Abstract
Purpose:
We sought to determine whether there is a subset of men who can avoid prostate biopsy based on multiparametric magnetic resonance imaging and clinical characteristics.
Materials and Methods:
Of 1,149 consecutive men who underwent prostate biopsy from October 2011 to March 2017, 135 had prebiopsy negative multi-parametric magnetic resonance imaging with PI-RADS™ (Prostate Imaging Reporting and Data System) score less than 3. The detection rate of clinically significant prostate cancer was evaluated according to prostate specific antigen density and prior biopsy history. Clinically significant prostate cancer was defined as Grade Group 2 or greater. Multivariable logistic regression analysis was performed to identify predictors of nonclinically significant prostate cancer on biopsy.
Results:
The prostate cancer and clinically significant prostate cancer detection rates were 38% and 18%, respectively. Men with biopsy detected, clinically significant prostate cancer had a smaller prostate (p = 0.004), higher prostate specific antigen density (p = 0.02) and no history of prior negative biopsy (p = 0.01) compared to the nonclinically significant prostate cancer cohort. Prostate specific antigen density less than 0.15 ng/ml/cc (p <0.001) and prior negative biopsy (p = 0.005) were independent predictors of absent clinically significant prostate cancer on biopsy. The negative predictive value of multiparametric magnetic resonance imaging for biopsy detection of clinically significant prostate cancer improved with decreasing prostate specific antigen density, primarily in men with prior negative biopsy (p = 0.001) but not in biopsy naïve men. Of the men 32% had the combination of negative multiparametric magnetic resonance imaging, prostate specific antigen density less than 0.15 ng/ml/cc and negative prior biopsy, and none had clinically significant prostate cancer on repeat biopsy. The incidence of biopsy identified, clinically significant prostate cancer was 18%, 10% and 0% in men with negative multiparametric magnetic resonance imaging only, men with negative multiparametric magnetic resonance imaging and prostate specific antigen density less than 0.15 ng/ml/cc, and men with negative multiparametric magnetic resonance imaging, prostate specific antigen density less than 0.15 ng/ml/cc and negative prior biopsy, respectively.
Conclusions:
We propose that a subset of men with negative multiparametric magnetic resonance imaging, prostate specific antigen density less than 0.15 ng/ml/cc and prior negative biopsy may safely avoid rebiopsy. Conversely prostate biopsy should be considered in biopsy naïve men regardless of negative multiparametric magnetic resonance imaging, particularly those with prostate specific antigen density greater than 0.15 ng/ml/cc.
Keywords: prostatic neoplasms, biopsy, magnetic resonance imaging, prostate-specific antigen, predictive value of tests
MULTIPARAMETRIC MRI of the prostate combined with TRUS fusion PBx is a promising tool to identify CSPCa.1,2 However, interpretation of negative mpMRI findings remains debatable. Whether men without suspicious lesions on mpMRI can safely omit unnecessary prostate biopsy remains controversial. It has been suggested that including additional negative predictors of CSPCa may help refine the decision algorithm to more confidently determine whether men with negative mpMRI need to undergo prostate biopsy.3–6
We evaluated a prospective cohort of men with negative prebiopsy mpMRI to determine CSPCa detection rates based on PSAD and biopsy history. In a subset of patients biopsy histology was correlated with the gold standard, serially sectioned RP specimens.
MATERIALS AND METHODS
From a prospectively maintained, institutional review board approved PBx database we identified consecutive men who underwent prebiopsy mpMRI at our institution from October 2011 to March 2017. Patients were included in study if they had negative mpMRI, defined as PI-RADS v1 or v2 score less than 3.7,8 We excluded patients with mpMRI done elsewhere and those who had undergone any prior surgical or medical treatment for PCa or BPH. The study received Institutional Review Board approval (IRB No. HS-13–00663).
Multiparametric MRI was performed on a 3 Tesla MRI system (GE Healthcare, San Antonio, Texas) using a multichannel phased array coil. The MRI acquisition protocol included high resolution T2-weighted, diffusion-weighted and T1-weighted dynamic contrast enhanced sequences. Parametric apparent diffusion coefficient maps were calculated from the diffusion weighted images.
In 2016 we reevaluated and updated our prostate MRI protocol from PI-RADS v1 to meet the recommendations of PI-RADS v2. Prostate volume was calculated using the ellipsoid formula based on MRI measurements. All mpMRIs were evaluated by experienced radiologists with a minimum of 5 years of experience with prostate MRI.
TRUS guided transrectal PBx was performed by 2 experienced urologists with the patient under local anesthesia using 3-dimensional mapping on a Urostation (Koelis®). Systematic extended sextant 12-core biopsies were obtained with an 18 gauge needle and each core was independently labeled and submitted to histology in separate containers. No MRI directed biopsies were performed in cases with lesions with PI-RADS score less than 3.
In patients who underwent RP serially sectioned RP specimens were evaluated by a fellowship trained uropathologist. PCa mapping and detailed characteristics of PCa foci were recorded using the Stanford protocol, the AJCC Staging Manual, 7th edition, and ISUP (International Society of Urological Pathology) standards.9,10 CSPCa was defined as GG 2 or greater (GS score 3 + 4 or greater). CIPCa was defined as GG 1 (GS score 6). CSPCa and CIPCa were based on PBx histology. Prior negative biopsy was defined as at least 1 PBx prior to the current biopsy.
To analyze demographic data the patients were divided into 3 cohorts, including CSPCa, CIPCa and benign groups. To assess predictors of absent CSPCa on prostate biopsy patients were compared as CSPCa vs nonCSPCa group with the latter including patients with CIPCa and benign findings. ROC curve analysis was performed to detect nonCSPCa on PBx. Multivariable logistic regression analysis was done with clinically relevant parameters and statistically significant variables on univariate analysis.
Statistical analysis was performed with EZR, version 1.35,11 which is a graphic user interface for R (https://www.r-project.org/). The Mann-Whitney U test was used for 2 continuous variables and the chi-square or Fisher exact test was used for categorical variables. The Kruskal-Wallis and post hoc tests were used for 3 continuous variables. Statistical significance was considered at p <0.05.
RESULTS
Of the 147 consecutive patients with negative prebiopsy mpMRI we excluded 5 who underwent transurethral prostate resection, 6 who underwent mpMRI elsewhere and 1 who underwent non-contrast MRI (fig. 1). Therefore, 135 patients met the inclusion criteria for this study. One case in which a PSA test was performed more than 6 months prior to PBx was excluded from all PSA and PSAD analyses.
Figure 1.
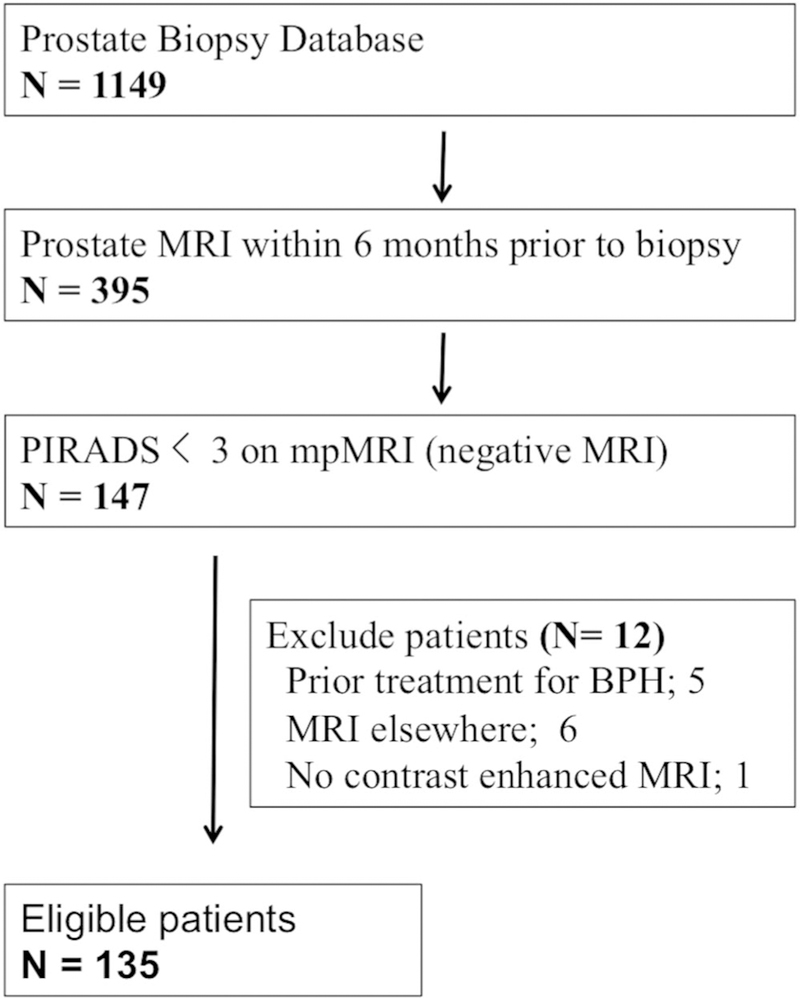
Schematic tree of study cohort
Table 1 lists demographics. Of the men 48 (36%) were PBx naïve and 60 (44%) had undergone previous negative PBx with a median of 29 months from the last biopsy, including 38 (63%) with 1 prior biopsy and 22 (37%) with more than 1 prior biopsy. A total of 27 men (20%) had undergone previous positive PBx a median of 12 months from the last biopsy, including 20 on active surveillance and 7 with biopsy proven GG 2 for restaging biopsy (table 1). Prostate biopsy detected PCa (any GG) in 51 men (38%) and CSPCa in 24 (18%).
Table 1.
Demographics of patients with negative MRI who underwent prostate biopsy
| Biopsy Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Clinically Significant Prostate Ca* |
Clinically Insignificant Prostate Ca † |
Benign | p Value | |||||
| No. pts | 135 | 24 | 27 | 84 | – | ||||
| Median age (IQR) | 64 | (58–69) | 66 | (58–71) | 67 | (59–70) | 63 | (57–69) | 0.5 |
| Median ng/ml PSA (IQR) | 5.9 | (4.1–8.0) | 6.1 | (4.9–8.1) | 5.2 | (3.4–8.4) | 5.9 | (4.5–7.9) | 0.5 |
| Median cc prostate vol (IQR) | 55 | (38–79) | 36 | (27–50) | 43 | (34–58) | 64 | (48–81) | <0.001 |
| Median ng/ml/cc PSAD (IQR) | 0.1 | (0.073–0.15) | 0.16 | (0.13–0.20) | 0.10 | (0.09–0.15) | 0.09 | (0.07–0.12) | <0.001 |
| No. prostate Ca (%): | |||||||||
| Suspicious digital rectal examination | 11 | (8.1) | 0 | 2 | (7) | 9 | (10) | 0.3 | |
| Family history | 33 | (24) | 6 | (25) | 7 | (26) | 20 | (24) | 1.0 |
| Median No. biopsy cores/pt (IQR) | 13 | (12–14) | 13 | (12–14) | 13 | (12–14) | 13 | (12–14) | 0.4 |
| No. prostate biopsy status (%): | |||||||||
| Naïve | 48 | (36) | 10 | (42) | 8 | (30) | 30 | (36) | 0.6 |
| Previous neg | 60 | (44) | 5 | (21) | 7 | (26) | 48 | (57) | <0.001 |
| Previous pos | 27 | (20) | 9 | (38) | 12 | (44) | 6 | (7) | <0.001 |
Grade Group 2 or greater.
Grade Group 1.
Comparison among the CSPCa, CIPCa and benign groups revealed that prostate volume, PSAD, prior negative biopsy and prior positive biopsy statistically differed among the 3 groups (all p <0.001, table 1, and figs. 2 and 3). On biopsy CSPCa cases showed a higher number of positive cores, longer cancer core length and greater cancer core percent than those with CIPCa (all p <0.001, supplementary table 1, http://jurology.com/).
Figure 2.
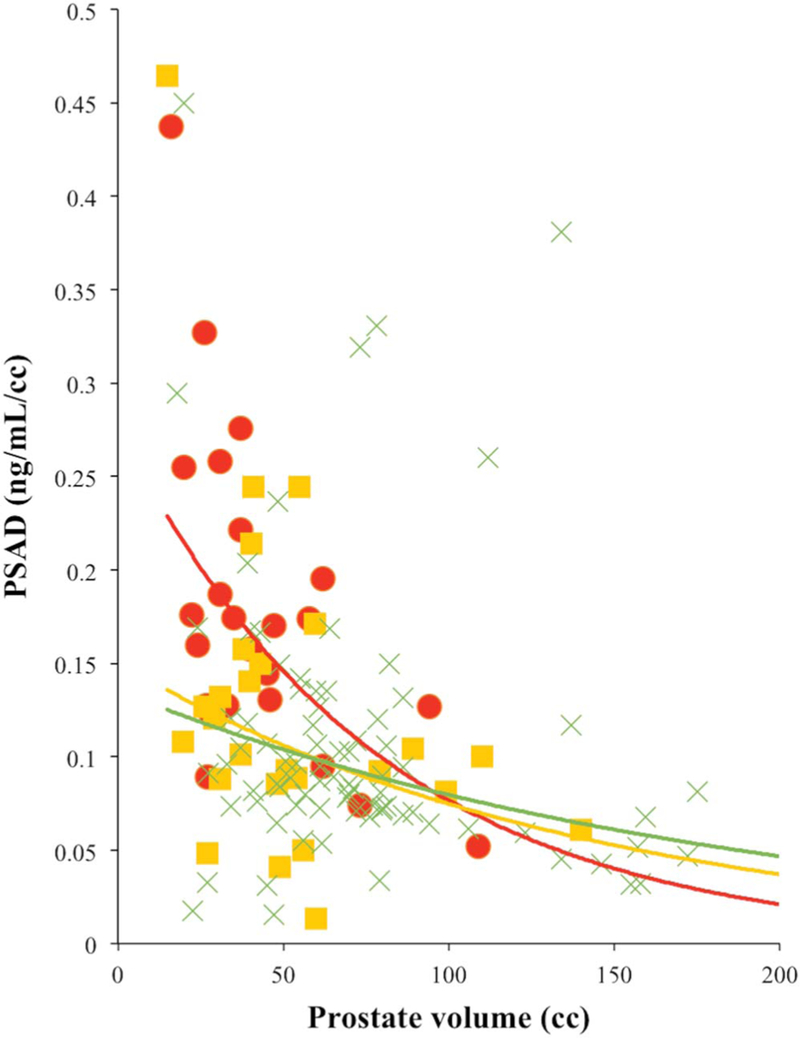
Scatterplot of PSAD vs prostate volume. Red circles represent CSPCa. Yellow squares represent CIPCa. Green crosses represent benign finding. Red curve represents exponent (CSPCa). Yellow curve represents exponent (CIPCa). Green curve represents exponent (benign).
Figure 3.
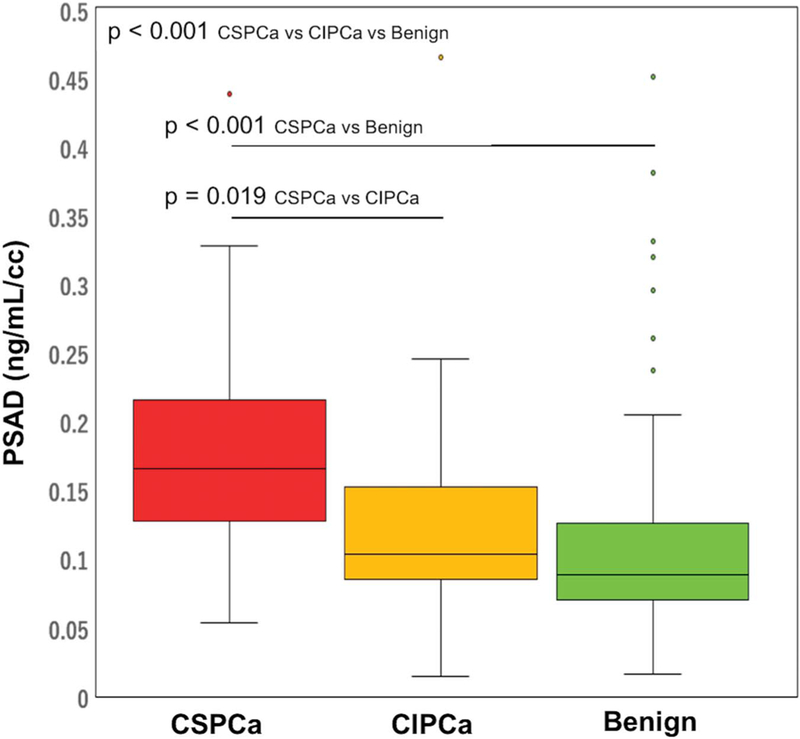
PSAD by PBx histology in patients with negative MRI
When comparing 24 patients with CSPCa to 111 with nonCSPCa, univariate analysis revealed that greater prostate volume (p = 0.004), lower PSAD (p = 0.02) and prior negative PBx (p = 0.01) were predictors of biopsy detection of nonCSPCa in patients with negative MRI. Multivariate analysis confirmed that PSAD less than 0.15 ng/ml/cc (p <0.001) and prior negative PBx (p = 0.005) were independent predictors of biopsy detection of non-CSPCa (table 2). ROC curve analysis of nonCSPCa detection showed a larger AUC for PSAD compared to prostate volume or other parameters (0.77 vs 0.73) (fig. 4). All 43 patients (32%) with PSAD less than 0.15 ng/ml/cc and negative biopsy history had no CSPCa on repeat PBx. Conversely 9 of the 18 patients (50%) with PSAD 0.15 ng/ml/cc or greater and no history of negative biopsy had CSPCa on PBx (supplementary table 2, http://jurology.com/).
Table 2.
Predictors of absent biopsy detected, clinically significant prostate cancer in men with negative MRI
| Univariate | Multivariable | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | ||
| Age | 0.99 | (0.94–1.06) | 0.97 | – | – |
| PSA | 1.02 | (0.93–1.11) | 0.7 | – | – |
| Prostate vol | 1.03 | (1.01–1.06) | 0.004 | – | – |
| PSAD | 0.01 | (0.0002–0.55) | 0.02 | – | – |
| PSAD less than 0.15 ng/ml/cc | 5.93 | (2.32–15.2) | <0.001 | 7.7 (2.8–21.3) | <0.001 |
| DRE suspicious for PCa (yes vs no) | 0 (0–1.81) | 0.2 | – | – | |
| Prostate Ca family history (yes vs no) | 0.96 | (0.35–2.68) | 0.9 | – | – |
| Biopsy naïve (yes vs no) | 0.73 | (0.30–1.79) | 0.5 | – | – |
| Previous biopsy (yes vs no): | |||||
| Neg | 3.73 | (1.30–10.7) | 0.01 | 5.2 (1.6–16.5) | 0.005 |
| Pos | 0.32 | (0.12–0.85) | 0.02 | – | – |
| PI-RADS version 1 vs 2 | 0.62 | (0.24–1.56) | 0.31 | – | – |
Figure 4.
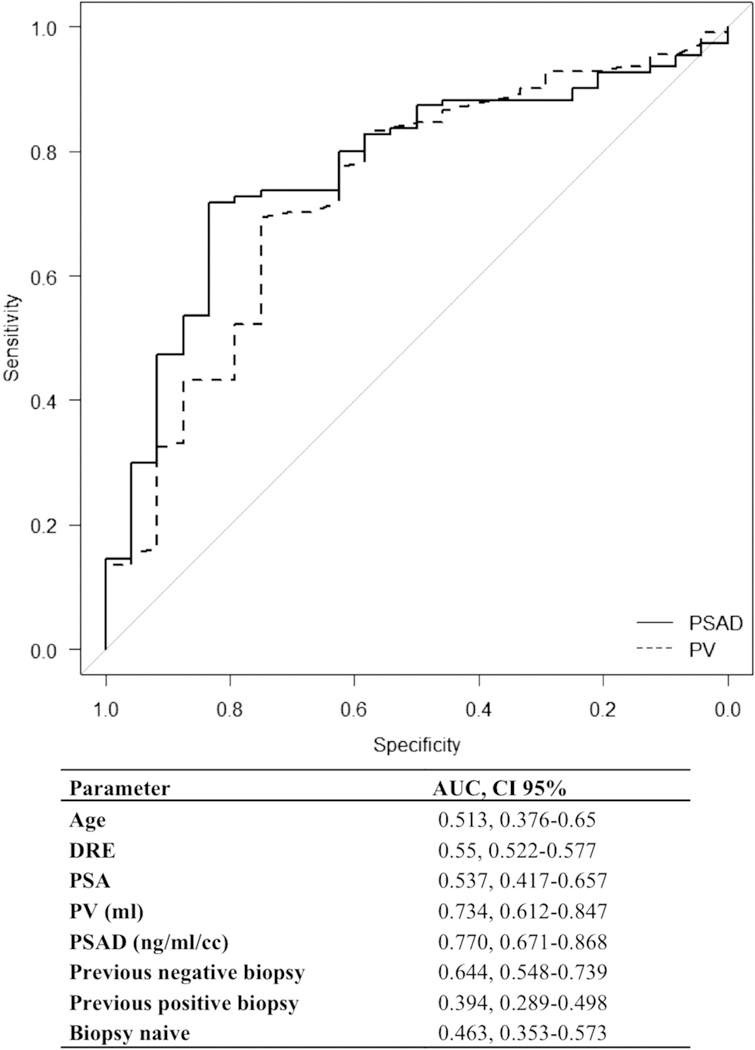
ROC curve analysis of absent CSPCa in patients with negative MRI. PV, prostate volume.
The NPV of mpMRI for biopsy detection of CSPCa improved with decreasing PSAD (table 3 and fig. 5). The NPV of negative MRI improved from 82% in all patients to 90% in patients with PSAD less than 0.15 ng/ml/cc. Additional analyses by PBx status showed further improvement in the NPV of mpMRI with decreasing PSAD in patients with prior PBx. There was mild improvement in the NPV of mpMRI in biopsy naïve patients from 71% in those with PSAD 0.15 ng/ml/cc or greater to 80% in those with PSAD less than 0.15 ng/ml/cc (p = 0.63, table 3 and fig. 5).
Table 3.
MRI negative predictive value of clinically significant, Grade Group 2 or greater prostate cancer detection according to PSAD and biopsy history
| PSAD (ng/ml/cc) | No. MRI Neg Predictive Value/Total No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Biopsy Naïve |
Prior Neg Biopsy |
Prior Pos Biopsy |
|||||
| 0.15 or Greater | 14/35 | (60) | 2/7 | (71) | 5/17 | (71) | 7/11 | (36) |
| Less than 0.15 | 10/99 | (90) | 8/40 | (80) | 0/43 | (100) | 2/16 | (88) |
| p Value | <0.001 | 0.63 | 0.001 | 0.012 | ||||
| 0.15 or Greater | 14/35 | (60) | 2/7 | (71) | 5/17 | (71) | 7/11 | (36) |
| 0.10—Less than 0.15 | 6/33 | (82) | 4/13 | (69) | 0/13 | (100) | 2/7 | (71) |
| Less than 0.10 | 4/66 | (94) | 4/27 | (85) | 0/30 | (100) | 0/9 | (100) |
| p Value | <0.001 | 0.53 | 0.001 | 0.009 | ||||
Figure 5.
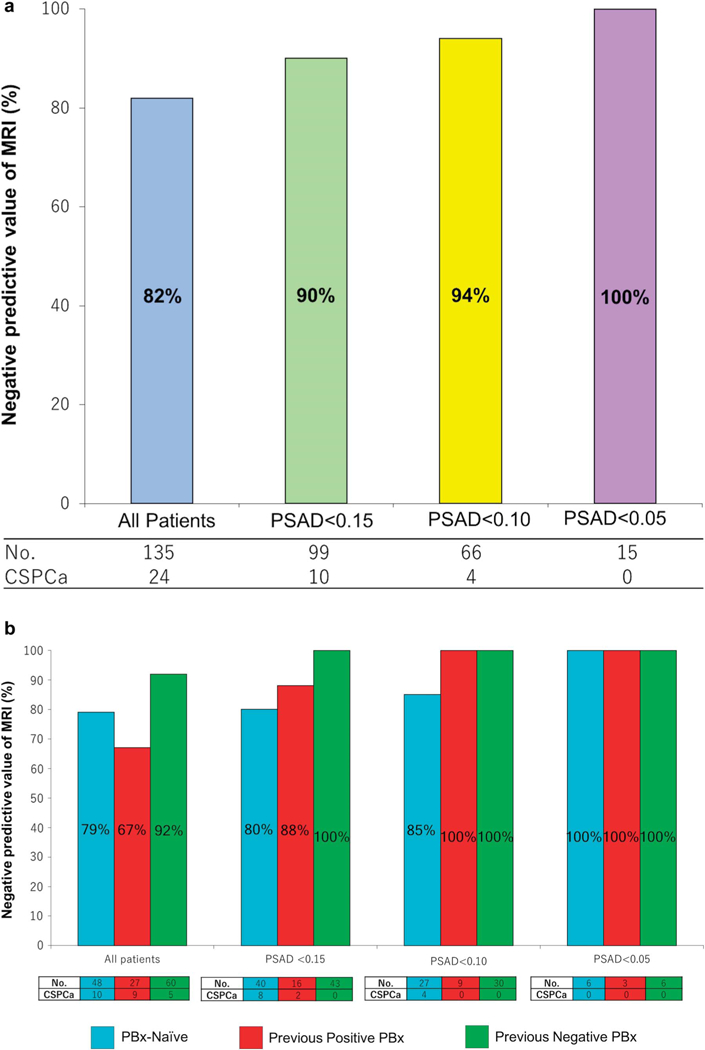
Negative predictive value of MRI for clinically significant prostate cancer. a, by PSAD in ng/ml/cc. b, by PSAD in ng/ml/cc and PBx status.
Of the 51 patients diagnosed with PCa on PBx 26 underwent RP, including 19 of 24 with CSPCa and 7 of 27 with CIPCa (supplementary table 3, http://jurology.com/). Biopsy and serially sectioned RP histology were concordant in 77% of cases (supplementary table 4, http://jurology.com/). Four of the 7 CIPCa cases on biopsy in which RP was done were upgraded to CSPCa on RP histology. Extraprostatic extension (pT3a) was identified in 5 patients by RP histology, corresponding to 5 of all 24 biopsy detected CSPCa cases (21%). On RP histology 21 patients had CSPCa, which was GG 2 in 16 and GG 3 in 5. Pathological stage was pT2a in 5 patients (19%), pT2c in 16 (62%) and pT3a in 5 (19%) (supplementary table 5, http://jurology.com/).
DISCUSSION
In this single institution study patients were included if there were no suspicious lesions on prebiopsy mpMRI as defined by PI-RADS criteria.3–6,12,13 Patients with prior PCa or BPH treatment were excluded because that could alter PSA, introducing an artifact. Patients with mpMRI performed elsewhere were also excluded to maintain MRI protocol uniformity.
There was an evolution of our MRI protocol and assessment from PI-RADS v1 to PI-RADS v2. However, these evolutionary changes did not impact the detection of CSPCa (table 2). Thus, of the 1,149 men in our database we identified this homogenous cohort of 135, comprising 35% of all 395 patients who underwent MRI prior to biopsy. Based on this cohort we sought to identify predictors of nonCSPCa on PBx with the aim of clarifying the NPV of mpMRI when combined with other negative predictors of CSCPa.3–6 In fact, patients with negative MRI comprise around 27% to 44% of all patients with MRI prior to PBx.13–15
To date at our institution we have performed PBx if clinically indicated by elevated PSA, suspicious DRE and active surveillance regardless of MRI findings. A total of 27 patients (20%) with prior positive PBx underwent mpMRI and repeat biopsy, including 20 (15%) per active surveillance protocol and 7 (5%) for restaging biopsy in whom GG 2 was identified in PBx performed elsewhere and who sought ablation therapy at our institution. The CSPCa and PCa detection rates were 18% and 38%, corresponding to NPV of 82% and 62%, respectively. These results are compatible with recent literature and similar to those reported by Hansen et al using PSAD less than 0.1 ng/ml/cc (supplementary table 6, http://jurology.com/).3–6, 12–14, 16
However, in most published reports predictors of absent CSPCa on biopsy were not assessed in patients with negative MRI. Some studies were underpowered or had only a few events, precluding further evaluation. In fact, the multi-institutional PROMIS (Prostate MR Imaging Study) trial provided level 1B evidence when evaluating 576 patients who underwent mpMRI and PBx with saturation transperineal PBx as the reference standard.17 Multiparametric MRI was negative in 158 patients (27%) and the NPV of GS 3 + 4 or greater was 76%. However, in this study mpMRI was performed at 1.5 Tesla strength and the investigators did not evaluate predictors of nonCSPCa on PBx in the setting of negative MRI.
A recent retrospective study described outcomes in 1,255 patients with negative mpMRI who were followed a median of 52 months.18 The 48-month PCa and CSPCa-free survival rates were 84% and 95% in 659 PBx naïve patients while in 596 with prior negative PBx the rates were 96% and 96%, respectively. However, in some cases with initial negative MRI the PBx was deferred and performed for cause during followup. Therefore, PBx was not routinely performed since some patients harboring PCa and CSPCa might not have been diagnosed. Nevertheless, the investigators reported that prior negative PBx and PSAD were predictors of CSPCa on PBx. However, on multivariate analysis PSAD but not prior negative PBx was an independent predictor of CSPCa. The group concluded that PBx should be recommended even after negative mpMRI, especially in young patients or patients with high or rising PSA.18 These NPVs seem slightly higher than those in our report. However, to date we have routinely biopsied all patients with clinical indication for PBx regardless of negative mpMRI.
Adding clinical information to mpMRI may help improve the mpMRI NPV.3–6 Washino et al biopsied 288 men, including 127 (44%) with negative MRI.3 Biopsy naïve men were divided into 3 groups, including those with PSAD less than 0.15 ng/ml/cc, 0.15 to 0.29 and 0.3 or greater, respectively. Only of the 51 patients with PSAD less than 0.15 ng/ml/cc had CSPCa. Furthermore, none of the 38 men with PSAD less than 0.15 ng/ml/cc and negative MRI (PI-RADS less than 3) had PCa (supplementary table 6, http://jurology.com/). It is important to note the high median PSAD of 0.26 ng/ml/cc in this cohort and the fact that only 52% of patients underwent 3 Tesla MRI with 1.5 Tesla MRI performed in 49%. Further, the CSPCa definition of GS 3 + 4 or greater, or any PCa with core length of 4 mm or greater was not as restrictive as the GS 3 + 4 or greater cutoff reported by many others.12–14
Distler et al used PI-RADS v1 to assess MRI and performed transperineal biopsies with a median of27 biopsy cores per patient.4 A total of 1,040 consecutive men were enrolled in that study, including 344 (33%) without suspicious lesions on mpMRI. Patients were stratified into 3 PSAD groups, including less than 0.07, 0.07 to 0.15 and greater than 0.15 ng/ ml/cc, respectively. When combined with PSAD 0.15 ng/ml/cc or less, the NPV of mpMRI for CSPCa (GS 3 + 4 or greater) increased from 79% to 89%. When combining PSAD and mpMRI, approximately 20% of biopsies were deemed to be unnecessary. The use of other markers such as the PHI (Prostate Health Index) and PCA3 (prostate cancer antigen 3) were also explored and reported to improve the NPV of MRI to detect CSPCa (supplementary table 6, http://jurology.com/).5,6
We used PSAD and the history of negative biopsy, and excluded prostate volume from multivariable analysis to avoid confounding factors. We applied a PSAD threshold of 0.15 ng/ml/cc for analysis based on several reports and as recommended by the NCCN Guidelines®.3, 4, 19 In fact, analysis of 285 RP specimens revealed that PSAD was a predictor superior to PSA and GS for positive surgical margins, extracapsular disease, lymph node invasion and/or seminal vesicle invasion.20 Conversely PSAD less than 0.1 ng/ml/cc or PSAD 0.1 to 0.15 ng/ml/cc with less than 3 mm PCa found in only 1 positive core on PBx were predictors of CIPCa in RP specimens.21
A recent consensus suggested that mpMRI should be strongly considered in patients with previous negative biopsy who have persistent suspicion for PCa and are being considered for repeat PBx.22 Furthermore, in patients with negative MRI other markers, including PSAD, might add value in identifying those warranting repeat biopsy. Patients with a history of prior negative biopsy have lower chance of PCa detection on repeat biopsy. Ploussard et al analyzed the records of 617 men who underwent repeat biopsy with a mean of 19 months of followup.15 The investigators documented 16.7%, 16.9% and 12.5% PCa detection rates for the second, third and fourth sets of repeat biopsies, respectively. They also found that PSAD greater than 0.15 ng/ml/cc more than doubled PCa detection on repeat biopsy.
Interestingly no men in our study had CSPCa on repeat biopsy when combining negative MRI, PSAD less than 0.15 ng/ml/cc and prior negative biopsy history. Conversely, patients with PSAD 0.15 ng/ml/cc or greater and no prior negative biopsy history should consider undergoing systematic PBx even if mpMRI shows no lesion (supplementary table 2, http://jurology.com/).
We defined CSPCa as GG 2 or greater as reported previously.4, 12–14, 17 Biopsy and serially sectioned RP histology were reviewed by a single dedicated uropathologist. For patients who underwent RP we compared biopsy outcomes with RP histology. Notably there was pT3a disease on RP that was missed by MRI in 5 of 24 biopsy proven CSPCa cases (21%). Biopsy and RP histology were concordant in 77% of cases and 4 of 7 cases diagnosed with CIPCa on biopsy were upgraded to CSPCa on RP histology (supplementary table 4, http://jurology.com/). Thus, even in the setting of a negative MRI and CIPCa on PBx some patients may still harbor CSPCa on RP specimens.
Our study was limited by the relatively small number of patients. However, our cohort size is in line with those in prior reports (supplementary table 6, http://jurology.com/). The patients with prior negative biopsies underwent varying biopsy protocols with a varying number of cores taken, of which the details were not available from reports done elsewhere. Nevertheless, we report the number of prior biopsies and time from the last biopsy. We used biopsies rather than RP specimens as the reference standard but to our knowledge no prior publication has done so. Patients without PCa on biopsy do not undergo RP, precluding routine correlation with RP specimens. Nevertheless, we found concordance of PBx with RP histology in those who underwent RP. High quality mpMRIs were performed, interpreted and reported by experienced uroradiologists according to PI-RADS criteria and, thus, our study is in accordance with the AUA (American Urological Association)/SAR (Society of Abdominal Radiology) consensus recommendation.22 However, mpMRI scans were not specifically rereviewed in this study.
Our data reflect real world practice. Prostate biopsies were performed by experienced urologists and histology findings were reviewed by a dedicated uropathologist.
CONCLUSIONS
The NPV of mpMRI for biopsy detected CSPCa was 82%, which improved to 90% when combined with PSAD less than 0.15 ng/ml/cc. Men with a combination of negative mpMRI and PSAD less than 0.15 ng/ml/cc with prior negative biopsy may safely avoid rebiopsy as none of these men had CSPCa on biopsy. Conversely PBx should be considered in biopsy naïve men regardless of negative mpMRI, particularly those with PSAD greater than 0.15 ng/ml/cc.
Supplementary Material
Acknowledgments
Supported by NIH (National Institutes of Health)/NCI (National Cancer Institute) Grant R01 CA205058–01 (MCS, ISG, ALdCA).No direct or indirect commercial incentive associated with publishing this article.
Abbreviations and Acronyms
- BPH
benign prostatic hyperplasia
- CIPCa
clinically insignificant prostate cancer
- CSPCa
clinically significant prostate cancer
- DRE
digital rectal examination
- GG
Grade Group
- GS
Gleason score
- mpMRI
multiparametric MRI
- MRI
magnetic resonance imaging
- NPV
negative predictive value
- PBx
prostate biopsy
- PCa
prostate cancer
- PI-RADStm
Prostate Imaging Reporting and Data System
- PSA
prostate specific antigen
- PSAD
PSA density
- RP
radical prostatectomy
- TRUS
transrectal ultrasound
- v1
version 1
- v2
version 2
Footnotes
The corresponding author certifies that, when applicable, statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
REFERENCES
- 1.Kasivisvanathan V, Rannikko AS, Borghi M et al. : MRI-targeted or standard biopsy for prostatecancer diagnosis. N Engl J Med 2018; 378: 1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoots IG, Roobol MJ, Nieboer D et al. : Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: systematic review and meta-analysis. Eur Urol 2015; 68: 438. [DOI] [PubMed] [Google Scholar]
- 3.Washino S, Okochi T, Saito K et al. : Combination of Prostate Imaging Reporting and Data System (PI-RADS) score and prostate-specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naïve patients. BJU Int 2017; 119: 225. [DOI] [PubMed] [Google Scholar]
- 4.Distler FA, Radtke JP, Bonekamp D et al. : The value of PSA density in combination with PI-RADS™ for the accuracy of prostate cancer prediction. J Urol 2017; 198: 575. [DOI] [PubMed] [Google Scholar]
- 5.Druskin SC, Tosoian JJ, Young et al: Combining Prostate Health Index density, magnetic resonance imaging and prior negative biopsy status to improve the detection of clinically significant prostate cancer. BJU Int 2018; 121: 619. [DOI] [PubMed] [Google Scholar]
- 6.Perlis N, Al-Kasab T, Ahmadet al: Defining cohort that may not require repeat prostate biopsy based on PCA3 score and magnetic resonance imaging: the dual negative effect. J Urol 2018; 199: 1182. [DOI] [PubMed] [Google Scholar]
- 7.Barentsz JO, Richenberg J, Clements R et al. : ESUR prostate MR guidelines 2012. Eur Radiol 2012; 22: 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinreb JC, Barentsz JO, Choyke PL et al. : PI-RADS Prostate Imaging - Reporting and Data System: 2015, version 2. Eur Urol 2016; 69: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edge S, Byrd D, Compton C et al. : AJCC Cancer Staging Manual, 7th ed New York: Springer 2010. [Google Scholar]
- 10.Villers A, McNeal JE, Freiha FS et al. : Multiple cancers in the prostate. Morphologic features of clinically recognized versus incidental tumors. Cancer 1992; 70: 2313. [DOI] [PubMed] [Google Scholar]
- 11.Kanda Y: Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 2013; 48: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wysock JS, Mendhiratta N, Zattoni F et al. : Predictive value of negative 3T multiparametric magnetic resonance imaging of the prostate on 12-core biopsy results. BJU Int 2016; 118: 515. [DOI] [PubMed] [Google Scholar]
- 13.Wang RS, Kim EH, Vetter JM et al. : Determination of the role of negative magnetic resonance imaging of the prostate in clinical practice: is biopsy still necessary? Urology 2017; 102: 190. [DOI] [PubMed] [Google Scholar]
- 14.Lu AJ, Syed JS, Nguyen KA et al. : Negative multiparametric magnetic resonance imaging of the prostate predicts absence of clinically significant prostate cancer on 12-core template prostate biopsy. Urology 2017; 105: 118. [DOI] [PubMed] [Google Scholar]
- 15.Ploussard G, Nicolaiew N, Marchand C et al. : Risk of repeat biopsy and prostate cancer detection after an initial extended negative biopsy: longitudinal follow-up from prospective trial. BJU Int 2013; 111: 988. [DOI] [PubMed] [Google Scholar]
- 16.Hansen NL, Barrett T, Kesch C et al. : Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy-naïve men with suspicion of prostate cancer. BJU Int 2018; 122: 40. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed HU, El-Shater Bosaily A, Brown LC et al. : Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): paired validating confirmatory study. Lancet 2017; 389: 815. [DOI] [PubMed] [Google Scholar]
- 18.Panebianco V, Barchetti G, Simone G et al. : Negative multiparametric magnetic, resonance imaging for prostate cancer: whats next? Eur Urol 2018; 74: 48. [DOI] [PubMed] [Google Scholar]
- 19.Carroll P Parsons J, Andriole G et al. : NCCN Guidelines Version 1.2017 Prostate Cancer Early Detection. Plymouth Meeting, Pennsylvania: National Comprehensive Cancer Network; 2017. [Google Scholar]
- 20.Sfoungaristos S and Perimenis P: PSA density is superior than PSA and Gleason score for adverse pathologic features prediction in patients with clinically localized prostate cancer. Can Urol Assoc J 2012; 6: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein JI, Walsh PC, Carmichael M et al. : Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA 1994; 271: 368. [PubMed] [Google Scholar]
- 22.Rosenkrantz AB, Verma S, Choyke P et al. : Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with prior negative biopsy: consensus statement by AUA and SAR. J Urol 2016; 196: 1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


