Abstract
Background:
Smoking during pregnancy is a serious public health problem in need of better treatments. Nicotine replacement treatment (NRT) (patch or gum) has not been shown in randomized placebo-controlled trials to be efficacious for smoking cessation during pregnancy. However, the nicotine inhaler may have advantages over other NRTs as it replicates some of the sensory effects of smoking.
Objective:
The purpose of the study was examine the efficacy and safety of the nicotine inhaler for smoking cessation during pregnancy. We hypothesized that the nicotine inhaler compared to placebo would increase quit rates and reduce smoking during treatment and at the end of pregnancy, result in a higher birth weight and gestational age in the offspring, and reduce the incidence of preterm birth and low birth weight infants.
Study Design:
We conducted a randomized, double-blind, placebo-controlled trial of the nicotine inhaler for smoking cessation during pregnancy. Pregnant women who smoked ≥5 cigarettes daily received behavioral counseling and random assignment to a 6-week treatment with nicotine or placebo inhaler, followed by a 6-week taper period. Throughout treatment, we assessed tobacco exposure biomarkers, cessation rates, and adverse events. We also obtained information on birth outcomes. The primary outcome was smoking cessation at 32–34 weeks gestation; secondary outcomes were smoking reduction, birth weight and gestational age, and the incidence of preterm birth or low birth weight infants. We compared treatment groups on these measures using t-tests, Fisher’s exact tests, and multivariate linear and logistic regression.
Results:
Participants in the placebo (n=67) and nicotine (n=70) groups were comparable on baseline characteristics, though women in the placebo group reported a higher motivation to quit (p=0.016). Biochemically-validated smoking cessation rates were similar with nicotine and placebo (after 6 weeks of treatment: 4% (3/70) vs. 3% (2/67), respectively, p< 0.99, and at 32–34 weeks gestation: 10% (7/70) vs. 18% (12/67), respectively, p=0.220). Cigarettes per day (CPD) decreased over time in both groups (p< 0.001), with the nicotine inhaler group having a greater decrease than the placebo group two (p=0.022) and six weeks after the quit date (p=0.042), but not at 32–34 weeks gestation (p=0.108). Serum cotinine levels, birth weight, gestational age and reductions in carbon monoxide did not differ by group. However, the incidence of preterm delivery was higher in the placebo than the nicotine group: 15% (10/67) vs. 4% (3/67), respectively, p=0.030). The incidence of delivering a low birth weight infant was also higher in the placebo than the nicotine group: 15% (10/67) vs. 6% (4/67), respectively, p=0.035, but not after adjusting for preterm delivery p=0.268.
Conclusions:
Although the nicotine inhaler group did not have a higher quit rate during pregnancy than the placebo group, the outcome of preterm delivery occurred less frequently in the nicotine group.
Keywords: nicotine inhaler, pregnancy, preterm delivery, smoking cessation
Introduction:
Cigarette smoking during pregnancy increases the risk of delivering a low birth weight (<2500 grams) or premature infant (<37 weeks gestation)1 and increases perinatal mortality and other health risks.2 Most pregnant women who smoke prior to becoming pregnant continue to smoke during pregnancy.3 Moreover, the majority of pregnant women do not quit smoking with behavioral interventions alone.4,5 Consequently, a need exists to examine the safety and efficacy of pharmacotherapy for smoking cessation during pregnancy. Nicotine replacement therapies (NRTs) approximately double quit rates relative to placebo in studies of non-pregnant individuals.6 However, to date, placebo-controlled NRT trials have not shown efficacy for smoking cessation during pregnancy.7
We examined the utility of the nicotine inhaler for smoking cessation during pregnancy based on favorable data using nicotine gum for smoking cessation during pregnancy (which has pharmacokinetics and overall nicotine exposure similar to those of the inhaler): namely, it reduced tobacco exposure and increased birth weight and gestational age compared to placebo gum.8 Moreover, the nicotine inhaler has never been examined as a treatment for smoking in pregnancy, and is more efficacious than the gum in non-pregnant smokers.6 The inhaler also provides some of the sensory and ritualized components of smoking (i.e., handling and oral inhalation)9 which may be particularly important for female smokers10,11 and was a preferred treatment among smokers who sampled different intermittent NRT products.12 Indeed, the nicotine patch is not as efficacious in non-pregnant women compared to men13 consistent with the finding that women may benefit from sensory components to quit. Thus, we hypothesized that the nicotine inhaler could improve cessation rates during pregnancy.
We conducted a randomized, double blind, placebo-controlled clinical trial of the efficacy and safety of the nicotine inhaler during pregnancy. Because smoking cessation during pregnancy optimizes maternal and perinatal outcomes2 the primary objective of the study was to determine the effect of the nicotine inhaler during pregnancy on smoking cessation rate at the end of pregnancy. Secondary objectives were to determine the effect of the intervention on birth weight, gestational age, and the incidence of low birth weight and preterm infants. Because smoking reduction during pregnancy is also beneficial,14 we also examined the impact of the intervention on reducing smoking during pregnancy.
Materials and Methods:
The primary outcome for the study was biochemically-confirmed, 7-day point prevalence abstinence rates at the end of pregnancy. Other endpoints included abstinence rates during treatment, smoking reduction, birth weight, and gestational age (absolute values and the percentage of subjects with low birth weight or preterm delivery).
Institutional Review Boards at UConn Health (Farmington, CT), Hartford Hospital (Hartford, CT), and Baystate Medical Center (Springfield, MA) approved the study. The study was conducted under an Investigational New Drug Application by the U.S. Food and Drug Administration (IND # 104914) and was registered on Clinicaltrials.gov (). Pfizer Pharmaceuticals donated the nicotine and placebo inhalers.
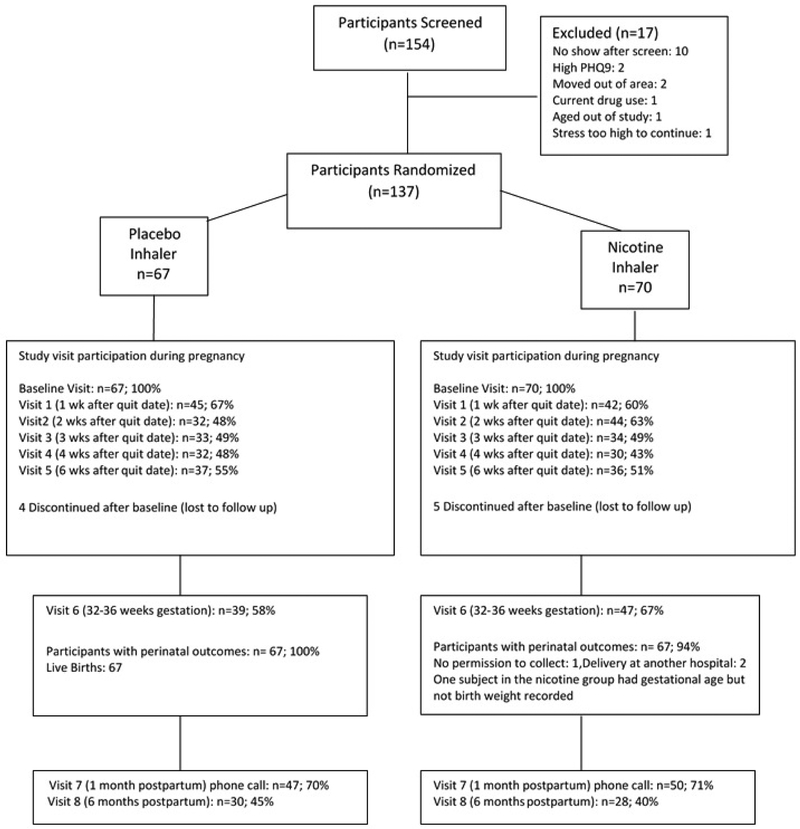
The protocol consisted of nine in-person visits and a phone call one month after delivery (Figure 1). At screening, we obtained written informed consent from participants (available in English or Spanish), and parental consent and participant assent from minors enrolled in Connecticut. We also obtained a medical and obstetrical history, background characteristics, smoking history, a score on the Fagerstrom Test for Cigarette Dependence (FTCD,- a measure of nicotine dependence for cigarette smokers ),15 and the PRIME-MD Health Questionnaire, used to diagnose the most common psychiatric disorders in primary care.16 Pregnant women were eligible if they were: a) smoking, at least 5 cigarettes per day (CPD); b) 13–26 weeks gestation; c) ≥16 years of age; d) able to speak English or Spanish; e) intending to carry their pregnancy to term; and f) living in a stable residence. Exclusion criteria were: a) current drug abuse or dependence by self report (other than methadone maintenance); b) twins or other multiple gestation; c) an unstable psychiatric (PRIME MD questionnaire indicating that a participant was suicidal, having significant depressive symptoms, or psychosis), or medical problem; and d) a congenital abnormality. If the participant met study criteria, the research pharmacy used an urn randomization procedure to balance subject assignment to the two treatment groups (nicotine or placebo inhaler) for each study site on the following variables: gestational age, history of preterm delivery and average number of cigarettes smoked per day (< 10 vs. ≥ 10). All study personnel and participants were blinded to treatment assignment.
Figure 1:
CONSORT diagram of the flow of participants through the study.
At the next two visits (baseline and one week after the quit date) participants received 35 minutes of individual smoking cessation counseling by a study nurse who was trained to deliver the counseling using a motivational interviewing approach.17 At the baseline visit we asked participants to pick a quit date (a date that they would completely stop smoking beginning at 12 am) sometime within the next week and to start using the inhaler on that date. We also provided written educational materials on smoking cessation during pregnancy and the package insert for the nicotine inhaler.
Study Medication:
As outlined in the package insert18 “ NICOTROL® Inhaler (nicotine inhalation system) consists of a mouthpiece and a plastic cartridge delivering 4 mg of nicotine from a porous plug containing 10 mg nicotine.” Nicotine is the active ingredient; inactive components of the product are menthol and a porous plug. Nicotine is released when air is inhaled through the inhaler.
The study nurse provided participants with instructions on how to use the nicotine inhaler based on the package insert, i.e., to puff on the inhaler 3 to 4 times per minute, for up to 20 minutes and to inhale deeply in short breaths as they would normally smoke a cigarette. They were also instructed on the number of inhalers to use per day (see below), based on their level of smoking. Nurses dispensed the inhaler (nicotine 10 mg or placebo) at baseline and at visits 2 and 4 to provide participants with a 6 week treatment of study medication. Participants were encouraged to continue use of the inhaler as long as they were actively trying to quit smoking. The inhaler was also dispensed at visit 6 for an additional 6 weeks only if the participant was abstinent from smoking, to avoid continued exposure to a medication that may not be beneficial for smoking cessation during pregnancy.
Inhalers were packaged in the same device to maintain the integrity of the blinded assignment. Participants who smoked ≥10 cigarettes per day (CPD) were instructed to begin with 4–12 cartridge inhalers per day, similar to other inhaler studies that have recommended a minimum of two,19 four,9 or six inhalers daily.20 Women who smoked 5–9 CPD were instructed to begin by using 1–4 cartridge inhalers per day, based on an estimated 1–2 mg of nicotine delivery per cigarette, with each cartridge inhaler estimated to release 4 mg of nicotine.20
As a safety measure, samples for cotinine (the major metabolite of nicotine and a measure of overall nicotine intake) were obtained at baseline and visit 2 and analyzed within two weeks to identify participants whose nicotine exposure was increased with study medication. Cotinine has a half life of 8 hours during pregnancy,21 so changes in cotinine levels with medication treatment would occur by visit 2. Participants were informed if their serum cotinine concentration during treatment exceeded the baseline cotinine measure by > 40% and they were instructed to stop smoking and/or reduce inhaler use until the cotinine concentration returned to near pretreatment values. If values continued to be elevated after another repeat assessment, participants were instructed to stop inhaler use.
Study Outcome Measurements:
At every visit, a study nurse monitored the patients’ smoking status and medication compliance. Quitting smoking at each visit was defined as not even a puff in the last seven days and was biochemically verified with exhaled breath carbon monoxide (CO) < 4ppm.22 CO, with a half-life of 2–3 hours is an inexpensive method to verify cigarette abstinence. At each visit, participants estimated the average number of cigarettes per day (CPD) they smoked in the past 7 days. If a study participant was not using the study medication as directed she was queried about her reasons for non-compliance and was counseled to be more adherent.
Serum samples for cotinine were collected at baseline two and six weeks after the quit date, and at 32–34 weeks gestation. Samples were analyzed by gas chromatography at Yale University.
Information on adverse events (AEs) and serious adverse events (SAEs) was collected at each visit. A checklist of AEs was comprised of the most common inhaler side effects included in the package insert (cough, throat and mouth irritation, rhinitis) and we recorded all other adverse effects.18 Study nurses abstracted data on pregnancy and neonatal outcomes from the medical chart after delivery for SAEs and birth outcomes. SAEs assessed by chart review included preterm (< 37 weeks gestation) delivery, low birth weight (<2500 grams), spontaneous abortion (unintended pregnancy loss < 28 weeks gestation), perinatal mortality, and any other SAEs.
A Data and Safety and Monitoring Board (DSMB) reviewed safety data throughout the trial and efficacy data after 137 participants were randomized to treatment. Participants were recruited beginning on August 20, 2012 and the last participant completed her participation on Jan 11, 2017. The a priori stopping rules were based on the primary endpoint of efficacy for quitting smoking as well as safety measures. We planned to recruit 360 subjects providing power in excess of 80% (with alpha = 0.05, two-sided) to detect a difference in quit rates between placebo and nicotine groups (14.9 % vs. 26.9%, respectively), a 200-gram difference in birth weight, and a lower incidence of low birth weight (5% vs. 18%) and preterm delivery (7% vs. 18%) in the nicotine vs. placebo groups. However, after the first review of the efficacy data at the 32-week time point after 137 participants had been randomized, the DSMB recommended that enrollment be stopped. At the time of the interim analysis, the quit rate was approximately 7/62 (11.3%) in the nicotine inhaler group, and 12/59 (20.3%) in the placebo group for participants who had completed the 32 week time point. The DSMB recommended discontinuing the trial due to futility in detecting differences in the primary outcome.
Statistical analyses:
Variables were descriptively summarized and compared between groups using a Wilcoxon rank-sum test or a Fisher’s exact test. Biochemically verified quit rates were examined at 6 weeks post-quit date and at 32 weeks gestation and compared by group using Fisher’s exact test. Participants who did not attend a follow-up visit were considered to be smokers. Changes from baseline measures to visits 2, 5 and 6 in measures of tobacco exposure (CPD, cotinine, and CO) and birth outcomes (birth weight, gestational age, and preterm delivery or birth weight < 2500 g) were compared between groups using a two-sample t-test assuming unequal variances or a Fisher’s exact test. In addition, changes in tobacco exposure and birth outcomes were examined using linear regression and logistic regression to adjust for site and potential confounders (variables that were unbalanced between groups and associated with the outcome of interest). We also examined the frequency of SAEs, compliance rates with the inhaler, and reasons for non-compliance. A p-value < 0.05 was deemed to be statistically significant. All the analyses were performed in R 3.4.3.
Results:
Seventy women were allocated to receive nicotine inhaler and 67 women were allocated to the placebo inhaler control group (Figure 1). Nicotine and placebo groups were comparable on most demographic, smoking, medical history, and obstetrical history variables (Table 1). However, there were baseline differences in motivation to quit and history of preterm delivery. The nicotine and placebo groups were equally likely to attend study visits (55.7% vs. 56.2%, respectively, p=0.931).
Table 1:
Baseline Characteristics, n (%) or mean (SD) (N=137)
| Placebo (n=67) | Nicotine (n=70) | P-Value* | |
|---|---|---|---|
| Site | |||
| Baystate Medical Center | 44 (66%) | 45 (64%) | 1.000 |
| Hartford Hospital | 23 (34%) | 25 (36%) | |
| Age in Years | 28.24 (6.30) | 26.97 (5.45) | 0.257 |
| Cigarettes smoked per day | 8.04 (4.59) | 8.66 (4.71) | 0.321 |
| FTCDa score | 4.27 (2.00) | 4.25 (1.79) | 0.922 |
| Race/Ethnicity | 0.378 | ||
| Hispanic or Latino | 33 (50%) | 28 (41%) | |
| Non Hispanic, White | 25 (38%) | 26 (38%) | |
| Non Hispanic, Black | 8 (12%) | 14 (20%) | |
| Other | 0 (0%) | 1 (1%) | |
| Highest level of education completed | 0.836 | ||
| Grade school completed | 20 (30%) | 18 (26%) | |
| High school completed | 31 (46%) | 36 (51%) | |
| College or post-college completed | 16 (24%) | 16 (23%) | |
| Insurance | 0.745 | ||
| Public | 63 (94%) | 64 (91%) | |
| Private | 4 (6%) | 6 (9%) | |
| Opioid Dependence Treatment | 13 (19%) | 12 (17%) | 0.826 |
| Medical History | |||
| Depression or anxiety | 39 (58%) | 44 (63%) | 0.604 |
| Substance abuse | 23 (34%) | 26 (37%) | 0.859 |
| Progesterone use | 5 (7%) | 6 (9%) | 0.999 |
| Pregnancy | |||
| Number of previous (median) pregnancies | 2 | 2 | 0.733 |
| Gestational age at entry (week) | 17.38 (3.88) | 17.54 (3.91) | 0.880 |
| Gestational age starting inhaler use | 19.39 (4.10) | 19.73 (4.08) | 0.629 |
| History of preterm delivery | 6 (9%) | 15 (21%) | 0.057 |
| First pregnancy | 14 (21%) | 12 (17%) | 0.665 |
| Motivation to quit smoking (0–10) | 8.76 (1.53) | 8.13 (1.61) | 0.016 |
| Self-efficacy to quit smoking (0–10) | 7.54 (2.1) | 6.90 (2.37) | 0.135 |
Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous/discrete variables.
Fagertsrom test for nicotine dependence
Smoking Outcomes:
The proportion of participants who quit smoking at six weeks post-quit date and 32 weeks gestation did not differ by treatment assignment. At six weeks, 3/70 (4%) of participants in the nicotine group compared to 2/67 (3%) in the placebo group had quit (p >0.99). The proportion was higher at 32 weeks gestation for the placebo (12/67 or 18%) and nicotine groups (7/70 or 10%), but did not differ significantly (p=0.220). Re-examining the data using information only for subjects who attended study visits also yielded non-significant results.
A difference in baseline motivation to quit by treatment assignment may explain the nominal difference in quit rates at 32 weeks gestation (placebo: 18%, nicotine group: 10%). In a post-hoc analysis, we found that the average (± SD) level of motivation to quit was higher in participants who had quit than those who had not quit (9.5 ± 0.8 vs. 8.2 ± 1.6, respectively, p<0.001).
Among participants with follow-up data (completer analysis), we compared multiple measures of tobacco exposure that were collected throughout the study (Table 2). In the unadjusted analysis, the nicotine group showed significantly greater reductions in CPD from baseline than the placebo group at all study visits. After adjusting for study site, motivation to quit smoking, the difference in reported CPD was only significant during medication treatment (2 and 6 weeks after the quit date). Thus, during the treatment period, the nicotine inhaler group reduced cigarette smoking nominally more than the placebo group. Two women in the nicotine group were discontinued from inhaler use due to repeated elevations in cotinine values.
Table 2:
Changes in Measures of Tobacco Exposure
| Placebo/Nicotine # of Subjects | Placebo Mean (SD) | Nicotine Mean (SD) | P-Value* | P-Value** | |
|---|---|---|---|---|---|
| Cigarettes Per Day (Survey) | |||||
| Baseline | 67/70 | 8.04 (4.59) | 8.66 (4.71) | ||
| Visit 2 | 32/43 | 5.03 (4.69) | 3.48 (3.29) | 0.009 | 0.022 |
| Visit 5 | 37/35 | 3.51 (2.69) | 2.37 (1.78) | 0.038 | 0.042 |
| Visit 6 | 41/47 | 2.85 (3.50) | 2.43 (2.22) | 0.041 | 0.108 |
| Cotinine (ng/mL) | |||||
| Baseline | 59/65 | 108.45 (62.65) | 117.59 (77.40) | ||
| Visit 2 | 38/47 | 89.87 (62.35) | 88.09 (54.66) | 0.143 | 0.131 |
| Visit 5 | 30/27 | 99.19 (62.20) | 103.64 (66.91) | 0.435 | 0.492 |
| Visit 6 | 26/39 | 85.48 (58.84) | 87.97 (56.36) | 0.290 | 0.229 |
| Exhaled CO (ppm) | |||||
| Baseline | 66/70 | 10.80 (7.28) | 11.39 (8.34) | ||
| Visit 2 | 33/44 | 7.94 (6.77) | 9.14 (7.01) | 0.883 | 0.637 |
| Visit 5 | 37/36 | 8.43 (7.33) | 7.25 (5.23) | 0.961 | 0.959 |
| Visit 6 | 40/46 | 5.50 (5.42) | 6.7 (7.4) | 0.754 | 0.907 |
Analysis of change scores for participants with follow-up data (completer analyses) by two-sample t-test assuming unequal variances and no adjustment for potential confounders.
Analysis of change scores for participants with follow-up data (completer analyses) by linear regression with adjustment for site and motivation to quit smoking.
Perinatal outcomes:
The mean (SD) birth weight was 3036.78 (583.8) g in the placebo group and 3141.12 (561.94) g in the nicotine group (p=0.128). Gestational age was 38.61 (2.53) weeks in the placebo group and 39.11 (2.42) weeks in the nicotine group (p=0.123). More women in the placebo group had a preterm delivery or low birth weight infant than in the nicotine group (Table 3). Given that more women in the nicotine group had a history of preterm delivery, we controlled for this variable in birth weight and gestational age outcomes. Further, when we used logistic regression to analyze the effect of treatment (nicotine vs. placebo) on the incidence of low birth weight, controlling for site, preterm delivery history, and gestational age less than 37 weeks, we found that the treatment effect was no longer statistically significant for birth weight (p=0.268). Of the variables that could impact the incidence of preterm delivery, we controlled only for a history of preterm delivery and study site because age, race/ethnicity, and progesterone therapy were similar between groups (Table 1). No other outcomes differed by group.
Table 3:
Frequency of Adverse Maternal and Perinatal outcomes*
| Placebo (N=67) |
Nicotine (N=67) |
P-valuea | P-valueb | |
|---|---|---|---|---|
| Maternal Hospitalization for Medical Condition | 1 (1%) | 3 (4%) | 0.620 | 0.383 |
| Low Birth Weight <2500 grams | 10 (15%) | 4 (6%) | 0.094 | 0.035 |
| Preterm Delivery <37 weeks gestation | 10 (15%) | 3 (4%) | 0.042 | 0.030 |
| Spontaneous Abortion | 0 (0%) | 1 (1%) | >0.99 | 0.552 |
| Perinatal mortality | 0 (0%) | 0 (0%) | >0.99 | NA |
| Neonatal Abstinence Syndrome | 11 (16%) | 13 (19%) | 0.824 | 0.598 |
| Any other infant SAEc | 8 (12%) | 4 (6%) | 0.237 | 0.305 |
Outcomes obtained by chart review
P-value by Fisher’s exact test, without adjustment for confounders
Logistic regression: P-value adjusted for site and history of preterm history delivery <37 weeks
Placebo group (fetal cardiac anomaly, right club foot, 5 respiratory distress, low blood sugar); nicotine group (2 respiratory distress, bradycardia, hypoglycemia).
Moderate AEs known to be associated with nicotine inhaler use were reported by eight participants during treatment, including throat irritation (n=8), cough (n=1), and “other” (n=3) (feeling nauseous, bad taste, and increased mucus in throat). All reports of throat irritation were in the nicotine group. The incidence of individuals experiencing AEs after baseline was significantly higher in the nicotine (8/70 = 11%) than in the placebo group (0/67 = 0%) (p=0.008).
Study medication compliance:
Neither the number of days of cartridge use [placebo:34.11 (SD=20.54); nicotine: 36.39 (SD=23.92); p=0.587] nor the average number of cartridges used per day [placebo: 1.81 (SD=1.62); nicotine: 1.70 (SD=1.19); p=0.701] differed significantly by treatment group. Compliance with the inhaler during treatment was 69% in the placebo group and 70% in the nicotine group, which was not significant (p>0.99). There was no relationship between baseline levels of cigarette smoking and inhaler use during the first two weeks of treatment in either the nicotine r= 0.116 (p=0.476) or the placebo group r=−0.125 (p=0.503).
Reasons for medication non-compliance did not differ statistically by group. “Does not get relief of cravings from use of inhaler” was the primary reason for non-compliance in both groups (44% placebo vs. 32% nicotine); “Believes she is taking the placebo inhaler” (28% placebo vs. 14% nicotine). “Forgets to take or loses inhaler” (20% placebo vs. 13% nicotine). Few women (< 10%) reported the following reasons for non-compliance: worried about side effects, effects on baby, tired of using inhaler, taste, or difficulty using the inhaler.
Comment:
We found that smoking cessation counseling with adjunctive use of nicotine inhaler was associated with a modest reduction in smoking during treatment, but it did not increase quit rates. Consistent with this finding, many women in the active inhaler group reported an inadequate relief of cravings. However, the nicotine inhaler was associated with a lower risk of delivering a preterm delivery and a low birth weight infant. These findings suggest that the nicotine inhaler has a favorable safety profile, but is not efficacious for smoking cessation during pregnancy.
Our study population consisted primarily of women on public assistance, including some with a history of mental health and/or substance use disorders. These factors may have increased the external validity of the study, but also may have lowered overall efficacy rates. Nonetheless, our findings agree with a large number of placebo-controlled trials showing that NRT (gum or patch) does not increase smoking cessation during pregnancy.7
Our study differs from previous studies in that we evaluated the nicotine inhaler, which has unique features that are designed to replace the sensory aspects of smoking, which is important because women in particular may respond well to treatments that address the sensory aspects of smoking.11 However, we did not find that the nicotine inhaler increased quit rates, in contrast to studies in non-pregnant smokers. In one meta-analysis, the nicotine inhaler produced an approximate doubling of quit rates relative to placebo.6 The lack of efficacy may be due in part to accelerated nicotine metabolism during pregnancy, such that pregnant smokers may need higher nicotine doses to quit.21 Indeed, one study showed that pregnant women who used both the patch and another intermittent nicotine formulation had higher quit rates than a single form of NRT which had no effect.23 It is also likely that pregnant women who enter clinical trials for smoking cessation have a harder time quitting than the general population of smokers, as many pregnant women quit smoking before their first perinatal visit.24 A slightly higher quit rate found in our placebo group at the end of pregnancy, was likely due to differences between groups on motivation to quit smoking.
Although the nicotine inhaler did not increase quit rates, during treatment it reduced the number of cigarettes per day that pregnant women smoked compared to the placebo inhaler. It is likely that the inhaler relieved some but not all of the desire to smoke, resulting in reduced smoking by study participants in the nicotine group but not smoking cessation. Indeed, the primary reason for non-compliance in our study appeared to be that the inhaler did not decrease cravings adequately, consistent with our finding of a lack of efficacy. Another common reason reported for not using the inhaler was that the participant forgot or lost the inhaler, suggesting that the inhaler was not highly valued by the participant. Interestingly, a few participants asked “where is the smoke?” after first using the inhaler, suggesting they may have confused the inhaler with electronic cigarettes (which are not FDA approved for smoking cessation, come in a variety of flavors, heat to aerosolize nicotine, and can produce higher levels of nicotine than smoking). The nicotine inhaler has better sensory qualities than other NRT formulations, though in non-pregnant smokers electronic cigarettes or conventional cigarettes are rated better than the inhaler on satisfaction, taste, likelihood to induce cigarette abstinence, and positive and negative reinforcing effects.25 Survey responses suggest that side effects were not a major reason for poorer compliance with the medication, though the frequency of adverse effects was higher in the nicotine group. Although we were concerned that women might not use the inhaler because of a concern that it could have harmful effects on the baby, this reason was endorsed by a minority of women.
One of the reasons to treat pregnant smokers aggressively is to improve perinatal outcomes. The lower incidence of delivering a preterm with the nicotine inhaler may have been due to a greater reduction in smoking in that group during treatment. We also observed a lower incidence of infants born with low birth weight (<2500 grams) in the nicotine group, but this was likely because they were also born preterm (<37 weeks). These findings are consistent with our larger placebo-controlled study of nicotine gum for smoking cessation during pregnancy, which showed that nicotine reduced the number of cigarettes smoked and had a beneficial impact on birth weight and gestational age, as well as the incidence of preterm delivery and low birth weight.8 Although meta-analyses by Coleman et al.,7 suggests that NRT for smoking cessation during pregnancy does not improve birthweight and gestational age; most of these studies examined the nicotine patch; whereas we have evaluated other NRT formulations (i.e., gum and inhaler) for smoking cessation during pregnancy. These treatments typically provide intermittent nicotine delivery and a lower overall nicotine dose. Also, we monitored nicotine exposure in our study to ensure that women did not have greater exposure during treatment than during usual smoking. Caution is advised in using the nicotine inhaler during pregnancy, as there is a risk of perinatal smoking-related morbidity may be attributable to nicotine. There is not adequate evidence to conclude whether NRT has a negative or positive effect on the risk of miscarriage, stillbirth, or preterm delivery compared to cigarette smoking. However, nicotine is considered a neurobehavioral teratogen and linked to sudden infant death syndrome and has effects on lung development in animal models.2 We discontinued the medication treatment based on an estimate of normal fluctuations of cotinine with smoking during pregnancy.26 Using an intermittent NRT formulation, cotinine monitoring, as well as a reduction in cigarette smoking in the inhaler compared to the placebo group, may be responsible for some improved infant outcomes in the nicotine group.
Our study has some limitations. Overall quit rates were low in both groups during medication treatment and at the end of pregnancy, leading to it being stopped prematurely because of futility. This reduced the sample size from that which was planned and thus our ability to detect differences that are potentially clinically meaningful such as birth weight, gestational age, and other secondary outcomes. Our sample was heterogeneous with some women also being treated for substance abuse issues or depression, which may have increased our external validity but resulted in overall lower quit rates. Finally, we observed a relatively low rate of treatment completion is a limitation; however, we were able to obtain birth outcomes on nearly all participants, which is important for a medication trial in pregnancy.
In summary, this study suggests that the nicotine inhaler does not significantly increase smoking cessation rates, but may reduce overall tobacco exposure during pregnancy. Nicotine inhaler use was associated with a lower incidence of preterm delivery and low birth weight infants. Future studies of smoking cessation in pregnant women should examine dosages and formulations of NRT or other medications that reduce cravings, promote cessation and reduction, and improve infant outcomes.
1). CONDENSATION:
Nicotine inhaler was not efficacious for smoking cessation during pregnancy, but the outcome of preterm delivery occurred less frequently in the nicotine group.
3). AJOG AT A GLANCE
Why was this study conducted?
This study was conducted to determine if the nicotine inhaler could help pregnant women quit smoking, and if it had a favorable risk/benefit profile on maternal and birth outcomes compared to placebo inhaler.
4). What are the key findings?
Smoking cessation rates were similar for the nicotine and placebo inhaler groups during treatment and at the end of pregnancy. However, during medication treatment, although cigarettes per day (CPD) decreased over time in both groups, the nicotine inhaler group had a greater decrease as well as fewer preterm deliveries.
5). What does this study add to what is already known?
This study adds to the literature as it is the only randomized placebo controlled trial examining the safety and efficacy of the nicotine inhaler for smoking cessation during pregnancy.
Acknowledgments
HRK is a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which was supported in the last three years by AbbVie, Alkermes, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, Pfizer, Arbor, and Amygdala Neurosciences and is named as an inventor on PCT patent application #15/878,640 entitled: “Genotype-guided dosing of opioid agonists,” filed January 24, 2018. The remaining authors report no conflict of interest.
The project was supported by NIH grant R01HD069314, and the Lowell P. Weicker Clinical Research at University of Connecticut School of Medicine. The study medication was donated by Pfizer pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Office of the Surgeon G, Office on S, Health. Reports of the Surgeon General. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2004. [PubMed] [Google Scholar]
- 2.National Center for Chronic Disease P, Health Promotion Office on S, Health. Reports of the Surgeon General. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Vol 9, Reproductive Outcomes. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. [Google Scholar]
- 3.Curtin SC, Matthews TJ. Smoking Prevalence and Cessation Before and During Pregnancy: Data From the Birth Certificate, 2014. Natl Vital Stat Rep. 2016;65(1): 1–14. [PubMed] [Google Scholar]
- 4.Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2009(3):CD001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamberlain C, O’Mara-Eves A, Porter J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev. 2017;2:CD001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiore MC. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am JPrevMed. 2008;35(2):158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2015(12):CD010078. [DOI] [PubMed] [Google Scholar]
- 8.Oncken C, Dornelas E, Greene J, et al. Nicotine gum for pregnant smokers: a randomized controlled trial. Obstet Gynecol. 2008;112(4):859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hjalmarson A, Nilsson F, Sjostrom L, Wiklund O. The nicotine inhaler in smoking cessation. Arch Intern Med. 1997;157(15): 1721–1728. [PubMed] [Google Scholar]
- 10.Benowitz NL, Hatsukami D. Gender differences in the pharmacology of nicotine addiction. Addict Biol. 1998;3(4):383–404. [DOI] [PubMed] [Google Scholar]
- 11.Allen AM, Oncken C, Hatsukami D. Women and Smoking: The Effect of Gender on the Epidemiology, Health Effects, and Cessation of Smoking. Curr Addict Rep. 2014; 1(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider NG, Olmstead RE, Nides M, et al. Comparative testing of 5 nicotine systems: initial use and preferences. Am J Health Behav. 2004;28(1):72–86. [DOI] [PubMed] [Google Scholar]
- 13.Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10(7):1245–1250. [DOI] [PubMed] [Google Scholar]
- 14.Li CQ, Windsor RA, Perkins L, Goldenberg RL, Lowe JB. The impact on infant birth weight and gestational age of cotinine-validated smoking reduction during pregnancy. JAMA. 1993;269(12): 1519–1524. [PubMed] [Google Scholar]
- 15.Fagerstrom K Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res. 2012;14(1):75–78. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. [DOI] [PubMed] [Google Scholar]
- 17.Dornelas EA, Magnavita J, Beazoglou T, et al. Efficacy and cost-effectiveness of a clinic-based counseling intervention tested in an ethnically diverse sample of pregnant smokers. PatientEduc Couns. 2006;64(1–3):342–349. [DOI] [PubMed] [Google Scholar]
- 18.Pfizer. Nicotrol® Inhaler https://www.pfizer.com/files/products/uspi_nicotrol_inhaler.pdf. Pharmacia and Upjohn Company LLC; 2008. [Google Scholar]
- 19.Tonnesen P, Norregaard J, Mikkelsen K, Jorgensen S, Nilsson F. A double-blind trial of a nicotine inhaler for smoking cessation. JAMA. 1993;269(10): 1268–1271. [PubMed] [Google Scholar]
- 20.Schneider NG, Olmstead RE, Franzon MA, Lunell E. The nicotine inhaler: clinical pharmacokinetics and comparison with other nicotine treatments. Clin Pharmacokinet. 2001;40(9):661–684. [DOI] [PubMed] [Google Scholar]
- 21.Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301(2):594–598. [DOI] [PubMed] [Google Scholar]
- 22.Cropsey KL, Trent LR, Clark CB, Stevens EN, Lahti AC, Hendricks PS. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine Tob Res.2014;16(10): 1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brose LS, McEwen A, West R. Association between nicotine replacement therapy use in pregnancy and smoking cessation. Drug Alcohol Depend. 2013;132(3):660–664. [DOI] [PubMed] [Google Scholar]
- 24.Solomon L, Quinn V. Spontaneous quitting: self-initiated smoking cessation in early pregnancy. Nicotine Tob Res. 2004;6 Suppl 2:S203–216. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg MB, Zimmermann MH, Delnevo CD, et al. E-cigarette versus nicotine inhaler: comparing the perceptions and experiences of inhaled nicotine devices. J Gen Intern Med. 2014;29(11):1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oncken CA, Hatsukami DK, Lupo VR, Lando HA, Gibeau LM, Hansen RJ. Effects of short-term use of nicotine gum in pregnant smokers. Clin Pharmacol Ther. 1996;59(6):654–661. [DOI] [PubMed] [Google Scholar]