Abstract
Content:
Clinician prescribing of off-label medications is common due to a lack of pediatric-specific data regarding the dosing, efficacy and safety of medications regularly prescribed to children.
Objective:
This systematic review summarizes the published incidence of off-label medication use in children from the past 10 years. We also performed a retrospective chart review to determine the incidence of off-label prescriptions for children seen in the OU Physicians clinics.
Data Sources:
We conducted a literature search of PubMed and OVID Medline from 2007 to 2017. Search terms included off-label use of medications and all child. For the local review, the outpatient electronic medical record (EMR) was queried.
Study Selection:
Studies were eligible for inclusion if the study included children < 18 years of age, defined off-label use in the paper, and included the incidence of off-label drug use.
Data Extraction:
Each review author extracted the study data from their assigned studies. For the retrospective chart review, the EMR was queried for patients <21 years of age who had a clinic visit and received a new prescription during 2017.
Results:
We identified 31 studies, with off-label prescription rates from 3.2 % to 95%. The local retrospective chart review included 1,323 prescriptions; 504 were off-label (38.1%) and 819 were approved. The frequency of off-label prescriptions does not differ significantly between the meta-analysis from the systematic review and the local retrospective chart review (30.9% vs 38.1%).
Conclusions:
The use of off-label medications in children remains a common practice for pediatric providers.
Introduction
In comparison to adults, there is limited data pertaining to the dosing, efficacy, and safety of medications in children. This relative lack of data can be attributed to many causes, including unfamiliarity with age-related developmental pharmacology in pediatric patients, ethical considerations with conducting pediatric research, and a lack of financial incentive for the pharmaceutical industry. As a result, over the years there have been many significant therapeutic misadventures with off-label use of medications involving children with thalidomide and chloramphenicol being prominent examples. This lack of knowledge regarding pediatric specific drug use is an on-going area of concern in need of significant research. To address these concerns, Congress approved several pieces of legislation over the last 30 years. Table 1 provides an overview of the actions created by each bill,[1] which were designed to stimulate research related to drug pharmacology in pediatric populations. Following introduction of these bills in the United States over the last 15 years, 1200 studies have been submitted to the Food and Drug Administration (FDA), resulting in changes to over 700 medication labels[2]. Other countries have also adopted similar legislation. The European Medicines Agency created the European Pediatric regulation in 2007 in an effort to facilitate the development and availability of medicines for children while Canada created the Pediatric Expert Advisory Committee in 2009 in order to promote the development and licensing of drugs for children.
Table 1:
Overview of Federal Legislation to Promote Pediatric Studies (Data from U.S. Drug and Food Administration1)
| Legislation [Year Enacted] |
Implications of Legislation |
|---|---|
| Food and Drug Modernization Act (FDAMA) [1997] | • Encouraged pharmaceutical
manufacturers to perform pediatric studies • Offered 6 months patent exclusivity as financial incentive |
| Pediatric Rule [1998] | • Required efficacy & safety
testing for NDAs if medication could be used in children • Studies under Pediatric Rule were eligible for 6 month patent extension under FDAMA • Federal court overturned in 2002 because FDA did not have authority |
| Best Pharmaceuticals Act for Children (BCPA) [2002] | • Authorized FDA to request pediatric
studies with NDAs (including orphan drugs) for new
indications • Extended 6 month patent exclusivity through 2007 • Required NIH to publish list of needs for future study in children |
| Pediatric Research Equality Act (PREA) [2003] | • Required pediatric assessment &
development of PSP with NDAs (expanded version of Pediatric Rule) for
certain drugs (NOT orphan drugs) • Allowed for modifications to existing indications • Pediatric plan must be developed before approval in adults |
| Food and Drug Administration Amendments Act (FDAAA) [2007] | • Reauthorized PREA & BPCA ×
5 years • Expanded the BCPA so FDA could issue request for > 1 indication (i.e., “on” & “off-label” use) • Introduced Pediatric Medical Device Safety & Improvement Act |
| Food and Drug Administration Safety and Administration Act (FDASIA) [2012] | • Made BPCA & PREA permanent |
Despite the passage of these bills and subsequent research, there is still a significant lack of both pediatric-specific drug information and governmental approvals for on-label pediatric prescribing. Until further research and applications occur, many clinicians are forced to prescribe medications off-label. In response to this practice gap, The American Academy of Pediatrics adopted a policy statement on the use of off-label medications in children; they defined off-label use as “use of a drug that is not included in the package insert (FDA-approved labeling) [and] does not imply improper, illegal contraindicated or investigational use”[3]. This statement also emphasizes that off-label use does not necessarily require prescribers to obtain informed consent if the decision to use the medication is supported by scientific or anecdotal evidence and is not investigational in nature. For example, enalapril has an FDA-approved indication for hypertension, heart failure, and asymptomatic left ventricular dysfunction in adults but only has a FDA-labeled indication for hypertension in children.[4] Despite this, enalapril is a commonly used to treat heart failure in pediatric patients and informed consent is not required in these situations. It is important to recognize that designation of off-label use can refer not only to the clinical indication for which a drug is prescribed but also includes administration of a medication by any route or dosing scheme that is not included in the package labeling approved by the FDA. For example, dexmedetomidine, a commonly used sedative is labeled for administration via the IV route only in adults but is used off-label when delivered via the inhaled route in adults and children.
Despite the adoption of the previous legislation and subsequent studies, many medications continue to be used off-label in children. The Department of Pediatrics at The University of Oklahoma Health Sciences Center (OUHSC) is in the process of joining an ongoing national study, entitled “Pharmacokinetics of Understudied Drugs Administered to Children Per Standard of Care” (POPS). This trial aims to provide more data regarding drug administration in children in an effort to decrease off-label use of medications and improve FDA-labeling of medications for pediatric patients. As the study enrolls patients and gathers medication-specific data, study medications change over time; currently POPS is studying 32 drugs, which are regularly prescribed in an off-label manner. The purpose of this systematic review is to determine the incidence of off-label medication use in children across varying health care settings over the previous 10 years. We also evaluated the off-label use of medications in the outpatient pediatric clinics as part of our center’s preparation for participation in the POPS study.
Methods
First, we conducted a literature review inclusive of prospective and retrospective studies on off-label use of medications in pediatric patients in any healthcare setting using PubMed (National Center for Biotechnology Information, U.S. National Library of Medicine, Bethesda MD), and OVID Medline (National Library of Medicine, Bethesda MD) databases. The initial search was performed April 6, 2018, and a second search was performed June 12, 2018. Search terms included all child (0–18 years), off-label use of medications with publication dates of 2007 to 2017. The studies included were limited to those written in English with the full article available through the OUHSC Bird Library (Appendix 1). Literature reviews, letters to the editor, or opinion papers were excluded. Studies were eligible for final inclusion if the study population included children, defined off-label use in the paper, and included the incidence of off-label drug use. As our objective was to report the overall incidence of off-label use in varying health care settings, studies were included if they focused on multiple classes of medications in any health care setting and excluded if they reported the incidence of off-label use of only a single medication or medication class. Eligibility assessment was done by two authors (CA &SD); article titles and abstracts were reviewed in an unblinded, standardized manner. Disagreements between the primary reviewers were resolved by consensus opinion of four of the authors (CA, SD, PJ, & JM). Studies from all countries were included, and we relied on the individual authors’ interpretation of the local regulatory agency’s ruling to determine off-label use of medications in the country the article was published.
The included studies were then divided amongst five of the authors (CA, SD, HC, NA, & JL) for review and extraction of study variables. A data extraction tool was created and pilot tested on 5 randomly selected articles; no changes were made to the data extraction tool after the pilot testing. Each review author extracted the study data from their assigned included studies. A single author (CA) checked extracted data, and disagreements were presented to a third author (SD) for resolution. Study variables included year of publication, years of data collection or duration of data collection, study design, age range of patient population included in the study, setting of the study (i.e. inpatient unit vs. outpatient clinic), geographic location of study, total number of prescriptions, the rate of off-label medication use, and study definition of off-label use. The reason (i.e. dose, indication, frequency or route) drugs were determined to be off-label, adverse drug reactions, and handling of parental consent for off-label drug use was also collected if clearly stated in the paper.
There was no separate assessment of bias in the systematic review as all studies were either retrospective or prospective observational. There is the inherent bias due to the nature of the study design of retrospective and prospective observational studies.
Next we conducted a retrospective chart review of our electronic medical record system (Centricity EMR, GE Healthcare) to determine the prevalence of off-label mediation use in children at our institution. Data were collected for patients 0 to 20 years of age, as POPS is enrolling patients through age 20, who had at least one outpatient visit at our institution during 2017, and received a prescription with a start date in 2017 for one of the POPS current 32 drugs of interest listed in Table 2. Data collected included the generic name of the drug prescribed, date of prescription, zip code to determine rural/urban status, patient race, patient ethnicity, and determination of off-label or approved use of the drug; if the drug was used in an off-label manner, the reason was noted. IBM Micromedex (IBM Corporation) was used to determine the Food and Drug Administration approved age range, route, dose and indication for medications. Two authors (CA and SD) reviewed and discussed unclear cases until consensus reached. The local retrospective study was approved by the Institutional Review Board at OUHSC.
Table 2:
List of 32 POPS drugs of interest
| 1. Alfentanil |
| 2. Amikacin |
| 3. Atropine |
| 4. Cefepime |
| 5. Ceftazidime |
| 6. Cidofovir |
| 7. Ciprofloxacin |
| 8. Clozapine |
| 9. Dexmedetomidine |
| 10. Diazepam |
| 11. Etomidate |
| 12. Fosphenytoin |
| 13. Haloperidol |
| 14. Low Molecular Weight Heparin |
| 15. Hydromorphone |
| 16. Lidocaine |
| 17. Lurasidone |
| 18. Meropenem |
| 19. Methadone |
| 20. Methylprednisolone |
| 21. Midazolam |
| 22. Molindone |
| 23. Nafcillin |
| 24. Pentobarbital |
| 25. Piperacillin |
| 26. Timolol |
| 27. Tobramycin |
| 28. Valproic Acid |
| 29. Vancomycin |
| 30. Vecuronium |
| 31. Warfarin |
| 32. Ziprasidone |
Descriptive statistics for the local data were calculated for demographic variables and outcome of interest, prescription off-label status (approved vs. off-label). Chi squares were calculated for categorical demographics (sex, rural/urban status, race, ethnicity), and log binomial regression was used for continuous predictors (age). A p value of < 0.05 was considered significant. Zip codes were used to determine rural/urban designation with rural urban commuting area (RUCA) codes, using the four category classification as defined by the Rural Health Research Center[5]. Comparisons of meta-analyses from the systematic review and local data were performed by observing proportions and confidence intervals of off-label prescriptions, and the frequencies of reasons for being off-label (age, dose, indication, and route), among off-label prescriptions. Meta-analyses were performed using MedCalc software’s meta-analysis function for proportions for the systematic review. Analyses on local data were performed using SAS 9.4. Comparisons between Meta-analyses and local data were performed by observing estimated proportions of off-label prescriptions and their respective 95% confidence intervals.
Results
During the literature review, 285 publications were initially identified. Eight duplicates were removed, leaving 277 studies for initial screening. 54 studies met initial screening criteria and were sent on for full review. From these, 23 studies were ultimately excluded; five studies were not available in full manuscript, eleven were specific for a single medication or medication class, and seven published no incidence of off-label prescriptions (Diagram 1). Thirty-one studies were included in final analysis, including16 retrospective studies and 15 prospective observational studies. Pediatric patients from infants to adolescents were included in 24 studies, while 7 studies included only neonates. A total of 19 of the studies were conducted in inpatient populations, 11 studies were outpatient and one study included both inpatient and outpatient locations at a single center. The majority of the studies were conducted in Europe (n=19), Asia (n=6) and Australia (n=3). The number of patients involved in each study varied between 81 and 1.9 million patients. The 31 studies in the systematic review had varied age ranges but overall included patients that were preterm infants to 19 years old
Off-label use was described in the included 31 studies. Use of the medication outside the package insert recommendations for indication, age, route, dose and frequency was the most common definition of off-label use. Four studies further defined off-label as “use of medications that had no pediatric indication or were contraindicated in children” [6–8]. The rate of off-label prescriptions varied widely in the 31 reviewed studies and was reported to be between 3.2% and 95%. Seven studies in our literature review specifically evaluated the use of off-label medications in the neonate population, with off-label drug use ranging from 26% to 95%. See Table 3 for individual article details.
Table 3:
List of studies included in the literature review.
| Author | Year Published |
Type of Report (# Patients) |
Age Range |
Location | Country of Origin |
% Off-Label Prescriptions |
|---|---|---|---|---|---|---|
| Lass et al [6] | 2011 | Retrospective (n=151,476) | < 19 years | Multi-center outpatient clinics | Estonia | 31% |
| Carnovale et al. [7] | 2013 | Retrospective database search (n=1,708,755) | 0–18 year | Single-center outpatient clinics | Italy | 3.3% |
| Olsson et al. [8] | 2011 | Retrospective database search (n=1,911,417) | 0–18 years | Multi-center outpatient clinics | Sweden | 13.5% |
| Muhlbauer et al [12] | 2009 | Retrospective database search (n=289,000) | 0–16 years | Multi-center outpatient clinics | Germany | 3.2% |
| Ballard et al. [9] | 2013 | Retrospective (n=300) | < 12 years | Single center general pediatric wards | Australia | 32% |
| Palmaro et al.[10] | 2015 | Prospective (n=2,313) | 0–16 years | Multi-center outpatient clinics | France | 37.6% |
| Maltz et al.[13] | 2013 | Retrospective (n=82) | < 18 years | Single-center CVICU3 | USA | 36% |
| Morales-Capri et al.[14] | 2010 | Prospective observational (n=462) | < 14 years | Single center emergency department | Spain | 27% |
| Ribeiro et al. [15] | 2013 | Retrospective (n=700) | < 18 years | Single-center emergency department | Portugal | 32.2% |
| Lee et al. [16] | 2013 | Prospective observational (n=192) | Preterm to 18 years | Single-center PICUs | Malaysia | 34.1% |
| Blanco-Reina et al. [17] | 2014 | Prospective observational (n=81) | Birth to 14 years | Single-center PICU and NICU4 | Spain | 52% |
| Berdkan et al.[18] | 2016 | Retrospective (n=500) | 1 day to 16 years | Multi-center including multiple wards | Lebanon | 30.2% |
| Lindell- Osuagwu et al.[19] | 2014 | Retrospective (n=123) | < 18 years | Single-center including multiple wards | Finland | 42% |
| Aamir et al.[20] | 2017 | Prospective observational (n=895) | < 18 years | Multi-center Surgical wards | Pakistan | 48.2% |
| Czarniak et al.[21] | 2015 | Retrospective (n=699) | All ages | Single Center inpatient and outpatient | Australia | 25.7% |
| Teigen et al. [22] | 2017 | Prospective (n=400) | 0–17 years | Multi-center including multiple wards | Norway | 44% |
| Joret-Descout et al. [23] | 2015 | Retrospective (n=120) | 0–18 years | Single-center including multiple wards | France | 36.5% |
| Taylor et al. [24] | 2015 | Retrospective (n=3343) | 0–17 years | Multi-center outpatient | Australia | 30.5% |
| Hsien et al. [25] | 2008 | Prospective observational (n=417) | All ages | Single-center including multiple wards | Germany | 31% |
| Langerova et al.[11] | 2014 | Prospective (n=4,282) | 0–15 years | Single-center outpatient clinics | Czech Republic | 9.01% |
| Abdulah et al.[26] | 2015 | Retrospective (n=4,936) | 0–5 years | Multi-center outpatient clinics | Indonesia | 18.6% |
| Knopf et al. [27] | 2013 | Retrospective (n=17,450) | 0–17 years | Single-center outpatient clinics | Germany | 40.2% |
| Palcevski et al. [28] | 2012 | Prospective (n=691) | 0–19 years | Single-center including multiple wards | Croatia | 13.3% |
| Cuzzolin et al.[29] | 2016 | Prospective (n=220) | Preterm/neonates | Multi-center NICUs | Italy | 59% |
| Kieran et al.[30] | 2014 | Prospective (n=110) | Neonates | Single-center NICU | Ireland | 39% |
| Schweigertova et al.[31] | 2016 | Prospective (n=202) | < 29 days old | Multi-center NICUs | Slovakia | 43% |
| Jain et al.[32] | 2014 | Prospective (n=156) | Neonates | Multi-center NICUs | India | 26% |
| de Souza et al. [33] | 2016 | Retrospective (n=192) | < 28 days old | Single-center NICU | Brazil | 95.6% |
| Silva et al. [34] | 2015 | Retrospective (n=218) | < 28 days old | Single-center NICU | Portugal | 25.7% |
| Chauthankar et al.[35] | 2017 | Prospective observational (n=460) | Preterm/neonates | Single-center NICU | India | 12.3% |
| Pereira Gomes et al. [36] | 2015 | Prospective observational (n=320) | 2–18 years | Single- center inpatient ward | Brazil | 57.2% |
ND = Not defined in study;
PICU = Pediatric Intensive Care Unit;
CVICU = Cardiac Intensive Care Unit;
NICU = Neonatal Intensive Care Unit
Only two studies reported adverse drug reactions related to off-label use of medications. One study did not define or elaborate on the nature of the adverse drug reaction, however, the second study reported fever, diarrhea and rash as an adverse drug reaction secondary to off-label use of medications [9, 10]. No study commented on obtaining parental consent for the use of off-label medications.
In our local retrospective chart review 22 of the 32 POPS drugs of interest were prescribed as a new prescription in 2017, resulting in a total of 1,323 prescriptions for 1079 patients. Of the 1,323 prescriptions, 504 prescriptions were off-label (38.1%) and 819 prescriptions were on-label. Table 4 provides a list of the 22 included drugs and the off-label versus approved use prescription percentages. The reasons the prescriptions were classified as off-label are listed in Table 5, with patient age being the most common reason. Table 6 displays patient demographics and frequency of off-label prescriptions defined by demographic grouping. There was no significant difference in the frequency of off-label prescriptions between male and female patients (38.6%, 37.6%). Off-label prescription frequency did not significantly differ between urban and rural groups (37.7%, 39.1) or by ethnicity (Hispanic/Latino vs. Not Hispanic/Latino 41.4% v. 38.2%). Though ostensibly there is large range in off-label frequency between racial groups (27.8%−46.6%), this was not statistically significant. There is, however, a significant association between age (in years) and frequency of being prescribed an off-label drug (p<0.001). The probability of receiving an off-label prescription is reduced by 3% for every year increase in age, with the probability at less than a year old being 51.6% and the probability at 20 years old being 29.3%.
Table 4:
Summary of Prescription Off-label Status by Drug (N=1323 prescriptions)
| Off-label N (%) | Approved N (%) | |
|---|---|---|
| Drug | ||
| amikacin | 1 (50.0) | 1 (50.0) |
| atropine | 4 (100) | - |
| cefepime | 2 (66.7) | 1(33.3) |
| ceftazidime | - | 1 (100) |
| ciprofloxacin | 120 (66.7) | 60 (33.3) |
| clozapine | 3 (100) | - |
| diazepam | 43 (22.3) | 150 (77.7) |
| haloperidol | 6 (54.6) | 5 (45.4) |
| heparin | 95 (100) | - |
| hydromorphone | 6 (100) | - |
| lidocaine | 9 (4.3) | 201 (95.7) |
| lurasidone | 18 (85.7) | 3 (14.3) |
| methylprednisolone | - | 285 (100) |
| midazolam | 2 (66.7) | 1 (33.3) |
| nafcillin | 1 (100) | - |
| piperacillin | 4 (100) | - |
| timolol | 28 (100) | - |
| tobramycin | 31 (31.3) | 68 (68.7) |
| valproic acid | 13 (61.9) | 8 (38.1) |
| vancomycin | 1 (4.3) | 22 (95.7) |
| warfarin | 89 (89.9) | 10 (10.1) |
| ziprasidone | 28 (90.3) | 3 (9.7) |
Table 5:
Summary of Off-label Status Frequency and Off-label Frequency by Reason
| n (%) | |
|---|---|
| Off-label Status | |
| Approved | 819 (61.9) |
| Off-label | 504 (38.1) |
| Reason for Off-label Status* | |
| Age | 338 (74.5) |
| Dose | 52 (10.3) |
| Indication | 108 (21.4) |
| Route | 16 (3.2) |
Four “Off-label” missing reason. 14 prescriptions contributing to more than one reason
Table 6:
Summary of Demographic characteristics by Prescription Off-label Status (N=1,323 prescriptions)
| Off-label N (%) | Approved N (%) | ||
|---|---|---|---|
| Sex | p=0.71 | ||
| Male | 252 (38.6) | 401 (61.4) | |
| Female | 252 (37.6) | 418 (62.4) | |
| RUCA* | p=0.63 | ||
| Rural | 144 (39.1) | 224 (60.9) | |
| Urban | 360 (37.7) | 595 (62.3) | |
| Race | Ref=White | ||
| American Indian/ Alaska Native | 27 (46.6) | 31 (53.4) | p=0.21 |
| Asian | 5 (27.8) | 13 (72.2) | p=0.27 |
| Black/ African American | 56 (36.1) | 99 (63.9) | p=0.63 |
| Native Hawaiian/ Pacific Islander | 5 (45.4) | 6 (54.6) | p=0.63 |
| White | 400 (38.1) | 649 (61.9) | |
| Ethnicity | p=0.43 | ||
| Hispanic or Latino | 72 (41.4) | 102 (58.6) | |
| Not Hispanic or Latino | 421 (38.2) | 680 (61.8) | |
| Age (years) | p<0.001** | ||
| Mean (Standard Deviation) | 10.2 (6.1) | 11.8 (5.7) | |
| Median | 12 | 13 | |
| Min, Max (Interquartile Range) | <1 month, 19 (5,16) | <1 month, 20 (7,17) | |
Rural Urban Commuting Area
Age in years: RR 0.97 (0.96–0.98)
To estimate a combined proportion of off-label prescriptions from the systematic review for comparison with our own data, we performed a meta-analysis for proportions (Figure 1). The I2 statistic indicated that over 99% of the variation in proportions across studies was due to heterogeneity, and thusly the estimated proportion derived from the random effects model used was 30.9%, (95% CI: 26.0–36.0) for the systematic review. Comparing the results of local data (38.1%, 95%CI: 35.5, 40.8) with the meta-analysis from the systematic review, the frequency of off-label prescription does not differ significantly (Table 7). When comparing the reasons for off-label prescription (among those studies that listed them), OU Physicians pediatric outpatient clinics showed a significantly larger proportion of off-label prescriptions due to age (74.5%, 25.6%), and a significantly smaller proportion due to dose (10.3%, 48.3%). There was no significant difference between proportions of off-label prescriptions due to indication (21.4%, 19.5%) or route (3.2%, 3.4%).
Figure 1:
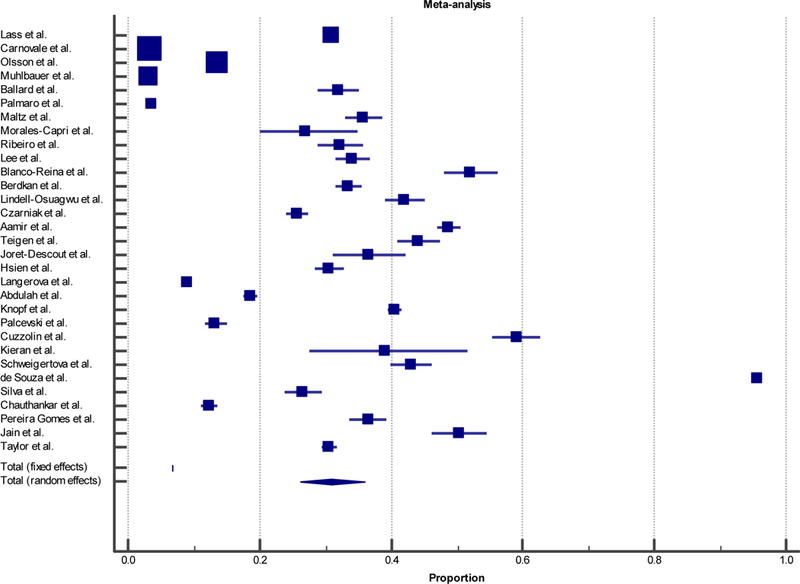
Meta-analysis Forest Plot
Table 7:
Summary of Off-label Prescription Frequency and Off-label Prescription Frequency by Reason:
OUHSC Children’s Hospital Outpatient compared to Meta-Analysis
| OU Physicians Percent Off-label (95% CI) |
Meta-Analysis Percent Off-label (95% CI) |
|
|---|---|---|
| Off-label | 38.1 (35.5, 40.8) | 30.9 (26.0, 36.0) |
| Reason for Off-label | ||
| Age | 74.5 (70.2, 78.4) | 25.6 (15.4, 37.3) |
| Dose | 10.3 (7.8, 13.3) | 48.3 (35.7, 61) |
| Indication | 21.4 (17.9, 25.3) | 19.5 (12.4, 27.8) |
| Route | 3.2 (1.8, 5.1) | 3.4 (0.6, 8.3) |
Discussion
Our study and literature review demonstrate that off-label use of medications in pediatric patients is a common practice, with a significant number of children, inpatient and outpatient, receiving an off-label medication. With the exception of three of the reviewed studies [7, 11, 12], all studies demonstrate >12% of prescriptions are off-label. Though most of the articles reviewed were from European countries, all continents were represented. The historical practice of using off-label medications in children is a frequent worldwide occurrence and continues to be an issue despite the increased awareness and passed legislation. Eighteen of the reviewed studies were conducted in European countries and published after the European Union Pediatric Regulation was established in 2007, which proposed to stimulate pediatric specific research much like the United States regulations reviewed in the introduction. The single included United States study in children was published more than 10 years after FDAMA and Pediatric Rule, yet it continues to report a high incidence of off-label medication prescriptions (36%).[13] Our local study for new prescriptions in 2017 also demonstrated a high incidence of off-label use at 38.1%. Despite international government efforts to mandate further research for pediatric drug labeling, there continues to be a high rate of off-label use of medications.
The reason for off-label use varies among studies. Our local study had a significantly higher proportion of patients that were given a medication off-label due to age and fewer that were off-label due to dose when compared to the meta-analysis from the systematic review. As the majority of the studies included in the systematic review were outside of the United States, this may in part be due to different approved dosing regimens from the local regulatory entities in the various countries that the systematic review studies were conducted. In addition, we only included the 32 POPS drugs of interest in our local study, this may account for the disparity between age and dosage between our study and the meta-analysis.
The literature review demonstrates the incidence of off-label use is higher among younger populations, especially neonates as reflected in the neonate intensive care unit-specific articles with off-label prescription rates of at least 26%. While our local study was not focused primarily on the neonate population, we did find a significant number of off-label prescriptions were due to patient age and the risk of receiving an off-label prescription decreased as age increased.
Finally, none of the studies we reviewed addressed the issue of parental consent for off-label use despite two studies reporting an adverse drug reaction secondary to off-label medication use. This may in part be secondary to the above-referenced American Academy of Pediatrics adopted policy that specifically states providers do not need parental consent if the medication use is supported by current evidence in the literature or a practitioners experience with the medication. Additionally, there may be a lack of knowledge among prescribers about the on label indications for commonly used pediatric medications. Practitioners should be aware of the off-label medications they commonly use in children and be mindful of known potential adverse reactions and side effects but also be wary of possible new unreported drug reactions or side effects.
The real answer to this prescribing dilemma is more research in the pediatric population. Hopefully, the POPS study will generate pediatric safety data and result in FDA approval for many of the commonly prescribed medications currently used off-label, thereby eliminating the conflict providers may feel in the current prescribing environment.
Our literature review was limited to the previous ten years and excluded studies evaluating off-label use of a single specific drug or a specific class of drugs. Non-English language articles were not included in the final analysis. Exclusion of these types of studies may have limited the scope of our review. The systematic review included studies from multiple countries, a broad age range and settings such as inpatient and outpatient, which we felt would give us a current global perspective on off-label medication use but perhaps introduced bias as each setting and country defined off-label based on their local regulatory board. The studies included in the systematic review were also either retrospective or prospective observational studies, which have inherent increased risk of bias compared to randomized trials.
The local retrospective study was limited to the 32 drugs of interest in the POPs study that are frequently prescribed off-label to children. The local prevalence of off-label use could be significantly different in our patient population if inpatient prescriptions or all prescriptions were included in analysis.
Conclusions
Off-label medication use is common in the pediatric population, especially in neonates and younger age groups. More age-specific research is needed to provide adequate drug safety and effectiveness for children. Until more data is provided, clinical decision making should be guided by the best available evidence.
Supplementary Material
Acknowledegements
Research reported in this publication was supported by the Office of the Director, National Institutes of Health under Award Numbers UG1OD024950 and US4GM104938. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest and Disclosures
The authors declare no pertinent conflict of interest and did not receive funding for this project.
References
- 1.[cited 2018 May 10]; Pediatric Product Development]. Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm049867.htm..
- 2.Green DJ ZI, Burckart GJ, Pediatric Drug Development: outlook for science-based innovation. Clinical Pharmacology and Therapeutics 2018. 103: p. 376–8. [DOI] [PubMed] [Google Scholar]
- 3.Frattarelli DA GJ, Green TP et al. , Off-Label drugs in children. Pediatrics, 2014. 133: p. 563–7. [DOI] [PubMed] [Google Scholar]
- 4.in EXPANED (package insert). Greenwood Village, CO: Silvergate Pharmaceuticals, Inc. [Google Scholar]
- 5.http://depts.washington.edu/uwruca/ruca-maps.php[7/6/201810:57:06AM
- 6.Lass J, et al. , Off label use of prescription medicines in children in outpatient setting in Estonia is common. Pharmacoepidemiol Drug Saf, 2011. 20(5): p. 474–81. [DOI] [PubMed] [Google Scholar]
- 7.Carnovale C, et al. , Paediatric drug use with focus on off-label prescriptions in Lombardy and implications for therapeutic approaches. Eur J Pediatr, 2013. 172(12): p. 1679–85. [DOI] [PubMed] [Google Scholar]
- 8.Olsson J, et al. , Paediatric drug use with focus on off-label prescriptions in Swedish outpatient care--a nationwide study. Acta Paediatr, 2011. 100(9): p. 1272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballard CD, et al. , Off-label use of medicines in paediatric inpatients at an Australian teaching hospital. J Paediatr Child Health, 2013. 49(1): p. 38–42. [DOI] [PubMed] [Google Scholar]
- 10.Palmaro A, et al. , Off-label prescribing in pediatric outpatients. Pediatrics, 2015. 135(1): p. 49–58. [DOI] [PubMed] [Google Scholar]
- 11.Langerova P, Vrtal J, and Urbanek K, Incidence of unlicensed and off-label prescription in children. Ital J Pediatr, 2014. 40: p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muhlbauer B JK, Pichler J, Schoettler P, Off-label use of prescription drugs in childhood and adolescence: an analysis of prescription patterns in Germany. Dtsch Arztebl Int 2009. 106(3): p. 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maltz LA, et al. , Off-label drug use in a single-center pediatric cardiac intensive care unit. World J Pediatr Congenit Heart Surg, 2013. 4(3): p. 262–6. [DOI] [PubMed] [Google Scholar]
- 14.Morales-Carpi C, et al. , Drug utilization and off-label drug use among Spanish emergency room paediatric patients. Eur J Clin Pharmacol, 2010. 66(3): p. 315–20. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro M, Jorge A, and Macedo AF, Off-label drug prescribing in a Portuguese paediatric emergency unit. Int J Clin Pharm, 2013. 35(1): p. 30–6. [DOI] [PubMed] [Google Scholar]
- 16.Lee JL, Redzuan AM, and Shah NM, Unlicensed and off-label use of medicines in children admitted to the intensive care units of a hospital in Malaysia. Int J Clin Pharm, 2013. 35(6): p. 1025–9. [DOI] [PubMed] [Google Scholar]
- 17.Blanco-Reina E M-CA, Vega-Jimenez MA, Ocana-Riola R, Marquez-Romero EI, Ruiz-Extremera A, Drug Utilization pattern in children and off-label use of medicines in a pediatric intensive care unit. Medicina Intensiva 2014. 40(1): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 18.Berdkan S RL, Hajj A, Eid B, Jabbour H, Osta NE, Karam L, Khabbaz LR. , Comparative Assessment of Off-Label and Unlicensed Drug Prescriptions in Children: FDA versus ANSM Guidlines Clin Ther, 2016. 38(8): p. 1833–44. [DOI] [PubMed] [Google Scholar]
- 19.Lindell-Osuagwu L, et al. , Prescribing for off-label use and unauthorized medicines in three paediatric wards in Finland, the status before and after the European Union Paediatric Regulation. J Clin Pharm Ther, 2014. 39(2): p. 144–53. [DOI] [PubMed] [Google Scholar]
- 20.Aamir M, et al. , Unlicensed and off-label use of drugs in pediatric surgical units at tertiary care hospitals of Pakistan. Int J Clin Pharm, 2017. 39(4): p. 860–866. [DOI] [PubMed] [Google Scholar]
- 21.Czarniak P, et al. , Clinical setting influences off-label and unlicensed prescribing in a paediatric teaching hospital. PLoS One, 2015. 10(3): p. e0120630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teigen A, et al. , Off-label and unlicensed medicines to hospitalised children in Norway. J Pharm Pharmacol, 2017. 69(4): p. 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joret-Descout P, et al. , Off-label and unlicensed utilisation of medicines in a French paediatric hospital. Int J Clin Pharm, 2015. 37(6): p. 1222–7. [DOI] [PubMed] [Google Scholar]
- 24.Taylor D JP, Taylor SE., Jones A, Cheek JA, Craig SS, Graudins A, Dhir R, Krieser D, Babl FE, , Off-Label and unlicensed medicine adminsitration to pediatric emergency department patients. Emergency Medicine Australasia, 2015. 27: p. 440–446. [DOI] [PubMed] [Google Scholar]
- 25.Hsien L, et al. , Off-label drug use among hospitalised children: identifying areas with the highest need for research. Pharm World Sci, 2008. 30(5): p. 497–502. [DOI] [PubMed] [Google Scholar]
- 26.Abdulah R, et al. , Off-label paediatric drug use in an Indonesian community setting. J Clin Pharm Ther, 2015. 40(4): p. 409–12. [DOI] [PubMed] [Google Scholar]
- 27.Knopf H, et al. , Off-label medicine use in children and adolescents: results of a population-based study in Germany. BMC Public Health, 2013. 13: p. 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palcevski G, Skocibusic N, and Vlahovic-Palcevski V, Unlicensed and off-label drug use in hospitalized children in Croatia: a cross-sectional survey. Eur J Clin Pharmacol, 2012. 68(7): p. 1073–7. [DOI] [PubMed] [Google Scholar]
- 29.Cuzzolin L AR, Off-lable and unlicensed drug treatments in Neonatal INtensive Care Units: an Italian multicentere study. Eur J Clin Pharmacol, 2016. 72(1): p. 117–23. [DOI] [PubMed] [Google Scholar]
- 30.Kieran EA, O’Callaghan N, and O’Donnell CP, Unlicensed and off-label drug use in an Irish neonatal intensive care unit: a prospective cohort study. Acta Paediatr, 2014. 103(4): p. e139–42. [DOI] [PubMed] [Google Scholar]
- 31.Schweigertova J DA, Doinikova D, Ondriasova E, Balazova M, Slezakova V, Kuzelova M, Off Label and unlicensed use of medicinal products in the neonatal setting in the SLovak Republic. Pediatri Int, 2016. 58(2): p. 126–31. [DOI] [PubMed] [Google Scholar]
- 32.Jain S, et al. , Off-label use of drugs in neonatal intensive care units. Indian Pediatr, 2014. 51(8): p. 644–6. [DOI] [PubMed] [Google Scholar]
- 33.de Souza AS Jr., et al. , Off-label use and harmful potential of drugs in a NICU in Brazil: A descriptive study. BMC Pediatr, 2016. 16: p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva J F.-d.-L.F., Soares H, Guimaraes H, Off-Label and Unlicensed Drug Use in Neonatology: Reality in a Portugese University. Acta Med Port, 2015. 28(3): p. 297–306. [DOI] [PubMed] [Google Scholar]
- 35.Chauthankar SA,MP, Potey AV, Nanavati RN , Drug Utilization in Neonatal Intensive Care Unit of a Tertiary-care Hospital in Mumbai India. Indian Pediatrics 2017. 54: p. 931–934. [DOI] [PubMed] [Google Scholar]
- 36.Pereira Gomes V, et al. , Off-label and unlicensed utilization of drugs in a Brazilian pediatric hospital. Farm Hosp, 2015. 39(3): p. 176–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


