Abstract
Clinically approved cancer therapies include small molecules, antibodies, and nanoparticles. There has been major progress in the treatment of several cancer types over recent decades. However, many challenges remain for optimal use of conventional and nanoparticle-based therapies in oncology including poor drug delivery, rapid clearance, and drug resistance. The antimalarial agent chloroquine has been found to mitigate some of these challenges by modulating cancer cells and the tissue microenvironment. Particularly, chloroquine was recently found to reduce immunological clearance of nanoparticles by resident macrophages in the liver, leading to increased tumor accumulation of nanodrugs. Additionally, chloroquine has been shown to improve drug delivery and efficacy through normalization of tumor vasculature and suppression of several oncogenic and stress-tolerance pathways, such as autophagy, that protect cancer cells from cytotoxic agents. This review will discuss the use of chloroquine as combination therapy to improve cancer treatment.
Keywords: autophagy, cancer, chloroquine, liver, nanomedicine, vasculature
1. Introduction
Cancer is one of the most challenging diseases to treat and a leading cause of morbidity and mortality (Gao, 2008). Effective treatment of cancer requires the ability to overcome challenges such as poor drug delivery, rapid clearance, drug resistance, and patient tolerability (Gao, 2008). Although cancer therapeutics seek to interfere with biological processes that are more predominant in cancer cells, normal cells are also impacted, leading to toxic effects in patients (Gao, 2008). Additionally, conventional anti-cancer drugs are distributed throughout the body, as opposed to preferentially accumulating in cancerous tissues (Wolfram, Shen, et al. 2015). Consequently, administration of lower drug doses is often necessary, which limits therapeutic efficacy and may promote drug resistance. There is a pressing need for higher potency and site-specific delivery strategies. Multipronged solutions that reduce non-tumor tissue accumulation, modulate the tumor microenvironment for improved drug delivery, and prevent protective cancer cell processes should be developed for effective treatment.
Various nanodrugs have been developed, several clinically approved, to improve site-specific drug delivery and in many instances enhance safety and potency of therapy (Anselmo & Mitragotri, 2016; Ferrari, 2010; Gentile, et al., 2013; Wilhelm, et al., 2016; Wolfram, Zhu, et al., 2015). Nanodelivery is thought to increase site-specific accumulation of drugs from 0.001–0.01% to 1% of the systemically injected dose (Wolfram, Shen, et al., 2015). Despite the potential of nanomedicine, there are major challenges that remain (S. Shen, et al., 2017; Venuta, et al., 2017). For example, over 90% of intravenously injected nanoparticles are taken up by the liver (Bae & Park, 2011; Borrelli, et al., 2018; Zhang, et al., 2016), primarily by tissue resident Kupffer cells (Gustafson, et al., 2015; Samuelsson, et al., 2017). Certain properties of the tumor microenvironment can also limit effective drug delivery. For instance, tumors typically have abnormal and disorganized vasculature that contributes to insufficient and heterogeneous intratumoral drug delivery (Jain, 2012; Mazzone, et al., 2009). Cancer cells have also developed protective mechanisms to avoid cell death upon exposure to drugs, which remains an issue despite use of nanocarriers. For example, enhancement of autophagy in response to cell stress can have anti-apoptotic effects that desensitize cancer cells to therapy (Chen, et al., 2010; White & DiPaola, 2009).
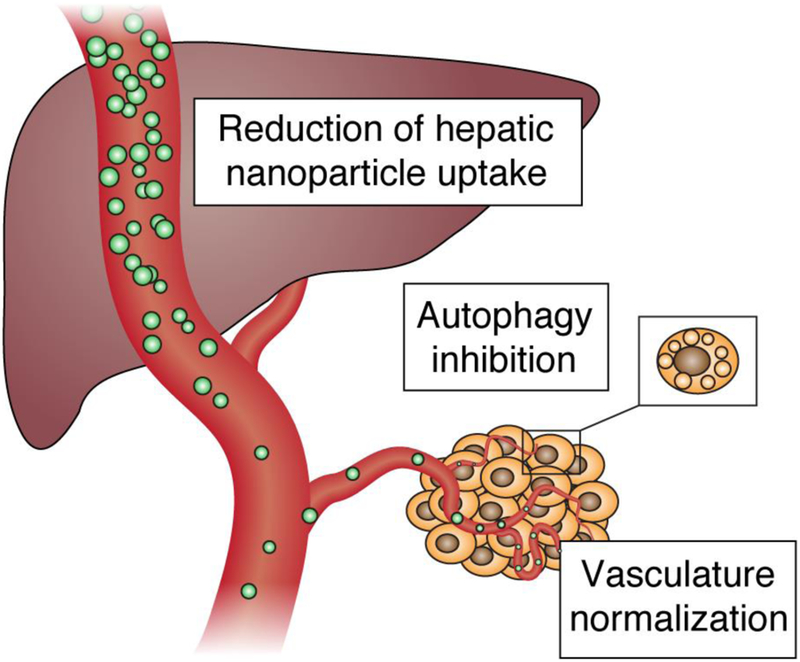
One promising strategy to overcome some of the current limitation with cancer therapeutics involves the use of the clinically approved antimalarial agent chloroquine, which has been administered to patients for over 60 years (Wolfram, et al., 2017). In malaria infection, chloroquine inhibits heme polymerase activity in Plasmodium parasites, thereby leading to the buildup of free heme, a substance toxic to the parasite (Hempelmann, 2007). Chloroquine has also over the years demonstrated an ability to interfere with physiological processes in mammalian cells, a property that can be exploited for cancer therapy (Fig. 1). For example, chloroquine blocks cell autophagy through prevention of the final steps of this process (Gonzalez-Polo, et al., 2005; Matthew Redmann, 2017), subsequently making tumor cells more susceptible to drug-induced apoptosis (Amaravadi, et al., 2007). Chloroquine also causes normalization of tumor vasculature, which improves drug delivery (Maes, et al., 2014). In regard to nanomedicines, chloroquine has more recently been shown to substantially reduce nanoparticle accumulation in the liver by affecting macrophage function (Wolfram, et al., 2017). The safety profile of chloroquine is well known due to decades of use in malaria treatment and prophylaxis. Chloroquine has also been studied in combination therapy with chemotherapeutic agents in cancer patients and has shown acceptable toxicity levels (Montanari, et al., 2014).
Fig. 1.
Chloroquine effects that can be exploited for cancer therapy. Chloroquine reduces the uptake of nanoparticles by Kupffer cells, inhibits survival-promoting autophagy, and normalizes tumor vasculature for improved drug delivery.
2. Chloroquine-induced inhibition of autophagy
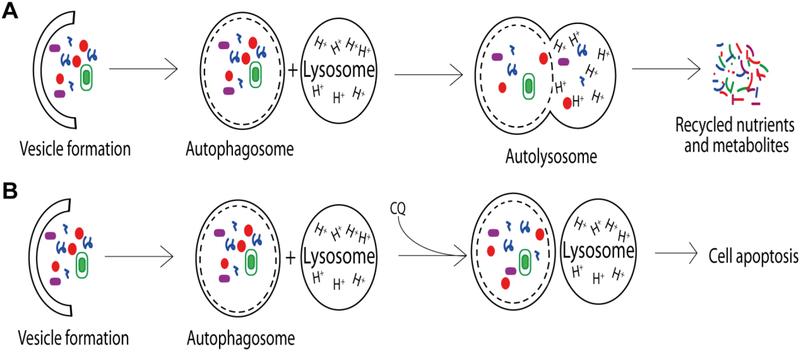
Autophagy is a cellular process that involves clearance and recycling of cytoplasmic components, including protein aggregates and damaged organelles, via transport in autophagic vesicles (AVs) to lysosomes for degradation (Fig. 2) (White, 2012). This process occurs in most living cells and frequently takes place as a result of cell damage, pathogen exposure, or starvation (Amaravadi, et al., 2011). It is believed that autophagy enhances cell survival by converting cell debris into recyclable nutrients and metabolites (Cicchini, et al., 2015; Kuma & Mizushima, 2010).
Fig. 2.
The autophagy processes. (A) Defective biomolecules and organelles are degraded through the autophagy pathway. A vesicle forms around particles in the cytosol, forming an autophagosome. An acidic lysosome fuses with the autophagosome to form an autolysosome in which degradation occurs. The pathway produces recyclable nutrients and metabolites that promote cell viability in stressful environments. (B) Chloroquine disrupts the autophagy pathway by decreasing the acidity of the lysosome, preventing the formation of the autolysosome. Inhibition of autophagy sensitizes cancer cells to environmental stress and increases rates of apoptosis.
Cancer cells with functional autophagy pathways have displayed increased cell viability compared to cells with defective autophagy (Amaravadi, et al., 2011). In cancer cells autophagy is enhanced to increase availability of metabolic precursors and provide protection from high-stress microenvironments within tumors, such as those with hypoxia and low levels of nutrients (Amaravadi, et al., 2011; Cicchini, et al., 2015). For example, autophagy increases metabolites for ATP production, which are required for proper DNA replication and repair.
Chloroquine is able to function as an effective autophagy inhibitor (Amaravadi, et al., 2007), as this drug is a weak base that becomes diprotonated, and entrapped in lysosomes, causing an increase in lysosomal pH (Amaravadi, et al., 2011). This pH elevation inhibits the final steps in the autophagy pathway, leading to buildup of autophagic vesicles in the cytoplasm, which prevents the production and recycling of important nutrients and metabolites and leads to cell damage and ultimately cell death (Amaravadi, et al., 2011; Gonzalez-Polo, et al., 2005). Therefore, exposure of cancer cells to chloroquine can lead to inhibition of tumor growth and apoptosis (Carew, et al., 2007; Degenhardt, et al., 2006; Ding, et al., 2009; Katayama M, et al., 2007; Torgersen, et al., 2013; White & DiPaola, 2009). Tumor types that have a V600E mutation in v-Raf murine sarcoma viral oncogene homolog B (BRAF) exploit autophagy as a metabolic advantage. For example, several central nervous system (CNS) cancer cell lines with the BRAF mutation displayed enhanced sensitivity to vemurafenib, cisplatin, or vinblastin when combined with chloroquine (Levy, et al., 2014). The same study also demonstrated that chloroquine was able to overcome drug resistance in a vemurafenib resistant cancer cell line (Levy, et al., 2014). Chloroquine has been shown to increase the therapeutic efficacy of anticancer agents that increase autophagy, such as histone deacetylase inhibitors (Torgersen, et al., 2013). For example, in Kasumi-1 acute myeloid leukemia (AML) cells, chloroquine caused an additional 30–40% reduction in the viability of cells exposed to the histone deacetylase inhibitors valproic acid or vorinostat (Torgersen, et al., 2013). In summary, chloroquine is a promising anticancer agent for combination therapy due the ability of this drug to improve the therapeutic efficacy of conventional cancer drugs through synergistic effects, such as prevention of undesirable therapy-induced autophagy.
However, there is currently a pressing need for a context-dependent understanding of autophagy, as this process may also have tumor suppressing effects. It has been shown that autophagy is a mediator of cell death in certain instances (Qu, et al., 2003) and has anti-inflammatory properties, as degradation of defective proteins and debris prevents inflammation (Hara, et al., 2006; Levine, et al., 2011). Thus, it is important to elucidate the role of autophagy in different tumor subtypes for optimization of cancer therapy.
3. Chloroquine-induced normalization of tumor vasculature
Tumors typically have a vascular architecture that differs from that of normal tissues, which have well-organized and homogenous vasculature (Baluk, et al., 2005; Nagy, et al., 2009; Nagy, et al., 2010). For example, tumors contain many irregularly branched tortuous blood vessels that lack pericytes, which are contractile cells that synthetize basal membrane and provide structural support for endothelial cells (Nagy, et al., 2009). As a result, there is enhanced permeability and inadequate blood flow to some tumor regions, causing hypoxia, starvation, and necrosis (Carmeliet & Jain, 2011). In addition to preventing efficient drug delivery, these vascular network abnormalities have other disadvantages, such as increased cancer cell invasion and impaired immune responses due to hypoxia (Carmeliet & Jain, 2011). Studies have shown that normalization of tumor vasculature can increase drug delivery and reduce metastasis (Chauhan, et al., 2012; Hamzah, et al., 2008; Jiang, et al., 2015; Khalid, et al., 2017; Mazzone, et al., 2009).
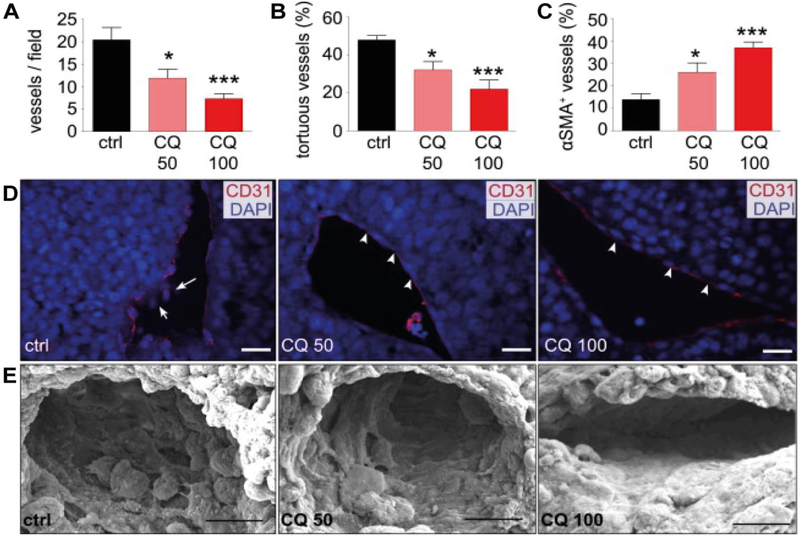
Chloroquine has been shown to cause tumor vasculature normalization in a dose-dependent manner in a murine model of melanoma (Maes, et al., 2014). In particular, chloroquine decreased tumor vasculature density (up to 70%) and tortuosity (up to 50%), and increased the presence of alpha-smooth muscle actin (α-SMA) (Maes, et al., 2014), which is a contractile protein characteristic of pericytes (Fig. 3 A – C) (Bergers & Song, 2005). Chloroquine treatment also promoted vessel differentiation as demonstrated by immunostaining for the endothelial cell marker cluster differentiation 31 (CD31), which displayed a more organized pattern in response to chloroquine (Fig. 3D). In addition, scanning electron microscopy (SEM) imaging revealed that the blood vessel wall had a smoother and more continuous structure following treatment (Fig. 3 E) (Maes, et al., 2014).
Fig. 3.
The effect of chloroquine (CQ) on tumor blood vessels. B16-F10 melanoma tumor-bearing mice were treated with chloroquine (CQ) or phosphate buffered saline (PBS) (control, ctrl). (A) Tumor vessel density. (B) Percentage of tortuous tumor vessels. (C) Percentage of pericyte-covered tumor vessels. (D) Confocal microscopy images of tumors stained with CD31 (endothelial cell marker, red) and DAPI (nuclei marker, blue). (E) Scanning electron microscopy (SEM) images of tumors. Chloroquine doses of 50 mg/kg (CQ50) or 100 mg/kg (CQ100) were administered daily. Data is presented as mean ± standard error of mean.*, P< 0.05;***, P< 0.001. Reproduced from (Maes, et al., 2014) with permission. α-SMA, alpha-smooth muscle actin; CD31, cluster differentiation 31; DAPI, 4’,6-diamidino-2-phenylindole.
A major consequence of the chloroqune-induced vessel normalization process was improved intratumoral delivery of chemotherapeutic agents (Maes, et al., 2014). Chloroquine also reduced metastasis due to vasculature normalization (Maes, et al., 2014). Although this study did not investigate the effect of chloroquine on nanodelivery (Maes, et al., 2014), several other studies have shown that vasculature normalization improves intratumoral accumulation of nanoparticles (Chauhan, et al., 2012; Jiang, et al., 2015; Khalid, et al., 2017). Other clinically approved antiangiogenic drugs that normalize vasculature target the vascular endothelial growth factor (VEGF) signaling pathway, which is a major mediator of angiogenesis and is highly activated during tumor growth (Niu & Chen, 2010; Goel, et al., 2011; Jain, 2005). Chloroquine provides an alternative mechanism to VEGF modulation for obtaining tumor vasculature normalization, which could have distinct benefits. Specifically, chloroquine upregulates the neurogenic locus notch homolog protein 1 (NOTCH1) signaling pathway (Maes, et al., 2014). NOTCH1 is a transmembrane angiogenic protein that cycles between the cell surface and endosomal compartments (Gridley, 2010). Chloroquine induces the accumulation of a NOTCH1 fragment in multivesicular bodies, leading to the release of a transcription factor that modulates vascular function (Maes, et al., 2014). In a Notch1 endothelial cell knockout mouse model, chloroquine did not cause normalization of tumor vasculature, indicating that the NOTCH1 pathway was responsible for therapeutic efficacy (Maes, et al., 2014).
4. Chloroquine-induced reduction of hepatic nanoparticle clearance
The liver is responsible for the clearance of endogenous and exogenous materials. Hepatic tropism and clearance limits the therapeutic efficacy of nanoparticles, and the liver represents a pivotal obstacle in drug delivery. Various design strategies have been attempted to avoid immunological clearance of nanoparticles. For instance, the coating of nanocarrier surfaces with polyethylene glycol (PEG) to form a hydration layer that reduces nanoparticle interactions with resident macrophages is currently in clinical use (Parr, et al., 1994; Pasut, et al., 2015; Walkey & Chan, 2012; Wolfram, Suri, et al., 2014; Wolfram, Yang, et al., 2014). However, nanoparticle pegylation fails to extensively prevent hepatic tropism due to incomplete coverage of the surface, loss of nanoparticle integrity in the circulation, and immunological activation and destruction after repeated injections (Hatakeyama, et al., 2013; Moghimi, 2017; Yang & Lai, 2015). There are also several preclinical approaches that prolong systemic circulation of nanoparticles, such as the use of cell membrane coatings (Hu, et al., 2011; Parodi, et al., 2013) and peptides for self-recognition (Rodriguez, et al., 2013). Nevertheless, these preclinical strategies have several drawbacks, such as laborious processes for coating nanoparticles with cell surfaces and the triggering of immune responses related to the source material and processing steps. There remains a pressing need to develop improved strategies to reduce nanoparticle clearance by the liver, given the detrimental impact of hepatic uptake on circulating nanodrugs.
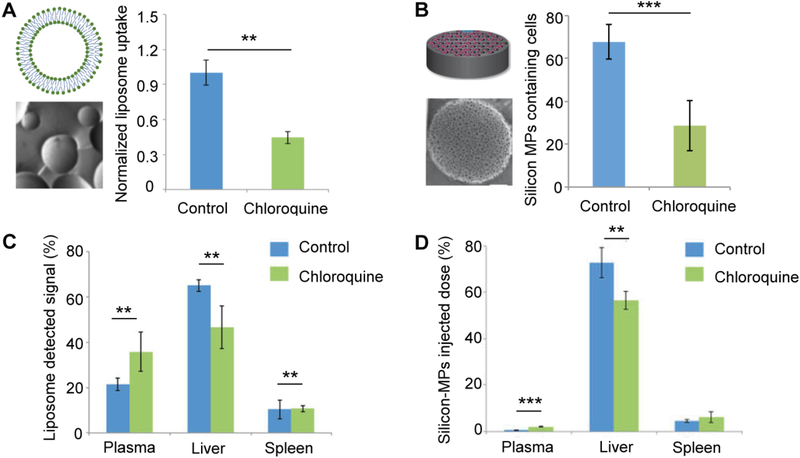
A complementary approach to nanoparticle surface modification strategies is modulation of the liver microenvironment to reduce the ability of resident immune cells to take up nanoparticles (Khalid, et al., 2017; Wolfram, et al., 2017). These strategies are broadly applicable to various types of drug delivery vehicles, ranging from nanosized liposomes to discoidal silicon microparticles. In a screen to identify clinically approved drugs that modulate the ability of Kupffer cells to internalize nanoparticles, chloroquine was recognized as a promising candidate (Wolfram, et al., 2017). Pretreatment of Kupffer cells with chloroquine led to reduced cellular uptake of various nanodelivery systems (Wolfram, et al., 2017). On the other hand, nanoparticle uptake in various cancer cell lines exposed to chloroquine remained unchanged, suggesting that this strategy prevents nanoparticle internalization by macrophages without affecting cancer cell uptake mechanisms (Wolfram, et al., 2017). Biodistribution studies conducted in mouse models showed that pretreatment with a clinically relevant dose of chloroquine, caused a 28.5% and 22% reduction in liver accumulation of systemically administered liposomes and disk-shaped silicon microparticles, respectively (Fig. 4) (Wolfram, et al., 2017). The ability of chloroquine to decrease both soft (liposomes) and hard (silicon) nanomaterial deposition in the liver highlights the versatility of this strategy in improving nanodelivery of both lipid-based nanoparticles that are already approved for clinical use (Barenholz, 2012; Gentile, et al., 2013) and discoidal particles that represent the next frontier of organotropic nanomedicine (Mi, Mu, et al., 2016; Mi, Wolfram, et al., 2016; J. Shen, et al., 2015; Venuta, et al., 2017; Wolfram, Shen, et al., 2015).
Fig. 4.
Effect of chloroquine on particle uptake by Kupffer cells. Kupffer cells and mice were treated with chloroquine (CQ) or phosphate buffered saline (PBS) (control, ctrl). Schematics, transmission electron microscopy (TEM) images, and charts of Kupffer cell uptake of liposomes (A) or silicon microparticles (MPs) (B). Biodistribution of fluorescently labeled liposomes (C) and radiolabeled silicon MPs (D). Data is presented as mean ± standard deviation of three replicates (A), 16 randomly selected regions (B), or n = 5 (C-D). *, P < 0.05; **, P < 0.01; ***, P < 0.001. Reproduced from (Paolino, et al., 2014; Wolfram, et al., 2017; Wolfram, Shen, et al., 2015) with permission.
A proposed mechanism for chloroquine-induced inhibition of nanoparticle uptake in macrophages is suppression of phosphatidylinositol binding clathrin assembly protein (PICALM) (Wolfram, et al., 2017). Expression of PICALM is thought to be essential for clathrin-mediated endocytosis (S. E. Miller, et al., 2015), which is one of the main internalization pathways for cellular uptake of nanoparticles (Sahay, et al., 2010). Although suppression of PICALM is likely to be a predominant mechanism by which nanoparticle internalization is inhibited, other pathways are also involved as pharmacological inhibition of clathrin-dependent endocytosis failed to prevent nanoparticle uptake to the same extent as chloroquine (Wolfram, et al., 2017). In fact, endocytic vesicles frequently fuse with the lysosome, a function which is impaired by chloroquine, potentially causing upstream processes to become affected (Wolfram, et al., 2017).
The promising benefits of chloroquine priming should spur further interest in the development of other agents that precondition the innate immune system for improved drug delivery. Nevertheless, there is concern that suppression of macrophage immune responses might impede the defense system against pathogens, which poses a safety issue (Wolfram, et al., 2017). However, there is typically a three to four-week interval between the injection of nanodrugs (Rose, 2005), and chloroquine administration would follow a similar schedule, enabling macrophage function to return to normal between dosages. Another concern with chloroquine pretreatment is potential suppression of tumor-associated macrophages (TAMs), which play a role in drug accumulation in certain types of tumors (M. A. Miller, et al., 2015; Tanei, et al., 2016). Therefore, the effect of chloroquine on drug delivery may vary, as the prevalence of TAMs differ depending on tumor type (Jung, et al., 2015). Besides having an impact on intratumoral nanoparticle accumulation, chloroquine may also display anticancer activity by interfering with the function of TAMs, which have been shown to promote cell survival, invasion, and angiogenesis (Noy, 2014).
5. Other chloroquine-induced anticancer mechanisms
Chloroquine offers additional potential uses for cancer treatment by interfering with oncogenic signaling pathways through mechanisms other than inhibition of autophagy, normalization of tumor vasculature, and suppression of liver macrophages (Table 1). For example, chloroquine has been shown to suppress pancreatic ductal adenocarcinoma (PDAC) cancer stem cells (CSCs), which are highly drug resistant and promote tumor progression and metastasis (Balic, et al., 2014). In mouse models of human PDAC, chloroquine was shown to preferentially target CSCs via inhibition of signaling pathways driven by chemokines, resulting in suppression of signal transducer and activator of transcription 3 (STAT3) and extracellular signal-regulated kinase (ERK), which play an important role in metastatic spread (Balic, et al., 2014). Chloroquine also blocked epithelial to mesenchymal transition (EMT) in CSCs, though suppression of sonic hedgehog (SHH)-driven chemotaxis (Balic, et al., 2014). Chloroquine-mediated inhibitory effects on SHH signaling and chemokine ligand-receptor interactions suppressed the metastatic capacity of CSCs, resulting in increased cure rates in mice (Balic, et al., 2014). Chloroquine is also able to target CSCs in triple-negative breast cancer by downregulating various signaling pathways, such as STAT3, resulting in a reduction of CSCs (Choi, et al., 2014). The ability of chloroquine to eliminate CSCs makes it a promising candidate for combination therapy with cytotoxic agents for prevention of CSC-driven tumor progression (Balic, et al., 2014). Notably, other strategies to inhibit cancer stemness have shown promising results. For instance, napabucasin, a first-in-class cancer stemness inhibitor that suppresses STAT3 signaling, inhibited tumor growth when administered in combination with chemotherapeutics in a phase 1b/2 study in patients with PDAC. A disease control rate of 92% was reported, with 43% of patients displaying a 30% or greater reduction in tumor burden (Bekaii-Saab, 2017), and a pivotal phase 3 trial with napabucasin is now ongoing (). As chloroquine also interferes with the STAT3 signaling pathway in CSCs, this drug may display similar clinical efficacy as napabucasin.
Table 1.
Examples of anticancer effects of chloroquine mediated by mechanisms other than autophagy inhibition, tumor blood vessel normalization, and Kupffer cell suppression.
| Agents | Cancer | Mechanism |
|---|---|---|
| Chloroquine, gemcitabine | Pancreatic ductal adenocarcinoma (PDAC) | Inhibits CXCR4/CXCL12 and sonic hedgehog signaling in cancer stem cells (CSCs), leading to anticancer activity both in vitro and in vivo (Balic, et al., 2014) |
| Chloroquine, cisplatin, rapamycin | Breast cancer | Autophagy-independent mechanism (potentially DNA intercalation or activation of ATM and p53) that improves therapeutic efficacy (Maycotte, et al., 2012) |
| Chloroquine, rapamycin, AKTi, and mTORi | Bladder cancer (FGFR3 mutant) | Inhibits cholesterol uptake resulting in enhanced permeability of the lysosomal membrane and apoptosis (King, Ganley, & Flemington, 2016) |
| Chloroquine and paclitaxel | Triple negative breast cancer (TNBC) | Inhibits JAK2-STAT3 signaling in CSCs, leading to anticancer activity both in vitro and in vivo (Choi, et al., 2014) |
AKTi, protein kinase B inhibitor; ATM, ATM serine threonine kinase; CXCL12, C-X-C motif chemokine ligand 12; CXCR4, C-X-C chemokine receptor type 4; FGFR3, fibroblast growth factor receptor 3; JAK2, janus kinase 2; mTORi, mammalian target of rapamycin inhibitor; p53, tumor protein p53; STAT3, signal transducer and activator of transcription 3.
Chloroquine is also able to sensitize cancer cells to therapeutic agents through modulation of non-CSC-specific signaling pathways. For instance, chloroquine treatment has been shown to improve the therapeutic efficacy of the DNA damaging agent cisplatin and the mammalian target of rapamycin (mTOR) inhibitor rapamycin in breast cancer cells (Maycotte, et al., 2012). This chemo-sensitization occurred independent of autophagy inhibition, as exposure to the autophagy inhibitor bafilomycin failed to reduce cell viability (Maycotte, et al., 2012). Additionally, knockdown of genes that promote autophagy, such as autophagy-related protein 12 (Atg12) and Beclin1, was unable to mimic the effects of chloroquine (Maycotte, et al., 2012). Potential mechanisms for this chloroquine-induced drug sensitization are thought to include DNA intercalation and activation of ataxia telangiectasia mutated (ATM) and p53 (Bakkenist & Kastan, 2003; Maycotte, et al., 2012; Wenzel, et al., 2010).
6. Conclusion
Chloroquine is an orally administered, clinically-approved drug that is used to prevent or treat malaria. This water-soluble drug can efficiently cross the cell membrane and deposit in acidic intracellular organelles, leading to antimalarial therapeutic effects. Chloroquine can also affect several mammalian physiological processes and signaling pathways. In particular, chloroquine is promising for combination cancer therapy, as this drug affects four distinct cell populations: Kupffer cells, tumor endothelial cells, cancer cells, and CSCs. Chloroquine sensitizes cancer cells to conventional therapeutic agents through prevention of autophagy; normalizes tumor vasculature, leading to improved drug delivery and decreased metastasis; reduces nanoparticle accumulation in the liver through interfering with endocytosis in Kupffer cells; suppresses cancer stem cells; and inhibits other oncogenic signaling pathways. Chloroquine has a well-known safety profile and displays acceptable toxicity when administered in combination with cancer drugs. A favorable safety profile together with versatile anticancer effects make chloroquine a promising candidate for various combination therapy strategies with both conventional anticancer drugs and novel nanotherapeutics.
Acknowledgements
The authors acknowledge financial support from the following sources: Mayo Clinic, the Ernest Cockrell Jr. Presidential Distinguished Chair, and the National Cancer Institute Physical Sciences-Oncology Network of the National Institutes of Health, under award number U54CA210181.
Abbreviations:
- α-SMA
alpha-smooth muscle actin
- AML
acute myeloid leukemia
- Atg12
autophagy-related protein 12
- ATM
ataxia telangiectasia mutated
- AVs
autophagic vesicles
- BRAF
v-Raf murine sarcoma viral oncogene homolog B
- CD31
cluster differentiation 31
- CNS
central nervous system
- CSCs
cancer stem cells
- ERK
extracellular signal-regulated kinase
- mTOR
mammalian target of rapamycin
- PDAC
pancreatic ductal adenocarcinoma
- PICALM
phosphatidylinositol binding clathrin assembly protein
- NOTCH1
notch homolog protein 1
- SHH
sonic hedgehog
- SEM
scanning electron microscopy
- STAT3
signal transducer and activator of transcription 3
- TAMs
tumor-associated macrophages
- VEGF
vascular endothelial growth factor
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, & White E (2011). Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res, 17, 654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, & Thompson CB (2007). Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest, 117, 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmo AC, & Mitragotri S (2016). Nanoparticles in the clinic. Bioeng Transl Med, 1, 10–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YH, & Park K (2011). Targeted drug delivery to tumors: myths, reality and possibility. J Control Release, 153, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkenist CJ, & Kastan MB (2003). DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature, 421, 499–506. [DOI] [PubMed] [Google Scholar]
- Balic A, Sorensen MD, Trabulo SM, Sainz B Jr., Cioffi M, Vieira CR, Miranda-Lorenzo I, Hidalgo M, Kleeff J, Erkan M, & Heeschen C (2014). Chloroquine targets pancreatic cancer stem cells via inhibition of CXCR4 and hedgehog signaling. Mol Cancer Ther, 13, 1758–1771. [DOI] [PubMed] [Google Scholar]
- Baluk P, Hashizume H, & McDonald DM (2005). Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev, 15, 102–111. [DOI] [PubMed] [Google Scholar]
- Barenholz Y (2012). Doxil(R)--the first FDA-approved nano-drug: lessons learned. J Control Release, 160, 117–134. [DOI] [PubMed] [Google Scholar]
- Bekaii-Saab T, Starodub A, El-Rayes BF, O’Neil BH, Shahda S, Keon Ciombor K, Noonan AM, Tewfik Hanna W, Sehdev A, Labib Shaib W, Mikhail S, Neki AS, Oh C, Li Y, Li W, Borodyansky L, Li C (2017). A phase Ib/II study of cancer stemness inhibitor napabucasin (BBI-608) in combination with gemcitabine (gem) and nab-paclitaxel (nabPTX) in metastatic pancreatic adenocarcinoma (mPDAC) patients (pts). J Clin Oncol, 35, 4106–4106. [Google Scholar]
- Bergers G, & Song S (2005). The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol, 7, 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli DA, Yankson K, Shukla N, Vilanilam G, Ticer T, & Wolfram J (2018). Extracellular vesicle therapeutics for liver disease. J Control Release, 273, 86–98. [DOI] [PubMed] [Google Scholar]
- Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, Houghton JA, Huang P, Giles FJ, & Cleveland JL (2007). Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood, 110, 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, & Jain RK (2011). Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov, 10, 417–427. [DOI] [PubMed] [Google Scholar]
- Chan S DN, Juozaityte E, Erdkamp F, Pluzanska A, Azarnia N, Lee LW (2004). Phase III trial of liposomal doxorubicin and cyclophosphamide compared with epirubicin and cyclophosphamide as first-line therapy for metastatic breast cancer. Ann Oncol, 15, 8. [DOI] [PubMed] [Google Scholar]
- Chauhan VP, Stylianopoulos T, Martin JD, Popović Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, & Jain RK (2012). Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol, 7, 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Rehman SK, Zhang W, Wen A, Yao L, & Zhang J (2010). Autophagy is a therapeutic target in anticancer drug resistance. Biochim Biophys Acta, 1806, 220–229. [DOI] [PubMed] [Google Scholar]
- Choi DS, Blanco E, Kim YS, Rodriguez AA, Zhao H, Huang TH, Chen CL, Jin G, Landis MD, Burey LA, Qian W, Granados SM, Dave B, Wong HH, Ferrari M, Wong ST, & Chang JC (2014). Chloroquine eliminates cancer stem cells through deregulation of Jak2 and DNMT1. Stem Cells, 32, 2309–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchini M, Karantza V, & Xia B (2015). Molecular pathways: autophagy in cancer--a matter of timing and context. Clin Cancer Res, 21, 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gelinas C, Fan Y, Nelson DA, Jin S, & White E (2006). Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell, 10, 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Gao W, Chen X, Kang JH, Stolz DB, Liu J, & Yin XM (2009). Oncogenic transformation confers a selective susceptibility to the combined suppression of the proteasome and autophagy. Mol Cancer Ther, 8, 2036–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M (2010). Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol, 28, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao BSJ (2008). Theranostic nanomedicine for cancer. Nanomedicine, 3, 137–140. [DOI] [PubMed] [Google Scholar]
- Gentile E, Cilurzo F, Di Marzio L, Carafa M, Ventura CA, Wolfram J, Paolino D, & Celia C (2013). Liposomal chemotherapeutics. Future Oncol, 9, 1849–1859. [DOI] [PubMed] [Google Scholar]
- Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, & Jain RK (2011). Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev, 91, 1071–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Polo RA, Boya P, Pauleau AL, Jalil A, Larochette N, Souquere S, Eskelinen EL, Pierron G, Saftig P, & Kroemer G (2005). The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci, 118, 3091–3102. [DOI] [PubMed] [Google Scholar]
- Gridley T (2010). Notch signaling in the vasculature. Curr Top Dev Biol, 92, 277–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson HH, Holt-Casper D, Grainger DW, & Ghandehari H (2015). Nanoparticle Uptake: The Phagocyte Problem. Nano Today, 10, 487–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, Rabie T, Kaden S, Grone HJ, Hammerling GJ, Arnold B, & Ganss R (2008). Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature, 453, 410–414. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, & Mizushima N (2006). Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature, 441, 885–889. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Akita H, & Harashima H (2013). The polyethyleneglycol dilemma: advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol Pharm Bull, 36, 892–899. [DOI] [PubMed] [Google Scholar]
- Hempelmann E (2007). Hemozoin biocrystallization in Plasmodium falciparum and the antimalarial activity of crystallization inhibitors. Parasitol Res, 100, 671–676. [DOI] [PubMed] [Google Scholar]
- Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, & Zhang L (2011). Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A, 108, 10980–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK (2005). Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science, 307, 58–62. [DOI] [PubMed] [Google Scholar]
- Jain RK (2012). Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev, 64, 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Huang Y, An Y, & Kim BY (2015). Remodeling Tumor Vasculature to Enhance Delivery of Intermediate-Sized Nanoparticles. ACS Nano, 9, 8689–8696. [DOI] [PubMed] [Google Scholar]
- Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park DJ, & Park YJ (2015). Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates. J Pathol Transl Med, 49, 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama M KT, Berger MS, Pieper RO. (2007). DNA damaging agent-induced autophagy produces a cytoprotective adenosine triphosphate surge in malignant glioma cells. Cell Death Differ, 14, 11. [DOI] [PubMed] [Google Scholar]
- Khalid A, Persano S, Shen H, Zhao Y, Blanco E, Ferrari M, & Wolfram J (2017). Strategies for improving drug delivery: nanocarriers and microenvironmental priming. Expert Opin Drug Deliv, 14, 865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MA, Ganley IG, & Flemington V (2016). Inhibition of cholesterol metabolism underlies synergy between mTOR pathway inhibition and chloroquine in bladder cancer cells. Oncogene, 35, 4518–4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, & Mizushima N (2010). Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Semin Cell Dev Biol, 21, 683–690. [DOI] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW (2011). Autophagy in immunity and inflammation. Nature, 469, 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JM, Thompson JC, Griesinger AM, Amani V, Donson AM, Birks DK, Morgan MJ, Mirsky DM, Handler MH, Foreman NK, & Thorburn A (2014). Autophagy inhibition improves chemosensitivity in BRAF(V600E) brain tumors. Cancer Discov, 4, 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K, Quaegebeur A, Schoors S, Georgiadou M, Wouters J, Vinckier S, Vankelecom H, Garmyn M, Vion AC, Radtke F, Boulanger C, Gerhardt H, Dejana E, Dewerchin M, Ghesquiere B, Annaert W, Agostinis P, & Carmeliet P (2014). Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell, 26, 190–206. [DOI] [PubMed] [Google Scholar]
- Redmann Matthew, B. GA, Berryhill Taylor F., Wani Willayat Y., Ouyang Xiaosen, Johnson Michelle S., Ravi Saranya, Barnes Stephen, Darley-Usmar Victor M., and Zhanga Jianhua. (2017). Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol, 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycotte P, Aryal S, Cummings CT, Thorburn J, Morgan MJ, & Thorburn A (2012). Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy, 8, 200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone M, Dettori D, de Oliveira RL, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, de Almodovar CR, De Smet F, Vinckier S, Aragones J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, & Carmeliet P (2009). Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell, 136, 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Y, Mu C, Wolfram J, Deng Z, Hu TY, Liu X, Blanco E, Shen H, & Ferrari M (2016). A Micro/Nano Composite for Combination Treatment of Melanoma Lung Metastasis. Adv Healthc Mater, 5, 936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Y, Wolfram J, Mu C, Liu X, Blanco E, Shen H, & Ferrari M (2016). Enzyme-responsive multistage vector for drug delivery to tumor tissue. Pharmacol Res, 113, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Zheng YR, Gadde S, Pfirschke C, Zope H, Engblom C, Kohler RH, Iwamoto Y, Yang KS, Askevold B, Kolishetti N, Pittet M, Lippard SJ, Farokhzad OC, & Weissleder R (2015). Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat Commun, 6, 8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SE, Mathiasen S, Bright NA, Pierre F, Kelly BT, Kladt N, Schauss A, Merrifield CJ, Stamou D, Honing S, & Owen DJ (2015). CALM regulates clathrin-coated vesicle size and maturation by directly sensing and driving membrane curvature. Dev Cell, 33, 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghimi SM S. D. (2017). Complement activation turnover on surfaces of nanoparticles. Nano Today, 15, 8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanari F, Lu M, Marcus S, Saran A, Malankar A, & Mazumder A (2014). A Phase II Trial of Chloroquine in Combination with Bortezomib and Cyclophosphamide in Patients with Relapsed and Refractory Multiple Myeloma. Blood, 124, 5775. [Google Scholar]
- Nagy JA, Chang SH, Dvorak AM, & Dvorak HF (2009). Why are tumour blood vessels abnormal and why is it important to know? Br J Cancer, 100, 865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JA, Chang SH, Shih SC, Dvorak AM, & Dvorak HF (2010). Heterogeneity of the tumor vasculature. Semin Thromb Hemost, 36, 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G, & Chen X (2010). Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets, 11, 1000–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy R (2014). Tumor-associated macrophages: from mechanisms to therapy. Immunity, 41, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolino D, Cosco D, Gaspari M, Celano M, Wolfram J, Voce P, Puxeddu E, Filetti S, Celia C, Ferrari M, Russo D, & Fresta M (2014). Targeting the thyroid gland with thyroid-stimulating hormone (TSH)-nanoliposomes. Biomaterials, 35, 7101–7109. [DOI] [PubMed] [Google Scholar]
- Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, & Tasciotti E (2013). Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol, 8, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr MJ, Ansell SM, Choi LS, & Cullis PR (1994). Factors influencing the retention and chemical stability of poly(ethylene glycol)-lipid conjugates incorporated into large unilamellar vesicles. Biochim Biophys Acta, 1195, 21–30. [DOI] [PubMed] [Google Scholar]
- Pasut G, Paolino D, Celia C, Mero A, Joseph AS, Wolfram J, Cosco D, Schiavon O, Shen H, & Fresta M (2015). Polyethylene glycol (PEG)-dendron phospholipids as innovative constructs for the preparation of super stealth liposomes for anticancer therapy. J Control Release, 199, 106–113. [DOI] [PubMed] [Google Scholar]
- Rose PG (2005). Pegylated liposomal doxorubicin: optimizing the dosing schedule in ovarian cancer. Oncologist, 10, 10. [DOI] [PubMed] [Google Scholar]
- Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, & Levine B (2003). Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest, 112, 1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, & Discher DE (2013). Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science, 339, 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay G, Alakhova DY, & Kabanov AV (2010). Endocytosis of nanomedicines. J Control Release, 145, 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson E, Shen H, Blanco E, Ferrari M, & Wolfram J (2017). Contribution of Kupffer cells to liposome accumulation in the liver. Colloids Surf B: Biointerfaces, 158, 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Wu X, Lee Y, Wolfram J, Yang Z, Mao ZW, Ferrari M, & Shen H (2015). Porous silicon microparticles for delivery of siRNA therapeutics. J Vis Exp, 52075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Wu Y, Liu Y, & Wu D (2017). High drug-loading nanomedicines: progress, current status, and prospects. Int J Nanomedicine, 12, 4085–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanei T, Leonard F, Liu X, Alexander JF, Saito Y, Ferrari M, Godin B, & Yokoi K (2016). Redirecting Transport of Nanoparticle Albumin-Bound Paclitaxel to Macrophages Enhances Therapeutic Efficacy against Liver Metastases. Cancer Res, 76, 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen ML, Engedal N, Boe SO, Hokland P, & Simonsen A (2013). Targeting autophagy potentiates the apoptotic effect of histone deacetylase inhibitors in t(8;21) AML cells. Blood, 122, 2467–2476. [DOI] [PubMed] [Google Scholar]
- U.S. National Library of Medicine. (2016). ClinicalTrials.gov,, https://clinicaltrials.gov/ct2/show/NCT02993731.
- Venuta A, Wolfram J, Shen H, & Ferrari M (2017). Post-nano strategies for drug delivery: Multistage porous silicon microvectors. J Mater Chem B, 5, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkey CD, & Chan WC (2012). Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev, 41, 2780–2799. [DOI] [PubMed] [Google Scholar]
- Wenzel NI, Chavain N, Wang Y, Friebolin W, Maes L, Pradines B, Lanzer M, Yardley V, Brun R, Herold-Mende C, Biot C, Toth K, & Davioud-Charvet E (2010). Antimalarial versus cytotoxic properties of dual drugs derived from 4-aminoquinolines and Mannich bases: interaction with DNA. J Med Chem, 53, 3214–3226. [DOI] [PubMed] [Google Scholar]
- White E (2012). Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer, 12, 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E, & DiPaola RS (2009). The double-edged sword of autophagy modulation in cancer. Clin Cancer Res, 15, 5308–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, & Chan WCW (2016). Analysis of nanoparticle delivery to tumours. Nat Rev Mater, 1, 16014. [Google Scholar]
- Wolfram J, Nizzero S, Liu H, Li F, Zhang G, Li Z, Shen H, Blanco E, & Ferrari M (2017). A chloroquine-induced macrophage-preconditioning strategy for improved nanodelivery. Sci Rep, 7, 13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram J, Shen H, & Ferrari M (2015). Multistage vector (MSV) therapeutics. J Control Release, 219, 406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram J, Suri K, Yang Y, Shen J, Celia C, Fresta M, Zhao Y, Shen H, & Ferrari M (2014). Shrinkage of pegylated and non-pegylated liposomes in serum. Colloids Surf B: Biointerfaces, 114C, 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram J, Yang Y, Shen J, Moten A, Chen C, Shen H, Ferrari M, & Zhao Y (2014). The nano-plasma interface: Implications of the protein corona. Colloids Surf B: Biointerfaces, 124, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram J, Zhu M, Yang Y, Shen J, Gentile E, Paolino D, Fresta M, Nie G, Chen C, Shen H, Ferrari M, & Zhao Y (2015). Safety of Nanoparticles in Medicine. Curr Drug Targets, 16, 1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, & Lai SK (2015). Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 7, 655–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YN, Poon W, Tavares AJ, McGilvray ID, & Chan WCW (2016). Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J Control Release, 240, 332–348. [DOI] [PubMed] [Google Scholar]