Abstract
αB-Crystallin is a member of the small heat shock protein family. It is a molecular chaperone and an anti-apoptotic protein. Previous studies have shown that the peptide (73DRFSVNLDVKHFSPEELKVKV93, hereafter referred to as peptain-1) from the core domain of αB-crystallin exhibits both chaperone and anti-apoptotic properties similar to the parent protein. We developed a mouse monoclonal antibody against peptain-1 with the aim of blocking the functions of αB-crystallin. The antibody reacted with peptain-1, it did not react with the chaperone peptide of αA-crystallin. The antibody strongly reacted with human recombinant αB-crystallin but weakly with Hsp20; it did not react with αA-crystallin or Hsp27. The antibody specifically reacted with αB-crystallin in human and mouse lens proteins but not with αA-crystallin. The antibody reacted with αB-crystallin in human lens epithelial cells, human retinal endothelial cells, and with peptain-1 in peptain-1-transduced cells. Unlike the commercial antibodies against αB-crystallin, the antibody against peptain-1 inhibited the chaperone and anti-apoptotic activities of peptain-1. The antibody might find use in inhibiting αB-crystallin’s chaperone and anti-apoptotic activities in diseases where αB-crystallin is a causative or contributing factor.
Keywords: Peptain-1, monoclonal antibody, αB-crystallin, chaperone activity, apoptosis
1. Introduction
α-Crystallin is a small heat shock protein and consists of αA- and αB- subunits. They have 55% sequence homology between them, and both are molecular chaperones (Kappe et al., 2003) and anti-apoptotic proteins (Andley et al., 2000). αB-Crystallin is stress-inducible and is present in several tissues including lens, retina, heart, skeletal muscles and kidney, but αA-crystallin, which is non-stress inducible, is present mainly in lens (Arrigo, 2013). αB-crystallin performs important roles in tissues by protecting them against various forms of stress. Previous studies showed that αB-crystallin protects cells from hyperthermia, UV light, hydrogen peroxide-induced oxidative stress, and chemically induced apoptosis (Liu et al., 2004; Dou et al., 2012; Christopher et al., 2014; Tang et al., 2014). Similarly, administration of αB-crystallin blocks ischemic injury, brain stroke, multiple sclerosis, optic nerve crush injury, spinal cord contusion injury and acute hypertension in experimental animals (Ying et al., 2008; Arac et al., 2011; Klopstein et al., 2012; Rothbard et al., 2012; Wu et al., 2012; Yan et al., 2017). Other studies have shown that αB-crystallin promotes pathological angiogenesis in retina (Kase et al., 2010) and epithelial to mesenchymal transition during fibrotic diseases (Bellaye et al., 2015; Ishikawa et al., 2016; Nahomi et al., 2016; Nam and Nagaraj, 2018). In addition, it is overexpressed in many cancers, suggestive of its causative or contributing role (Koletsa et al., 2014; Shi et al., 2014). One previous study showed inhibition of tumor progression in human breast cancer xenografted mice by treatment with an inhibitor of αB-crystallin (Chen et al., 2014). Together, these observations point to αB-crystallin as a therapeutic target in diseases. Whether extracellular αB-crystallin plays a role in the pathogenesis of diseases is not known. Rothbard et al. (Rothbard et al., 2012) showed that αB-crystallin levels in plasma are elevated ~5 fold in patients with multiple sclerosis relative to normal individuals, suggesting a possible pathological role for extracellular αB-crystallin. An αB-crystallin related small heat shock protein Hsp27 has been found to be increased in the serum of patients in several diseases, among them are, chronic pancreatitis (Liao et al., 2009), multiple sclerosis (Ce et al., 2011), gastric adenocarcinoma (Huang et al., 2010) and insulin-resistance associated macrovascular complications (Burut et al., 2010). Whether such an increase in Hsp27 contributes to the pathogenesis is currently not known. Thus, the role of extracellular small heat shock proteins in disease needs to be established.
All small heat shock proteins (sHSPs) have a conserved α-crystallin core domain containing ~90 amino acids. In recent years, several researchers have identified core domain peptides from sHSPs, including those in αA- and αB-crystallin that can function similar to their parent molecules (Sharma et al., 2000; Bhattacharyya et al., 2006; Ghosh et al., 2007; Nahomi et al., 2013b; Nahomi et al., 2015). We found that transfer of the peptides of αA- and αB-crystallin into cells effectively inhibited chemical-induced apoptosis and treatment in rats prevented cataract development (Nahomi et al., 2013b). Hinton and his group showed αB-crystallin-derived peptide is internalized into cells by an amino acid transporter, and such internalized peptide was able to block oxidative stress-induced apoptosis in retinal pigment epithelial cells (Sreekumar et al., 2013). Steinman’s group reported that intraperitoneally injected αB-crystallin peptide was effective in the treatment of experimental autoimmune encephalomyelitis in mice (Kurnellas et al., 2012). These observations suggest that sHSP-derived peptides may have therapeutic benefits.
Monoclonal antibodies are widely used for the detection of proteins, protein modifications and as therapeutics. Several monoclonal antibodies (humanized) are now FDA-approved drugs. The most notable are anti-TNFα (Humira) and anti-VEGF-A (Lucentis). There are others for treatment of ulcerative colitis, cancer, angiogenesis, inflammation, etc. Our idea was to develop a monoclonal antibody against peptain-1, so the antibody will neutralize the two major functions of αB-crystallin and inhibit diseases promoted by αB-crystallin. We report development of a monoclonal antibody that preferentially reacts with peptain-1 and inhibits the chaperone and anti-apoptotic activities of peptian-1 and the chaperone activity of αB-crystallin.
2. Material and methods
2.1. Immunization
All peptides used for this study were purchased from Peptide 2.0. VA (HPLC and mass spectrometry data for all peptides are provided in Supplemental Fig. S1). All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of Colorado and were performed in accordance with published National Institutes of Health guidelines and ARVO guidelines. Peptain-1 (2 mg) was conjugated with 2 mg of Imject Mariculture Keyhole Limpet Hemocyanin (mcKLH, Thermo Scientific, MA, Cat#77600), mixed with Freund’s complete adjuvant (Sigma, MO, Cat#5881), and injected intraperitoneally (i.p.) to Balb/c mice, and then after three booster injections (i.p.) in Freund’s incomplete adjuvant (Sigma, MO, Cat#5506) as previously described (Mailankot et al., 2009). Ten days after the final injection, blood was collected from the saphenous vein, and serum was separated for ELISA.
2.2. Generation of monoclonal antibody
Spleen cells were fused with myeloma cells FOX-NY cells (ATCC, VA) using polyethylene glycol, and hybridomas were cultured in a selection medium. ELISAs were performed either with peptain-1, Imject bovine serum albumin (Thermo Scientific, Cat#77110) conjugated peptain-1, scrambled peptain-1 (FEPSVRFSKVDHLVKENDLVK, Peptide 2.0, VA) or human recombinant αB-crystallin to select positive clones by ELISA. Hybridoma clones with high reactivity to peptain-1 and αB-crystallin and no reactivity against scrambled peptide were selected by subcloning. Immunoglobulin G (IgG) subclass was isotyped using Mouse Typer isotyping kit (Bio-Rad, CA, Cat#172–2051). The hybridoma clone that showed strongest immunoreactivity was grown in hybridoma serum free media (Life Technologies, NY, Cat#12045076) for antibody production. The monoclonal antibody was purified on a Protein G-Sepharose column (GE Healthcare, PA) and stored at −80°C until use.
2.3. Coupling of BSA with peptain-1
Peptain-1 (4 mg) was dissolved in 100 μl of DMSO and made up to 0.85 ml with the conjugation buffer (Thermo Scientific, Cat#77162). This was mixed with Imject bovine serum albumin (4 mg in 0.65 ml conjugation buffer). To this mixture, 0.165 ml of EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride) (Thermo Scientific, Cat#77149, 1.65 mg) was added, incubated at room temperature (RT) for 2 h and dialyzed overnight at 4°C against PBS.
2.4. Western blotting
Water-soluble protein was prepared from human and mouse lenses by the procedure previously described (Nagaraj et al., 2012). Recombinant human Hsp20 was purchased from (Biotang Inc, MA, Cat# BT-HSP-020), and αA- and αB-crystallin, and Hsp27 were purified as described before (Nagaraj et al., 2012). All proteins or peptides (1 to 30 μg) were resolved on 12% SDS-PAGE and transferred electrophoretically to a nitrocellulose membrane. Membranes were blocked with 5% NFDM (non-fat dry milk, Bio-Rad, Cat#170–6404) in TBST, incubated overnight at 4°C with either the peptain-1 antibody (1:1,000 dilution), or monoclonal αB-crystallin antibody (mouse, Developmental Studies Hybridoma Bank, University of Iowa, diluted 1:500,000) or a polyclonal αB-crystallin antibody (rabbit, Millipore, Billerica, MA, Cat#ABN185, 1:1,000 dilution) and treated with an HRP-conjugated horse-anti-mouse IgG (Cell Signaling, MA, Cat#7076S, 1:5,000 dilution) or HRP conjugated goat-anti-rabbit IgG (Cell Signaling, MA, Cat#7074S, 1:5,000 dilution) for 1 h at RT. Membranes were developed using the SuperSignal West Pico or Femto Kit (Pierce Chemicals, IL, Cat#34580 and Cat#34096). For stripping membranes, we used Restore Western Blot Stripping Buffer (Thermo Scientific, Cat#21059) as per manufacture’s instruction.
2.5. Direct ELISA
The ELISA plate wells were coated with peptain-1 or truncated peptain-1 peptides (5 μg/well) or BSA-conjugated peptain-1 (2 μg/well) in 50 mM sodium carbonate buffer, pH 9.6 overnight at 4°C. The wells were blocked with 5% NFDM-PBST for 2 h at RT in a humidified chamber. The wells were washed with 1X PBST and treated with the peptain-1 antibody (1:50 dilution) for 2 h at 37°C. For the ELISAs in Fig.1E, primary antibodies (peptain-1 or αB-crystallin mouse monoclonal or αB-crystallin rabbit polyclonal) were diluted 1:50 and incubated for 1 h at 37°C. The wells were washed and treated with HRP-conjugated horse-anti-mouse IgG (Cell Signaling, MA, Cat#7076, 1:5,000 dilution) and incubated for 1 h at 37°C. After washing, the wells were treated with 3,3′,5,5′-tetramethylbenzidine substrate (Sigma, Cat# T3405–50TAB) in 50 mM phosphate citrate buffer, pH 5.0, for 45 min at 37°C. The reaction was stopped by the addition of 2N H2SO4, and the absorbance was measured at 450 nm.
Figure 1: The peptain-1 monoclonal antibody reacts with peptain-1 but the two commercial antibodies (against human αB-crystallin) do not.
Peptain-1 and αB-crystallin were subjected to SDS-PAGE on 12% gel and Western blotted. First, we probed with a commercially available mouse monoclonal antibody (A). The membrane was stripped and re-probed with a commercially available rabbit polyclonal antibody (B), the membrane was stripped again and probed with the peptain-1 antibody (C). The gel run in parallel was stained with Coomassie blue (D). M=molecular weight markers, a= peptain-1, b= human αB-crystallin. The solid arrow indicates αB-crystallin and the dotted arrow peptain-1. A direct ELISA against BSA-conjugated peptain-1 was performed to test the immunoreactivity of peptain-1 antibody and commercial antibodies (E). 1= peptain-1 antibody, 2= mouse monoclonal antibody, 3= rabbit polyclonal antibody.
2.6. Competitive ELISA
The ELISA plate wells were coated with BSA-conjugated peptain-1 (2 μg/well) in 50 mM carbonate buffer, pH 9.6, overnight at 4°C. Varying concentrations of peptain-1, αA-crystallin, Hsp20 and Hsp27-derived peptides (0–20 μg) were incubated with the peptain-1 antibody (10 μg) in PBS overnight in a shaker at 4°C. The wells were blocked with 5% NFDM-PBST for 2 h at RT in a humidified chamber. The wells were then washed with 1X PBST and treated with the antibody-peptide mixtures for 2 h at 37°C. The wells were washed and treated with HRP-conjugated horse-anti-mouse IgG (1:5,000 dilution) and incubated for 1 h at 37°C. After washing, wells were treated with 3,3′,5,5′-tetramethylbenzidine substrate in 50 mM phosphate citrate buffer, pH 5.0, for 45 min at 37°C. The reaction was stopped by the addition of 2N H2SO4, and absorbance was measured at 450 nm. The results are expressed as the ratio between B (absorbance in the presence of competitor) divided by B0 (absorbance in the absence of competitor).
2.7. Immunofluorescence and immunohistochemistry
For immunofluorescence, human lens epithelial cells (FHL124 cells from Dr. Michael Wormstone, University of East Anglia, UK) were cultured in chamber slides (Lab-Tek II, NY) and incubated with or without human recombinant TGFβ2 (Peprotech, NJ, Cat#100–35B, 10 ng/ml) for 48 h to stimulate αB-crystallin expression as previously described (Nahomi et al., 2016). Human retinal endothelial cells (HRECs) were isolated and cultured on chamber slides as previously described (Busik et al., 2008). Cells were fixed with 4% paraformaldehyde, permeabilized with ice cold 80% methanol, blocked with 5% goat serum, and treated with peptain-1 antibody (20 μg/ml) in goat serum overnight at 4°C. Cells were then incubated with goat anti-mouse Alexa Fluor 488 conjugated secondary antibody (Life Technologies, Cat#A1100,1:250 dilution) for 1 h at 37°C. For immunohistochemistry, mouse eye sections were deparaffinized and blocked with 5% goat serum. Sections were then treated with antibodies as described above. Negative controls were prepared by omitting the primary antibody. DAPI was used to visualize nuclei, and images were taken in a confocal microscope (ECLIPSE Ti) at 20X magnification.
2.8. Effect of the antibody against the chaperone activity of peptain-1 and αB-crystallin
The chaperone activity of peptain-1 and αB-crystallin was determined using citrate synthase (CS) and alcohol dehydrogenase (ADH). All assays were performed using UV microplate reader (Spectramax 190, Molecular devices, San Jose). For the CS assay, peptain-1 (300 μg/ml) or αB-crystallin (167 μg/ml) was pre-incubated in the absence or presence of either peptain-1 antibody or αB-crystallin mouse monoclonal antibody or αB-crystallin rabbit polyclonal antibody (42 μg/ml in the case of peptain-1 and 54.4 μg/ml in the case of αB-crystallin) at RT for 4 h in 40 mM HEPES buffer, pH 7.4. Aggregation of CS (167 μg/ml) at 43°C was monitored at 400 nm for 1 h in a kinetic mode. For the ADH assay, peptain-1 (500 μg/ml) and αB-crystallin (60 μg/ml) were pre-incubated with or without either peptain-1 antibody or αB-crystallin mouse monoclonal antibody or αB-crystallin rabbit polyclonal antibody (120 μg/ml in the case of peptain-1 and 20 μg/ml in the case of αB-crystallin) for 4 h at RT in PBS. Aggregation of ADH (1 mg/ml in PBS containing 2 mM EDTA) was induced by incubating at 43°C and monitored at 400 nm for 1 h in a kinetic mode. To determine specific effects of the peptain-1 antibody on the chaperone activity, we tested a naïve antibody in the ADH assay. Peptain-1 (500 μg/ml) or αB-crystallin (60 μg/ml) was pre-incubated with the peptain-1 antibody or naïve mouse IgG (Sigma, Cat # I5381) (120 μg/ml in the case of peptain-1 and 20 μg/ml in the case of αB-crystallin) for 4 h at RT in PBS. The ADH aggregation assay was carried out as above. Percent protection by the chaperone was calculated by taking O.D. at 60 min and using the formula:
2.9. Detection of peptain-1 in HeLa cells
HeLa cells were cultured on chamber slides as previously described (Nahomi et al., 2016). Cells were incubated with or without peptain-1 (100 μg/well in 250 μl serum free medium) for 24 h, fixed as described above and permeabilized with 0.1% Triton-X 100 for 5 min at RT. The cells were processed as described above except that in these experiments, a Texas Red conjugated secondary antibody (Life Technologies, Cat #T6390) was used.
2.10. Effect of the antibody against the anti-apoptotic activity of peptain-1
Peptain-1 with or without prior incubation with antibodies was transferred into HeLa cells using PULSin protein delivery reagent (Polyplus-transfection-Bioparc, France, Cat # 501–04) as per manufacturer’s instructions. In brief, peptain-1 (35 or 50 μg) was incubated with or without peptain-1 or αB-crystallin mouse monoclonal or αB-crystallin rabbit polyclonal antibody (all 10 μg) in 150 μl 20mM HEPES buffer pH 7.4 for 16 h at 4°C in a shaker. Next, 7.5 μl of the protein delivery reagent was added and incubated at RT for 15 min. To the mixture, 550 μl of serum free media was added and incubated with HeLa cells in six well plates for 4 h 37°C. The wells were washed once with PBS and then treated with staurosporine (Sigma, Cat # S6942–200UL) at 20 nM for 16 h at 37°C. The wells were then washed once with PBS and the cells were lysed using 1X RIPA buffer (Thermo scientific, Cat # 89900) containing a protease inhibitor cocktail (Sigma, Cat # P8340, 1:100 dilution) and processed for Western blotting. Ten μg protein was used for Western blotting. Cleaved caspase-3 level, an indicator of apoptosis, was determined by Western blotting of cell lysates using a cleaved caspase-3 antibody (Cell Signaling, Cat# 9664S, 1:1000 dilution).
2.11. Statistical analysis
The data were analyzed with GraphPad Prism software (Version 7) by one-way ANOVA and presented as the means ± SD of the specific number of experiments indicated in figure legends. The differences were considered significant at p<0.05.
3. Results
3.1. Production of peptain-1 monoclonal antibody
The culture media from the selected clone (2000 ml) was collected, passed through a 1.5 ml Protein G-Sepharose column, and purified according to the manufacturer’s protocol (GE Healthcare). The isotyping ELISA showed that the purified antibody was of IgG1 subtype.
3.2. Immunoreactivity of the antibodies
First, we tested the immunoreactivity of peptain-1 antibody and the two commercially available antibodies against human αB-crystallin in Western blotting. The commercial mouse monoclonal antibody reacted with human recombinant αB-crystallin but not with peptain-1 (Fig. 1A). Similar results were obtained when we stripped the same membrane and probed with a rabbit polyclonal antibody (Fig. 1B). However, when we probed the same membrane with the peptain-1 antibody, we observed immunoreactivity for both peptain-1 (below 10 kDa) and αB-crystallin (Fig. 1C). SDSPAGE run in parallel showed peptide/protein loading (Fig. 1D). Similar results were obtained in direct ELISAs in which we coated the wells with BSA-conjugated peptain-1 (Fig. 1E).
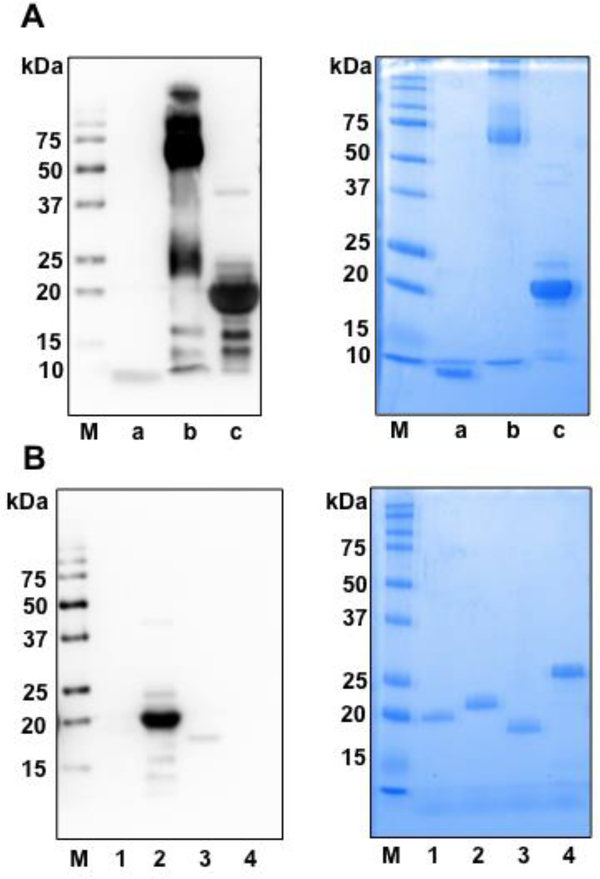
The antibody reacted with BSA-conjugated peptain-1 and recombinant human αB-crystallin (Fig. 2A). BSA-conjugated peptain-1 showed an intense band between 50 to 75 kDa and less intense bands with several high molecular weight proteins, possibly because of crosslinking of BSA during the conjugation of peptain-1 to BSA. Human recombinant αB-crystallin showed a major band, as expected at 20 kDa. The peptain-1 antibody was then tested by Western blotting against other human small heat shock proteins, αA-crystallin, Hsp20 and Hsp27. As seen in Fig. 2B, the antibody strongly reacted with αB-crystallin, weakly with Hsp20, and did not react with either αA-crystallin or Hsp27.
Figure 2: The monoclonal antibody reacts with peptain-1 and human αB-crystallin.
Peptain-1, BSA-conjugated peptain-1 and αB-crystallin were subjected to Western blotting using the peptain-1 antibody (A, left panel). M=molecular weight markers, a= peptain-1, b= BSA-conjugated peptain-1, c= αB-crystallin. Recombinant small heat shock proteins were also tested for peptain-1 immunoreactivity (B, left panel). The gels run in parallel were stained with Coomassie blue (A and B right panels), 1= human αA-crystallin, 2= human αB-crystallin, 3= human Hsp20 and 4= human Hsp27.
3.3. Immunoreactivity of the antibody against peptain-1 and truncated peptain-1 peptides
Peptain-1 used in this study was a 21 amino acid peptide (73DRFSVNLDVKHFSPEELKVKV93). We tested three truncated peptides by direct ELISAs to determine the minimum amino acid sequence required for the antibody recognition. The direct ELISAs results showed that the 11 amino acids at the C-terminus (83HFSPEELKVKV93), 10 amino acids at the N-terminus (73DRFSVNLDVK82) and 10 amino acids in the middle (78NLDVKHFSPE87) all had no immunoreaction (Fig. 3). This suggests that the antibody requires the full-length peptide for immunoreaction. We could not test this by Western blotting since truncated peptides ran as broad bands in SDS-PAGE gel unlike peptain-1.
Figure 3: The antibody reacts only with the full-length peptain-1 but not with truncated peptain-1.
A direct ELISA was performed to test the immunoreactivity of antibody against peptain-1 and truncated peptides. 1= peptain-1, 2= 11 amino acids at the C-terminus (83HFSPEELKVKV93), 3= 10 amino acids at N-terminus (73DRFSVNLDVK82), 4= 10 amino acids in the middle (78NLDVKHFSPE87).
3.4. Immunoreactivity of the antibody against chaperone peptides of small heat shock proteins
We performed competitive ELISAs for peptain-1 and the core domain peptides of αA-crystallin (70KFVIFLDVKHFSPEDLTVK88), Hsp20 (71GHFSVLLDVKHFSPEEIAVK91) and Hsp27 (93DRWRVSLDVNHFAPDELTVK113). For this assay, we incubated various concentrations of the peptides with peptain-1 antibody and used the reaction mixtures in competitive ELISAs. We found peptain-1 to be the strongest competitor for antibody binding (Fig. 4A). Hsp20 peptide also showed strong competition, but it was lower than peptain-1. The Hsp27 peptide showed only weak competition, whereas the αA-crystallin peptide was non-reactive.
Figure 4: Peptain-1 is the preferred chaperone peptide for the antibody binding.
Peptain-1 and the chaperone peptides of other small heat shock proteins were tested by a competitive ELISA (A) and Western blotting (B, left panel) as described in Methods. The gel run in parallel was stained with Coomassie blue (B, right panel). M=molecular weight markers, 1= peptain-1, 2=αA-crystallin, 3=Hsp20, and 4=Hsp27.
Next, we tested the antibody by Western blotting. The antibody strongly reacted with peptain-1, weakly reacted with the peptide of Hsp20 and showed no reaction with the other two peptides (Fig. 4B). The strong immunoreactivity of Hsp20 in the competitive ELISA, but weak reaction by Western blotting, is possibly due to the two different ways the antigen is presented to the antibody in the two assays.
3.5. Detection of αB-crystallin in lens proteins and mouse lenses
We tested the antibody against human and mouse lens proteins by Western blotting, as they contain high levels of αB-crystallin and are expected to react strongly with the antibody. We used water-soluble proteins from three different human lenses of age 18, 30 and 46 years. All three samples reacted with the antibody (Fig. 5A). As the lens age increased, the immunoreactivity in the high molecular weight region increased (between 25 and 200 kDa), which was expected as α-crystallin covalently crosslinks with itself and other proteins with age. We observed reaction against αB-crystallin in three wild-type (WT) mouse lenses but not in αB-crystallin knockout (αB−/−) lenses.
Figure 5: The antibody shows immunoreactivity with αB-crystallin in human lens proteins and with WT but not in αB −/− mouse lenses and lens proteins.
Water-soluble protein from human lenses (age 18, 30 and 46) and from WT and αB−/− mouse lenses were subjected to SDS-PAGE on 12% gel and subjected to Western blotting using peptain-1 antibody (A, top panel). Coomassie staining of the gel after transfer to the nitrocellullose membrane show comparable protein loading (A, bottom panel). Immunohistochemical analyses of the WT mouse lens showed strong immunoreactivity for αB-crystallin (B, green in top left panels, +Ab). Omission of the primary antibody (−Ab) resulted in no immunoreactivity (top right panels). Similar staining was not observed in the lens of αB−/− mouse (bottom panels). The nucleus was stained with DAPI (blue). The images shown are representative from three independent experiments. Ab= antibody, M=molecular weight markers, WT= wild type, αB−/− = αB-crystallin KO. The solid arrow denotes the cortical region and the dotted arrow the nuclear region of the lens. The scale bar =100 μm.
We then tested the antibody by immunohistochemistry in WT and αB-crystallin knockout (KO) mouse lenses. We observed that the antibody showed immunoreaction in WT lens but not in KO lens (Fig. 5B). Negative control, where we omitted the primary antibody, showed no immunoreaction in WT lenses. In mouse lenses, the immunoreactivity was seen predominantly in the outer cortex but not the nucleus. This could be due to inaccessibility of the epitope for the antibody or the inability of the antibody to react with the post-translationally modified αB-crystallin. Nonetheless, these data confirm high specificity of the antibody toward αB-crystallin in lenses.
3.6. Immunoreaction of the antibody in cultured cells
We have previously shown that αB-crystallin is highly upregulated in FHL124 cells upon treatment with 10 ng/ml of TGF-β2 for 48 h (Nahomi et al., 2016). The antibody showed immunoreactivity against αB-crystallin (observed in cytoplasm and nucleus) in the TGF-β2 treated cells (Fig. 6A). HRECs inherently express αB-crystallin, which was evident by the immunoreaction throughout cells (Fig. 6B). In negative controls, where the primary antibody was omitted, immunoreaction was absent in both FHL124 and HRECs.
Figure 6: Peptain-1 antibody reacts with αB-crystallin in cultured cells.
FHL124 cells were seeded on chamber slides and treated with TGF-β2 (to upregulate αB-crystallin) as described in Methods. Immunoreactivity for αB-crystallin (green) was observed in TGFβ2-treated cells (A, bottom left panel, +Ab). No significant immunoreactivity was observed in untreated control cells (A, top left panels). αB-crystallin was detected in HREC (B). Cell nuclei were stained with DAPI (blue). Immunoreactivity was not observed in cells in which the primary antibody was omitted (−Ab in A and B). The images shown are representative from two independent experiments. Ab= antibody. The scale bar =100 μm.
A previous study showed that peptain-1, when incubated with cultured cells, enters cells (Sreekumar et al., 2013). Furthermore, our previous study also showed cellular effects of peptain-1 when incubated with lens epithelial cells, possibly because of its entry into cells (Nahomi et al., 2013b). We detected peptain-1 in HeLa cells treated with peptain-1 for 24 h (Fig. 7B), which was mainly in the cytoplasm. Cells not treated with peptain-1 (Fig. 7A) or cells in which the primary antibody was omitted (Fig. 7A and B, right panels) did not show immunoreaction.
Figure 7: The antibody detects peptain-1 in HeLa cells.
HeLa cells were treated with or without peptain-1 as described in Methods. Peptain-1 immunoreaction (red) was observed only in treated cells (B, +Ab) but not in untreated control cells (A, +Ab). Immunoreactions were absent where the primary antibody was omitted (−Ab, in A and B). Cell nuclei were stained with DAPI (blue). Ab= antibody. The scale bar =100 μm.
3.7. Inhibition of the chaperone activity of peptain-1 and αB-crystallin by the peptain-1 antibody
The antibody’s ability to block the chaperone activity of peptain-1 was tested in chaperone assays. We used chemical aggregation of CS (Fig. 8A, B, E and F), thermal aggregation of ADH (Fig. 8C, D, G and H). Peptain-1 alone inhibited the aggregation of CS and ADH up to 61.3% and 69.4%, respectively (Fig. 8A and C). We noticed that the antibodies alone had some protection against client protein aggregation. This was considered when calculating percent protection, as mentioned in Methods (Section 2.8). However, when pre-incubated with the peptain-1 antibody, peptain-1 was able to protect aggregation only by 6.2% and 16.5%, respectively. Thus, the peptain-1 antibody treatment resulted almost complete loss in the chaperone activity of peptain-1. The results of Western blotting (Fig. 1B) and ELISA (Fig. 1E) indicated that the polyclonal antibody did not bind to peptain-1 and therefore the inhibition of the chaperone activity of peptain-1 by the polyclonal antibody in the ADH aggregation assay (Fig. 8C) (and not in the CS aggregation assay) is puzzling and needs further investigation. To determine whether the antibody blocks the chaperone activity of αB-crystallin as well, we tested the antibody in chaperone assays using CS (Fig. 8B and F) and ADH (Fig. 8D and H) as client proteins. In the absence of the antibody, αB-crystallin protected the aggregation of CS and ADH by 58.4% and 62.5%, respectively (Fig. 8B and D). However, in the presence of the antibody, while the protection of ADH was reduced by 16%, the aggregation of CS remained unaltered. The reason for this is not known, a possibility is that αB-crystallin binds to CS through other chaperone sites (in addition to peptain-1) and inhibits CS aggregation in the presence of the peptain-1 antibody. To check the specificity of the antibody towards peptain-1 or αB-crystallin in these assays, we used the commercial monoclonal antibody against αB crystallin and a polyclonal antibody against αB crystallin. All chaperone assays were performed using these two antibodies at the same concentration as the peptain-1 antibody. In contrast to the peptain-1 antibody, the other two antibodies did not block the chaperone activity of either peptain-1 or αB-crystallin, except in the case of polyclonal antibody, which blocked the chaperone activity of peptain-1 in the ADH aggregation assay (Fig. 8C and G).
Figure 8: Antibody inhibits the chaperone activity of peptain-1 and αB-crystallin.
The chaperone activity of peptain-1 and αB-crystallin was assayed in the presence or absence of the antibody using CS (A and B) or ADH (C and D). 1= + peptain-1 (in A and C) or αB-crystallin (in B and D), 2= + peptain-1 (in A and C) or αB-crystallin (in B and D) prior treated with mouse monoclonal antibody, 3= + peptain-1 (in A and C) or αB-crystallin (in B and D) prior treated with rabbit polyclonal antibody, 4= + peptain-1 (in A and C) or αB-crystallin (in B and D) prior treated with peptain-1 antibody. The bar graphs represent the means ± SD of triplicate measurements. NS= Not significant, *p<0.05, ***p< 0.0005. Aggregation profiles of CS at 43°C in the presence or absence of peptain-1 and αB- crystallin and chemically-induced aggregation of ADH at 43°C are shown in panels E, F, G and H, respectively. Ab= antibody. The monoclonal antibody refers to the monoclonal antibody to αB-crystallin from University of Iowa Hybridoma Bank and the polyclonal antibody refers to the rabbit polyclonal antibody from Millipore.
To confirm specificity of the peptain-1 antibody against the chaperone activity of peptain-1 and αB-crystallin, we compared it with a naïve antibody in the ADH aggregation assay. Pepatin-1 blocked the aggregation of ADH by 53.5%, but when pre-incubated with the antibody, this protective ability was reduced to 13% (Fig. 9A and B). Such a loss in the chaperone activity was not observed with the naïve antibody. αB-Crystallin prevented the aggregation of ADH by 77.6%, but in the presence of peptain-1 antibody, it was reduced to 68.2%. Such a reduction was not observed with the naïve antibody (Fig. 9C and D). The absence of a complete inhibition of the chaperone activity by the peptain-1 antibody could be due to lower amounts of the antibody compared to chaperones. Nonetheless, these data suggest that the peptain-1 antibody binds to the core domain of αB-crystallin and inhibits its chaperone activity.
Figure 9: The peptain-1 antibody but not a naïve antibody is able to inhibit the chaperone activity of αB-crystallin.
The chaperone activity of peptain-1 and αB-crystallin was assessed in the ADH aggregation assay. The aggregation profiles of ADH with peptain-1 (A) and αB-crystallin (C) in the presence or absence of peptain-1 antibody or naïve mouse IgG are shown. Percent protection corresponding to panels A and C are shown in panels B and D. The bar graphs represent the means ± SD of triplicate measurements. 1= peptain-1 Ab (in B and D), 2= naïve Ab (in B and D), 3= peptain-1 (in B) or αB-crystallin (in D), 4= peptain-1 + peptain-1 Ab (in B) or αB-crystallin + peptain-1 Ab (in D), 5= peptain-1 + naïve Ab (in B) or αB-crystallin + naïve Ab (in D). Ab, antibody. NS, Not significant, **p< 0.005 and ****p< 0.0001.
3.8. Inhibition of the anti-apoptotic activity of peptain-1 by the antibody
Finally we tested the ability of the antibody to block the anti-apoptotic function of peptain-1 during staurosporine induced apoptosis in HeLa cells. Caspase-3 activation is an indicator of apoptosis in cells (Porter and Janicke, 1999). We checked the levels of cleaved caspase-3 (active caspase-3) by Western blotting. We found a large increase in cleaved caspase-3 levels in cells treated with staurosporine and that was significantly reduced by peptain-1 treatment, the inhibition with 50 μg (53%, Fig. S2) was 33% greater than 35 μg of peptain-1 (20%, Fig. 10). This effect of peptain-1 was inhibited by prior incubation with the peptain-1 antibody (26% and 100% against 50 μg and 35 μg peptain-1). In contrast, the two commercial antibodies did not inhibit the anti-apoptotic activity of peptain-1. However, we cannot completely rule out the loss of inhibitory effect of peptain-1 due to lower levels of transduction in peptain-1 + antibody transduced cells. We could not test the effect of antibodies on the anti-apoptotic activity of αB-crystallin in this experimental set up because of the difficulty in delivering two proteins simultaneously into cells.
Figure 10: Peptain-1 antibody inhibits the anti-apoptotic function of peptain-1.
HeLa cells were transduced with either peptain-1 alone (35 μg) or peptain-1 incubated with antibody using PULSin protein delivery reagent as described in Methods. Cells were then treated with 20 nM staurosporine for 16 h to induce apoptosis. Treatment with peptain-1 reduced the activation of caspase-3, which was not observed in cells treated with peptain-1 incubated with the peptain-1 antibody. The commercial antibodies did not inhibit the anti-apoptotic activity of peptain-1. Representative Western blot for cleaved caspase-3 (Clvd.Casp-3) is shown, β-actin was used as a loading control. The bar graphs are densitometric plots for the Clvd.Casp-3 levels and are means ± SD of triplicate measurements. 1= Control, 2= staurosporine alone, 3= staurosporine + peptain-1, 4= staurosporine + peptain-1 prior treated with peptain-1 Ab, 5= staurosporine + peptain-1 prior treated with a mouse monoclonal Ab, 6= staurosporine + peptain-1 prior treated with rabbit polyclonal Ab. Ab, antibody, NS= Not significant, *p<0.05, **p< 0.005 and ****p< 0.0001.
4. Discussion
The purpose of this study was to develop a monoclonal antibody against peptain-1, which could be used to block the chaperone and anti-apoptotic activities, two major functional attributes of αB-crystallin. The backdrop for this idea was that αB-crystallin is highly elevated in several diseases or disease processes, and its inhibition would be beneficial to inhibit disease or disease progression. For example, αB-crystallin is highly expressed in triple negative basal-type breast cancers and during metastasis into the brain (Kim et al., 2011; Malin et al., 2014; Voduc et al., 2015). In fact, its higher expression levels are directly related to poor prognosis in breast cancer. In addition, αB-crystallin is highly expressed and obligatory for fibrosis in lungs, retina and lens (Bellaye et al., 2015; Ishikawa et al., 2016; Nahomi et al., 2016). It is also involved in retinal angiogenesis (Kase et al., 2010).
αB-Crystallin is a cytosolic protein, but studies have shown that αB-crystallin can be exported from cells through exosome as a full-length protein (Sreekumar et al., 2010; Gangalum et al., 2011). Whether exosome-encapsulated protein is released extracellularly is not known; it is likely that exosomes are taken up by neighboring cells and that their contents are released inside those cells. Many investigators have studied whether extracellular αB-crystallin has any cellular effect. One study showed that exogenous αB-crystallin protects brain astrocytes from staurosporine and C2-ceramide-induced cell death and inhibits reactive oxygen species (ROS) generation in brain mitochondria (Arac et al., 2011; Zhu et al., 2015) and another recent study showed suppression of inflammatory response in astrocytes and microglia by exogenous αB-crystallin (Guo et al., 2019). Several studies have shown effects of exogenous αB-crystallin on retinal ganglion cells and have convincingly shown that intravitreally injected protein protects retinal ganglion cells against ischemia/reperfusion, inflammation and oxidative stress-mediated apoptosis and suggested that αB-crystallin could be used as a therapeutic protein in glaucoma (Fort and Lampi, 2011; Yan et al., 2017). In addition, work from Steinman’s group has shown that plasma levels of αB-crystallin are increased in patients with multiple sclerosis when compared normal individuals (Rothbard et al., 2012) and αB-crystallin administered intravenously can inhibit neuronal damage in experimental models for stroke and autoimmune encephalomyelitis (Arac et al., 2011; Rothbard et al., 2012). Taken together these observations point to cellular effects of exogenous αB-crystallin, possibly through its interaction with cell membrane proteins (receptors). Thus, it is conceivable that in diseases where αB-crystallin exhibits promotional activity, its blockade could be beneficial, and in such conditions peptain-1 antibody might find use.
Sharma et al. first showed that peptain-1 is a molecular chaperone, and their study together with others has shown that αB-crystallin can inhibit protein aggregation caused by various stresses (Sharma et al., 2000; Bhattacharyya et al., 2006). Our study showed that peptain-1, when injected intraperitoneally in rats, could inhibit selenite-induced cataracts by blocking protein insolubilization and lens epithelial cell apoptosis (Nahomi et al., 2013b). Steinman’s group and Hinton’s group have shown that peptain-1 or αB-crystallin can inhibit cellular death in animals (Kurnellas et al., 2012; Zhou et al., 2014). Given these results, it can be inferred that peptain-1 enters cells on its own to bring about its effects. In fact, Sreekumar et al. have shown that peptain-1 is transported into cells through an amino acid transporter (Sreekumar et al., 2013). The ability of exogenous peptain-1 to bring about cellular effects further underscores the importance of methods to block αB-crystallin activities where it participates in promotion of disease.
Peptain-1 antibody recognized αB-crystallin, but not αA-crystallin in lens proteins. The antibody only showed weak reaction with Hsp20. This could be due to subtle differences in amino acid sequence in the region corresponding to peptain-1 in small heat shock proteins. The ability of the antibody to inhibit the chaperone activity of αB-crystallin clearly suggests that the antibody binds to the peptain-1 sequence within αB-crystallin during such inhibition. Given that the chaperone and anti-apoptotic activities are interrelated in α-crystallin (Pasupuleti et al., 2010; Nahomi et al., 2013a), it can be assumed that peptain-1 antibody would block the anti-apoptotic activity of αB-crystallin as well. Further work is needed to verify this possibility. The antibody might find use in detection and quantification of peptain-1 in experiments where peptain-1 is injected in experimental animals or treated in cells to determine its effects. If the perceived beneficial effects of peptain-1 antibody come to fruition, a humanized version of the antibody could find an application in clinical settings.
Supplementary Material
Highlights.
A monoclonal antibody was developed against a major chaperone peptide (peptain-1) of αB-crystallin.
The monoclonal antibody reacted with peptain-1 but commercial antibodies against αB-crystallin did not.
The antibody reacted strongly with αB-crystallin but not with αA-crystallin or Hsp27.
The antibody inhibited the chaperone and anti-apoptotic activities of peptain-1 and the chaperone activity of αB-crystallin.
Acknowledgments
We thank Dr. Micheal Wormstone for providing FHL124 cells (originally from Prof. John Reddan, Oakland University, MI), Michael DiMauro for helping with immunization of animals and Drs. Johanna Rankenberg and Mi-hyun Nam for critical reading of the manuscript. We thank the staff at the Protein Production/MoAB/Tissue Culture Core Facility, University of Colorado Cancer Center, Anschutz Medical campus, for their help with the production of monoclonal antibody.
Funding
This work was supported by the National Institutes of Health Grants EY022061 and EY028836 and a challenge grant by Research to Prevent Blindness, NY to the Department of Ophthalmology, University of Colorado School of Medicine.
Abbreviations
- Peptain-1
αB-crystallin peptide
- ELISA
enzyme-linked immunosorbent assay
- EDC
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride
- DMSO
dimethyl sulfoxide
- RT
room temperature
- TBST
Tris-buffered saline – Tween 20
- HRP
horseradish peroxidase
- ROS
reactive oxygen species
- DAPI
2-(4-amidinophenyl)-1H-indole-6-carboxamidine
- HRECs
human retinal endothelial cells
- ADH
alcohol dehydrogenase
- CS
citrate synthase
- Hsp20
human small heat shock protein 20
- Hsp27
human small heat shock protein 27
- HEPES
(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)
- Ab
antibody
- IgG
Immunoglobulin G
- PBS
Phosphate-buffered saline
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andley UP, Song Z, Wawrousek EF, Fleming TP and Bassnett S, 2000, Differential protective activity of alpha A- and alphaB-crystallin in lens epithelial cells. J Biol Chem 275, 36823–31. [DOI] [PubMed] [Google Scholar]
- Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L and Steinberg GK, 2011, Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc Natl Acad Sci U S A 108, 13287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP, 2013, Human small heat shock proteins: protein interactomes of homo- and hetero-oligomeric complexes: an update. FEBS Lett 587, 1959–69. [DOI] [PubMed] [Google Scholar]
- Bellaye PS, Burgy O, Colas J, Fabre A, Marchal-Somme J, Crestani B, Kolb M, Camus P, Garrido C and Bonniaud P, 2015, Antifibrotic role of alphaB-crystallin inhibition in pleural and subpleural fibrosis. Am J Respir Cell Mol Biol 52, 244–52. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya J, Padmanabha Udupa EG, Wang J and Sharma KK, 2006, Mini-alphaB-crystallin: a functional element of alphaB-crystallin with chaperone-like activity. Biochemistry 45, 3069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burut DF, Borai A, Livingstone C and Ferns G, 2010, Serum heat shock protein 27 antigen and antibody levels appear to be related to the macrovascular complications associated with insulin resistance: a pilot study. Cell Stress Chaperones 15, 379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busik JV, Mohr S and Grant MB, 2008, Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes 57, 1952–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ce P, Erkizan O and Gedizlioglu M, 2011, Elevated HSP27 levels during attacks in patients with multiple sclerosis. Acta Neurol Scand 124, 317–20. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ruan Q, Han S, Xi L, Jiang W, Jiang H, Ostrov DA and Cai J, 2014, Discovery of structure-based small molecular inhibitor of alphaB-crystallin against basal-like/triple-negative breast cancer development in vitro and in vivo. Breast Cancer Res Treat 145, 45–59. [DOI] [PubMed] [Google Scholar]
- Christopher KL, Pedler MG, Shieh B, Ammar DA, Petrash JM and Mueller NH, 2014, Alpha-crystallin-mediated protection of lens cells against heat and oxidative stress-induced cell death. Biochim Biophys Acta 1843, 309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou G, Sreekumar PG, Spee C, He S, Ryan SJ, Kannan R and Hinton DR, 2012, Deficiency of alphaB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic Biol Med 53, 1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort PE and Lampi KJ, 2011, New focus on alpha-crystallins in retinal neurodegenerative diseases. Exp Eye Res 92, 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangalum RK, Atanasov IC, Zhou ZH and Bhat SP, 2011, AlphaB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J Biol Chem 286, 3261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JG, Houck SA and Clark JI, 2007, Interactive sequences in the stress protein and molecular chaperone human alphaB crystallin recognize and modulate the assembly of filaments. Int J Biochem Cell Biol 39, 1804–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YS, Liang PZ, Lu SZ, Chen R, Yin YQ and Zhou JW, 2019, Extracellular alphaB-crystallin modulates the inflammatory responses. Biochem Biophys Res Commun 508, 282–288. [DOI] [PubMed] [Google Scholar]
- Huang Q, Ye J, Huang Q, Chen W, Wang L, Lin W, Lin J and Lin X, 2010, Heat shock protein 27 is over-expressed in tumor tissues and increased in sera of patients with gastric adenocarcinoma. Clin Chem Lab Med 48, 263–9. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Sreekumar PG, Spee C, Nazari H, Zhu D, Kannan R and Hinton DR, 2016, alphaB-Crystallin Regulates Subretinal Fibrosis by Modulation of Epithelial-Mesenchymal Transition. Am J Pathol 186, 859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JA and de Jong WW, 2003, The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1–10. Cell Stress Chaperones 8, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase S, He S, Sonoda S, Kitamura M, Spee C, Wawrousek E, Ryan SJ, Kannan R and Hinton DR, 2010, alphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood 115, 3398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Lee Y, Lim YA, Kang HJ and Kim LS, 2011, alphaB-Crystallin is a Novel Oncoprotein Associated with Poor Prognosis in Breast Cancer. J Breast Cancer 14, 14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopstein A, Santos-Nogueira E, Francos-Quijorna I, Redensek A, David S, Navarro X and Lopez-Vales R, 2012, Beneficial effects of alphaB-crystallin in spinal cord contusion injury. J Neurosci 32, 14478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletsa T, Stavridi F, Bobos M, Kostopoulos I, Kotoula V, Eleftheraki AG, Konstantopoulou I, Papadimitriou C, Batistatou A, Gogas H, Koutras A, Skarlos DV, Pentheroudakis G, Efstratiou I, Pectasides D and Fountzilas G, 2014, alphaB-crystallin is a marker of aggressive breast cancer behavior but does not independently predict for patient outcome: a combined analysis of two randomized studies. BMC Clin Pathol 14, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnellas MP, Brownell SE, Su L, Malkovskiy AV, Rajadas J, Dolganov G, Chopra S, Schoolnik GK, Sobel RA, Webster J, Ousman SS, Becker RA, Steinman L and Rothbard JB, 2012, Chaperone activity of small heat shock proteins underlies therapeutic efficacy in experimental autoimmune encephalomyelitis. J Biol Chem 287, 36423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WC, Wu MS, Wang HP, Tien YW and Lin JT, 2009, Serum heat shock protein 27 is increased in chronic pancreatitis and pancreatic carcinoma. Pancreas 38, 422–6. [DOI] [PubMed] [Google Scholar]
- Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Lui L, Huang XQ, Liu Y and Li DW, 2004, Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res 79, 393–403. [PubMed] [Google Scholar]
- Mailankot M, Padmanabha S, Pasupuleti N, Major D, Howell S and Nagaraj RH, 2009, Glyoxalase I activity and immunoreactivity in the aging human lens. Biogerontology 10, 711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin D, Strekalova E, Petrovic V, Deal AM, Al Ahmad A, Adamo B, Miller CR, Ugolkov A, Livasy C, Fritchie K, Hamilton E, Blackwell K, Geradts J, Ewend M, Carey L, Shusta EV, Anders CK and Cryns VL, 2014, alphaB-crystallin: a novel regulator of breast cancer metastasis to the brain. Clin Cancer Res 20, 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj RH, Nahomi RB, Shanthakumar S, Linetsky M, Padmanabha S, Pasupuleti N, Wang B, Santhoshkumar P, Panda AK and Biswas A, 2012, Acetylation of alphaA-crystallin in the human lens: effects on structure and chaperone function. Biochim Biophys Acta 1822, 120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahomi RB, DiMauro MA, Wang B and Nagaraj RH, 2015, Identification of peptides in human Hsp20 and Hsp27 that possess molecular chaperone and anti-apoptotic activities. Biochem J 465, 115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahomi RB, Huang R, Nandi SK, Wang B, Padmanabha S, Santhoshkumar P, Filipek S, Biswas A and Nagaraj RH, 2013a, Acetylation of lysine 92 improves the chaperone and anti-apoptotic activities of human alphaB-crystallin. Biochemistry 52, 8126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahomi RB, Pantcheva MB and Nagaraj RH, 2016, alphaB-crystallin is essential for the TGF-beta2-mediated epithelial to mesenchymal transition of lens epithelial cells. Biochem J 473, 1455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahomi RB, Wang B, Raghavan CT, Voss O, Doseff AI, Santhoshkumar P and Nagaraj RH, 2013b, Chaperone peptides of alpha-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J Biol Chem 288, 13022–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam MH and Nagaraj RH, 2018, Matrix-bound AGEs enhance TGFbeta2-mediated mesenchymal transition of lens epithelial cells via the noncanonical pathway: implications for secondary cataract formation. Biochem J 475, 1427–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupuleti N, Matsuyama S, Voss O, Doseff AI, Song K, Danielpour D and Nagaraj RH, 2010, The anti-apoptotic function of human alphaA-crystallin is directly related to its chaperone activity. Cell Death Dis 1, e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AG and Janicke RU, 1999, Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6, 99–104. [DOI] [PubMed] [Google Scholar]
- Rothbard JB, Kurnellas MP, Brownell S, Adams CM, Su L, Axtell RC, Chen R, Fathman CG, Robinson WH and Steinman L, 2012, Therapeutic effects of systemic administration of chaperone alphaB-crystallin associated with binding proinflammatory plasma proteins. J Biol Chem 287, 9708–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KK, Kumar RS, Kumar GS and Quinn PT, 2000, Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. J Biol Chem 275, 3767–71. [DOI] [PubMed] [Google Scholar]
- Shi C, He Z, Hou N, Ni Y, Xiong L and Chen P, 2014, Alpha B-crystallin correlates with poor survival in colorectal cancer. Int J Clin Exp Pathol 7, 6056–63. [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Chothe P, Sharma KK, Baid R, Kompella U, Spee C, Kannan N, Manh C, Ryan SJ, Ganapathy V, Kannan R and Hinton DR, 2013, Antiapoptotic properties of alpha-crystallin-derived peptide chaperones and characterization of their uptake transporters in human RPE cells. Invest Ophthalmol Vis Sci 54, 2787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ and Hinton DR, 2010, alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One 5, e12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Lv Y, Chen H, Adam A, Cheng Y, Hartung J and Bao E, 2014, Comparative analysis of alphaB-crystallin expression in heat-stressed myocardial cells in vivo and in vitro. PLoS One 9, e86937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voduc KD, Nielsen TO, Perou CM, Harrell JC, Fan C, Kennecke H, Minn AJ, Cryns VL and Cheang MCU, 2015, alphaB-crystallin Expression in Breast Cancer is Associated with Brain Metastasis. NPJ Breast Cancer 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Wang L and Hou S, 2012, Alpha B-crystallin improved survival of retinal ganglion cells in a rat model of acute ocular hypertension. Neural Regen Res 7, 1493–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Peng Y, Huang W, Gong L and Li L, 2017, The Protective Effects of alphaB-Crystallin on Ischemia-Reperfusion Injury in the Rat Retina. J Ophthalmol 2017, 7205408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying X, Zhang J, Wang Y, Wu N, Wang Y and Yew DT, 2008, Alpha-crystallin protected axons from optic nerve degeneration after crushing in rats. J Mol Neurosci 35, 253–8. [DOI] [PubMed] [Google Scholar]
- Zhou P, Kannan R, Spee C, Sreekumar PG, Dou G and Hinton DR, 2014, Protection of retina by alphaB crystallin in sodium iodate induced retinal degeneration. PLoS One 9, e98275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Li R, Stricker R and Reiser G, 2015, Extracellular alpha-crystallin protects astrocytes from cell death through activation of MAPK, PI3K/Akt signaling pathway and blockade of ROS release from mitochondria. Brain Res 1620, 17–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.