Abstract
Objective:
Tourette Syndrome is polygenic and highly heritable. Genome-wide association (GWAS) approaches are useful for interrogating the genetic architecture and determinants of Tourette syndrome and other tic disorders.
Method:
GWAS meta-analysis, gene-based association, and genetic enrichment analyses were conducted in 4,819 Tourette syndrome cases and 9,488 controls. Replication of top loci was conducted in an independent, population-based sample (706 cases; 6,068 controls). Relationships between Tourette polygenic risk scores (PRS), other tic disorders, ascertainment, and tic severity were examined.
Results:
GWAS and gene-based analyses identified one genome-wide significant locus within FLT3 on chromosome 13, rs2504235 (SNP p=2.1×10−8; Gene p=8.9×10−7), though this association was not replicated in the population-based sample. Genetic variants spanning evolutionarily-conserved regions explained 92.4% of Tourette syndrome heritability (Bonferroni corrected p-value=0.005). Tourette-associated genes were preferentially expressed in human dorsolateral prefrontal cortex (p=1.2×10−4). Tourette PRS predicted both Tourette syndrome (p=5.3×10−9) and tic spectrum disorders (p=4.2 ×10−4) status in the population-based sample. Tourette PRS also correlated with worst-ever tic severity (p=0.026) and was higher in cases with a family history of tics than in simplex cases.
Conclusions:
Modulation of gene expression through non-coding variants, particularly within cortico-striatal circuits, is implicated as a fundamental mechanism in Tourette syndrome pathogenesis. At a genetic level, tic disorders represent a continuous spectrum of disease, supporting the unification of Tourette syndrome and other tic disorders in future diagnostic schema. Tourette PRS derived from sufficiently large samples may be useful in the future for predicting conversion of transient tics to chronic tic disorders, as well as tic persistence and lifetime tic severity.
INTRODUCTION
Tourette syndrome is a complex neuropsychiatric disorder that occurs along a phenotypic spectrum that also includes chronic (persistent) motor or vocal tic disorder (chronic tics) and transient (provisional) tic disorder (1). Although Tourette syndrome is highly heritable (2), variants in known Tourette risk genes (e.g., CNTN6, NRXN1, SLITRK1, HDC, and CELSR3) account for fewer than 2% of affected individuals (3–6). Tourette syndrome is highly polygenic, with a demonstrated role for multiple common genetic variants of small effect distributed widely across the genome (7). Thus, genome-wide association studies (8) will be of benefit in further elucidating its underlying genetic etiology.
To date, only one Tourette GWAS has been published (9). Although no single nucleotide polymorphisms (SNPs) met criteria for genome-wide significance (p<5×10−8), in aggregate, the top SNPs (p-values <1×10−3) were enriched for expression quantitative trait loci (eQTLs) in frontal cortex and for methylation quantitative trait loci (mQTLs) in the cerebellum, indicating that a significant proportion of these variants have biological relevance to Tourette syndrome, and perhaps also to other tic disorders. However, as with other neuropsychiatric disorders, much larger sample sizes are needed to elucidate the disorder’s genetic underpinnings. Here, we report the results of a GWAS meta-analysis from the Psychiatric Genomics Consortium (PGC) Tourette Syndrome Workgroup in a sample that is nearly four times larger than the initial GWAS. We also probe aggregated Tourette syndrome polygenic risk to test two specific hypotheses: 1) whether Tourette and related tic disorders have an underlying shared genetic etiology; and 2) whether Tourette polygenic risk scores correlate with worst-ever tic severity and might represent a future potential predictor of disease severity.
METHOD
Subjects
The primary GWAS meta-analysis consisted of four European ancestry (EU) GWAS datasets: 1) 969 cases and 3,923 ancestry-matched controls from the initial Tourette syndrome GWAS (GWAS1) (9); 2) 2,711 additional EU ancestry Tourette cases (4) and 3,762 ancestry-matched controls (GWAS2); 3) Tourette probands from GWAS1 and one or more of their Tourette syndrome-affected family members (10) (N=548) plus 597 ancestry-matched controls (GWAS2 FAM); 4) 591 independent EU Tourette probands from the TIC Genetics consortium (TIC)(11) and 1,206 unselected ancestry-matched controls. Genotyping details are provided in the online Data Supplement (Tables S1 and S2).
GWAS1:
969 EU cases were collected from Tourette syndrome specialty clinics in the US, Canada, the UK, the Netherlands, and via recruitment from the Tourette Association of America membership. Tourette diagnoses were assigned based on DSM-IV-TR criteria plus observation of tics by an experienced clinician. 3,923 controls were identified primarily from previously genotyped unselected population controls and ancestry matched to the cases (9).
GWAS2:
2,871 EU cases with DSM-5 Tourette syndrome were identified by email/online recruitment combined with validated, web-based phenotypic assessments (12, 13) (N=1,264), or from Tourette syndrome specialty clinics in the US, Canada, and Europe (N=1,607) (Supplemental Methods). All subjects were genotyped at the UCLA Neuroscience Genomics Core (UNGC). After quality control, 2,711 cases were retained for analysis.
GWAS2 FAM:
The family sample consisted of 548 probands and first-degree relatives with Tourette syndrome from 207 independent families(10). 175 probands came from the original Tourette GWAS1 sample; these cases were removed from the GWAS1 analysis along with ancestry-matched controls and re-analyzed with the family-based sample. 32 Tourette probands and 341 additional Tourette-affected family members (total N=373) were genotyped along with the GWAS2 case-control sample. 597 ancestry-matched controls were selected from a pool of previously genotyped controls (Supplemental Methods).
TIC:
The TIC Genetics sample consisted of 591 probands, 579 who met DSM-5 criteria for Tourette syndrome, and 12 with DSM-5 chronic motor or vocal tic disorder (Table S1, S2).
Controls:
6,920 EU controls were obtained from cohorts of previously genotyped, unselected population controls for the GWAS2 analyses; an additional 595 EU controls were genotyped with the Tourette cases at the UNGC (Table S1; Supplemental Methods).
deCODE:
An independent case-control replication sample from Iceland (deCODE genetics, Reykjavik, Iceland) consisted of 706 Icelandic Tourette syndrome cases and 466 cases with other tic disorders (chronic tics or unspecified tic disorder) (Supplemental Methods). 127,164 unscreened population-matched controls were also available; 6,068 of these were screened and reported no lifetime subclinical motor or vocal tics. Cases and controls were genotyped at deCODE on Illumina SNP arrays (Supplemental Methods).
Participants ages 18 years and older provided written informed consent. Individuals under 18 gave assent; parental permission was also obtained. The study was approved by the human subjects committees at all participating sites.
Quality Control
Genotyping quality control was performed in PLINK v1.9 (14) (Supplemental Methods). Duplicates and relatives were identified using genome-wide identity-by-descent estimates, and one member of each duplicate or relative pair was removed from the case-control sample. Relative pairs where both individuals had a Tourette diagnosis were removed from the case-control sample and moved to the family-based analysis.
Population stratification was assessed through multidimensional scaling (MDS) analysis; individuals of non-European ancestry and extreme outliers on each of the MDS components were removed (Supplemental Methods and Figure S1). Case-control matching was verified across all MDS components. The final post-quality control GWAS2 sample contained 2,711 cases and 3,762 ancestry-matched controls and 550,550 SNPs; the final GWAS2 FAM sample contained 548 cases/family members, 597 ancestry-matched controls and 236,748 SNPs. The final TIC sample included 591 cases and 1206 ancestry-matched controls and 581,774 SNPs (Table S2).
Imputation and Genome-wide Association
SNP imputation was conducted on all genotype data for the primary meta-analyses using the 1000 Genomes Project Phase 1 integrated haplotypes (December 2013 release, with singleton sites removed) as the reference panel (15). ShapeIT was used to phase genotype data, followed by imputation with IMPUTE v2. SNPs with Information score <0.6 or Certainty <0.9 were excluded.
Genome-wide association tests were performed on the imputed dosage data of GWAS2 and TIC samples separately in PLINK1.9, using logistic regression under an additive model with the first four MDS components and any additional MDS components associated with Tourette case-control status at p<0.05 included as covariates. A linear mixed model was used for the GWAS2 FAM association analysis in MMM v.1.0 (16) to control for familial relatedness. GWAS1 samples were re-imputed as described above; association tests were performed in four ancestry-based strata: Non-isolate European (GWAS1_EU), Ashkenazi Jewish (GWAS1_AJ), French Canadian (GWAS1_FC), and GWAS1 TIC (GWAS1_TIC) (Table S2).
A primary GWAS meta-analysis was conducted on the GWAS1, GWAS2, GWAS2 FAM, and TIC datasets using the inverse-variance method in METAL (17). Heterogeneity was assessed with Cochran’s I2 statistics. The genomic control factor (λ) was calculated for each individual GWAS and for the overall meta-analysis using all SNPs with MAF>0.01 to identify residual population stratification or systematic technical artifact (Figure S2). GWAS summary statistics were subjected to linkage disequilibrium (LD) score regression (LDSC) analyses on high quality, common SNPs (INFO>0.9 and MAF>0.01) to examine the LDSC intercept as a more specific measure of inflation of the GWAS test statistic (18) due to residual artifact or stratification. The genome-wide significant threshold for the GWAS (19, 20) was set at p=5.0×10−8.
Heritability Estimation
Tourette syndrome SNP-based heritability was estimated on the liability scale, assuming a population prevalence of 0.8% (21), using both LDSC (18) and, in GWAS1 and GWAS2 samples after excluding Ashkenazi Jewish samples, using genotype-level data in a linear mixed model framework (7). To compare the relative polygenic burden of Tourette syndrome samples collected with different ascertainment methods, the Tourette GWAS1 and GWAS2 datasets were separated into 3 groups: 1) GWAS1 cases (25% from affected sib-pair families)(10); 2) GWAS2 cases recruited through Tourette syndrome specialty clinics; 3) GWAS2 cases recruited via email from the membership of the Tourette Association of America and assessed with a web-based phenotyping instrument (12). Following additional stringent quality control of SNPs and samples (Supplemental Methods), the SNP-based heritability of each ascertainment group was estimated both separately and jointly.
Partitioned heritability analyses were conducted using LDSC to evaluate enrichment of Tourette SNP-based heritability from different functional annotation classes and different cell/ tissue types (22) and to examine genetic correlations between the GWAS1, GWAS2, and TIC datasets.
Targeted Replication
The population-based deCODE samples were used to: 1) independently replicate the 39 top LD-independent SNPs (r2<0.2 and MAF>0.01; p<1.0×10−5) in the primary meta-analysis, followed by a sign test to examine consistency in the direction of effects in these top SNPs across the two datasets, as well as a targeted meta-analysis of these 39 SNPs using the inverse-variance method (Supplemental Methods); and 2) examine the genetic relationships between Tourette and other tic disorders through polygenic risk score (PRS) analyses (Supplemental Methods) (23). Logistic regressions were performed to test the prediction power of PRS for Tourette syndrome and tic disorders cases versus controls, adjusted by sex, year of birth, and the first 20 principal components (24).
Polygenic Risk Score Analyses
Genome-wide Tourette PRS adjusted for ancestry principal components (aPRS) were generated for all subjects in the primary meta-analysis using the entire distribution (GWAS p≤1) of LD-independent SNPs (r2<0.2) through a cross-validation approach (23) and used to examine the relationship between Tourette aPRS and ascertainment, family history of Tourette/ chronic tics, and lifetime worst-ever tic severity (Yale Global Tic Severity Scale Total Tic Score (tic severity), range 0-50) (Supplemental Methods).
Gene-based and Gene Set Enrichment Analysis
Gene-based and competitive gene set enrichment analyses were conducted in MAGMA (25) (Supplemental Methods). Gene-based test statistics were derived using association summary statistics for all SNPs assigned to each gene including 50kb flanking regions after accounting for LD. P-values were adjusted with a Bonferroni correction for 18,079 genes genome-wide. Gene-based statistics were then analyzed for tissue expression enrichment in 53 distinct human tissues from 714 donors using GTEx RNA-seq data (26), and Bonferroni correction was applied for 53 tissue types (p=0.05/53=9.4×10−4). Tested gene sets included 107 probable autism spectrum disorder susceptibility genes from exome sequencing studies (27), evolutionarily constrained genes (probability of Loss-of-Function Intolerance (pLI) score >0.9), previously identified constrained genes harboring deleterious rare variants (large CNVs or de novo loss of function mutations) in Tourette syndrome cases (4, 5), and all Gene Ontology terms from MSigDB6.0 (Supplemental Methods). Bonferroni correction was applied for the number of gene sets tested.
RESULTS
Genome-wide Association Study
The final GWAS meta-analysis consisted of 8,265,319 SNPs in 4,819 Tourette syndrome cases and 9,488 controls. No evidence for residual population stratification or systematic technical artifact was observed in any of the individual datasets (Figure S2) or in the final meta-analysis (λ=1.072, λ1000=1.011, Figure 1). LDSC indicated that 86% of the observed test statistic inflation was attributable to an underlying genome-wide polygenic signal (Figure S3). PRS analyses in each individual GWAS dataset derived using a “leave one out” approach, and in the deCODE sample, indicated genetic homogeneity across all contributing datasets (Figures S4 and S5).
Figure 1.
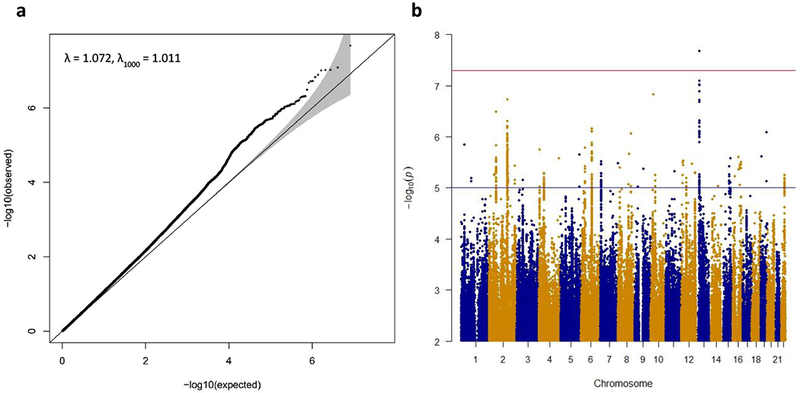
Results of the primary Tourette Syndrome GWAS meta-analysis of 4,819 cases and 9,488 controls.
The top SNP in the GWAS meta-analysis, rs2504235, located on chromosome 13q12.2, surpassed the genome-wide significance threshold (OR=1.16, p=2.1×10−8, Table 1; Figure S6). rs2504235 lies within an intron of FLT3, encoding FMS-like tyrosine kinase 3. No other SNPs achieved genome-wide significance, although rs1933437, a common FLT3 missense variant (Thr227Met) that lies 11.4 kb away from and is in strong LD with rs2504235 (r2=0.93), had a p-value of p=8.2×10−8 (Table S3). Across the genome, 39 LD-independent index SNPs with p<1×10−5 were identified by LD pruning (r2<0.2) followed by conditional association analyses controlling for the most significant SNP within each 2 Mb window and manual inspection of regional association plots to confirm the presence of supporting statistical evidence of association from nearby SNPs (Tables S3 and S4). The top 10 LD-independent index SNPs are presented in Table 1.
Table 1.
Top 10 linkage disequilibrium (LD) independent loci in the primary Tourette syndrome GWAS meta-analysis.
| SNP | CHR | BP | A1/A2 | Primary meta-analysis | deCODE | Primary + deCODE | LD block | GENES | GWAS catalog | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INFO | FRQ | OR | P | FRQ | OR | P | OR | P | |||||||
| rs2504235 | 13 | 28,612,886 | A/G | 0.99 | 0.38 | 1.16 | 2.1E-08 | 0.32 | 0.94 | 0.50 | 1.14 | 2.4E-07 | 28591318..28659473 | FLT3 | |
| rs191044310 | 10 | 23,705,451 | A/T | 0.83 | 0.02 | 0.54 | 1.5E-07 | 0.0024 | 2.27 | 0.25 | 0.56 | 5.9E-07 | 23661149..23815120 | OTUD1 | |
| rs13407215 | 2 | 161,544,891 | T/C | 1.00 | 0.02 | 2.21 | 1.9E-07 | 0.0001 | 0.02 | 0.85 | 2.21 | 1.9E-07 | 160090844..162912453 | AHCTF1P1,BAZ2B,CD302,DPP4,ITGB6,LOC643 072,LOC100505984,LOC100996579,LOC1019 29512,LY75,LY75-CD302,MARCH7,MIR4785,PLA2R1,PSMD14,RBMS1,SLC4A10,TANC1,TANK,TBR1,WDSUB1 | |
| rs2708146 | 2 | 58955953 | G/A | 1.009 | 0.46 | 0.88 | 3.2E-07 | 0.48 | 0.98 | 0.75 | 0.89 | 8.0E-07 | 58847953..59094609 | LINC01122 | |
| rs1906252 | 6 | 98,550,289 | A/C | 1.00 | 0.49 | 0.88 | 7.0E-07 | 0.50 | 0.90 | 0.17 | 0.88 | 2.8E-07 | 98214814..98664414 | MIR2113 | Bipolar disorder, Educational attainment, Gut microbiota, Intelligence |
| rs12459560 | 19 | 52,318,380 | T/G | 0.98 | 0.15 | 1.19 | 8.2E-07 | 0.16 | 1.08 | 0.45 | 1.18 | 9.1E-07 | 52266072..52606936 | FPR1,FPR2,FPR3,HCCAT3,LOC101928571,ZNF350,ZNF432,ZNF577,ZNF613,ZNF614,ZNF615,ZNF616,ZNF649,ZNF841 | |
| rs117648881 | 8 | 113,581,898 | A/G | 0.77 | 0.02 | 0.59 | 8.8E-07 | 0.01 | 0.72 | 0.32 | 0.60 | 6.2E-07 | 113581898..114612903 | CSMD3,MIR2053 | |
| rs6670211 | 1 | 29,576,784 | A/C | 1.00 | 0.47 | 0.88 | 1.4E-06 | 0.42 | 0.94 | 0.45 | 0.89 | 1.5E-06 | 29188630..29607279 | EPB41,MECR,OPRD1,PTPRU,SRSF4,TMEM200B | |
| rs72853320 | 6 | 36,623,338 | A/G | 1.00 | 0.13 | 1.20 | 1.7E-06 | 0.12 | 0.88 | 0.28 | 1.17 | 2.2E-05 | 36375986..36658092 | CDKN1A,KCTD20,MIR3925,PANDAR,PXT1,RAB44,SRSF3,STK38 | |
| rs73205493 | 4 | 2,460,571 | T/C | 0.89 | 0.34 | 1.16 | 1.8E-06 | 0.35 | 1.08 | 0.34 | 1.15 | 1.6E-06 | 2407263..2481088 | LOC402160,RNF4,ZFYVE28 | |
Targeted Replication
The 39 LD-independent index SNPs with p<1×10−5 were investigated for replication in the deCODE sample (706 cases, 6,068 controls). None of the individual SNPs were replicated after Bonferroni correction (replication threshold for 39 tests, p<0.0013; Table 1; Table S4); 23 of 39 putative Tourette syndrome risk alleles had the same direction of effect, though this was not statistically significant (Binomial two-way sign test, p=0.34).
Meta-analysis restricted to these 39 SNPs was conducted using summary statistics from the primary meta-analysis and the deCODE data with the inverse variance method in METAL. No SNPs achieved genome-wide significance; the SNP with the lowest p-value was rs13407215 on chromosome 2 (p=1.9×10−7). rs2504235 was not genome-wide significant in this analysis (p=2.4×10−7) (Table 1, Table S4).
Heritability and Polygenic Risk Score Analyses
Tourette syndrome SNP-based heritability (h2g) was estimated in the primary GWAS meta-analysis using LDSC (h2g=0.21, SE=0.024, p<2.0×10−16). Pairwise genetic correlations across the three independent case-control datasets (GWAS1, GWAS2, TIC) confirmed a significant, shared polygenic architecture (rgGWAS1-GWAS2=0.86 (SE=0.21), p=3.9×10−5; rgGWAS1-TIC=0.84 (SE=0.30), p=4.5×10−3; rgGWAS2-TIC=0.93 (SE=0.26), p=4×10−4).
As the previous estimate of Tourette syndrome h2g from the first Tourette GWAS (h2g LMM=0.58, SE=0.09) (7) was significantly higher than that observed in this study, additional heritability analyses were conducted in the individual datasets stratified on ascertainment status using linear mixed models (LMM)(7) (Table 2). These analyses confirmed both the high SNP-based heritability of the sib-pair enriched Tourette GWAS1 sample (h2g GWAS1-LMM=0.56, SE=0.10; p=1.2×10−9), and the lower heritability of the larger GWAS2 sample (h2g GWAS2-LMM=0.29, SE=0.04; p=5.5×10−14).
Table 2.
Single nucleotide polymorphism (SNP)-based heritability estimates for the Tourette syndrome GWAS1 and GWAS2 European ancestry case-control samples.
| Samplea | Cases(%) | Controls (%) | V(G)/Vp_Lc | SE | P |
|---|---|---|---|---|---|
| GWAS1 | 559 (14%) | 3400 (86%) | 0.565 | 0.096 | 1.2×10−9 |
| GWAS2b | 2146 (46%) | 2564 (54%) | 0.288 | 0.040 | 5.5×10−14 |
| GWAS2b web-based | 934 (27%) | 2564 (73%) | 0.294 | 0.067 | 2.4×10−6 |
| GWAS2b clinic-based | 1098 (30%) | 2564 (70%) | 0.284 | 0.059 | 4.0×10−7 |
To explore the hypothesis that the lower heritability of the Tourette GWAS2 sample might have arisen from the inclusion of Tourette cases diagnosed in the community and ascertained using a validated web-based screen (12, 13), the GWAS2 case-control sample was divided into clinic-based cases vs. web-based cases, and the LMM-based heritability analyses were repeated. Contrary to the predicted hypothesis, both subsets had the same heritability (h2g GWAS2-clinic=0.29, SE=0.07; p=1.2×10−9; h2g GWAS2-web=0.28, SE=0.10; p=1.2×10−9) (Table 2).
Tourette syndrome PRS in Multiplex vs. Simplex Families
Since a large proportion of Tourette GWAS1 cases were derived from affected sib-pair families, which might be expected to harbor higher Tourette syndrome polygenic risk than cases from simplex families without affected first-degree relatives, we examined the relationship between ancestry-adjusted PRS (aPRS) in cases from multiplex (first-degree relative family-history positive) versus simplex (first-degree relative family-history negative) families (Supplemental Methods).
As multiplex Tourette syndrome cases with a Tourette-affected parent or sibling (N=417) demonstrated similar mean aPRS to Tourette GWAS cases with a chronic tic-affected parent/sibling (N=111) (Fdf=1=0.12, p=0.73), we combined both Tourette case groups together for further analyses (Tourette/chronic tic family-history positive cases, N=528). The combined Tourette/ chronic tic multiplex cases had a significantly increased mean aPRS compared to aPRS from Tourette/ chronic tic simplex cases (N=346) (Fdf=1=4.90, p=0.027), confirming that multiplex cases were enriched for Tourette polygenic risk (Figure S7).
Tourette syndrome PRS and Tic Severity
Given the strong enrichment of Tourette aPRS in cases from multiplex families, Tourette/ chronic tic family-history positive Tourette cases were next examined to test whether Tourette aPRS might serve as a predictor of higher disease severity in these cases (Supplemental Methods). Following adjustment for the first 4 GWAS principal components, higher Tourette aPRS was significantly correlated with increased worst-ever tic severity (β=0.93, SE=0.42, p=0.026), with every 1 SD increase in Tourette aPRS corresponding to a 0.93 point increase in worst-ever tic severity (total range, 0-50).
Tourette syndrome and Tic Spectrum Phenotypes
Given the hypothesis that Tourette and other tic disorders represent a phenotypic spectrum with a shared genetic etiology, Tourette PRS derived from the GWAS meta-analysis was compared in Tourette and tic spectrum cases in the Icelandic deCODE sample (Figure 2 and Figure S5). Tourette PRS was significantly higher in both deCODE Tourette syndrome cases and tic spectrum cases compared to controls (OR=1.33, p=5.3×10−9 and OR=1.20, p=5.2 ×10−4, respectively), explaining 0.78% and 0.42% of the phenotypic variance, respectively. Direct comparison between case groups confirmed that deCODE Tourette cases carried a higher Tourette syndrome polygenic burden than subjects with other tic spectrum disorders (OR=1.14, p=0.05), representing an excess 0.37% of the phenotypic variance (Figure 2).
Figure 2.

Density plot demonstrating the distribution of Tourette syndrome polygenic risk scores (PRS) in population-based Icelandic Tourette cases (purple), tic disorder cases (blue), unscreened population controls (green), and tic negative population controls (orange).
Enrichment of Tourette syndrome heritability by functional annotation and gene expression
Tourette syndrome SNP-based heritability (h2g) from the GWAS meta-analysis was also used as a genome-wide probe to test whether aggregated Tourette syndrome genetic risk might be concentrated either in 52 specific functional genomic elements (e.g., promoters, enhancers, epigenetic marks) or in gene expression patterns from 10 grouped tissue/cell types using partitioned LDSC (22). Evolutionarily-conserved SNPs (2.6% of all SNPs) were enriched 16.5-fold for Tourette h2g, accounting for 42.3% of Tourette syndrome heritability (Pr(h2g)/Pr(SNPs)=16.5, SE=5.3, p=3.6×10−3, NS after correction). A parallel analysis including these evolutionarily-conserved SNPs plus 500-bp flanking windows (33% of all SNPs) was enriched 2.8-fold for Tourette h2g and accounted for 92.4% of Tourette syndrome heritability (Pr(hg2)/Pr(SNPs)=2.80, SE=0.46, p=1.0×10−4; p=0.005 after correction) (Figure S8). No other genomic annotations were significantly enriched for Tourette SNP-based heritability. In the cell-type analysis, significant enrichment was found only for CNS cell types, with 62.7% of Tourette syndrome heritability contributed by 14.8% of SNPs (p=4.2×10−8; p=4.2×10−7 after correction) (Figure S9).
Gene-based association and enrichment analyses
Gene-based association and enrichment tests were performed using meta-analysis summary statistics in MAGMA. FLT3 was identified with genome-wide significant association after correcting for 18,079 gene tests (p=8.9×10−7) (Figure S10). The most significant SNP in the FLT3 locus, rs2504235, was the only SNP surpassing genome-wide significance threshold in the primary meta-analysis, and was significantly associated with FLT3 expression level both in cerebellum (p=6.5×10−10) and cerebral cortex (p=2.6×10−11)(28). No gene set was significantly associated with Tourette syndrome after Bonferroni correction. In the gene expression enrichment analyses of 53 adult human tissues, only dorsolateral prefrontal cortex (Brodmann area 9 (BA9)) demonstrated significant enrichment of Tourette-associated genes after correction (β=0.023, SE=0.0069, p=1.2×10−4) (Figure 3, Supplemental Methods).
Figure 3.

Gene expression enrichment analysis of genome-wide Tourette syndrome polygenic risk in 53 adult human tissues.
DISCUSSION
Tourette syndrome has long been conceptualized as part of a spectrum of developmental tic disorders, with transient tics at one end (1), and severe Tourette syndrome with multiple psychiatric comorbidities at the other. However, until recently, potential biological relationships between the various tic disorders were unknown, as were the underlying genetic contributions to tic severity. The results of this study further illuminate the genetic architecture of Tourette syndrome and its relationships to phenotypic expression. First, the PRS analyses probing the genetic architecture of tic disorders in the population-based Icelandic sample demonstrates that individuals with Tourette syndrome share the same underlying polygenic risk as those with other tic disorders. Furthermore, the observation that Tourette syndrome cases have a significantly higher mean PRS than those with non-Tourette tic disorders provides evidence for a liability spectrum of genetic risk within tic disorders. Lastly, within Tourette syndrome cases, the finding that higher Tourette PRS was associated with increased tic severity also builds on our previous analyses demonstrating a relationship between higher Tourette PRS and the presence of complex symmetry and socially inappropriate tics (29). These relationships, although hypothesized based on clinical observations, have not previously been demonstrated at the molecular genetic level, and ultimately will help to provide insight into the molecular mechanisms of tic development and expression.
These observations have direct biological and clinical relevance. First, they support previous efforts to conceptualize Tourette and chronic tics as a unified condition and to combine them into a single tic spectrum disorder in future diagnostic schemas (1). Although traditionally separated clinically into distinct disorders, chronic/persistent tic disorders, whether consisting of motor tics, vocal tics, or both, appear to be due to the same underlying genetic causes. Second, while the small proportion of explained variance in worst-ever tic severity is a limitation of the current study, work in other polygenic psychiatric disorders such as schizophrenia has repeatedly demonstrated that, as GWAS sample sizes increase, the proportion of phenotype explained by polygenic risk scores increases markedly (30). It is therefore possible that in the future, Tourette PRS might be a potential candidate for predicting both conversion to chronic tics in the 20-25% of children who present with transient tics (1), and, at the other end of the phenotypic spectrum, tic persistence and lifetime tic severity in those with Tourette syndrome. Finally, particularly important in the context of the very large sample sizes required for the success of GWAS efforts, our results suggest that future genetic association studies may benefit from expanding disease definitions to include both Tourette and chronic tic cases.
Our genome-wide cell and tissue-based enrichment analyses implicate modulation of gene expression through non-coding variants as a fundamental mechanism in Tourette syndrome pathogenesis. All of the top tissues in the enrichment analyses were derived from brain, although dorsolateral pre-frontal cortex (BA9) was the only tissue in which eQTL enrichment surpassed Bonferroni correction. The 5 tissues with the strongest eQTL enrichment (frontal cortex, caudate, putamen, nucleus accumbens and cerebellum) all represent key nodes within the cortico-striatal and cortico-cerebellar circuits that have been implicated in Tourette pathophysiology (1). These results support the hypothesis that Tourette syndrome is a developmental circuit disorder affecting motor, cognitive and behavioral control (as manifested by tics, ADHD, and OCD symptoms), and suggest that future GWAS analyses in larger datasets should aid in identifying not only the individual genes underlying Tourette syndrome susceptibility, but also core pathways in development and/or regulation of these circuits that could serve as targets for modulation-based therapies.
LIMITATIONS
This study has several potential limitations, the most significant of which is the sample size. Although the largest Tourette syndrome GWAS conducted to date, our sample of under 5,000 cases is clearly not yet sufficient to identify definitive Tourette susceptibility variants, as demonstrated by the failure of the top GWAS SNP to replicate in the deCODE sample. Additional potential limitations are also related to sample size, including reduced power to examine additional clinical variables of interest such as age of onset of tics and co-occurring psychiatric illnesses such as obsessive compulsive disorder and attention deficit hyperactivity disorder. However, we anticipate that most, if not all, of these limitations can be resolved by substantial increases in the number of Tourette syndrome cases collected for GWAS, an effort that is currently underway.
Supplementary Material
Acknowledgments
The authors thank the patients with Tourette Syndrome and their families, and all the volunteers who participated in this study. This study was supported by NIH grants U01 NS040024 to Drs. Pauls, Mathews, Scharf and the TAAICG, ARRA Grant NS040024-09S1, K23 MH085057, and K02 NS085048 to Dr. Scharf, ARRA Grants NS040024-07S1 and NS016648 to Dr. Pauls, MH096767 to Dr. Mathews and NINDS Informatics Center for Neurogenetics and Neurogenomics grant P30 NS062691 to Drs. Coppola and Freimer, and by grants from the Tourette Association of America to Drs. Paschou, Pauls, Mathews, and Scharf. This study was also funded in part by grants from NIH R01MH092290 to Dr. Brown, R01MH092291 to Dr. Kuperman, R01MH092292 to Dr. Coffey, R01MH092293 to Dr. Heiman, R01MH092513 to Dr. Zinner, R01MH092516 to Dr. Grice, R01MH092520 to Dr. Gilbert, R01MH092289 to Dr. State, grants P01AG021654 and the Nathan Shock Center of Excellence for the Biology of Aging P30AG038072 to Dr. Barzilai, R01AG042188 to Dr. Atzmon, and a grant from the German Research Society to Dr. Hebebrand. Funding support for the Study of Addiction: Genetics and Environment (SAGE) was provided through the NIH Genes, Environment and Health Initiative [GEI] (U01 HG004422). SAGE is one of the genome-wide association studies funded as part of the Gene Environment Association Studies (GENEVA) under GEI. Assistance with phenotype harmonization and genotype cleaning, as well as with general study coordination, was provided by the GENEVA Coordinating Center (U01 HG004446). Assistance with data cleaning was provided by the National Center for Biotechnology Information. Support for collection of datasets and samples was provided by the Collaborative Study on the Genetics of Alcoholism (COGA; U10 AA008401), the Collaborative Genetic Study of Nicotine Dependence (COGEND; P01 CA089392), and the Family Study of Cocaine Dependence (FSCD; R01 DA013423). Funding support for genotyping, which was performed at the Johns Hopkins University Center for Inherited Disease Research, was provided by the NIH GEI (U01HG004438), the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the NIH contract “High throughput genotyping for studying the genetic contributions to human disease” (HHSN268200782096C). The datasets used for the analyses described in this manuscript were obtained from dbGaP at http://www.ncbi.nlm.nih.gov/projects/gap/cgibin/study.cgi?study_id=phs000092.v1.p1 through dbGaP accession number phs000092.v1.p. None of the funding agencies for this project had any influence or played any role in a) the design or conduct of the study; b) management, analysis, or interpretation of the data; or c) preparation, review, or approval of the manuscript. The views expressed in this publication are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
Disclosures
Drs. Budman, Cath, Dion, Gilbert, Grados, Lee, Lyon, King, McMahon, Malaty, Mathews, Pauls, Sandor, Scharf, and Singer have received research support from the Tourette Association of America (TAA). Dr. Muller-Vahl received funding for research from the German Research Society (DFG: GZ MU 1527/3-1), the German Ministry of Education and Research (BMBF: 01KG1421), GW, Almirall, Abide Therapeutics, and Therapix Biosciences. Dr. Muller-Vahl receives honoraria from Abide Therapeutics, Fundacion Canna, Tilray, and Therapix Biosiences, and is member of the advisory board of Therapix Biosiences. Dr. Sandor reports shares in Alnylam Pharmaceuticals, Extendicare Inc, Illumina, and Neurozone MSH Inc, and was a member of the data safety monitoring committee for Psyadon Pharmaceuticals. Dr. Singer receives book royalties from Elsevier and is a consultant for Teva Pharmaceuticals. Dr. Nöthen reports receiving fees for memberships in the Scientific Advisory Boards for the Lundbeck Foundation and the Robert-Bosch-Stiftung, and for membership in the Medical-Scientific Editorial Office of the Deutsches Ärzteblatt. He also reports travel support from Shire Deutschland GmbH, and receives salary from and holds shares in Life & Brain GmbH. Dr. Fernandez reports research funding from the NIH, Simons Foundation, and Shire, and is a consultant for Cogstate and Guidepoint. Dr. McMahon receives salary from the University of Utah for serving as Autism Community Outreach Director, has received royalties from LineaGen, and is a paid consultant for Pathways to Careers. Dr. Batterson receives stipend funding from the American Psychiatric Association (APA), and is the Speaker of the APA Assembly. Dr. Malaty has received travel and grant support from the TAA and the National Parkinson Foundation (NPF), and has participated in research funded by the Parkinson Foundation, TAA, Dystonia Coalition, Abbvie, Auspex, Biogen, Biotie, Intrepid, Lily, Lundbeck, Merz, Neurocrine, Nuvelution Pfizer, and Revance, but has no owner interest in any pharmaceutical company. Dr. Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH (NR014852 and R01NS096008), NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Association of America, and the UF Foundation. Dr. Okun has also received royalties from Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge for movement disorders books, and is an associate editor for New England Journal of Medicine Journal Watch Neurology; he has participated in CME and educational activities on movement disorders sponsored by PeerView, Prime, QuantiaMD, WebMD, Medicus, MedNet, Henry Stewart, and by Vanderbilt University; he receives grants from Medtronic, Abbvie, Allergan, and ANS/St. Jude, and the PI has no financial interest in these grants; he has participated as a site PI and/or co-PI for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria. Dr. Woods reports receiving royalties from Oxford University Press and Guilford Press and speaker fees from the TAA’s partnership with the Center for Disease Control (CDC). Dr. Jankovic reports research and/or training grants from: Adamas Pharmaceuticals, Inc, Allergan, Inc, CHDI Foundation, Civitas/Acorda Therapeutics, Dystonia Coalition, Dystonia Medical Research Foundation, F. Hoffmann-La Roche Ltd, Huntington Study Group, Medtronic Neuromodulation, Merz Pharmaceuticals, Michael J Fox Foundation for Parkinson Research, National Institutes of Health, Neurocrine Biosciences, Parkinson’s Foundation, Nuvelution, Parkinson Study Group, Pfizer Inc, Prothena Biosciences Inc, Psyadon Pharmaceuticals, Inc, Revance Therapeutics, Inc, Teva Pharmaceutical Industries Ltd, and US WorldMeds. Dr. Jankovic has served on an advisory or data monitoring committee or as a consultant for: Adamas Pharmaceuticals, Inc, Allergan, Inc, Merz Pharmaceuticals, Prothena Biosciences Inc, Retrophin, Inc-Parexel, Revance Therapeutics, Inc, and Teva Pharmaceutical Industries Ltd. Dr. Jankovic has received royalties from Cambridge, Elsevier, Future Science Group, Hodder Arnold, Medlink: Neurology, Lippincott Williams and Wilkins, and Wiley-Blackwell. He is on the Editorial Boards for Expert Review of Neurotherapeutics, Medlink, Neurology in Clinical Practice, The Botulinum Journal, PeerJ, Therapeutic Advances in Neurological Disorders, Neurotherapeutics, Tremor and Other Hyperkinetic Movements, and the Journal of Parkinson’s Disease. Dr. Heiman has received funding from the New Jersey Center for Tourette Syndrome & Associated Disorders (NJCTS). Dr. Gilbert has received honoraria and/or travel support from the TAA/CDC, the American Academy of Pediatrics, and the Child Neurology Society. Dr. Gilbert has received compensation for expert testimony for the U.S. National Vaccine Injury Compensation Program, through the Department of Health and Human Services. He has received research support from the NIH (NIMH, NINDS), funding from Ecopipam Pharmaceuticals (clinical trial, Tourette Syndrome) and EryDel (clinical trial, Ataxia Telangiectasia), and has received book royalties from Elsevier and Wolters Kluwer. Dr. Wagner has received a nonprofit grant from the German Research Foundation, DFG grant Number Wa 731/10-1. Dr. Kuperman is involved with Neurocrine Incorporated for the purpose of recruiting a small number of individuals with Tourette Syndrome for enrollment in a drug trial for a new medication to treat severe Tourette Syndrome; he has no other financial relationships with this company including any equity, ownership, profit-sharing agreement, royalties, or patents. Dr. Smoller is an unpaid member of the Bipolar/Depression Research Community Advisory Panel of 23andMe. Drs. Nawaz, H. Stefansson, and K. Stefansson are employees of deCODE genetics/Amgen. Dr. Neale is a member of the Scientific Advisory Board for Deep Genomics, and serves as a consultant for Camp4 Therapeutics Corporation and Merck & Co. Dr. Coffey is on the Scientific Advisory Board for Abide Therapeutics and Genco Sciences; she receives honoraria from the American Academy of Child and Adolescent Psychiatry; she receives research support from Catalyst Pharmaceuticals, Neurocrine Biosciences, Otsuka, Shire, and NIMH/UCSF; she is on the Scientific Advisory Board and receives research support from Auspex, Teva, and Nuvelution. Dr. Coffey is a Co-Chair and on the Medical Advisory Board for the TAA, TAA-CDC Partnership. Dr. Mathews has received research support, honoraria, and travel support from the TAA and is the co-chair of the TAA Scientific Advisory Board. Dr. Scharf has received consulting fees from Nuvelation Pharma and Abide Pharmaceuticals, has received travel and grant support from the TAA and the TLC Foundation for Body-Focused Repetitive Behaviors, and is a member of the Scientific Advisory Board for the TAA and the TLC Foundation for Body-Focused Repetitive Behaviors.
Ms. Yu, Dr. Sul, Dr. Tsetsos, Dr. Huang, Dr. Zelaya, Dr. Illmann, Ms. Osiecki, Dr. Darrow, Dr. Hirschtritt, Dr. Greenberg, Dr. Stuhrmann, Dr. Rouleau, Dr. Aschauer, Dr. Stamenkovic, Ms. Schlögelhofer, Dr. Barr, Dr. Hebebrand, Dr. Hinney, Dr. Barta, Dr. Tarnok, Dr. Nagy, Dr. Depienne, Dr. Worbe, Dr. Hartmann, Dr. Rizzo, Dr. Berlin, Dr. Robertson, Dr. Brown, Dr. Dietrich, Dr. Hoekstra, Dr. Zinner, Dr. Luðvigsson, Dr. Sæmundsen, Dr. Thorarensen, Dr. Atzmon, Dr. Barzilai, Dr. Moessner, Dr. Ophoff, Dr. C. Pato, Dr. M. Pato, Dr. Knowles, Dr. Roffman, Dr. Buckner, Dr. Willsey, Dr. Tischfield, Dr. Posthuma, Dr. Cox, Dr. Freimer, Dr. Davis, Dr. Paschou, and Dr. Coppola report no competing interests.
REFERENCES
- 1.Robertson MM, Eapen V, Singer HS, Martino D, Scharf JM, Paschou P, et al. Gilles de la Tourette syndrome. Nature reviews Disease primers. 2017;3:16097. [DOI] [PubMed] [Google Scholar]
- 2.Mataix-Cols D, Isomura K, Perez-Vigil A, Chang Z, Ruck C, Larsson KJ, et al. Familial Risks of Tourette Syndrome and Chronic Tic Disorders. A Population-Based Cohort Study. JAMA Psychiatry. 2015;72(8):787–93. [DOI] [PubMed] [Google Scholar]
- 3.Pauls D, Fernandez T, Mathews CA, State M, Scharf JM. The inheritance of Tourette Disorder: A review. Journal of obsessive-compulsive and related disorders. 2014;3:380–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang AY, Yu D, Davis LK, Sul JH, Tsetsos F, Ramensky V, et al. Rare Copy Number Variants in NRXN1 and CNTN6 Increase Risk for Tourette Syndrome. Neuron. 2017;94(6):1101–11 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willsey AJ, Fernandez TV, Yu D, King RA, Dietrich A, Xing J, et al. De Novo Coding Variants Are Strongly Associated with Tourette Disorder. Neuron. 2017;94(3):486–99 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Mandell JD, Kumar Y, Sun N, Morris MT, Arbelaez J, et al. De Novo Sequence and Copy Number Variants Are Strongly Associated with Tourette Disorder and Implicate Cell Polarity in Pathogenesis. Cell reports. 2018;24(13):3441–54 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis LK, Yu D, Keenan CL, Gamazon ER, Konkashbaev AI, Derks EM, et al. Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS Genet. 2013;9(10):e1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Psychiatric GWAS Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43(10):969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scharf JM, Yu D, Mathews CA, Neale BM, Stewart SE, Fagerness JA, et al. Genome-wide association study of Tourette’s syndrome. Molecular psychiatry. 2013;18(6):721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tourette Syndrome Association International Consortium for Genetics. Genome scan for Tourette disorder in affected-sibling-pair and multigenerational families. Am J Hum Genet. 2007;80(2):265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietrich A, Fernandez TV, King RA, State MW, Tischfield JA, Hoekstra PJ, et al. The Tourette International Collaborative Genetics (TIC Genetics) study, finding the genes causing Tourette syndrome: objectives and methods. Eur Child Adolesc Psychiatry. 2015;24(2):141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darrow SM, Illmann C, Gauvin C, Osiecki L, Egan CA, Greenberg E, et al. Web-based phenotyping for Tourette Syndrome: Reliability of common co-morbid diagnoses. Psychiatry Res. 2015;228(3):816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan CA, Marakovitz SE, O’Rourke JA, Osiecki L, Illmann C, Barton L, et al. Effectiveness of a web-based protocol for the screening and phenotyping of individuals with Tourette syndrome for genetic studies. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(8):987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nature methods. 2011;9(2):179–81. [DOI] [PubMed] [Google Scholar]
- 16.Pirinen M, Donnelly P, Spencer CCA. Efficient computation with a linear mixed model on large-scale data sets with applications to genetic studies. Annals of Applied Statistics. 2012;7(1):369–90. [Google Scholar]
- 17.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pe’er I, Yelensky R, Altshuler D, Daly MJ. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol. 2008;32(4):381–5. [DOI] [PubMed] [Google Scholar]
- 20.Dudbridge F, Gusnanto A. Estimation of significance thresholds for genomewide association scans. Genet Epidemiol. 2008;32(3):227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. 2012;47(2):77–90. [DOI] [PubMed] [Google Scholar]
- 22.Finucane HK, Bulik-Sullivan B, Gusev A, Trynka G, Reshef Y, Loh PR, et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet. 2015;47(11):1228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu D, Mathews CA, Scharf JM, Neale BM, Davis LK, Gamazon ER, et al. Cross-disorder genome-wide analyses suggest a complex genetic relationship between Tourette’s syndrome and OCD. Am J Psychiatry. 2015;172(1):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vilhjalmsson BJ, Yang J, Finucane HK, Gusev A, Lindstrom S, Ripke S, et al. Modeling Linkage Disequilibrium Increases Accuracy of Polygenic Risk Scores. Am J Hum Genet. 2015;97(4):576–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS computational biology. 2015;11(4):e1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GTEx Consortium, Data Analysis Laboratory, Coordinating Center-Analysis Working Group, Statistical Methods groups-Analysis Working Group, Enhancing GTEx groups, NIH Common Fund, et al. Genetic effects on gene expression across human tissues. Nature. 2017;550(7675):204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515(7526):209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Broad Institute of MIT and Harvard. GTEx Portal. 2017; Available from: https://www.gtexportal.org/home/.
- 29.Darrow SM, Hirschtritt ME, Davis LK, Illmann C, Osiecki L, Grados M, et al. Identification of Two Heritable Cross-Disorder Endophenotypes for Tourette Syndrome. Am J Psychiatry. 2017;174(4):387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


