al.30 | UK | BD, ICG | 50 | 48 | Consecutive |
| Polom et al.31 | Poland | Tc, ICG | 13 | 55 | Consecutive |
| Rauch et al.32 | Austria | BD, Tc, ICG | 98 | 61·9 | Consecutive |
| Rubio et al.33 | Spain | Tc, SPIO | 118 | n.a. | Non‐consecutive |
| Samorani et al.34 | Japan | Tc, ICG | 301 | n.a. | Consecutive |
| Schaafsma et al.35 | Netherlands | Tc, BD, ICG | 32 | 56 | Consecutive |
| Sever et al.36 | UK | BD, Tc, CEUS | 54 | 60·5 | Consecutive |
| Shiozawa et al.37 | Japan | BD, SPIO | 30 | 58·2 | Non‐consecutive |
| Stoffels et al.38 | Germany | Tc, ICG | 80 | 55 | Non‐consecutive |
| Sugie et al.39 | Japan | BD, ICG | 99 | 60 | Non‐consecutive |
| Sugie et al.40 | Japan | Tc, ICG | 821 | 55 | Consecutive |
| Tagaya et al.41 | Japan | BD, ICG | 25 | n.a. | Consecutive |
| Thill et al.42 | Germany | Tc, SPIO | 150 | 57·6 | Non‐consecutive |
| Tong et al.43 | China | BD, ICG | 96 | 54·2 | Non‐consecutive |
| van der Vorst et al.44 | Netherlands | Tc, BD, ICG | 24 | 60·5 | Consecutive |
| Verbeek et al.45 | Netherlands, USA | Tc, BD, ICG | 95 | 57 | Non‐consecutive |
| Wang et al.46 | China | BD, CEUS | 46 | 50·5 | Consecutive |
| Wishart et al.47 | UK | Tc, BD, ICG | 99 | n.a. | Non‐consecutive |
| Xie et al.48 | China | BD, CEUS | 101 | n.a. | Consecutive |
| Yamamoto et al.49 | Japan | BD, ICG | 258 | 57 | Consecutive |
BD, blue dye alone; ICG, indocyanine green; Tc, technetium‐99m; Tc/BD, combined use of technetium‐99m and blue dye; SPIO, superparamagnetic iron oxide; n.a., not available; CEUS, contrast‐enhanced ultrasound imaging.
The network geometry summarized the studies visually (Fig. 2). Tc, BD and ICG were the three most reported detection methods. Tc, BD and ICG fluorescence groups included most of the subjects. There were no direct comparisons between ICG, SPIO and CEUS.
Figure 2.
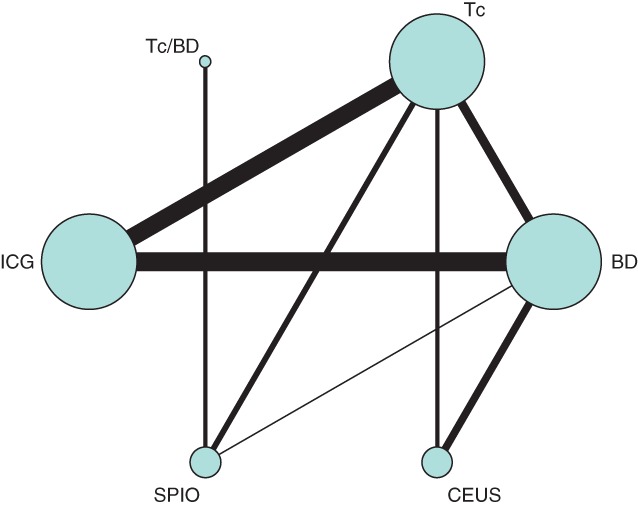
Network geometry of included studies. Tc/BD, combined use of technetium‐99m and blue dye; Tc, technetium‐99m; ICG, indocyanine green; BD, blue dye; SPIO, superparamagnetic iron oxide; CEUS, contrast‐enhanced ultrasound imaging
Quality evaluation of all the studies demonstrated a low to moderate risk of bias in the seven domains assessed, and 20 of 35 had a moderate risk of bias on overall assessment (Table S2, supporting information). Moderate risk of bias was assigned in terms of patient selection to studies where the details of recruitment were not clearly reported.
Detection rate
A total of 35 studies15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 involving 4244 subjects were included in the analysis of detection rate. Pooled detection rates for each method are shown in Table 2. Pooled RRs for Tc (1·09, 95 per cent c.i. 1·04 to 1·15), ICG (1·12, 1·07 to 1·16) and SPIO (1·09, 1·01 to 1·18) showed statistically better performance in detecting the sentinel lymph node, approximately 1·1 times better, than BD alone (Table 3). Risk differences (RD) compared with BD were 8·0 (95 per cent c.i. 3·7 to 12·3), 10·5 (6·8 to 14·2) and 6·8 (–0·3 to 14·0) per cent for Tc, ICG and SPIO respectively. RRs for the dual‐modality technique (Tc/BD) and CEUS were 1·09 (0·98 to 1·16) and 1·03 (0·98 to 1·11) respectively compared with BD, but were not statistically significant. RDs were 5·9 (–2·4 to 14·2) and 3·6 (–2·5 to 9·6) per cent respectively.
Table 2.
Pooled estimates of detection rate and false‐negative rate for each method
| Detection rate (%) | False‐negative rate (%) | |
|---|---|---|
| ICG | 97·9 (96·9, 98·9) | 0·6 (–0·3, 1·5) |
| SPIO | 97·4 (96·3, 98·6) | 4·0 (1·9, 6·1) |
| CEUS | 92·8 (86·7, 98·8) | 10·5 (1·7, 19·4) |
| Tc | 96·5 (95·2, 97·9) | 2·6 (0·7, 4·6) |
| Tc/BD | 96·7 (94·3, 99·1) | 5·5 (0·9, 10·2) |
| BD | 86·8 (82·7, 91·0) | 18·4 (11·9, 24·9) |
Values in parentheses are 95 per cent confidence intervals. ICG, indocyanine green; SPIO, superparamagnetic iron oxide; CEUS, contrast‐enhanced ultrasound imaging; Tc, technetium‐99m; Tc/BD, combined use of technetium‐99m and blue dye; BD, blue dye alone.
Table 3.
Pooled risk ratios for detection and false‐negative rates from network meta‐analysis
| Test method | Reference method | ||||
|---|---|---|---|---|---|
| SPIO | CEUS | Tc | Tc/BD | BD | |
| ICG | |||||
| RR for detection | 1·03 (0·96, 1·11) 0·67 (0·21, 2·08) | 1·08 (1·00, 1·15) 0·16 (0·52, 0·05) | 1·03 (0·99, 1·06) 0·64 (0·36, 1·13) | 1·05 (0·96, 1·14) 0·56 (0·14, 2·23) | 1·12 (1·07, 1·16)* 0·29 (0·16, 0·54)* |
| RR for FN rate | |||||
| SPIO | |||||
| RR for detection | 1·03 (0·95, 1·12) 0·24 (0·05, 1·08) | 0·99 (0·93, 1·06) 0·96 (0·34, 2·75) | 1·01 (0·95, 1·07) 0·84 (0·36, 1·93) | 1·09 (1·01, 1·18)* 0·45 (0·14, 1·45) | |
| RR for FN rate | |||||
| CEUS | |||||
| RR for detection | 0·96 (0·89, 1·02) 4·00 (1·17, 13·7)* | 0·98 (0·90, 1·06) 3·49 (0·67, 18·2) | 1·03 (0·98, 1·11) 1·85 (0·68, 6·06) | ||
| RR for FN rate | |||||
| Tc | |||||
| RR for detection | 1·00 (0·91, 1·10) 0·87 (0·24, 3·23) | 1·09 (1·04, 1·15)* 0·44 (0·20, 0·96)* | |||
| RR for FN rate | |||||
| Tc/BD | |||||
| RR for detection | 1·09 (0·98, 1·16) | ||||
| RR for FN rate | 0·57 (0·13, 2·51) | ||||
Values in parentheses are 95 per cent confidence intervals. Risk ratios (RRs) are shown for test versus reference technique. SPIO, superparamagnetic iron oxide; CEUS, contrast‐enhanced ultrasound imaging; Tc, technetium‐99m; Tc/BD, combined use of technetium‐99m and blue dye; BD, blue dye alone; ICG, indocyanine green; FN, false‐negative. *P < 0·050 (multivariable metaregression model with random effects).
ICG had a relatively better detection performance than CEUS (RR 1·08, 1·00 to 1·15), but not SPIO (RR 1·03, 0·96 to 1·11). Absolute RDs were 3·7 (–3·4 to 10·7) per cent for ICG over SPIO, and 7·0 (0·4 to 13·5) per cent for ICG over CEUS.
The ranking for performance in terms of detection rate, using SUCRA values, is shown in Table 4. The rank from highest to lowest was ICG, SPIO, Tc, Tc/BD, CEUS and BD. Of note, Tc/BD had almost the same SUCRA as Tc (60·9 and 61·3 per cent respectively).
Table 4.
Summary of SUCRA values and mean ranks by detection and false‐negative rates
| Detection rate | False‐negative rate | |||
|---|---|---|---|---|
| SUCRA (%) | Rank | SUCRA (%) | Rank | |
| ICG | 85·7 | 1 | 90·7 | 1 |
| SPIO | 63·8 | 2 | 65·6 | 2 |
| Tc | 61·3 | 3 | 64·1 | 3 |
| Tc/BD | 60·9 | 4 | 50·4 | 4 |
| CEUS | 23·0 | 5 | 4·5 | 6 |
| BD | 5·3 | 6 | 24·6 | 5 |
SUCRA, surface under the cumulative ranking; ICG, indocyanine green; SPIO, superparamagnetic iron oxide; Tc, technetium‐99m; Tc/BD, combined use of technetium‐99m and blue dye; CEUS, contrast‐enhanced ultrasound imaging; BD, blue dye alone.
False‐negative rate
Some 25 studies15, 17, 19, 20, 21, 22, 23, 24, 25, 27, 28, 29, 32, 33, 34, 35, 37, 38, 39, 40, 41, 42, 43, 45, 48 were included in the analysis of false‐negative rate. Pooled false‐negative rates for each technique are shown in Table 2. The lowest RR was seen in the ICG group (0·29, 95 per cent c.i. 0·16 to 0·54) followed by Tc (RR 0·44, 0·20 to 0·96) and SPIO (RR 0·45, 0·14 to 1·45), using BD as the reference group (Table 3). Similarly, RDs showed a reduction of 18·1 (95 per cent c.i. –24·2 to –12·0) per cent for ICG versus BD, 16·1 (–22·8 to –9·4) per cent for Tc versus BD and 15·5 (–23·2 to 7·9) per cent for SPIO versus BD. The risk of a false‐negative result with CEUS was twice that for BD (RR 1·85, 0·68 to 6·06).
The SUCRA results for false‐negative rate showed a similar pattern to those for detection rate; CEUS and BD had a low SUCRA rank of 6 and 5 respectively, which translates to higher false‐negative rate compared with other modalities (Table 4).
Network consistency
Forest plots comparing pooled estimates from both direct comparison and network meta‐analysis were reported for detection rate (Fig. S1, supporting information) and false‐negative rate (Fig. S2, supporting information). On visual check, greater consistency existed among pooled estimates from all two‐arm studies, all multiarm studies and network meta‐analysis. The consistency assumption was examined statistically using the χ2 test, which yielded P = 0·316 for detection rate and P = 0·948 for false‐negative rate, indicating no evidence for the potential violation of assumption. There was, therefore, no statistically significant evidence of inconsistency.
Discussion
In this review, the ICG fluorescence technique ranked first in terms of detection, as well as having the lowest false‐negative rate of the techniques compared. The ranking of other techniques in descending order was SPIO, Tc, dual modality (Tc/BD), CEUS and BD in terms of detection rate.
The use of ICG fluorescence for sentinel lymph node biopsy in patients with breast cancer was first described in 1999 by Motomura and colleagues50, who reported an identification rate of 73·8 per cent. The main advantages of this method are accurate placement of the skin incision as subcutaneous lymphatic channels can be identified on the skin before operation, and accuracy is improved with the use of fluorescence as there is high sensitivity with illumination even if the nodes do not stain green. On the contrary, problems and technical difficulties associated with this technique include difficulty with ICG fluorescence detection for lymph nodes more than 1 cm below the skin, need for operating theatre lights to be turned off during fluorescence navigation, and diffusion of ICG into subcutaneous tissue if the procedure takes more than 30 min, reducing the sensitivity of fluorescence detection. In a 2016 meta‐analysis51, the pooled detection rate for this technique was 0·98 (95 per cent c.i. 0·96 to 0·99) with a false‐negative rate of 8 per cent. The main limitation of the meta‐analysis was that only six studies were included in the final analysis. The present meta‐analysis included 22 studies that used ICG. This technique achieved a significant 12 per cent improvement in terms of detection rate and a 71 per cent lower false‐negative rate compared with BD.
SPIO nanoparticles (Sienna+®; Sysmex Europe, Hamburg, Germany) have gained interest since the first description of their use in 201352. SPIO nanoparticles stay in the tissue for a prolonged period and can be detected with a magnetometer25. This characteristic has made it feasible for SPIO to be injected in the outpatient clinic 3–15 days before surgery, with a success rate comparable to that of Tc/BD53. A meta‐analysis54 of seven randomized trials in 2016 showed that the SPIO method was not inferior to the standard technique in identification rate (97·1 versus 96·8 per cent), total lymph nodes retrieved (1·9 versus 1·8 nodes per patient) or false‐negative rate (8·4 versus 10·9 per cent). Using both direct and indirect comparisons, the present analysis found that SPIO performed significantly better than BD in terms of detection rate. Although the RR compared with BD for false‐negative rate was not statistically significant, a 55 per cent decrease on the point estimate suggested a promising direction for the use of SPIO in sentinel lymph node biopsy.
CEUS with microbubbles, based on the use of dispersion with sulphur hexafluoride gas injected intradermally around the areola, for sentinel node biopsy was first reported in 201355. In a subsequent meta‐analysis of five studies56, this technique identified sentinel lymph nodes in 9·3–55·2 per cent of patients, with a sensitivity of 61–89 per cent and a false‐negative rate of 6·6–39 per cent. No comparative study of CEUS against the dual‐modality technique exists to validate this method. In the present analysis, there was no statistical difference between CEUS and BD in terms of detection rate, but the risk of a false‐negative result was four times that for the Tc method (RR 4·00, 95 per cent c.i. 1·17 to 13·70). The high false‐negative rate might be attributable to inconsistent identification of sentinel lymph nodes owing to interobserver variability, even when the same ultrasound equipment is used. The introduction of super‐resolution imaging, ultrafast ultrasound imaging and improved microbubble transit may improve the visualization of lymphatics and sentinel lymph nodes with this technique55.
It is acknowledged that this review has inherent limitations as evaluation of the performance of each technique was limited to detection and false‐negative rates. From a clinical standpoint, the strength of a particular modality is also dependent on other factors such as ease of use and side‐effects. It would be of interest to investigate other details pertaining to the use of these techniques, such as dose and site of injection, to see how these affect performance. Limitations in terms of statistical analysis related to the lack of RCTs, although the risk of bias was generally low to moderate among the included studies.
The strength of the present review is the use of network meta‐analysis to allow direct and indirect comparisons between techniques for sentinel lymph node identification and false‐negative rates. Previous systematic reviews evaluated various techniques qualitatively, rather than quantitatively, making conclusions less convincing. By synthesizing direct and indirect evidence using network meta‐analysis, this study was able to rank the detection and false‐negative rates across all techniques quantitatively.
Both ICG and SPIO consistently performed better than BD alone in this review. Based on this finding, these methods could provide a reliable alternative in centres where BD is used as a single modality, as the use of either technique would result in better detection and false‐negative rates. Although it is interesting to note that these two novel methods had similar performance to radioactive colloid in terms of detection as well as false‐negative rates, technical, logistical and economic factors should be taken into consideration. Studies comparing novel methods with standard dual‐modality techniques are still needed, in the hope of demonstrating superior performance.
In this network meta‐analysis, Tc, ICG and SPIO demonstrated superior performance over BD in terms of detection and false‐negative rate. Future cost‐effectiveness analyses will be crucial to determine whether the novel techniques are sustainable in the long run.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1. Search strategies
Table S2. Risk of bias assessment
Fig. S1. Forest plot of all direct comparison on detection rate together with pooled estimates from network meta‐analysis
Fig. S2. Forest plot of all direct comparison on false negative rate together with pooled estimates from network meta‐analysis
References
- 1. Noguchi M, Tsugawa K, Bando E, Kawahara F, Miwa K, Yokoyama K et al Sentinel lymphadenectomy in breast cancer: identification of sentinel lymph node and detection of metastases. Breast Cancer Res Treat 1999; 53: 97–104. [DOI] [PubMed] [Google Scholar]
- 2. Kurtzman SH. The role of sentinel node biopsy in breast cancer. Conn Med 1999; 63: 5–6. [PubMed] [Google Scholar]
- 3. Reintgen D, Giuliano R, Cox CE. Sentinel node biopsy in breast cancer: an overview. Breast J 2000; 6: 299–305. [DOI] [PubMed] [Google Scholar]
- 4. Teal CB, Slocum JP, Akin EA. Evaluation of the benefit of using blue dye in addition to radioisotope for sentinel lymph node biopsy in patients with breast cancer. Breast J 2005; 11: 391–393. [DOI] [PubMed] [Google Scholar]
- 5. Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early‐stage breast carcinoma: a metaanalysis. Cancer 2006; 106: 4–16. [DOI] [PubMed] [Google Scholar]
- 6. Pesek S, Ashikaga T, Krag LE, Krag D. The false‐negative rate of sentinel node biopsy in patients with breast cancer: a meta‐analysis. World J Surg 2012; 36: 2239–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol 2014; 15: e351–e362. [DOI] [PubMed] [Google Scholar]
- 8. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C et al The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–784. [DOI] [PubMed] [Google Scholar]
- 9. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaimani A, Salanti G. Visualizing assumptions and results in network meta‐analysis: the network graphs package. Stata J 2015; 15: 905–950. [Google Scholar]
- 11. White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Res Synth Methods 2012; 3: 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS One 2013; 8: e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. StataCorp . Stata 13. StataCorp: College Station, 2014. [Google Scholar]
- 14. White IR. Network meta‐analysis. Stata J 2015; 15: 951–985. [Google Scholar]
- 15. Abe H, Mori T, Umeda T, Tanaka M, Kawai Y, Shimizu T et al Indocyanine green fluorescence imaging system for sentinel lymph node biopsies in early breast cancer patients. Surg Today 2011; 41: 197–202. [DOI] [PubMed] [Google Scholar]
- 16. Ballardini B, Santoro L, Sangalli C, Gentilini O, Renne G, Lissidini G et al The indocyanine green method is equivalent to the 99mTc‐labeled radiotracer method for identifying the sentinel node in breast cancer: a concordance and validation study. Eur J Surg Oncol 2013; 39: 1332–1336. [DOI] [PubMed] [Google Scholar]
- 17. Douek M, Klaase J, Monypenny I, Kothari A, Zechmeister K, Brown D et al; SentiMAG Trialists Group. Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG Multicentre Trial. Ann Surg Oncol 2014; 21: 1237–1245. [DOI] [PubMed] [Google Scholar]
- 18. Esfehani MH, Yazdankhah‐Kenari A, Omranipour R, Mahmoudzadeh HA, Shahriaran S, Zafarghandi MR et al Validation of contrast enhanced ultrasound technique to wire localization of sentinel lymph node in patients with early breast cancer. Indian J Surg Oncol 2015; 6: 370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghilli M, Carretta E, Di Filippo F, Battaglia C, Fustaino L, Galanou I et al The superparamagnetic iron oxide tracer: a valid alternative in sentinel node biopsy for breast cancer treatment. Eur J Cancer Care (Engl) 2017; 26. [DOI] [PubMed] [Google Scholar]
- 20. Grischke EM, Röhm C, Hahn M, Helms G, Brucker S, Wallwiener D. ICG fluorescence technique for the detection of sentinel lymph nodes in breast cancer: results of a prospective open‐label clinical trial. Geburtshilfe Frauenheilkd 2015; 75: 935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo W, Zhang L, Ji J, Gao W, Liu J, Tong M. Evaluation of the benefit of using blue dye in addition to indocyanine green fluorescence for sentinel lymph node biopsy in patients with breast cancer. World J Surg Oncol 2014; 12: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirano A, Kamimura M, Ogura K, Kim N, Hattori A, Setoguchi Y et al A comparison of indocyanine green fluorescence imaging plus blue dye and blue dye alone for sentinel node navigation surgery in breast cancer patients. Ann Surg Oncol 2012; 19: 4112–4116. [DOI] [PubMed] [Google Scholar]
- 23. Hojo T, Nagao T, Kikuyama M, Akashi S, Kinoshita T. Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast 2010; 19: 210–213. [DOI] [PubMed] [Google Scholar]
- 24. Houpeau JL, Chauvet MP, Guillemin F, Bendavid‐Athias C, Charitansky H, Kramar A et al Sentinel lymph node identification using superparamagnetic iron oxide particles versus radioisotope: the French Sentimag feasibility trial. J Surg Oncol 2016; 113: 501–507. [DOI] [PubMed] [Google Scholar]
- 25. Karakatsanis A, Christiansen PM, Fischer L, Hedin C, Pistioli L, Sund M et al The Nordic SentiMag trial: a comparison of super paramagnetic iron oxide (SPIO) nanoparticles versus Tc99 and patent blue in the detection of sentinel node (SN) in patients with breast cancer and a meta‐analysis of earlier studies. Breast Cancer Res Treat 2016; 157: 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mieog JS, Troyan SL, Hutteman M, Donohoe KJ, van der Vorst JR, Stockdale A et al Toward optimization of imaging system and lymphatic tracer for near‐infrared fluorescent sentinel lymph node mapping in breast cancer. Ann Surg Oncol 2011; 18: 2483–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murawa D, Hirche C, Dresel S, Hünerbein M. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg 2009; 96: 1289–1294. [DOI] [PubMed] [Google Scholar]
- 28. Omoto K, Matsunaga H, Take N, Hozumi Y, Takehara M, Omoto Y et al Sentinel node detection method using contrast‐enhanced ultrasonography with sonazoid in breast cancer: preliminary clinical study. Ultrasound Med Biol 2009; 35: 1249–1256. [DOI] [PubMed] [Google Scholar]
- 29. Piñero‐Madrona A, Torró‐Richart JA, De León‐Carrillo JM, de Castro‐Parga G, Navarro‐Cecilia J, Domínguez‐Cunchillos F et al; Grupo de Estudios Senológicos de la Sociedad Española de Patologia Mamaria (SESPM). Superparamagnetic iron oxide as a tracer for sentinel node biopsy in breast cancer: a comparative non‐inferiority study. Eur J Surg Oncol 2015; 41: 991–997. [DOI] [PubMed] [Google Scholar]
- 30. Pitsinis V, Provenzano E, Kaklamanis L, Wishart GC, Benson JR. Indocyanine green fluorescence mapping for sentinel lymph node biopsy in early breast cancer. Surg Oncol 2015; 24: 375–379. [DOI] [PubMed] [Google Scholar]
- 31. Polom K, Murawa D, Michalak M, Murawa P. Sentinel node biopsy in breast cancer using infrared laser system first experience with PDE camera. Rep Pract Oncol Radiother 2011; 16: 82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rauch S, Haid A, Jasarevic Z, Saely CH, Becherer A, Wenzl E. Does BMI affect the detection of sentinel lymph nodes with indocyanine green in early breast cancer patients? Eur Surg 2017; 49: 165–170. [Google Scholar]
- 33. Rubio IT, Diaz‐Botero S, Esgueva A, Rodriguez R, Cortadellas T, Cordoba O et al The superparamagnetic iron oxide is equivalent to the Tc99 radiotracer method for identifying the sentinel lymph node in breast cancer. Eur J Surg Oncol 2015; 41: 46–51. [DOI] [PubMed] [Google Scholar]
- 34. Samorani D, Fogacci T, Panzini I, Frisoni G, Accardi FG, Ricci M et al The use of indocyanine green to detect sentinel nodes in breast cancer: a prospective study. Eur J Surg Oncol 2015; 41: 64–70. [DOI] [PubMed] [Google Scholar]
- 35. Schaafsma BE, Verbeek FP, Rietbergen DD, van der Hiel B, van der Vorst JR, Liefers GJ et al Clinical trial of combined radio‐ and fluorescence‐guided sentinel lymph node biopsy in breast cancer. Br J Surg 2013; 100: 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sever A, Jones S, Cox K, Weeks J, Mills P, Jones P. Preoperative localization of sentinel lymph nodes using intradermal microbubbles and contrast‐enhanced ultrasonography in patients with breast cancer. Br J Surg 2009; 96: 1295–1299. [DOI] [PubMed] [Google Scholar]
- 37. Shiozawa M, Lefor AT, Hozumi Y, Kurihara K, Sata N, Yasuda Y et al Sentinel lymph node biopsy in patients with breast cancer using superparamagnetic iron oxide and a magnetometer. Breast Cancer 2013; 20: 223–229. [DOI] [PubMed] [Google Scholar]
- 38. Stoffels I, Dissemond J, Pöppel T, Schadendorf D, Klode J. Intraoperative fluorescence imaging for sentinel lymph node detection: prospective clinical trial to compare the usefulness of indocyanine green vs technetium Tc 99m for identification of sentinel lymph nodes. JAMA Surg 2015; 150: 617–623. [DOI] [PubMed] [Google Scholar]
- 39. Sugie T, Sawada T, Tagaya N, Kinoshita T, Yamagami K, Suwa H et al Comparison of the indocyanine green fluorescence and blue dye methods in detection of sentinel lymph nodes in early‐stage breast cancer. Ann Surg Oncol 2013; 20: 2213–2218 [DOI] [PubMed] [Google Scholar]
- 40. Sugie T, Kinoshita T, Masuda N, Sawada T, Yamauchi A, Kuroi K et al Evaluation of the clinical utility of the ICG fluorescence method compared with the radioisotope method for sentinel lymph node biopsy in breast cancer. Ann Surg Oncol 2016; 23: 44–50. [DOI] [PubMed] [Google Scholar]
- 41. Tagaya N, Yamazaki R, Nakagawa A, Abe A, Hamada K, Kubota K et al Intraoperative identification of sentinel lymph nodes by near‐infrared fluorescence imaging in patients with breast cancer. Am J Surg 2008; 195: 850–853. [DOI] [PubMed] [Google Scholar]
- 42. Thill M, Kurylcio A, Welter R, van Haasteren V, Grosse B, Berclaz G et al The Central‐European SentiMag study: sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast 2014; 23: 175–179. [DOI] [PubMed] [Google Scholar]
- 43. Tong M, Guo W, Gao W. Use of fluorescence imaging in combination with patent blue dye versus patent blue dye alone in sentinel lymph node biopsy in breast cancer. J Breast Cancer 2014; 17: 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van der Vorst JR, Schaafsma BE, Verbeek FP, Hutteman M, Mieog JS, Lowik CW et al Randomized comparison of near‐infrared fluorescence imaging using indocyanine green and 99m technetium with or without patent blue for the sentinel lymph node procedure in breast cancer patients. Ann Surg Oncol 2012; 19: 4104–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Verbeek FP, Troyan SL, Mieog JS, Liefers GJ, Moffitt LA, Rosenberg M et al Near‐infrared fluorescence sentinel lymph node mapping in breast cancer: a multicenter experience. Breast Cancer Res Treat 2014; 143: 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y, Zhou W, Li C, Gong H, Li C, Yang N et al Variation of sentinel lymphatic channels (SLCs) and sentinel lymph nodes (SLNs) assessed by contrast‐enhanced ultrasound (CEUS) in breast cancer patients. World J Surg Oncol 2017; 15: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wishart GC, Loh SW, Jones L, Benson JR. A feasibility study (ICG‐10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur J Surg Oncol 2012; 38: 651–656. [DOI] [PubMed] [Google Scholar]
- 48. Xie F, Zhang D, Cheng L, Yu L, Yang L, Tong F et al Intradermal microbubbles and contrast‐enhanced ultrasound (CEUS) is a feasible approach for sentinel lymph node identification in early‐stage breast cancer. World J Surg Oncol 2015; 13 : 319 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamamoto S, Maeda N, Yoshimura K, Oka M. Intraoperative detection of sentinel lymph nodes in breast cancer patients using ultrasonography‐guided direct indocyanine green dye‐marking by real‐time virtual sonography constructed with three‐dimensional computed tomography–lymphography. Breast 2013; 22: 933–937. [DOI] [PubMed] [Google Scholar]
- 50. Motomura K, Inaji H, Komoike Y, Kasugai T, Noguchi S, Koyama H. Sentinel node biopsy guided by indocyanine green dye in breast cancer patients. Jpn J Clin Oncol 1999; 29: 604–607. [DOI] [PubMed] [Google Scholar]
- 51. Zhang X, Li Y, Zhou Y, Mao F, Lin Y, Guan J et al Diagnostic performance of indocyanine green‐guided sentinel lymph node biopsy in breast cancer: a meta‐analysis. PLoS One 2016; 11: e0155597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thill M, Kurylcio A, Blechmann R Van Haasteren V, Grosse B, Berclaz G et al The SentiMag Study: sentinel node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Eur J Cancer 2013; 49: S260–S261. [DOI] [PubMed] [Google Scholar]
- 53. Karakatsanis A, Oloffson H, Bergkvist LA, Abdsaleh S, Sund M, Wärnberg F. How to avoid unnecessary sentinel node biopsy in patients with ductal cancer in situ . Breast 2015; 24: S133–S134. [Google Scholar]
- 54. Zada A, Peek MC, Ahmed M, Anninga B, Baker R, Kusakabe M et al Meta‐analysis of sentinel lymph node biopsy in breast cancer using the magnetic technique. Br J Surg 2016; 103: 1409–1419. [DOI] [PubMed] [Google Scholar]
- 55. Cox K, Sever A, Jones S, Weeks J, Mills P, Devalia H et al Validation of a technique using microbubbles and contrast enhanced ultrasound (CEUS) to biopsy sentinel lymph nodes (SLN) in pre‐operative breast cancer patients with a normal grey‐scale axillary ultrasound. Eur J Surg Oncol 2013; 39: 760–765. [DOI] [PubMed] [Google Scholar]
- 56. Gkegkes ID, Iavazzo C. Contrast enhanced ultrasound (CEU) using microbubbles for sentinel lymph node biopsy in breast cancer: a systematic review. Acta Chir Belg 2015; 115: 212–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strategies
Table S2. Risk of bias assessment
Fig. S1. Forest plot of all direct comparison on detection rate together with pooled estimates from network meta‐analysis
Fig. S2. Forest plot of all direct comparison on false negative rate together with pooled estimates from network meta‐analysis


