Abstract
Four novel lupane-type lupane-type triterpenoids (including three norlupane-type triterpenoids), 17β-hydroxy-28-norlup-20(29)-en-3-one (1), 3β,17β−dihydroxy-28,30-bisnorlupan-20-one (2), 3β-hydroxy-20-oxo-30-norlupan-28-al (3) and lup-20(29)-ene-3,23,30-triol (4), were isolated together with ten known lupane triterpenoids (5~14) from the bark of Euonymus alatus forma ciliato-dentatus. Their structures were determined from 1D- and 2D-NMR analysis and comparison of their spectroscopic data with literature values. The known compounds (5~14) were reported for the first time from this plant.
Keywords: Euonymus alatus forma ciliato-dentatus, Celastraceae, triterpenoid, lupane
Graphical abstract
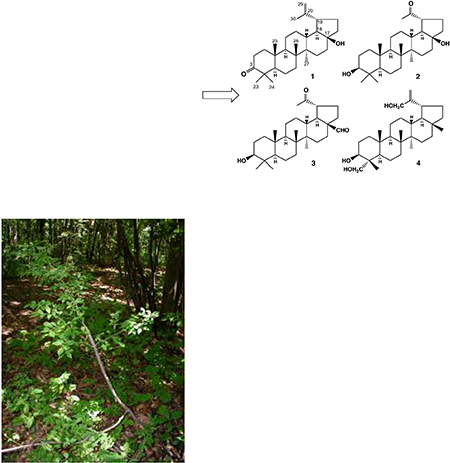
Four previously undescribed lupane-type triterpenoids (1 – 4) were isolated together with ten known triterpenoids from the bark of Euonymus alatus forma ciliato-dentatus.
1. Introduction
The genus Euonymus (family Celastraceae), found mainly in temperate and subtropical regions, contains popular ornamental shrubs used in landscaping. Moreover, Euonymus species generate a variety of secondary metabolites and have been used as folkloric medicines in several parts of the world [Alvarenga, 2006; Kitanaka, 1996; Baek, 1994; Descoins, 2002]. Specifically, the bark of E. alatus forma ciliatodentatus has been used as an analgesic for toothache by the Ainu tribe (AINU-people), an indigenous people of Japan [Kinoshita, 1983]. In our studies on the natural drug resources used by the Ainu, we became interested in the chemical constituents and morphological differences among Euonymus species. E. alatus forma ciliatodentatus grows naturally from northern Japan to Korea and, in addition to the medicinal purpose, is used commonly as a craftwork material. To date, there have been very few reports about the chemical components of E. alatus forma ciliatodentatus, which further prompted our current investigation of this species. We isolated four new triterpenoids, viz. 17β-hydroxy-28-norlup-20(29)-en-3-one (1), 3β,17β−dihydroxy-28,30-bisnorlupan-20-one (2), 3β-hydroxy-20-oxo-30-norlupan-28-al (3) and lup-20(29)-ene-3,23,30-triol (4), along with ten known triterpenoids, lupeol (5), lupenone (6), betulin (7), (20S)-3-hydroxylupan-30-al (8), (20R)-3-hydroxylupan-29-al (9), lup-20(29)-ene-3,30-diol (10), 3-hydroxy-30-norlupan-20-one (11), 3,30-dihydroxylup-20(29)-en-28-al (12), lup-20(29)-en-3,28,30-triol (13) and 3-hydroxylup-20(29)-en-30-al (14) (Chart 1) [Puapairoj et al., 2005; Ito et al., 1997; Mutai et al., 2007; Corbett et al., 1985; Razdan et al., 1988; Zhou et al., 2014; Silvia et al., 2005; Chakravarty AK et al., 1994], from a ethanol extract of the dried bark of E. alatus forma ciliatodentatus. The ten known triterpenoids (5–14) were identified mainly from NMR spectroscopic data or comparison with literature values and isolated for the first time from this plant. Herein, we report the isolation and structure elucidation of the new lupane-type triterpenoids (1–4), as well as the known triterpenoids (5–14), by applying 2D-NMR techniques. Three of the known triterpenoids (5, 6, 14) were evaluated for cytotoxic activity against four human tumor cell lines (A549, DU145, KB, and KB-VIN).
2. Results and Discussion
All fourteen compounds (1–14) were obtained from the n-hexane or CHCl3 soluble fraction of the ethanol extract of dried bark. These fractions were separated by repeated column chromatography over silica gel, Al2O3 and florisil followed by preparative HPLC.
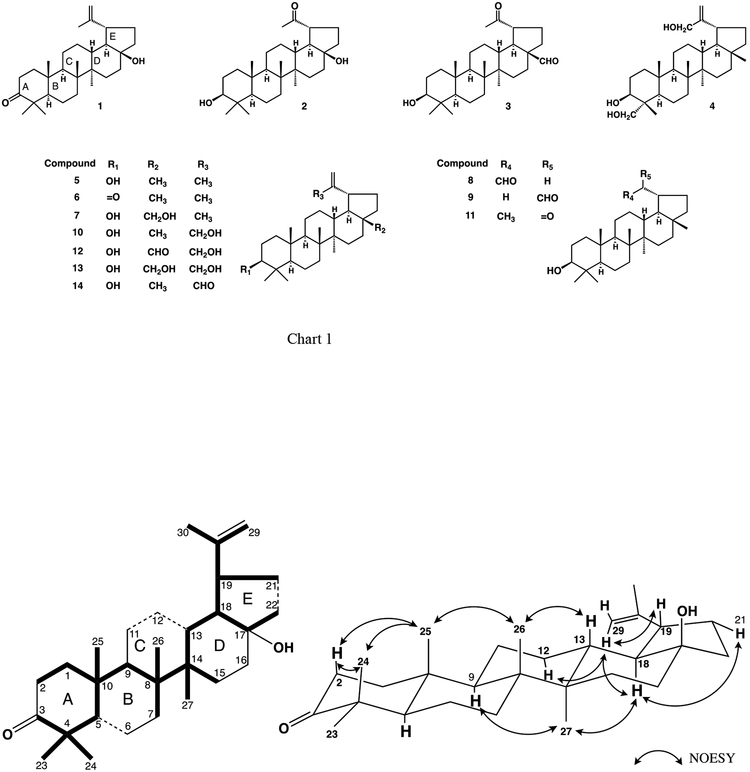
Compound 1, m.p. 177–178°C, [α]D + 42.4°, (c 0.1, CHCl3), was obtained as colorless needles. Based on HR-EIMS, the molecular formula of 1 is C29H46O2 ([M]+ m/z 426.3478; calcd. for 426.3498). The IR spectrum showed absorption bands at 3410 cm−1 for a hydroxyl group and 1698 cm−1 for a C=O group. The 1H-NMR spectrum of 1 indicated the presence of six tertiary methyl groups (δH 0.94, 0.96, 1.03, 1.08, 1.08 and 1.68) and one exo-methylene group (δH 4.61 and 4.73) (Table 1). The 13CNMR spectrum of 1, measured in CDCl3, showed signals for 29 carbon atoms, with one fewer methyl signal in comparison with the known compound 6 (lupenone). These signals were identified from a DEPT spectrum as six methyl, eleven methylene, five methine and seven quaternary carbons (Table 2). The EI-MS ion peaks at m/z 408, 365, 205 and 189 were characteristic fragment ions of a lupane skeleton [Shiojima et al., 1992]. After the five-ring lupane system was subtracted from the seven degrees of unsaturation determined from the molecular formula, two degrees of unsaturation remained. They were attributed to a carbonyl group based on a carbon signal at δC 217.70 and an isopropenyl group based on 1H-NMR signals for two vinyl protons at δH 4.61 and 4.73 and a methyl group at δH 1.68 together with 13C-NMR down-field signals at δC 149.6 and 109.7. Moreover, except for the absence of a resonance for methyl group and the presence of a resonance for hydroxyl group, the 1H-NMR chemical shifts of H3–23 to H3–27 and the 13C chemical shifts of C-1 to C-13 and C-23 to C-26 (Tables 1 and 2) of 1 were comparable with those of lup-20(29)-en-3-one (lupenone, 6). These observations clearly suggested that compounds 1 and 6 possess the same A, B, and C ring system. Based on the above observations, the planar structure of 1 was assigned as 17-hydroxy-28-norlup-20(29)-en-3-one. The 1H and 13C-NMR chemical shifts were found to be largely comparable to those of the related compounds 28-norlup-20(29)-ene-3β,17β-diol (15), except for rings A and B [Lee et al., 1998], and lup-20(29)-en-3-one (lupenone, 6), except for rings D and E. The carbon connectivity was established from the long-range 1H-13C correlations presented in the HMBC spectrum and shown by boldface lines in Fig. 1. Namely, correlations were observed between H3-23, 24 (δH 1.08, 1.03, respectively) and C-3, C-4, C-5; H3–25 (δH 0.94) and C-1, C-5, C-9 and C-10; H3-26 (δH 1.08) and C-7, C-8, C-9 and C-14; H3–27 (δH 0.96) and C-8, C-13, C-14 and C-15; H3–30 (δH 1.68) and C-19, C-20 and C-29; and H-19 (δH 2.61) and C-13, C-17, C-18, C-20, C-21, C-22, C-29 and C-30. In addition, the 1H-1H COSY correlations shown by dashed lines in Fig. 1 established the definitive connectivity of certain carbons. The relative stereochemistry was ascertained from the NOE interactions (Fig. 1) observed in the NOESY spectrum of 1. Significant NOE correlations were observed between H3-25 (δH 0.94) and H3-26 (δH 1.08), H3-26 (δH 1.08) and H-13 (δH 1.88), H-29 (δH 4.73) and H-19 (δH 2.61) / H-18 (δH 1.49) / H-12α (δH 1.72), H-18α (δH 1.49) and H3-27 (δH 0.96), and H-18α (δH 1.49) and H-21α (δH 2.07). These NOE interactions suggested a trans junction of the ring D/E system and a β-orientation of the hydroxyl group at C-17. Moreover, NOE correlations of H3-24 (δH 1.03) with H-2β (δH 2.50); H3-24 (δH 1.03) with H3-25 (δH 0.94); H-2β with H3-25 (δH 0.94) and H-9 (δH 1.39) with H3-27 (δH 0.96) were also observed. From the above evidence, compound 1 was established as 17β-hydroxy-28-norlup-20(29)-en-3-one.
Table 1.
1H-NMR Chemical Shifts for Compounds 1–4 (in CDCl3, 600MHz)
| position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 1.40 m, 1.91 m | 0.90 m, 1.68 m | 0.89 m, 1.65 m | 0.90 m, 1.65 m |
| 2 | 2.40 ddd (4.4, 7.2, 15.9) | 1.53 m, 1.65 m | 1.55 m, 1.61 m | 1.60 m, 1.60 m |
| 2.50 ddd (7.2, 8.8, 15.9) | ||||
| 3 | - | 3.19 dd (4.8, 11.2) | 3.19 dd (4.2, 11.4) | 3.62 dd (6.4, 9.6) |
| 5 | 1.33 m | 0.69 d (11.4) | 0.68 d (10.2) | 0.81m |
| 6 | 1.38 m, 1.50 m | 1.40 m, 1.53 m | 1.37 m, 1.51 m | 1.30 m, 1.30 m |
| 7 | 1.46 m, 1.46 m | 1.39 m, 1.39m | 1.35 m, 1.40 m | 1.35 m, 1.40 m |
| 9 | 1.39 m | 1.27 m | 1.28 m | 1.24 m |
| 11 | 1.30 m, 1.57 m | 1.24 m, 1.45 m | 1.26 m, 1.43 m | 1.18 m, 1.40 m |
| 12 | 1.12 m, 1.72 m | 1.56 m, 1.61 m | 1.07 m, 1.07 m | 1.08 m, 1.41 m |
| 13 | 1.88 m | 1.76 dd (4.1, 12.2) | 1.91 m | 1.66 m |
| 15 | 1.14 m, 1.79 m | 1.11 m, 1.78 m | 1.24 m, 1.38 m | 0.98 m, 1.60 m |
| 16 | 1.55 m, 1.76 m | 1.61 m, 1.71 m | 1.53 m, 2.10 m | 1.35 m, 1.48 m |
| 18 | 1.49 m | 1.96 t (11.3) | 2.20 m | 1.45 m |
| 19 | 2.61dt (6.2, 10.8) | 2.84 dt (5.6, 10.8) | 3.15 dt (5.6, 11.4) | 2.26 m |
| 21 | 1.41 m, 2.07 m | 1.49 m, 2.23 m | 1.53 m, 1.96 m | 1.33 m, 2.05 m |
| 22 | 1.50 m, 1.61 m | 1.58 m, 1.66 m | 1.42 m, 1.79 m | 1.22 m, 1.37 m |
| 23 | 1.08 s | 0.97 s | 0.96 s | 3.42 (d, 10.4) |
| 3.71 (d, 10.4) | ||||
| 24 | 1.03 s | 0.76 s | 0.75 s | 0.87 s |
| 25 | 0.94 s | 0.83 s | 0.81 s | 0.87 s |
| 26 | 1.08 s | 1.02 s | 0.89 s | 1.03 s |
| 27 | 0.96 s | 0.96 s | 1.00 s | 0.94 s |
| 28 | 9.56 d (1.8) | 0.78 s | ||
| 29 | 4.61 s | 2.18 s | 2.19 s | 4.90 s |
| 4.73 s | 4.94 s | |||
| 30 | 1.68 s | 4.08 (d, 14.4) | ||
| 4.11 (d, 14.4) |
Table 2.
13C-NMR chemical shifts for 1–4, 6 and 11 (in CDCl3, 150 MHz)a
| position | 1 | 2 | 3 | 4 | 6 | 11 |
|---|---|---|---|---|---|---|
| 1 | 39.61 | 38.59 | 38.65 | 38.36 | 39.64 | 38.64 |
| 2 | 34.09 | 27.38 | 27.33 | 27.02 | 34.18 | 27.37 |
| 3 | 217.69 | 78.90 | 78.86 | 76.80 | 218.13 | 78.91 |
| 4 | 47.25 | 38.86 | 38.83 | 41.89 | 47.35 | 38.85 |
| 5 | 54.86 | 55.28 | 55.21 | 49.90 | 54.95 | 55.24 |
| 6 | 19.64 | 18.28 | 18.22 | 18.44 | 19.70 | 18.28 |
| 7 | 33.62 | 34.28 | 34.20 | 34.04 | 33.59 | 34.17 |
| 8 | 40.65 | 40.65 | 40.67 | 40.82 | 40.81 | 40.72 |
| 9 | 49.83 | 50.36 | 50.31 | 50.36 | 49.82 | 50.25 |
| 10 | 36.86 | 37.20 | 37.15 | 37.04 | 36.91 | 37.16 |
| 11 | 21.50 | 20.96 | 20.75 | 21.37 | 21.50 | 20.89 |
| 12 | 25.15 | 27.27 | 27.51 | 26.62 | 25.18 | 27.31 |
| 13 | 37.73 | 36.63 | 37.24 | 37.95 | 38.20 | 37.01 |
| 14 | 41.93 | 41.80 | 42.29 | 42.80 | 42.92 | 42.66 |
| 15 | 26.86 | 26.79 | 28.98 | 27.39 | 27.45 | 27.17 |
| 16 | 33.13 | 32.85 | 28.73 | 35.43 | 35.54 | 34.96 |
| 17 | 80.17 | 80.29 | 59.27 | 43.00 | 43.02 | 43.05 |
| 18 | 48.30 | 49.17 | 47.97 | 48.85 | 48.27 | 49.68 |
| 19 | 48.03 | 52.25 | 51.06 | 43.79 | 47.98 | 52.61 |
| 20 | 149.64 | 212.20 | 211.78 | 154.73 | 150.59 | 212.91 |
| 21 | 29.39 | 27.55 | 27.64 | 31.75 | 29.86 | 27.64 |
| 22 | 38.47 | 38.70 | 32.42 | 39.83 | 39.99 | 39.84 |
| 23 | 26.63 | 27.99 | 27.96 | 72.11 | 26.67 | 27.97 |
| 24 | 21.00 | 15.38 | 15.36 | 11.23 | 21.05 | 15.37 |
| 25 | 16.04 | 16.17 | 16.13 | 16.46 | 15.98 | 16.07 |
| 26 | 15.90 | 16.03 | 15.82 | 16.00 | 15.81 | 15.90 |
| 27 | 13.76 | 13.17 | 14.23 | 14.56 | 14.50 | 14.46 |
| 28 | 206.08 | 17.70 | 18.03 | 17.97 | ||
| 29 | 109.65 | 29.94 | 30.23 | 106.82 | 109.40 | 29.17 |
| 30 | 19.28 | 65.01 | 19.33 |
The assignments were based on 1H-1H COSY, HMQC and HMBC experiments.
Fig. 1.
The partial structure solved by the HMBC and COSY and key correlations in the NOESY spectra in 1.
Compound 2, m.p. 171–178°C, [α]D + 7.7°, (c 0.1, CHCl3), was obtained as colorless needles. Based on HR-EIMS, the molecular formula of 2 is C28H46O3 ([M]+ m/z 430.3448; calcd. for 430.3447). The IR spectrum showed absorption bands at 3431 cm−1 for a hydroxyl group and 1692 cm−1 for a C=O group. Six tertiary methyl groups (Table 1) and two methine protons at δH 2.84 (dt, J = 10.8, 5.6 Hz) and 3.19 (dd, J = 11.2, 4.8 Hz) were seen in the 1H-NMR spectrum of 2; the latter methine proton was most likely on an oxygenated carbon. While the 1H-NMR spectra of 2 and 11 were similar, the spectrum of 2 lacked the signal for the tertiary methyl group at C-17 which was found in the spectrum of 11. The 28 carbon signals observed in the 13C-NMR spectrum of 2 were identified from a DEPT spectrum as six methyl, ten methylene, six methine and six quaternary carbons (Table 2).
The EI-MS ion peaks at m/z 207 and 189 (base peak) are characteristic fragment ions of the lupane skeleton [Shiojima et al., 1992]. The five-ring lupane skeleton accounts for five of the six degrees of unsaturation determined from the molecular formula. The remaining unsaturation was postulated to be a carbonyl group based on a typical IR absorption as well as 1H- and 13C-NMR data. No olefinic signals were observed in either NMR spectrum, while a three-proton singlet at δH 2.18 and a quaternary carbon signal at δC 212.20 were attributed to an acetyl side chain at C-19. Two down-field 13C-NMR signals at δC 78.90 and 80.29 were assigned to methine (C-3) and quaternary (C-17) carbons, respectively, attached to hydroxyl groups.
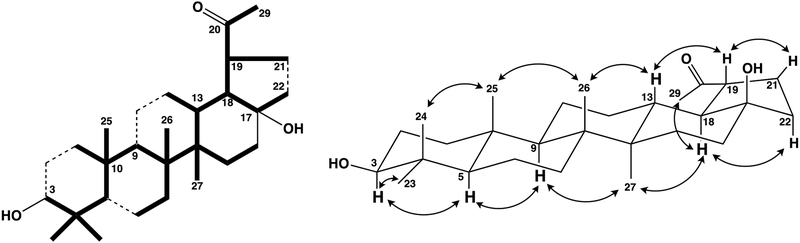
While the compound’s detailed structure could not be established completely from 1D NMR or mass spectroscopic data, the planar structure of 2 was postulated as shown in Fig. 2 and confirmed from 2D spectroscopic data as discussed below. The planar structure of 2 was solved from the HMBC correlations (shown by heavy lines) of the six methyl groups (Fig. 2). Detailed analysis of other correlations from H-1 (δH 1.55, 1.68), H-23 (δH 0.97) and H-24 (δH 0.76) with C-3 (δC 78.90) and H-13 (δH 1.76), H-18 (δH 1.96) and H-19 (δH 2.84) with C-17 (δC 80.29) could confirm the locations of substituents that two hydroxyl were located at C-3 and C-17. In the NOESY spectrum of 2 (Fig. 2), correlations of H3-27 (δH 0.96) and H-18 (δH 1.96), H-18 and H-22α (δH 1.58), H3-27 (δH 0.96) and H-18 (δH 1.96), H-13 (δH 1.76) and H-19 (δH 2.84), H-19 and H-21β (δH 2.23), H-3 (δH 3.19) and H-5 (δH 0.69), H-5 (δH 0.69) and H-9 (δH 1.27) were observed. In addition, the observed splitting pattern of H-18 was triplet (J = 11.3 Hz) and the large coupling constant indicated a dihedral angle of 180 degrees. This result also suggests a trans-fused stereochemistry of ring D/E. Therefore, the structure of 2 was assigned as 3β,17β−dihydroxy-28,30-bisnorlupan-20-one.
Fig. 2.
The partial structure solved by the HMBC and COSY and key correlations in the NOESY spectra in 2.
Compound 3 was obtained as a white amorphous solid. Based on HR-EIMS, the molecular formula of 3 is C29H46O3 ([M]+ m/z 442.3447; calcd. for 442.3445). The EI-MS ion peaks at m/z 207 (91) and 189 (100) are characteristic fragment ions of the lupane skeleton [Shiojima et al., 1992]. The IR spectrum showed absorption bands at 1690 cm−1 and 1710 cm−1 for a C=O group. The 1H-NMR spectrum of 3 indicated the presence of six tertiary methyl groups (Table 1). The downfield shift of the methyl singlet at δH 2.19 indicated the presence of a methyl ketone rather than an isopropenyl group as found in 1, and a signal observed at δH 9.56 (d, J = 1.8 Hz) was assigned as an aldehyde group. Correspondingly, among the 29 carbon signals in the 13C-NMR spectrum of 3, the ones at δC 211.78 and 206.08 were attributed to ketone and aldehyde carbons, respectively. These two unsaturated groups plus the pentacyclic lupane skeleton fulfilled the seven degrees of unsaturation calculated from the molecular formula. Furthermore, 2D NMR analysis indicated that 3 was a formyl analogue of 3β-hydroxy-30-norlupan-20-one (11). [Koul et. al., 2000] The aldehyde group was positioned at C-17 based on HMBC correlations of the aldehyde proton (δH 9.56) with C-16, 17, 18 and 22. A comparison of the 13C-NMR assignments of 3 with those of 2 and known compound 11 established the similarity of ring A, B and C in the three compounds. (Table 2)
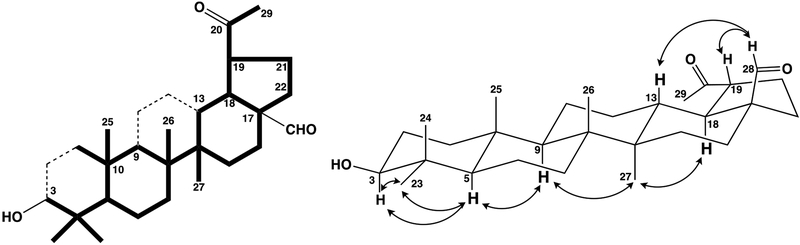
In the HMBC spectra of 3 (Fig. 3), the correlations of the proton at δH 9.56 (1H, s, H-28) with carbons at δC 28.73 (C-16), 32.42 and 47.97 (C-18) confirmed the presence of the formyl group at C-17. Correlations were also observed between protons at δH 0.75 (3H, s, H-24), 0.96 (3H, s, H-23) and 0.68 (1H, d, J = 10.2 Hz, H-5) with a carbon at δC 78.86 (C-3) as well as between the proton at δH 2.19 (3H, s, H-30) with the carbon at δC 51.06 (C-19). Key two- and three-bond heteronuclear connectivity also supported the assigned structure of 3. The relative configuration of 3 was established information from the NOESY data (Fig. 3). The observed cross peaks between H-3 and H3-23/H-5, H-5 and H-9, H-9 and H3-27, H3-27 and H-18, and H-28 and H-13/H-19 suggested that the C-28 aldehyde group was β-oriented and the side chain on C-19 was α-oriented. All above data and comparison with the corresponding data for compounds 2, 5, and 11 established the structure of 3 as 3β-hydroxy-20-oxo-30-norlupan-28-al.
Fig. 3.
The partial structure solved by the HMBC and COSY and key correlations in the NOESY spectra in 3.
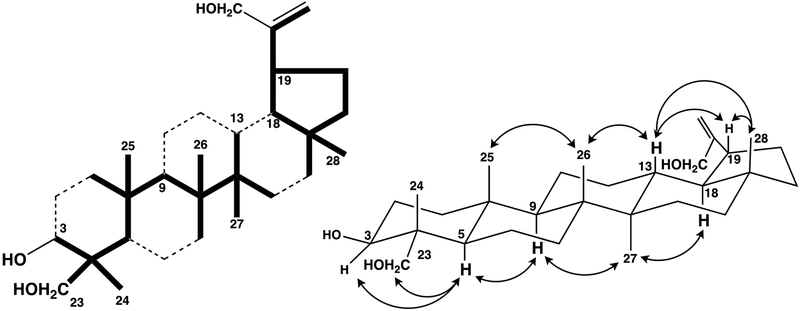
Compound 4 was isolated as colorless needles (m.p.: 200–209°C). Its molecular formula was determined to be C30H50O3 from the 13C-NMR data and molecular ion [M]+ at m/z 458.3786 (calcd. for 458.3760) in the HR-EIMS. The IR spectrum showed an absorption for a hydroxy group (3396 cm−1). The EI-MS fragment ion peaks at m/z 223 and 205 (base peak) indicated a lupane skeleton for compound 4. In the 1H- & 13C-NMR spectra of 4, signals were observed for five tertiary methyl groups, two primary hydroxyl groups, one secondary hydroxyl group and one exo-methylene (Table 1 and 2), and the spectra of 4 were very similar to those of 10. The planar structure of 4 was assigned from the HMBC correlations (Fig. 4) of the five methyl and two hydroxymethyl groups. Detailed analysis of other correlations, particularly H-19 (δH 2.26), H-3 (δH 3.62) and H-29a and H-29b (δH 4.90, 4.94), confirmed that the three hydroxyl groups were located at C-3, C-23 and C-30. The relative configuration of 4 was established from the NOESY data. The observed cross peaks between H-3 and H2-23 / H-5, H-5 and H-9, H-9 and H3-27, H-19 and H3-28 / H-13 and H3-27 and H-18 suggested that the C-4 hydroxymethyl group and C-19 side chain were α-oriented. Furthermore, comparison with the literature data for lup-20(29)-ene-3,30-diol (10) confirmed that compound 4 is lup-20(29)-ene-3,23,30-triol.
Fig. 4.
The partial structure solved by the HMBC and COSY and key correlations in the NOESY spectra in 4.
The three known lupane triterpenoids 5, 6 and 14 were evaluated for cytotoxic activity against four human tumor cell lines [lung carcinoma (A549), prostate carcinoma (DU145), HeLa derivative (KB), and multidrug resistant KB subline expressing P-glycoprotein (KB-VIN)]. However, these compounds were non-cytotoxic (IC50 > 20 μM) against the four tested cell lines.
3. Conclusion
We isolated four (1–4) novel lupane-type triterpenoids, including one 28-nor-lupane (1), one 28,30-bisnor-lupane (2), and one 30-nor-lupane (3), along with ten (5–14) known triterpenoids from the bark of E. alatus forma ciliato-dentatus. The structures of 1–4 were elucidated from 1D and 2D NMR spectroscopic data. Most likely, the biogenetic progression to the nor-type compounds is stepwise oxidation to carboxylic acid followed by decarboxylation.
Our chemotaxonomic study of Euonymus species has shown that E. alatus f. ciliatodentatus contains various novel lupane derivatives, including nor-lupane and bisnor-lupane compounds, which are commonly oxygenated at the D/E ring junction. In this plant, the 14 isolated triterpenoids contained oxygenated functionalities at C-17, C-20, C-28 or C-30, which could be a diagnostic distinction. The known compounds 5–14 are being reported for the first time from this plant. Further investigations on the triterpenoid constituents and a bioactivity study of this plant are in progress.
4. Material and methods
4.1. General
Melting points were measured on a Yanagimoto micro apparatus without correction. Measurement of optical rotation was carried out on a JASCO P-2300 in CHCl3 solution at 23 °C. IR spectra were recorded using a Perkin-Elmer Model Spectrum 100. 1H (400 or 600 MHz)- and 13C (100 or 150 MHz)-NMR spectra were recorded on a JEOL AL-400 or ECA-600 spectrometer using tetramethylsilane as an internal standard. The chemical shifts are expressed on the δ scale. EI-MS and HREI-MS were measured at 30–60 eV (direct inlet) with a JEOL Model JMS-700 and the relative intensities of peaks are reported with reference to the most intense peak higher than m/z 100. TLC was carried out on pre-coated silica gel 60 (Merck 5554) with n-hexane-EtOAc or CHCl3-MeOH as the developing phase. Detection was carried out by spraying with concentrated H2SO4 followed by heating. HPLC was performed on a Hitachi L-6000 equipped with a Hitachi L-7490 RI detector. The following column and solvents were used for elution: Mightysil, RP-18 GP 250–10 (25 cm x 10 mm i.d., 5μm) with MeOH-H2O or CH3CN-H2O.
4.2. Plant material
The bark of E. alatus forma ciliato-dentatus was collected in August 2001 at Nayoro, Hokkaido, Japan. A voucher specimen (010801) is on file in our laboratory. It was identified by one of the authors (T.S.), Tsukuba Division, Research Center for Medicinal Plant Resources, National Institute of Biomedical Innovation, Health and Nutrition.
4.3. Extraction and isolation
The dried bark of E. alatus forma ciliato-dentatus (1.85 kg) was extracted three times with EtOH at 60 °C for 6 hr, evaporated under reduced pressure to give a residue (99 g), and then suspended in 70% EtOH. This suspension was extracted successively with hexane, CHCl3, diethyl ether, BuOH and H2O. The hexane and CHCl3 soluble fractions were concentrated under reduced pressure to afford corresponding residues (10.0 g and 23.4 g, respectively).
The hexane extract was separated by silica gel column chromatography (C.C.) using a solvent system of n-hexane-benzene-diethyl ether into the following fractions: fr. 1 [eluted with n-hexane] (125 mg), fr. 2 [n-hexane-benzene (8 : 2)] (425 mg), fr. 3–6 [n-hexane-benzene (1 : 1)] (91, 181, 156, 292 mg, respectively), fr. 7, 8 [benzene] (531, 642 mg, respectively), fr. 9, 10 [benzene-ether (8 : 2)] (2657, 722 mg, respectively), fr. 11 [benzene-ether (1 : 1)] (356 mg). Likewise, the CHCl3 extract was separated by SiO2 gel C.C. using a CHCl3-MeOH solvent system. Each fraction was further purified by repeated chromatography (silica gel C.C., alumina C.C. and HPLC) and recrystallization to furnish pure 1–14.
4.3.1. 17β-Hydroxy-28-norlup-20(29)-en-3-one (1)
Yield 3.3 mg (0.0002%), Colorless needle (CHCl3-Hexane); m.p. 177–178 °C; [α]D24 +42.4 (c 0.1, CHCl3); HR-EIMS: m/z 426.3478 ([M]+; Calcd. for C29H46O2, 426.3498); IR: 3410, 1698 cm−1; 1HNMR (CDCl3) δ: 1.68 (3H, s, H-30), 1.08 (3H, s, H-23) and (3H, s, H-26), 1.03 (3H, s, H-24), 0.96 (3H, s, H-27), 0.94 (3H, s, H-25), 4.61 (1H, s, H-29a), 4.73 (1H, s, H-29b); LR-EIMS : m/z 426 ([M]+, 7), 408 (58), 365 (7), 205 (34)
4.3.2. 3β,17β−Dihydroxy-28, 30-bisnorlupan-20-one (2)
Yield 5.1 mg (0.0003%), Colorless needles (CHCl3-MeOH); m.p. 171–178 °C; [α]D24 +7.7 (c 0.1, CHCl3); HR-EIMS: m/z 430.3448 ([M]+; Calcd. for C28H46O3, 430.3447); IR: 3431, 2931, 1692 cm−1; 1H-NMR (CDCl3) δ: 2.18 (3H, s, H-29), 1.02 (3H, s, H-26), 0.97 (3H, s, H-23), 0.96 (3H, s, H-27), 0.83 (3H, s, H-25), 0.76 (3H, s, H-24), 3.19 (1H, dd, J = 11.2, 4.8 Hz, H-3), 2.84 (1H, dt, J = 10.8, 5.6 Hz, H-19); LR-EIMS : m/z 430 ([M]+,100), 369 (38), 207 (77), 189 (100)
4.3.3. 3β-Hydroxy-20-oxo-30-norlupan-28-al (3)
4.1 mg (0.0002%), amorphous solid; [α]D25 −8.1 (c 0.1, CHCl3); HR-EIMS: m/z 442.3447 ([M]+; Calcd. for C29H46O3, 442.3445); IR: 1710, 1690 cm−1; 1H-NMR (CDCl3) δ: 9.56 (1H, s, -CHO, H-28), 2.19 (3H, s, H-29), 1.00 (3H, s, H-27), 0.96 (3H, s, H-23), 0.89 (3H, s, H-26), 0.81 (3H, s, H-25), 0.75 (3H, s, H-24), 3.15 (1H, dt, J = 11.4, 5.6 Hz, H-19), 3.19 (1H, dd, J = 11.4, 4.2 Hz, H-3); LR-EIMS : m/z 442 ([M]+, 29), 424 (36), 207 (91), 189 (100)
4.3.4. Lup-20(29)-ene-3,23,30-triol (4)
5.2 mg (0.0003%), Colorless needles (CHCl3-MeOH); m.p. 208–209 °C; [α]D24 −25.1 (c 0.2, CHCl3); HR-EIMS : m/z 458.3786 ([M]+; Calcd. for C30H50O3, 458.3760); IR: 3396, 1454, 1387 cm−1; 1HNMR (CDCl3) δ: 4.94 (1H, s, H-29a), 4.90 (1H, s, H-29b), 4.11 (1H, d, J = 14.4 Hz, H-30a), 4.08 (1H, d, J = 14.4 Hz, H-30b), 3.71 (1H, d, J = 10.4 Hz, H-23a), 3.42 (1H, d, J = 10.4 Hz, H-23b), 1.03 (3H, s, H-26), 0.94 (3H, s, H-27), 0.87 (3H, s, H-25), 0.87 (3H, s, H-24), 0.78 (3H, s, H-28), 3.62 (H-3, dd, J = 6.4, 9.4 Hz); LR-EIMS : m/z 458 ([M]+, 58), 440 (45), 409 (35), 223 (78), 205 (100)
4.3.5. Lup-20(29)-en-3-ol (lupeol, 5)
1H-NMR (CDCl3) δ: 0.76 (3H, s, H-24), 0.79 (3H, s, H-28), 0.83 (3H, s, H-25), 0.95 (3H, s, H-23), 0.97 (3H, s, H-27), 1.03 (3H, s, H-26), 1.68 (3H, s, H-30), 4.57 (1H, s, H-29a), 4.69 (1H, s, H-29b)
4.3.6. Lup-20(29)-en-3-one (lupenone, 6)
0.9 mg (0.000049%), HR-EIMS: m/z 424.3686 ([M]+ calcd. for C30H48O, 424.3705). 1H-NMR (CDCl3) δ : 0.80 (3H, s, H-28), 0.93 (3H, s, H-25), 0.96 (3H, s, H-27), 1.03 (3H, s, H-24), 1.07 (3H, s, H-23), 1.07 (3H, s, H-26), 1.68 (3H, s, H-30), 4.57 (1H, bs, H-29a), 4.69 (1H, bs, H-29b). 13C-NMR (CDCl3) δ : 34.18 (C-1), 39.64 (C-2), 47.35 (C-4), 54.95 (C-5), 19.70 (C-6), 33.59 (C-7), 49.82 (C-9), 36.91 (C-10), 21.50 (C-11), 25.18 (C-12), 38.20 (C-13), 42.92 (C-14), 27.45 (C-15), 35.54 (C-16), 43.02 (C-17), 48.27 (C-18), 47.98 (C-19), 29.86 (C-21), 39.99 (C-22), 26.67 (C-23), 21.05 (C-24), 15.98 (C-25), 15.81 (C-26), 14.50 (C-27), 18.03 (C-28), 109.40 (C-29), 19.33 (C-30); LR-EIMS: m/z 424 ([M]+, 86), 409 (42), 381 (8), 205 (92), 204 (40), 203 (40), 189 (100).
4.3.7. Lup-20(29)-ene-3β,28-diol (betulin, 7)
3.7 mg (0.00020%), [α]: +11.0 (c 0.20, CHCl3); IR cm−1: 3366, 2928, 2868, 1452, 1026. HR-EIMS m/z: 442.3824 ([M]+; calcd. for C30H50O2, 442.3811); 1H-NMR (CDCl3) δ : 0.76 (3H, s, H-24), 0.83 (3H, s, H-25), 0.97 (3H, s, H-23), 0.98 (3H, s, H-27), 1.02 (3H, s, H-26), 1.68 (3H, s, H-30), 3.34 (d, J = 10.4 Hz, H-28a), 3.80 (d, J = 10.4 Hz, H-28b), 4.58 (dd, J = 2.4, 1.6 Hz, H-29a), 4.68 (d, J = 2.4 Hz, H-29b). 13C-NMR (CDCl3) δH: 38.69 (C-1), 27.39 (C-2), 78.98 (C-3), 38.86 (C-4), 55.28 (C-5), 18.29 (C-6), 34.22 (C-7), 40.91 (C-8), 50.39 (C-9), 37.15 (C-10), 20.82 (C-11), 25.20 (C-12), 37.30 (C-13), 42.71 (C-14), 27.04 (C-15), 29.16 (C-16), 47.78 (C-17,C-19), 48.75 (C-18), 150.47 (C-20), 29.74 (C-21), 33.96 (C-22), 27.97 (C-23), 15.35 (C-24), 16.09 (C-25), 15.97 (C-26), 14.75 (C-27), 60.56 (C-28), 109.68 (C-29), 16.07 (C-30); LR-EIMS m/z : 442 ([M]+, 37), 207 (76), 189 (100).
4.3.8. (20S)-3β-Hydroxylupan-30-al (30-oxo-lupanol, 8) 2.4 mg (0.00013 %). [α]:
+17.0 (c 0.1, CHCl3); IR cm−1: 2925, 2852, 1721, 1454, 1381; HR-EIMS m/z: 442.3800 ([M]+; calcd. for C30H50O2, 442.3811). 1H-NMR (CDCl3) δ: 0.77 (3H, s, H-24), 0.80 (3H, s, H-28), 0.85 (3H, s, H-25), 0.94 (3H, s, H-27), 0.98 (3H, s, H-23), 1.03 (3H, d, J = 7.2 Hz, H-29), 1.05 (3H, s, H-26), 9.63 (1H, s, H-30). 13C-NMR (CDCl3) δ: 38.71 (C-1), 27.37 (C-2), 78.97 (C-3), 38.87 (C-4), 55.24 (C-5), 18.31 (C-6), 34.31 (C-7), 40.83 (C-8), 50.00 (C-9), 37.14 (C-10), 20.77 (C-11), 26.53 (C-12), 37.78 (C-13), 42.86 (C-14), 27.23 (C-15), 35.33 (C-16), 43.06 (C-17), 47.10 (C-18), 37.41 (C-19), 49.68 (C-20), 23.64 (C-21), 40.47 (C-22), 27.99 (C-23), 15.39 (C-24), 16.05 (C-25), 15.95 (C-26), 14.34 (C-27), 17.91 (C-28), 7.35 (C-29), 205.09 (C-30); LR-EIMS m/z: 442 ([M]+, 71), 424 (96), 409 (92), 207 (99), 189 (98), 135 (100).
4.3.9. (20R)-3β-Hydroxylupan-29-al (29-oxo-lupanol, 9)
1H-NMR (CDCl3) δ: 0.76 (3H, s, H-28), 0.77 (3H, s, H-24), 0.85 (3H, s, H-25), 0.92 (3H, s, H-27), 0.98 (3H, s, H-23), 1.05 (3H, s, H-26), 1.08 (3H, d, J = 6.6 Hz, H-30), 9.86 (1H, s, H-29).
4.3.10. Lup-20(29)-ene-3β,30-diol (10)
29.7 mg (0.0016%), colorless needles; m.p. 224–226 °; [α]: −11 (c 0.10, CHCl3). IR cm−1: 3317, 2938, 2859, 1445, 1379; HR-EIMS m/z: 442.3830 ([M]+; calcd. for C30H50O2, 442.3811). 1H-NMR (CDCl3) δ : 0.76 (3H, s, H-24), 0.78 (3H, s, H-28), 0.83 (3H, s, H-25), 0.94 (3H, s, H-27), 0.97 (3H, s, H-23), 1.03 (3H, s, H-26), 2.29 (1H, ddd, J = 11.2, 11.2, 5.2 Hz, H-19), 3.19 (1H, ddd, J = 11.5, 5.6, 5.6 Hz, H-3), 4.10 (1H, dd, J = 14.4, 5.7 Hz, H-30a), 4.15 (1H, dd, J = 14.4, 5.7 Hz, H-30b). 13C-NMR (CDCl3) δ : 38.68 (C-1), 27.40 (C-2), 78.98 (C-3), 38.85 (C-4), 55.27 (C-5), 18.30 (C-6), 34.29 (C-7), 40.84 (C-8), 50.38 (C-9), 37.15 (C-10), 21.00 (C-11), 26.67 (C-12), 38.00 (C-13), 43.02 (C-14), 27.40 (C-15), 35.46 (C-16), 42.79 (C-17), 48.90 (C-18), 43.81 (C-19), 154.78 (C-20), 31.76 (C-21), 39.85 (C-22), 27.98 (C-23), 16.11 (C-24), 15.98 (C-25), 15.36 (C-26), 14.53 (C-27), 17.70 (C-28), 107.60 (C-29), 65.04 (C-30); LR-EIMS m/z: 442 ([M]+, 100), 424 (65), 409 (35), 207 (95), 189 (99).
4.3.11. 3-Hydroxy-30-norlupan-20-one (11)
3.7 mg (0.00020 %), [α]: −18 (c 0.17, CHCl3); IR cm−1: 2941, 2861, 1691. HR-EIMS m/z: 428.3639 ([M]+ calcd. for C29H48O2, 428.3654). 1H-NMR (CDCl3) δ: 0.76 (3H, s, H-24), 0.77 (3H, s, H-28), 0.83 (3H, s, H-25), 0.97 (3H, s, H-23), 1.02 (3H, s, H-26), 2.15 (3H, s, H-29). 13C-NMR (CDCl3) δ : 38.64 (C-1), 27.37 (C-2, C-15), 78.91 (C-3), 38.85 (C-4), 55.24 (C-5), 18.28 (C-6), 34.17 (C-7), 40.72 (C-8), 50.25 (C-9), 37.16 (C-10), 20.89 (C-11), 27.31 (C-12), 37.01 (C-13), 42.66 (C-14), 34.96 (C-16), 43.05 (C-17), 49.68 (C-18), 52.61 (C-19), 212.91 (C-20), 27.64 (C-21), 39.84 (C-22), 27.97 (C-23), 15.37 (C-24), 16.07 (C-25),15.90 (C-26), 14.46 (C-27), 17.97 (C-28), 29.17 (C-29) LR-EIMS m/z: 428 ([M]+, 36), 410 (85), 395 (30), 367 (23), 207 (88), 189 (100).
4.3.12. 3,30-Dihydroxylup-20(29)-en-28-al (12)
27.3 mg (0.0016%); 1H-NMR (CDCl3) δ: 0.75 (3H, s, H-24), 0.82 (3H, s, H-26), 0.91 (3H, s, H-25), 0.96 (3H, s, H-23), 0.98 (3H, s, H-27), 4.99 (1H, s, H-29a), 4.95 (1H, s, H-29b), 4.14 (2H, s, H-30), 9.64 (1H, s, H-28). 13C-NMR (CDCl3) δ: 38.71 (C-1), 26.93 (C-2), 78.94 (C-3), 38.84 (C-4), 55.28 (C-5), 18.24 (C-6), 34.34 (C-7), 40.83 (C-8), 50.43 (C-9), 37.15 (C-10), 20.83 (C-11), 27.37 (C-12), 38.54 (C-13), 42.50 (C-14), 29.18 (C-15), 31.73 (C-16), 59.32 (C-17), 48.63 (C-18), 43.19 (C-19), 154.06 (C-20), 28.86 (C-21), 32.93 (C-22), 27.97 (C-23), 15.35 (C-24), 15.91 (C-25), 16.14 (C-26), 14.26 (C-27), 206.30 (C-28), 107.38 (C-29), 65.01 (C-30)
4.3.13. Lup-20(29)-ene-3,28,30-triol (13)
1H-NMR (CDCl3) δ: 0.76 (3H, s, H-24), 0.82 (3H, s, H-25), 0.97 (3H, s, H-23), 0.98 (3H, s, H-27), 1.02 (3H, s, H-26), 3.79 (1H, d, J=10.8, H-28a), 3.32 (1H, d, J=10.8, H-28b), 4.14 (1H, d, J=14.4, H-30a), 4.10 (1H, d, J=14.4, H-30b), 4.90 (1H, s, H-29a), 4.95 (1H, s, H-29b); 13C-NMR (CDCl3) δ: 38.69 (C-1), 27.39 (C-2), 78.96 (C-3), 38.88 (C-4), 55.28 (C-5), 18.29 (C-6), 34.26 (C-7), 40.95 (C-8), 50.36 (C-9), 37.16 (C-10), 20.92 (C-11), 26.78 (C-12), 37.23 (C-13), 42.69 (C-14), 27.04 (C-15), 29.20 (C-16), 47.78 (C-17), 49.45 (C-18), 43.52 (C-19), 154.49 (C-20), 31.74 (C-21), 33.81 (C-22), 27.99 (C-23), 15.36 (C-24), 16.11 (C-25), 16.00 (C-26), 14.75 (C-27), 60.31 (C-28), 107.16 (C-29), 65.06 (C-30)
4.3.14. 3-Hydroxulup-20(29)-en-30-al (30-oxo-lupeol) (14)
2.2 mg (0.00012 %). [α]: +1.4 (c = 0.10 %). IR cm−1: 1735, 1462, 1379; HR-EIMS: m/z 440.3653 ([M]+ calcd. for C30H48O2, 440.3654); 1H-NMR (CDCl3) δ: 0.75 (3H, s, H-24), 0.81 (3H, s, H-25), 0.82 (3H, s, H-28), 0.92 (3H, s, H-27), 0.96 (3H, s, H-23), 1.01 (3H, s, H-26), 5.91 (1H, s, H-29a), 6.29 (1H, s, H-29b), 9.51 (1H, s, H-30).
LR-EIMS m/z: 440 ([M]+, 52), 428 (51), 207 (100), 189 (96).
4.4. Cell culture and cytotoxicity analysis
All cell lines were obtained from American Type Culture Collection (ATCC, Virginia, USA) or UNC Lineberger Comprehensive Cancer Center (North Carolina, USA), except for KB-VIN (vincristine-resistant KB subline), which was a gift from Professor Y.-C. Cheng (Yale University, Connecticut, USA). Cells were cultured in RPMI 1640 medium containing 25 mM HEPES and 2 mM L-glutamine (Corning, NY, USA), supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich, MO, USA), 100 IU penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (Corning). KB-VIN cells were maintained in media containing 100 nM vincristine (Sigma–Aldrich). Cells were maintained at 37 °C in a humidified 5% CO2 atmosphere. The cells were passaged every 3–4 days. Cytotoxicity was evaluated by the sulforhodamine B method as described previously [Nakagawa-Goto et al., 2015]. In brief, all compounds were prepared at 10 mM with DMSO, and the highest concentration of DMSO in the cultures (0.4% v/v) used for the cytotoxicity assay had no effect on cell growth. Freshly trypsinized cell suspensions were seeded in 96-well microtiter plates at densities of 8000–22,000 cells per well with compounds. After 72 h in culture with test compounds, cells were fixed in 10% trichloroacetic acid followed by staining with 0.04% sulforhodamine B. The protein-bound dye was solubilized by 10 mM Tris base and absorbance was measured at 515 nm using an EL×800 microplate reader with Gen5 software (BioTek, Vermont, USA). The IC50 was calculated from at least three independent experiments performed in duplicate.
Highlights.
Four previously undescribed lupane-triterpenoids were isolated from Euonymus plant.
Another 10 known compounds were reported for the first time from this plant.
Structures were elucidated on the basis of spectroscopic data.
Acknowledgement
We appreciate critical comments, suggestions, and editing of the manuscript by Dr. Susan L. Morris-Natschke (UNC-CH). This study was supported in part by NIH grant CA177584 from the National Cancer Institute awarded to K.H.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarenga N, Ferro EA, 2006. Bioactive triterpenes and related compounds from Celastraceae. Studies in Natural Products Chemistry. 33, 239–307. [Google Scholar]
- Baek NI, Lee YH, Park JD, Kim SI, Ahn BZ, 1994. Euonymoside A: a new cytotoxic cardenolide glycoside from the bark of Euonymus sieboldianus. Planta Med. 60 (1), 26–9. [DOI] [PubMed] [Google Scholar]
- Chakravarty AK, Masuda K, Suzuki H, Ageta H, 1994. Unambiguous assignment of 13C chemical shifts of some hopane and migrated hopane derivatives by 2D NMR. Tetrahedron. 50 (9), 2865–2876. [Google Scholar]
- Mutai C, Abatis D, Vagias C, Moreau D, Roussakis C, Roussis V, 2007. Lupane triterpenoids from Acacia mellifera with cytotoxic activity. Molecules. 12, 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett RE, Cong ANT, Wilkins AL, Thomson RA, 1985. Lichens and fungi. Part 17. The synthesis and absolute configuration at C-20 of the (R)- and (S)-epimers of some 29-substituted lupane derivatives and of some 30-norlupan-20-ol derivatives and the crystal structure of (20R)-3β-acetoxylupan-29-ol. J. Chem. Soc. Perkin Trans Ⅰ, 2051–2056. [Google Scholar]
- Descoins C Jr, Bazzocchi IL, Ravelo AG, 2002. New sesquiterpenes from Euonymus europaeus (Celastraceae) Chem Pharm Bull. 50 (2). 199–202. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y, 1983. Studies on the disease and medical treatments of the Ainu race. Hokkaido Institute of Public Health, 125–129. [Google Scholar]
- Ito J and Niwa M, 1997. Triterpenoids from the cork of Vitis vinifera “Kyohou” Nat. Med 51 (3), 269–271. [Google Scholar]
- Kitanaka S, Takido M, Mizoue K, Nakaike S, 1996. Cytotoxic cardenolides from woods of Euonymus alata. Chem Pharm Bull. 44, 615–617. [DOI] [PubMed] [Google Scholar]
- Koul S, Razdan TK, Andotra CS, Kalla AK, Taneja SC, Dhar KL, 2000. Koelpinin-A, B and C – three triterpenoids from Koelpinia linearis. Phytochemistry. 53 (2), 305–309. [DOI] [PubMed] [Google Scholar]
- Lee CK, 1998. A new norlupene from the leaves of Melaleuca leucadendron. J. Nat. Prod 61 (3), 375–376. [DOI] [PubMed] [Google Scholar]
- Nakagawa-Goto K, Oda A, Hamel E, Ohkoshi E, Lee KH, Goto M, 2015. Development of a novel class of tubulin inhibitor from desmosdumotin B with a hydroxylated bicyclic B-ring. J. Med. Chem 58, 2378–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puapairoj P, Naengchomnong W, Kijjoa A, Pinto MM, Pedro M, Nascimento MS, Silva AM, Herz W, 2005. Cytotoxic activity of lupane-type triterpenes from Glochidion sphaerogynum and Glochidion eriocarpum two of which induce apoptosis. Planta Med. 71 (3), 208–213. [DOI] [PubMed] [Google Scholar]
- Razdan TK, Harkara S, Qadri B, Qurishi MA, Khuroo MA, 1988. Lupene derivatives from Skimmia laureola. Phytochemistry. 27 (6), 1890–1892. [Google Scholar]
- Shiojima K, Arai Y, Masuda K, Takase Y, Ageta T, Ageta H, 1992. Mass spectra of pentacyclic triterpenoids. Chem Pharm Bull. 40 1683–1690. [Google Scholar]
- De Souza e Silva SR, de Fátima Silva GD, de Almeida Barbosa LC, Duarte LP, Filho SAV, 2005. Lupane pentacyclic triterpenes isolated from stems and branches of Maytenus imbricate (Celastraceae). Helv. Chim. Acta 88 (5), 1102–1109. [Google Scholar]
- Zhou J, Li CJ, Yang JZ, Ma J, Li Y, Bao XQ, Chen XG, Zhang D, Zhang DM, 2014. Lupane triterpenoids from the stems of Euonymus carnosus. J. Nat. Prod 77, 276–284. [DOI] [PubMed] [Google Scholar]