Abstract
Mucinous neoplasms of the ovary account for 10%–15% of ovarian neoplasms. They may be benign, borderline, or malignant. The large majority are benign or borderline, accounting for 80% and 16%–17%, respectively. Mucinous neoplasms of the ovary most commonly affect women in their 20s to 40s. The clinical manifestation is nonspecific, but most mucinous ovarian neoplasms manifest as large unilateral pelvic masses. At gross pathologic analysis, mucinous ovarian neoplasms appear as large multiloculated cystic masses. The contents of the cyst loculi vary on the basis of differences in internal mucin content. At histologic analysis, mucinous ovarian neoplasms are composed of multiple cysts lined by mucinous epithelium, often resembling gastrointestinal-type epithelium. Imaging evaluation most commonly includes US and/or MRI. The imaging findings parallel the gross pathologic features and include a large, unilateral, multiloculated cystic mass. The cyst loculi vary in echogenicity, attenuation, and signal intensity depending on the mucin content. Mucinous neoplasms of the ovary are staged surgically using the FIGO (International Federation of Gynecology and Obstetrics) staging system. Primary treatment is surgical, with adjuvant chemotherapy considered in the uncommon case of mucinous carcinoma with extraovarian disease. Since most mucinous ovarian neoplasms are benign or borderline, the overall prognosis is excellent.
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ List the pathologic subtypes of mucinous ovarian neoplasms.
■ Describe the main pathologic features of mucinous ovarian neoplasms.
■ Recognize the imaging features of mucinous ovarian neoplasms.
Introduction
The ovary is affected by a diverse set of neoplastic and nonneoplastic conditions. The World Health Organization (WHO) classifies ovarian neoplasms into 13 distinct categories (Table 1). Epithelial tumors are the most common category and include a heterogeneous collection of neoplasms. The subtypes of epithelial neoplasms are serous, mucinous, endometrioid, clear cell, Brenner, and seromucinous tumors and undifferentiated carcinoma (1).
Table 1:
WHO Classification of Tumors of the Ovary

Source.—Reference 1.
Mucinous neoplasms of the ovary represent 10%–15% of ovarian neoplasms (2). Mucinous ovarian neoplasms are divided into benign, borderline, and malignant groups. Benign mucinous neoplasms include mucinous cystadenoma and mucinous adenofibroma and account for 80% of cases. Borderline tumors (mucinous borderline tumor/atypical proliferative mucinous tumor) are the next most common type, accounting for 16%–17% of cases. The remaining 3%–4% of primary tumors are ovarian mucinous carcinomas (1,2). Metastatic mucinous tumors, particularly those of gastrointestinal or appendiceal origin, should be excluded when primary mucinous carcinoma of the ovary is considered (3).
In this article focused on radiologic-pathologic correlation, we use cases from the radiologic pathology archives of the AIRP (American Institute for Radiologic Pathology) to review important features of mucinous neoplasms of the ovary, including clinical manifestation, key pathologic features, multimodality imaging findings, staging, and treatment and prognosis considerations.
Clinical Considerations
Epidemiology
Mucinous ovarian tumors affect a wide age range of patients. Benign mucinous cystadenomas usually affect women in their 20s to 40s (4). Mucinous borderline tumor/atypical proliferative mucinous tumor has an average age at diagnosis of 40–49 years. Similarly, the mean age at diagnosis of mucinous carcinoma is 45 years (1).
Clinical Manifestation
Mucinous tumors of the ovary frequently manifest with clinical signs and symptoms of a large unilateral pelvic mass. The average size at presentation is 18 cm; however, these tumors can become very large and fill the entire abdominopelvic cavity (Fig 1). The resultant signs and symptoms are often secondary to the large size of the mass and may include pain, abdominal or pelvic fullness, or a palpable mass. The clinical signs and symptoms are nonspecific, but large size alone at physical examination is suggestive of a mucinous histologic type (5). This is important, as metastases to the ovary are more likely to be bilateral and smaller (6).
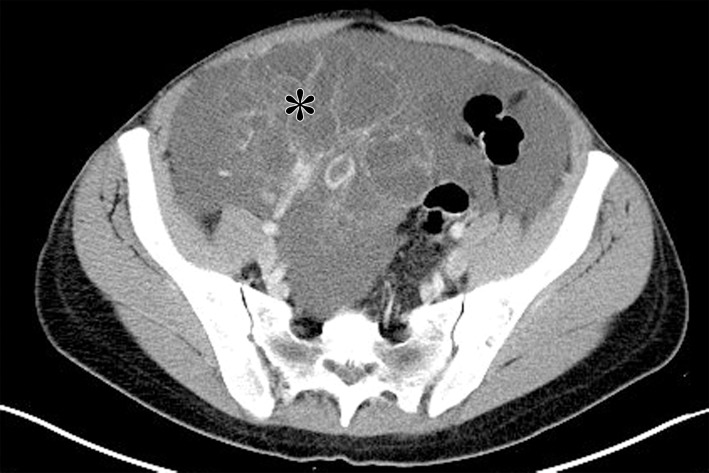
Figure 1a.
Bilateral mucinous cystadenoma in a 27-year-old woman with 1 year of weight gain and acute onset of abdominal pain. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a large cystic lesion (white *) with one thin septum arising from the left ovary. There is a smaller unilocular lesion in the right ovary (black * in b). (c) Gross photograph of the left ovarian lesion shows a smooth-walled cyst. (d) Photograph shows that the cyst was filled with over 7 L of serosanguineous fluid. (e) Photomicrograph of the left ovarian lesion shows simple mucinous cystic epithelium (arrow), characteristic of mucinous cystadenoma. (Hematoxylin-eosin [H-E] stain; original magnification, ×200.)
Figure 1b.

Bilateral mucinous cystadenoma in a 27-year-old woman with 1 year of weight gain and acute onset of abdominal pain. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a large cystic lesion (white *) with one thin septum arising from the left ovary. There is a smaller unilocular lesion in the right ovary (black * in b). (c) Gross photograph of the left ovarian lesion shows a smooth-walled cyst. (d) Photograph shows that the cyst was filled with over 7 L of serosanguineous fluid. (e) Photomicrograph of the left ovarian lesion shows simple mucinous cystic epithelium (arrow), characteristic of mucinous cystadenoma. (Hematoxylin-eosin [H-E] stain; original magnification, ×200.)
Figure 1c.
Bilateral mucinous cystadenoma in a 27-year-old woman with 1 year of weight gain and acute onset of abdominal pain. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a large cystic lesion (white *) with one thin septum arising from the left ovary. There is a smaller unilocular lesion in the right ovary (black * in b). (c) Gross photograph of the left ovarian lesion shows a smooth-walled cyst. (d) Photograph shows that the cyst was filled with over 7 L of serosanguineous fluid. (e) Photomicrograph of the left ovarian lesion shows simple mucinous cystic epithelium (arrow), characteristic of mucinous cystadenoma. (Hematoxylin-eosin [H-E] stain; original magnification, ×200.)
Figure 1d.
Bilateral mucinous cystadenoma in a 27-year-old woman with 1 year of weight gain and acute onset of abdominal pain. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a large cystic lesion (white *) with one thin septum arising from the left ovary. There is a smaller unilocular lesion in the right ovary (black * in b). (c) Gross photograph of the left ovarian lesion shows a smooth-walled cyst. (d) Photograph shows that the cyst was filled with over 7 L of serosanguineous fluid. (e) Photomicrograph of the left ovarian lesion shows simple mucinous cystic epithelium (arrow), characteristic of mucinous cystadenoma. (Hematoxylin-eosin [H-E] stain; original magnification, ×200.)
Figure 1e.
Bilateral mucinous cystadenoma in a 27-year-old woman with 1 year of weight gain and acute onset of abdominal pain. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a large cystic lesion (white *) with one thin septum arising from the left ovary. There is a smaller unilocular lesion in the right ovary (black * in b). (c) Gross photograph of the left ovarian lesion shows a smooth-walled cyst. (d) Photograph shows that the cyst was filled with over 7 L of serosanguineous fluid. (e) Photomicrograph of the left ovarian lesion shows simple mucinous cystic epithelium (arrow), characteristic of mucinous cystadenoma. (Hematoxylin-eosin [H-E] stain; original magnification, ×200.)
The most useful serum markers to identify mucinous ovarian carcinoma are CA 19-9 and CA-125. Levels of CA 19-9, CA-125, and carcinoembryonic antigen (CEA) may be used to identify the presence of tumor and may be followed postoperatively to assess treatment and recurrence (7,8).
Rarely, borderline mucinous tumors can manifest with pseudomyxoma peritonei, although this entity is usually related to tumors of appendiceal origin. Pseudomyxoma peritonei involves widespread seeding of mucin-producing cells in the peritoneal cavity, leading to significant accumulation of mucinous material. This can lead to bowel obstruction and tends to recur after treatment (9).
Staging and Treatment
As with all ovarian tumors, staging is surgical. However, mucinous tumors of the ovary are distinct from other epithelial ovarian tumors in that they are more likely than serous carcinomas to manifest at an early stage. Eighty-three percent of mucinous ovarian carcinomas are FIGO (International Federation of Gynecology and Obstetrics) stage I at presentation, in contrast to 4% of serous carcinomas (10).
Surgical treatment of mucinous tumors consists of intact removal of the involved adnexa. Oophorectomy, as opposed to cystectomy, decreases the rate of local recurrence. Great care must be taken, as spillage of the cyst contents could increase the risk of recurrence (11). The procedure is frequently done via laparotomy, given the large size of these tumors at presentation. However, in skilled hands, a minimally invasive approach is appropriate.
The remainder of the staging procedure depends on the pathologic diagnosis and if the patient desires future fertility. In benign tumors, intact removal of the adnexa is sufficient. However, if a borderline tumor or carcinoma is suspected and fertility is desired, staging should also include pelvic and peritoneal washings, peritoneal biopsies, and an omentectomy. If the patient is postmenopausal, a total hysterectomy and bilateral salpingo-oophorectomy may be performed (4).
Lymphadenectomy is unnecessary during staging because mucinous tumors grossly confined to the ovary do not have occult lymph node metastases (12). It was once thought that an appendectomy should be performed when a mucinous tumor is suspected; however, this is no longer considered necessary if the appendix appears to be grossly normal and there is no pseudomyxoma peritonei (13).
After surgical resection, both benign and borderline tumors should be adequately treated. In one Gynecologic Oncology Group study, there were no recurrences in 156 patients with stage I mucinous borderline tumors (14). For higher-stage borderline tumors, surgical resection of all visible disease still remains the best treatment (9). For mucinous carcinoma, surgical resection of all gross disease is critical.
Adjuvant chemotherapy can be considered in mucinous carcinoma for extraovarian disease. Typically, platinum-based chemotherapy in conjunction with a taxane is used. However, these tumors are not very chemosensitive, and many women with higher-stage tumors die of the disease.
Prognosis
Given the benign pathologic features of mucinous cystadenoma/adenofibroma, the prognosis of these tumors is excellent. Local recurrence can be minimized with oophorectomy as opposed to cystectomy (1). Mucinous borderline tumors also have an excellent prognosis, with overall survival of 98% at 5 years and 96% at 10 years (15). Only rare cases of malignant transformation have been described (9), but the current working hypothesis for the origin of ovarian mucinous carcinoma is multistep transformation from a borderline mucinous tumor (16).
Most mucinous carcinomas are confined to the ovary at the time of diagnosis, so the prognosis is favorable. Those with stage I mucinous carcinoma have a 91% 5-year survival rate (5). Unfortunately, higher-stage mucinous tumors have a worse prognosis than their serous counterparts (17).
In a study by the Gynecologic Oncology Group, progression-free survival for patients with mucinous tumors after optimal debulking and chemotherapy with cisplatin and paclitaxel was 10.5 months compared with 16.9 months for women with serous tumors (18). Overall survival was 14.8 months for women with mucinous tumors compared with 45.2 months for those with serous cancers. This discrepancy is due to high rates of platinum resistance in mucinous carcinomas, rendering them less chemosensitive (19). Recurrent tumors have a similarly poor prognosis and also respond poorly to chemotherapy and radiation therapy (1).
Pathologic Features
Mucinous Cystadenoma/Adenofibroma
The WHO defines mucinous cystadenoma as a benign cystic tumor lined by mucinous gastrointestinal-type epithelium. In the uncommon situation when the tumor demonstrates prominent fibrous stroma, the term adenofibroma is used (1).
The gross pathologic findings of mucinous cystadenoma are a large unilateral cystic mass with a smooth outer surface. The mass is unilateral in approximately 95% of cases. The cyst is usually multilocular but can be unilocular. The mass ranges in size from a few centimeters to more than 30 cm, with a mean size of 10 cm (1). Grossly, adenofibromas are usually smaller and solid (1).
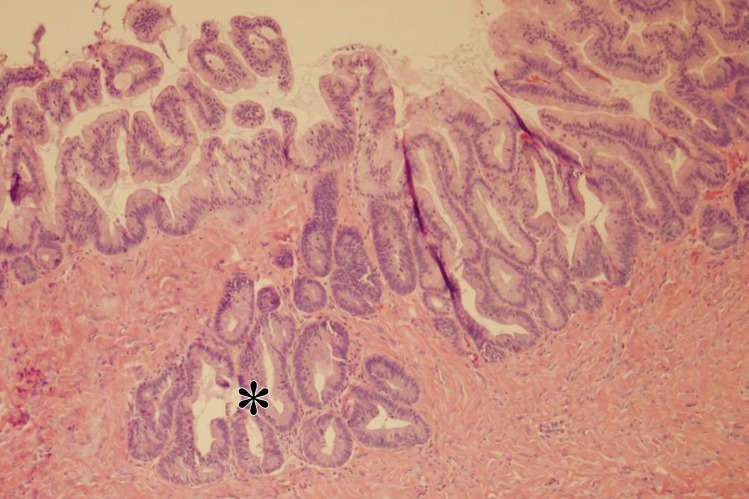
At histologic evaluation, mucinous cystadenomas are usually composed of multiple cysts and glands lined by simple nonstratified mucinous epithelium (Fig 2). The epithelium resembles gastric mucosa or intestinal epithelium with goblet cells (Fig 3). Neuroendocrine and Paneth cells may also be present. Focal papillae may be present. The ovarian stroma adjacent to the epithelial lining of the cyst(s) may be cellular and contain regions of stromal luteinization. Small regions of mucin or mucin granulomas may be present in the stroma if cyst rupture has occurred.
Figure 2a.
Mucinous cystadenoma in a 42-year-old woman with abdominal bloating. (a, b) Sagittal (a) and coronal (b) T2-weighted MR images show a large cystic mass (*) with minimal complexity. (c) Intraoperative photograph shows a large cyst with a smooth outer surface (*). (d) High-power photomicrograph shows cysts (arrow) and glands (*) lined by simple nonstratified mucinous epithelium, typical of mucinous cystadenoma. (H-E stain.)
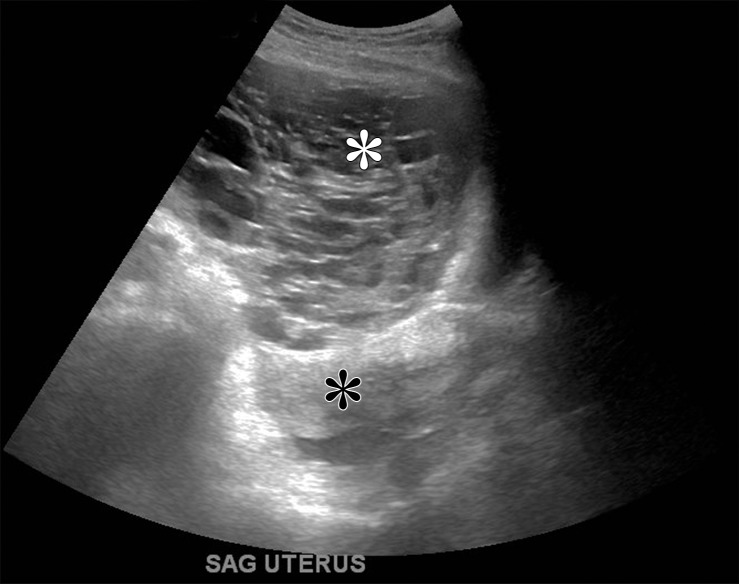
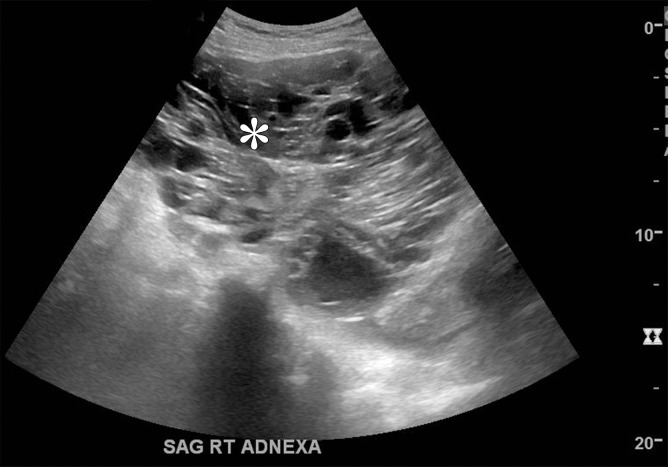
Figure 3a.
Mucinous cystadenoma in a 38-year-old woman with dysmenorrhea. (a) Sagittal transabdominal US image shows a cystic mass with multiple septa (*). (b) Axial contrast-enhanced CT image shows a cystic mass (white *) anterior to the uterus (black *). (c–e) Axial T2-weighted (c), sagittal T2-weighted (d), and sagittal contrast-enhanced fat-saturated T1-weighted (e) images show a cystic mass anterior to the uterus with multiple septa (black * in c and d). Enhancement of the septa is seen (arrow in e). Note the incidental left ovarian endometrioma (arrow in c) and adenomyosis (white * in c and d). (f) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (g) High-power photomicrograph shows intestinal differentiation of the lining with goblet cells (arrow), typical of mucinous cystadenoma. (H-E stain.)
Figure 2b.
Mucinous cystadenoma in a 42-year-old woman with abdominal bloating. (a, b) Sagittal (a) and coronal (b) T2-weighted MR images show a large cystic mass (*) with minimal complexity. (c) Intraoperative photograph shows a large cyst with a smooth outer surface (*). (d) High-power photomicrograph shows cysts (arrow) and glands (*) lined by simple nonstratified mucinous epithelium, typical of mucinous cystadenoma. (H-E stain.)
Figure 2c.
Mucinous cystadenoma in a 42-year-old woman with abdominal bloating. (a, b) Sagittal (a) and coronal (b) T2-weighted MR images show a large cystic mass (*) with minimal complexity. (c) Intraoperative photograph shows a large cyst with a smooth outer surface (*). (d) High-power photomicrograph shows cysts (arrow) and glands (*) lined by simple nonstratified mucinous epithelium, typical of mucinous cystadenoma. (H-E stain.)
Figure 2d.
Mucinous cystadenoma in a 42-year-old woman with abdominal bloating. (a, b) Sagittal (a) and coronal (b) T2-weighted MR images show a large cystic mass (*) with minimal complexity. (c) Intraoperative photograph shows a large cyst with a smooth outer surface (*). (d) High-power photomicrograph shows cysts (arrow) and glands (*) lined by simple nonstratified mucinous epithelium, typical of mucinous cystadenoma. (H-E stain.)
Figure 3b.
Mucinous cystadenoma in a 38-year-old woman with dysmenorrhea. (a) Sagittal transabdominal US image shows a cystic mass with multiple septa (*). (b) Axial contrast-enhanced CT image shows a cystic mass (white *) anterior to the uterus (black *). (c–e) Axial T2-weighted (c), sagittal T2-weighted (d), and sagittal contrast-enhanced fat-saturated T1-weighted (e) images show a cystic mass anterior to the uterus with multiple septa (black * in c and d). Enhancement of the septa is seen (arrow in e). Note the incidental left ovarian endometrioma (arrow in c) and adenomyosis (white * in c and d). (f) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (g) High-power photomicrograph shows intestinal differentiation of the lining with goblet cells (arrow), typical of mucinous cystadenoma. (H-E stain.)
Figure 3c.
Mucinous cystadenoma in a 38-year-old woman with dysmenorrhea. (a) Sagittal transabdominal US image shows a cystic mass with multiple septa (*). (b) Axial contrast-enhanced CT image shows a cystic mass (white *) anterior to the uterus (black *). (c–e) Axial T2-weighted (c), sagittal T2-weighted (d), and sagittal contrast-enhanced fat-saturated T1-weighted (e) images show a cystic mass anterior to the uterus with multiple septa (black * in c and d). Enhancement of the septa is seen (arrow in e). Note the incidental left ovarian endometrioma (arrow in c) and adenomyosis (white * in c and d). (f) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (g) High-power photomicrograph shows intestinal differentiation of the lining with goblet cells (arrow), typical of mucinous cystadenoma. (H-E stain.)
Figure 3d.

Mucinous cystadenoma in a 38-year-old woman with dysmenorrhea. (a) Sagittal transabdominal US image shows a cystic mass with multiple septa (*). (b) Axial contrast-enhanced CT image shows a cystic mass (white *) anterior to the uterus (black *). (c–e) Axial T2-weighted (c), sagittal T2-weighted (d), and sagittal contrast-enhanced fat-saturated T1-weighted (e) images show a cystic mass anterior to the uterus with multiple septa (black * in c and d). Enhancement of the septa is seen (arrow in e). Note the incidental left ovarian endometrioma (arrow in c) and adenomyosis (white * in c and d). (f) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (g) High-power photomicrograph shows intestinal differentiation of the lining with goblet cells (arrow), typical of mucinous cystadenoma. (H-E stain.)
Figure 3e.

Mucinous cystadenoma in a 38-year-old woman with dysmenorrhea. (a) Sagittal transabdominal US image shows a cystic mass with multiple septa (*). (b) Axial contrast-enhanced CT image shows a cystic mass (white *) anterior to the uterus (black *). (c–e) Axial T2-weighted (c), sagittal T2-weighted (d), and sagittal contrast-enhanced fat-saturated T1-weighted (e) images show a cystic mass anterior to the uterus with multiple septa (black * in c and d). Enhancement of the septa is seen (arrow in e). Note the incidental left ovarian endometrioma (arrow in c) and adenomyosis (white * in c and d). (f) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (g) High-power photomicrograph shows intestinal differentiation of the lining with goblet cells (arrow), typical of mucinous cystadenoma. (H-E stain.)
Figure 3f.

Mucinous cystadenoma in a 38-year-old woman with dysmenorrhea. (a) Sagittal transabdominal US image shows a cystic mass with multiple septa (*). (b) Axial contrast-enhanced CT image shows a cystic mass (white *) anterior to the uterus (black *). (c–e) Axial T2-weighted (c), sagittal T2-weighted (d), and sagittal contrast-enhanced fat-saturated T1-weighted (e) images show a cystic mass anterior to the uterus with multiple septa (black * in c and d). Enhancement of the septa is seen (arrow in e). Note the incidental left ovarian endometrioma (arrow in c) and adenomyosis (white * in c and d). (f) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (g) High-power photomicrograph shows intestinal differentiation of the lining with goblet cells (arrow), typical of mucinous cystadenoma. (H-E stain.)
Figure 3g.
Mucinous cystadenoma in a 38-year-old woman with dysmenorrhea. (a) Sagittal transabdominal US image shows a cystic mass with multiple septa (*). (b) Axial contrast-enhanced CT image shows a cystic mass (white *) anterior to the uterus (black *). (c–e) Axial T2-weighted (c), sagittal T2-weighted (d), and sagittal contrast-enhanced fat-saturated T1-weighted (e) images show a cystic mass anterior to the uterus with multiple septa (black * in c and d). Enhancement of the septa is seen (arrow in e). Note the incidental left ovarian endometrioma (arrow in c) and adenomyosis (white * in c and d). (f) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (g) High-power photomicrograph shows intestinal differentiation of the lining with goblet cells (arrow), typical of mucinous cystadenoma. (H-E stain.)
In approximately 10% of cases, mucinous cystadenomas may coexist with a dermoid cyst or Brenner tumor (Fig 4). In cases of mucinous adenofibroma, the stroma will appear dense and fibrous (1). KRAS mutations are the most common genetic alteration in benign mucinous tumors and are present in 58% of cases (1,20).
Figure 4a.
Mucinous cystadenoma and Brenner tumor in a 54-year-old woman with a palpable adnexal lesion at physical examination. (a–c) Coronal T2-weighted fat-saturated (a), axial T2-weighted (b), and axial contrast-enhanced fat-saturated T1-weighted (c) images show a multiloculated cystic mass (*). Enhancing soft tissue along the periphery of the lesion (arrow in c) is not typical in an isolated mucinous cystadenoma. (d) Low-power photomicrograph shows goblet cells lining the cyst wall (arrow) of the mucinous cystadenoma and adjacent Brenner tumor (*). (H-E stain.)
Figure 4b.
Mucinous cystadenoma and Brenner tumor in a 54-year-old woman with a palpable adnexal lesion at physical examination. (a–c) Coronal T2-weighted fat-saturated (a), axial T2-weighted (b), and axial contrast-enhanced fat-saturated T1-weighted (c) images show a multiloculated cystic mass (*). Enhancing soft tissue along the periphery of the lesion (arrow in c) is not typical in an isolated mucinous cystadenoma. (d) Low-power photomicrograph shows goblet cells lining the cyst wall (arrow) of the mucinous cystadenoma and adjacent Brenner tumor (*). (H-E stain.)
Figure 4c.
Mucinous cystadenoma and Brenner tumor in a 54-year-old woman with a palpable adnexal lesion at physical examination. (a–c) Coronal T2-weighted fat-saturated (a), axial T2-weighted (b), and axial contrast-enhanced fat-saturated T1-weighted (c) images show a multiloculated cystic mass (*). Enhancing soft tissue along the periphery of the lesion (arrow in c) is not typical in an isolated mucinous cystadenoma. (d) Low-power photomicrograph shows goblet cells lining the cyst wall (arrow) of the mucinous cystadenoma and adjacent Brenner tumor (*). (H-E stain.)
Figure 4d.
Mucinous cystadenoma and Brenner tumor in a 54-year-old woman with a palpable adnexal lesion at physical examination. (a–c) Coronal T2-weighted fat-saturated (a), axial T2-weighted (b), and axial contrast-enhanced fat-saturated T1-weighted (c) images show a multiloculated cystic mass (*). Enhancing soft tissue along the periphery of the lesion (arrow in c) is not typical in an isolated mucinous cystadenoma. (d) Low-power photomicrograph shows goblet cells lining the cyst wall (arrow) of the mucinous cystadenoma and adjacent Brenner tumor (*). (H-E stain.)
Mucinous Borderline Tumor/Atypical Proliferative Mucinous Tumor
The WHO defines mucinous borderline tumor/atypical proliferative mucinous tumor as composed of mild to moderately atypical, gastrointestinal-type, mucin-containing epithelial cells that show proliferation greater than that seen in benign mucinous tumors, but without stromal invasion (1). The analogous term mucinous tumor of low malignant potential is not recommended by the WHO.
The gross pathologic findings of mucinous borderline tumor/atypical proliferative mucinous tumor are a large unilateral tumor with a smooth external surface. Tumor size ranges from several centimeters to 50 cm with a mean of 21.5 cm (21). They consist of small to large cysts containing mucin. The cyst walls are usually smooth but may be ulcerated or contain areas of solid growth. These lesions are often heterogeneous and may contain small foci of intraepithelial or invasive carcinoma (1).
The histologic findings of mucinous borderline tumor/atypical proliferative mucinous tumor include a cystic lesion lined by gastrointestinal-type epithelium. The epithelium often resembles gastric-pyloric epithelium. Goblet cells, neuroendocrine cells, and Paneth cells may be present. The epithelium demonstrates varying degrees of stratification, tufting, and villous or slender filiform papillae (Fig 5).
Figure 5a.
Mucinous borderline tumor in a 63-year-old woman with minimal abdominal pain and urinary frequency. (a) Sagittal US image shows a cystic mass (*) superior and anterior to the bladder (BL). (b–d) Sagittal T2-weighted (b), coronal T2-weighted (c), and coronal contrast-enhanced fat-saturated T1-weighted (d) images show a cystic mass (*) with minimal complexity (arrow in c and d). (e) Photograph of the gross pathologic specimen shows a mass with minimal complexity (black arrow) filled with thick fluid (white arrow). (f) High-power photomicrograph shows epithelial proliferation, stratification, and atypia (*), consistent with mucinous borderline tumor. (H-E stain.)
Figure 5b.
Mucinous borderline tumor in a 63-year-old woman with minimal abdominal pain and urinary frequency. (a) Sagittal US image shows a cystic mass (*) superior and anterior to the bladder (BL). (b–d) Sagittal T2-weighted (b), coronal T2-weighted (c), and coronal contrast-enhanced fat-saturated T1-weighted (d) images show a cystic mass (*) with minimal complexity (arrow in c and d). (e) Photograph of the gross pathologic specimen shows a mass with minimal complexity (black arrow) filled with thick fluid (white arrow). (f) High-power photomicrograph shows epithelial proliferation, stratification, and atypia (*), consistent with mucinous borderline tumor. (H-E stain.)
Figure 5c.
Mucinous borderline tumor in a 63-year-old woman with minimal abdominal pain and urinary frequency. (a) Sagittal US image shows a cystic mass (*) superior and anterior to the bladder (BL). (b–d) Sagittal T2-weighted (b), coronal T2-weighted (c), and coronal contrast-enhanced fat-saturated T1-weighted (d) images show a cystic mass (*) with minimal complexity (arrow in c and d). (e) Photograph of the gross pathologic specimen shows a mass with minimal complexity (black arrow) filled with thick fluid (white arrow). (f) High-power photomicrograph shows epithelial proliferation, stratification, and atypia (*), consistent with mucinous borderline tumor. (H-E stain.)
Figure 5d.
Mucinous borderline tumor in a 63-year-old woman with minimal abdominal pain and urinary frequency. (a) Sagittal US image shows a cystic mass (*) superior and anterior to the bladder (BL). (b–d) Sagittal T2-weighted (b), coronal T2-weighted (c), and coronal contrast-enhanced fat-saturated T1-weighted (d) images show a cystic mass (*) with minimal complexity (arrow in c and d). (e) Photograph of the gross pathologic specimen shows a mass with minimal complexity (black arrow) filled with thick fluid (white arrow). (f) High-power photomicrograph shows epithelial proliferation, stratification, and atypia (*), consistent with mucinous borderline tumor. (H-E stain.)
Figure 5e.
Mucinous borderline tumor in a 63-year-old woman with minimal abdominal pain and urinary frequency. (a) Sagittal US image shows a cystic mass (*) superior and anterior to the bladder (BL). (b–d) Sagittal T2-weighted (b), coronal T2-weighted (c), and coronal contrast-enhanced fat-saturated T1-weighted (d) images show a cystic mass (*) with minimal complexity (arrow in c and d). (e) Photograph of the gross pathologic specimen shows a mass with minimal complexity (black arrow) filled with thick fluid (white arrow). (f) High-power photomicrograph shows epithelial proliferation, stratification, and atypia (*), consistent with mucinous borderline tumor. (H-E stain.)
Figure 5f.
Mucinous borderline tumor in a 63-year-old woman with minimal abdominal pain and urinary frequency. (a) Sagittal US image shows a cystic mass (*) superior and anterior to the bladder (BL). (b–d) Sagittal T2-weighted (b), coronal T2-weighted (c), and coronal contrast-enhanced fat-saturated T1-weighted (d) images show a cystic mass (*) with minimal complexity (arrow in c and d). (e) Photograph of the gross pathologic specimen shows a mass with minimal complexity (black arrow) filled with thick fluid (white arrow). (f) High-power photomicrograph shows epithelial proliferation, stratification, and atypia (*), consistent with mucinous borderline tumor. (H-E stain.)
The cytologic features include mild to moderate nuclear enlargement and hyperchromasia, but without high-grade nuclear features (Fig 6). For a tumor to be categorized as mucinous borderline tumor/atypical proliferative mucinous tumor, the proliferative areas must account for at least 10% of epithelial volume. Mitotic index is variable. As with the benign mucinous tumors, mucin granulomas and intrastromal mucin may be seen (1).
Figure 6a.
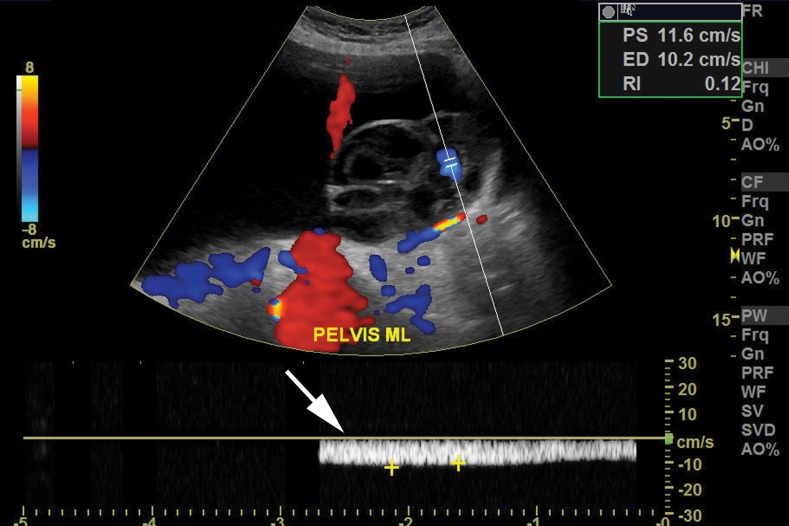
Mucinous borderline tumor in a 58-year-old woman with abdominal distention. (a–c) Sagittal gray-scale (a), color Doppler (b), and spectral (c) US images show a cystic mass (* in a) with vascular flow (arrows in b and c). (d) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (e) Photomicrograph shows nuclear enlargement and hyperchromatic nuclei (arrow), seen in mucinous borderline tumors. (H-E stain; original magnification, ×20.)
Figure 6b.
Mucinous borderline tumor in a 58-year-old woman with abdominal distention. (a–c) Sagittal gray-scale (a), color Doppler (b), and spectral (c) US images show a cystic mass (* in a) with vascular flow (arrows in b and c). (d) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (e) Photomicrograph shows nuclear enlargement and hyperchromatic nuclei (arrow), seen in mucinous borderline tumors. (H-E stain; original magnification, ×20.)
Figure 6c.
Mucinous borderline tumor in a 58-year-old woman with abdominal distention. (a–c) Sagittal gray-scale (a), color Doppler (b), and spectral (c) US images show a cystic mass (* in a) with vascular flow (arrows in b and c). (d) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (e) Photomicrograph shows nuclear enlargement and hyperchromatic nuclei (arrow), seen in mucinous borderline tumors. (H-E stain; original magnification, ×20.)
Figure 6d.
Mucinous borderline tumor in a 58-year-old woman with abdominal distention. (a–c) Sagittal gray-scale (a), color Doppler (b), and spectral (c) US images show a cystic mass (* in a) with vascular flow (arrows in b and c). (d) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (e) Photomicrograph shows nuclear enlargement and hyperchromatic nuclei (arrow), seen in mucinous borderline tumors. (H-E stain; original magnification, ×20.)
Figure 6e.
Mucinous borderline tumor in a 58-year-old woman with abdominal distention. (a–c) Sagittal gray-scale (a), color Doppler (b), and spectral (c) US images show a cystic mass (* in a) with vascular flow (arrows in b and c). (d) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (e) Photomicrograph shows nuclear enlargement and hyperchromatic nuclei (arrow), seen in mucinous borderline tumors. (H-E stain; original magnification, ×20.)
There is a subset of mucinous borderline tumors/atypical proliferative mucinous tumors that demonstrate foci with marked nuclear atypia or confluent growth patterns such as cribriform glands confined to the epithelium. This subset has been termed mucinous borderline tumor/atypical proliferative mucinous tumor with intraepithelial carcinoma (1). Areas of confluent growth must be less than 5 mm2 to be considered intraepithelial; larger areas of confluent proliferation are considered malignant.
Another subset of mucinous borderline tumors/atypical proliferative mucinous tumors show small foci of stromal invasion that measure less than 5 mm in greatest linear extent. This subset is termed mucinous borderline tumor/atypical proliferative mucinous tumor with microinvasion. The microinvasive areas may include single cells, glands, clusters, nests, or small foci of confluent glandular or cribiform growth displaying mild to moderate atypia (1). When the small foci of stromal invasion show marked atypia, the pathologic diagnosis is microinvasive carcinoma (1,22).
One or more mural nodules can be seen within mucinous borderline tumor/atypical proliferative mucinous tumor. The nodules are sharply demarcated from the mucinous epithelium and highly variable in size, from microscopic up to 10 cm. Three types of mural nodules have been described: reactive sarcoma-like nodules, anaplastic carcinoma, and sarcomatous nodules.
Reactive sarcoma-like nodules tend to be hemorrhagic, whereas neoplastic nodules are usually solid and white. Reactive sarcoma-like nodules contain a heterogeneous cell population including multinucleated giant cells, histiocytes, and mixed inflammatory cells in a background of atypical spindle cells. These nodules are benign, likely represent a response to hemorrhage or mucin, and do not affect prognosis (1,23). The neoplastic nodules are discussed in the mucinous carcinoma section.
As with benign mucinous tumors, KRAS mutations are the most frequent genetic abnormality, occurring in 30%–75% of tumors (1,20).
Mucinous Carcinoma
The WHO defines mucinous carcinoma as a malignant tumor composed of gastrointestinal-type cells containing intracytoplasmic mucin (1).
At gross analysis, mucinous carcinomas are large unilateral masses with both solid and cystic components. In most cases, the tumor is confined to the ovary. Large tumors may result in rupture, spilling tumor and mucin into the peritoneal cavity (1) (Fig 7). If pseudomyxoma peritonei is detected intraoperatively without tumor rupture, the most likely cause is an appendiceal mucinous neoplasm. Primary appendiceal mucinous neoplasms usually masquerade as unilateral primary ovarian mucinous borderline tumors with pseudomyxoma peritonei. The appendix can appear grossly normal, and the appendiceal primary tumor may be histologically subtle (24) (Fig 8).
Figure 7a.
Mucinous cystadenocarcinoma in a 31-year-old woman with acute severe abdominal pain. (a) Axial contrast-enhanced CT image shows an enhancing mass in the right adnexa (black *). Note the normal left ovary (arrow) and ascites (white *). (b) Intraoperative photograph shows rupture of the tumor capsule (arrow), as can be seen with mucinous cystadenocarcinoma. (c) Photograph of the gross pathologic specimen shows a solid and cystic mass containing viscous material (arrow). (d) High-power photomicrograph shows back-to-back glands lined by malignant-appearing cells with little or no intervening stroma (*), characteristic of the confluent glandular/expansile invasive pattern seen in mucinous cystadenocarcinoma. (H-E stain.)
Figure 8a.
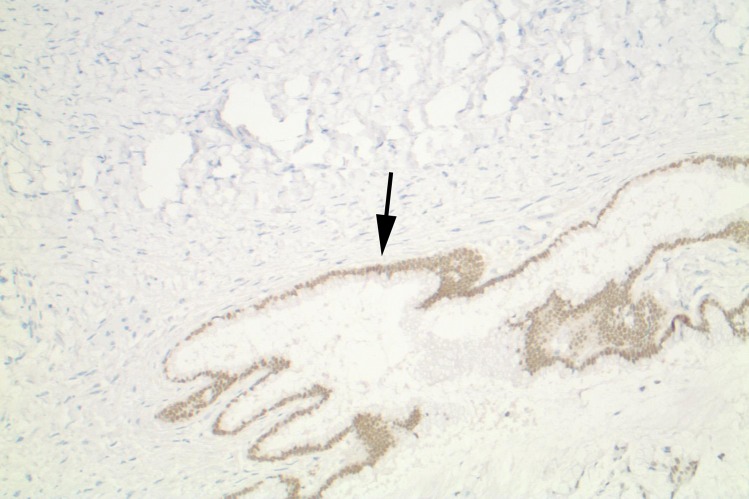
Appendiceal mucinous adenocarcinoma mimicking an ovarian mucinous neoplasm in a 58-year-old woman with increasing abdominal distention over the course of 1 year. (a, b) Sagittal gray-scale (a) and color Doppler (b) US images show a heterogeneous mass (* in a) in the midabdomen with internal flow (arrow in b). (c, d) Axial (c) and coronal (d) contrast-enhanced CT images show the extent of the cystic mass (white *) and areas suspicious for pseudomyxoma peritonei (black * in d). (e) Photograph of the gross pathologic specimen shows the cystic ovarian mass (*). (f) Photomicrograph shows mucin within glands (*). (H-E stain; original magnification, ×100.) (g) Photomicrograph shows positive CDX2 staining in the lining of the glands (arrow), which is specific for adenocarcinoma arising from the gastrointestinal tract. (Original magnification, ×100.)
Figure 7b.
Mucinous cystadenocarcinoma in a 31-year-old woman with acute severe abdominal pain. (a) Axial contrast-enhanced CT image shows an enhancing mass in the right adnexa (black *). Note the normal left ovary (arrow) and ascites (white *). (b) Intraoperative photograph shows rupture of the tumor capsule (arrow), as can be seen with mucinous cystadenocarcinoma. (c) Photograph of the gross pathologic specimen shows a solid and cystic mass containing viscous material (arrow). (d) High-power photomicrograph shows back-to-back glands lined by malignant-appearing cells with little or no intervening stroma (*), characteristic of the confluent glandular/expansile invasive pattern seen in mucinous cystadenocarcinoma. (H-E stain.)
Figure 7c.
Mucinous cystadenocarcinoma in a 31-year-old woman with acute severe abdominal pain. (a) Axial contrast-enhanced CT image shows an enhancing mass in the right adnexa (black *). Note the normal left ovary (arrow) and ascites (white *). (b) Intraoperative photograph shows rupture of the tumor capsule (arrow), as can be seen with mucinous cystadenocarcinoma. (c) Photograph of the gross pathologic specimen shows a solid and cystic mass containing viscous material (arrow). (d) High-power photomicrograph shows back-to-back glands lined by malignant-appearing cells with little or no intervening stroma (*), characteristic of the confluent glandular/expansile invasive pattern seen in mucinous cystadenocarcinoma. (H-E stain.)
Figure 7d.
Mucinous cystadenocarcinoma in a 31-year-old woman with acute severe abdominal pain. (a) Axial contrast-enhanced CT image shows an enhancing mass in the right adnexa (black *). Note the normal left ovary (arrow) and ascites (white *). (b) Intraoperative photograph shows rupture of the tumor capsule (arrow), as can be seen with mucinous cystadenocarcinoma. (c) Photograph of the gross pathologic specimen shows a solid and cystic mass containing viscous material (arrow). (d) High-power photomicrograph shows back-to-back glands lined by malignant-appearing cells with little or no intervening stroma (*), characteristic of the confluent glandular/expansile invasive pattern seen in mucinous cystadenocarcinoma. (H-E stain.)
Figure 8b.
Appendiceal mucinous adenocarcinoma mimicking an ovarian mucinous neoplasm in a 58-year-old woman with increasing abdominal distention over the course of 1 year. (a, b) Sagittal gray-scale (a) and color Doppler (b) US images show a heterogeneous mass (* in a) in the midabdomen with internal flow (arrow in b). (c, d) Axial (c) and coronal (d) contrast-enhanced CT images show the extent of the cystic mass (white *) and areas suspicious for pseudomyxoma peritonei (black * in d). (e) Photograph of the gross pathologic specimen shows the cystic ovarian mass (*). (f) Photomicrograph shows mucin within glands (*). (H-E stain; original magnification, ×100.) (g) Photomicrograph shows positive CDX2 staining in the lining of the glands (arrow), which is specific for adenocarcinoma arising from the gastrointestinal tract. (Original magnification, ×100.)
Figure 8c.
Appendiceal mucinous adenocarcinoma mimicking an ovarian mucinous neoplasm in a 58-year-old woman with increasing abdominal distention over the course of 1 year. (a, b) Sagittal gray-scale (a) and color Doppler (b) US images show a heterogeneous mass (* in a) in the midabdomen with internal flow (arrow in b). (c, d) Axial (c) and coronal (d) contrast-enhanced CT images show the extent of the cystic mass (white *) and areas suspicious for pseudomyxoma peritonei (black * in d). (e) Photograph of the gross pathologic specimen shows the cystic ovarian mass (*). (f) Photomicrograph shows mucin within glands (*). (H-E stain; original magnification, ×100.) (g) Photomicrograph shows positive CDX2 staining in the lining of the glands (arrow), which is specific for adenocarcinoma arising from the gastrointestinal tract. (Original magnification, ×100.)
Figure 8d.

Appendiceal mucinous adenocarcinoma mimicking an ovarian mucinous neoplasm in a 58-year-old woman with increasing abdominal distention over the course of 1 year. (a, b) Sagittal gray-scale (a) and color Doppler (b) US images show a heterogeneous mass (* in a) in the midabdomen with internal flow (arrow in b). (c, d) Axial (c) and coronal (d) contrast-enhanced CT images show the extent of the cystic mass (white *) and areas suspicious for pseudomyxoma peritonei (black * in d). (e) Photograph of the gross pathologic specimen shows the cystic ovarian mass (*). (f) Photomicrograph shows mucin within glands (*). (H-E stain; original magnification, ×100.) (g) Photomicrograph shows positive CDX2 staining in the lining of the glands (arrow), which is specific for adenocarcinoma arising from the gastrointestinal tract. (Original magnification, ×100.)
Figure 8e.

Appendiceal mucinous adenocarcinoma mimicking an ovarian mucinous neoplasm in a 58-year-old woman with increasing abdominal distention over the course of 1 year. (a, b) Sagittal gray-scale (a) and color Doppler (b) US images show a heterogeneous mass (* in a) in the midabdomen with internal flow (arrow in b). (c, d) Axial (c) and coronal (d) contrast-enhanced CT images show the extent of the cystic mass (white *) and areas suspicious for pseudomyxoma peritonei (black * in d). (e) Photograph of the gross pathologic specimen shows the cystic ovarian mass (*). (f) Photomicrograph shows mucin within glands (*). (H-E stain; original magnification, ×100.) (g) Photomicrograph shows positive CDX2 staining in the lining of the glands (arrow), which is specific for adenocarcinoma arising from the gastrointestinal tract. (Original magnification, ×100.)
Figure 8f.
Appendiceal mucinous adenocarcinoma mimicking an ovarian mucinous neoplasm in a 58-year-old woman with increasing abdominal distention over the course of 1 year. (a, b) Sagittal gray-scale (a) and color Doppler (b) US images show a heterogeneous mass (* in a) in the midabdomen with internal flow (arrow in b). (c, d) Axial (c) and coronal (d) contrast-enhanced CT images show the extent of the cystic mass (white *) and areas suspicious for pseudomyxoma peritonei (black * in d). (e) Photograph of the gross pathologic specimen shows the cystic ovarian mass (*). (f) Photomicrograph shows mucin within glands (*). (H-E stain; original magnification, ×100.) (g) Photomicrograph shows positive CDX2 staining in the lining of the glands (arrow), which is specific for adenocarcinoma arising from the gastrointestinal tract. (Original magnification, ×100.)
Figure 8g.
Appendiceal mucinous adenocarcinoma mimicking an ovarian mucinous neoplasm in a 58-year-old woman with increasing abdominal distention over the course of 1 year. (a, b) Sagittal gray-scale (a) and color Doppler (b) US images show a heterogeneous mass (* in a) in the midabdomen with internal flow (arrow in b). (c, d) Axial (c) and coronal (d) contrast-enhanced CT images show the extent of the cystic mass (white *) and areas suspicious for pseudomyxoma peritonei (black * in d). (e) Photograph of the gross pathologic specimen shows the cystic ovarian mass (*). (f) Photomicrograph shows mucin within glands (*). (H-E stain; original magnification, ×100.) (g) Photomicrograph shows positive CDX2 staining in the lining of the glands (arrow), which is specific for adenocarcinoma arising from the gastrointestinal tract. (Original magnification, ×100.)
At histologic evaluation, there is a continuum of architectural and cytologic atypia that includes benign, borderline, and carcinomatous elements. Two different types of invasion may be seen at microscopic evaluation of mucinous carcinomas: the confluent glandular/expansile invasive pattern and the destructive stromal invasive pattern. The confluent glandular/expansile invasive pattern shows marked glandular crowding with little intervening stroma. The destructive stromal invasive pattern shows irregular glands, nests, and single cells with malignant cytologic features infiltrating the stroma. The stroma may show desmoplastic changes.
The destructive stromal invasive pattern is the less common pattern. The presence of this pattern, especially in both ovaries, should prompt a search for an extraovarian primary mucinous neoplasm. High mitotic activity with abnormal mitotic figures is common in mucinous carcinoma (1).
As with mucinous borderline tumor/atypical proliferative mucinous tumor, anaplastic carcinoma or sarcomatous mural nodules may be seen. Mural nodules of anaplastic carcinoma show large rhabdoid cells with abundant eosinophilic cytoplasm, sarcomatoid spindle cells with a herringbone pattern, and pleomorphic cells. Anaplastic carcinoma may invade the adjacent tissues and less commonly the vascular spaces. Sarcomatous mural nodules are malignant spindle cell proliferations that do not express epithelial markers but may be rhabdomyosarcoma, fibrosarcoma, undifferentiated sarcoma, or mixed patterns.
There are reports of nodules with features of both anaplastic carcinoma and sarcoma. Reactivity to cytokeratin may help differentiate anaplastic carcinoma nodules from sarcomatous nodules (1). Sarcomatous nodules have a poorer prognosis than anaplastic carcinoma nodules for stage IA disease.
The heterogeneous nature of mucinous carcinoma, often coexisting with benign and borderline mucinous elements, suggests that mucinous carcinoma develops in a stepwise fashion from benign mucinous cystadenoma to mucinous borderline tumor/atypical proliferative mucinous tumor and then to mucinous carcinoma (Fig 9). Less commonly, mucinous carcinoma may arise from teratoma or Brenner tumor (1).
Figure 9a.
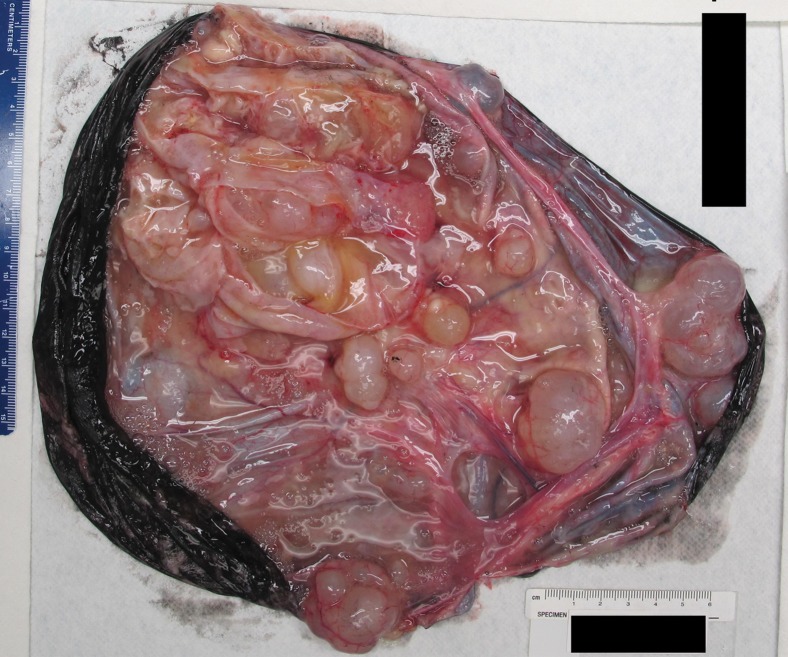
Borderline mucinous tumor arising from mucinous cystadenoma in a 41-year-old woman with abdominal distention. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a multiloculated cystic mass (black *). Note the ascites (white * in b). (c) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (d) Photomicrograph shows benign mucinous cystadenoma (arrow) and borderline mucinous cystadenoma (*). (H-E stain; original magnification, ×4.)
Figure 9b.
Borderline mucinous tumor arising from mucinous cystadenoma in a 41-year-old woman with abdominal distention. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a multiloculated cystic mass (black *). Note the ascites (white * in b). (c) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (d) Photomicrograph shows benign mucinous cystadenoma (arrow) and borderline mucinous cystadenoma (*). (H-E stain; original magnification, ×4.)
Figure 9c.
Borderline mucinous tumor arising from mucinous cystadenoma in a 41-year-old woman with abdominal distention. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a multiloculated cystic mass (black *). Note the ascites (white * in b). (c) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (d) Photomicrograph shows benign mucinous cystadenoma (arrow) and borderline mucinous cystadenoma (*). (H-E stain; original magnification, ×4.)
Figure 9d.
Borderline mucinous tumor arising from mucinous cystadenoma in a 41-year-old woman with abdominal distention. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a multiloculated cystic mass (black *). Note the ascites (white * in b). (c) Photograph of the gross pathologic specimen shows a multiloculated cystic mass. (d) Photomicrograph shows benign mucinous cystadenoma (arrow) and borderline mucinous cystadenoma (*). (H-E stain; original magnification, ×4.)
As in benign and borderline mucinous ovarian tumors, the most common genetic alteration in mucinous carcinoma is a somatic KRAS mutation (20). HER2 amplification is seen in 15%–20% of mucinous carcinomas, often in tumors without KRAS mutations (1,25).
Imaging Findings
Imaging is important in detection, characterization, and follow-up of mucinous ovarian neoplasms.
Detection and Characterization
The American College of Radiology (ACR) appropriateness criteria recommend pelvic US, typically using transabdominal, transvaginal, and color or power Doppler techniques, as the imaging modality of choice in patients with signs and symptoms suggestive of an adnexal mass (26). US is useful in determining the anatomic origin of the adnexal mass. Gynecologic organ–based adnexal masses may arise from the ovary, paraovarian tissues, or uterus. US also excels for determining the composition of a mass. Adnexal masses may be cystic, solid, or complex.
US is useful in diagnosis of common benign cystic adnexal masses, avoiding the need for surgical management. When characteristic imaging findings are present, US is excellent for diagnosing simple cysts, hemorrhagic cysts, endometriomas, and mature cystic teratomas (27). Transvaginal US is highly sensitive for detection of malignant adnexal masses, with reported sensitivity greater than 90% (28,29). Transvaginal pelvic US with color Doppler imaging may also be used to distinguish benign from malignant disease, with reported sensitivity of 99.1% and specificity of 85.9% (30).
The imaging findings of ovarian mucinous neoplasms reflect the findings at gross pathologic analysis. The US appearance of a mucinous ovarian neoplasm is a cystic mass of varying complexity (31–34) (Figs 3, 5). Mucinous ovarian neoplasms are often large and may require transabdominal imaging to view the full extent of the mass (Fig 6). They tend to be multilocular, containing small cystic components or honeycomb-like loculi (32) (Fig 10). The cysts tend to have smooth walls (34).
Figure 10a.
Mucinous borderline tumor in a 55-year-old woman with increasing abdominal bloating and early satiety. (a, b) Sagittal US images show a cystic mass (white *) in the right adnexa. Note the size of the mass compared with the uterus (black * in a), (c–f) Sagittal (c) and axial (d) T2-weighted, axial fat-saturated T1-weighted (e), and axial contrast-enhanced fat-saturated T1-weighted (f) images show a multiloculated cystic mass (* in c and d) with high-signal-intensity cysts at T1-weighted imaging (* in e) and enhancement (arrow in f). (g) Photomicrograph shows multiple cysts (*) filled with mucin, corresponding to the cysts seen at imaging. (H-E stain; original magnification, ×2.)
Figure 10b.
Mucinous borderline tumor in a 55-year-old woman with increasing abdominal bloating and early satiety. (a, b) Sagittal US images show a cystic mass (white *) in the right adnexa. Note the size of the mass compared with the uterus (black * in a), (c–f) Sagittal (c) and axial (d) T2-weighted, axial fat-saturated T1-weighted (e), and axial contrast-enhanced fat-saturated T1-weighted (f) images show a multiloculated cystic mass (* in c and d) with high-signal-intensity cysts at T1-weighted imaging (* in e) and enhancement (arrow in f). (g) Photomicrograph shows multiple cysts (*) filled with mucin, corresponding to the cysts seen at imaging. (H-E stain; original magnification, ×2.)
Figure 10c.
Mucinous borderline tumor in a 55-year-old woman with increasing abdominal bloating and early satiety. (a, b) Sagittal US images show a cystic mass (white *) in the right adnexa. Note the size of the mass compared with the uterus (black * in a), (c–f) Sagittal (c) and axial (d) T2-weighted, axial fat-saturated T1-weighted (e), and axial contrast-enhanced fat-saturated T1-weighted (f) images show a multiloculated cystic mass (* in c and d) with high-signal-intensity cysts at T1-weighted imaging (* in e) and enhancement (arrow in f). (g) Photomicrograph shows multiple cysts (*) filled with mucin, corresponding to the cysts seen at imaging. (H-E stain; original magnification, ×2.)
Figure 10d.
Mucinous borderline tumor in a 55-year-old woman with increasing abdominal bloating and early satiety. (a, b) Sagittal US images show a cystic mass (white *) in the right adnexa. Note the size of the mass compared with the uterus (black * in a), (c–f) Sagittal (c) and axial (d) T2-weighted, axial fat-saturated T1-weighted (e), and axial contrast-enhanced fat-saturated T1-weighted (f) images show a multiloculated cystic mass (* in c and d) with high-signal-intensity cysts at T1-weighted imaging (* in e) and enhancement (arrow in f). (g) Photomicrograph shows multiple cysts (*) filled with mucin, corresponding to the cysts seen at imaging. (H-E stain; original magnification, ×2.)
Figure 10e.
Mucinous borderline tumor in a 55-year-old woman with increasing abdominal bloating and early satiety. (a, b) Sagittal US images show a cystic mass (white *) in the right adnexa. Note the size of the mass compared with the uterus (black * in a), (c–f) Sagittal (c) and axial (d) T2-weighted, axial fat-saturated T1-weighted (e), and axial contrast-enhanced fat-saturated T1-weighted (f) images show a multiloculated cystic mass (* in c and d) with high-signal-intensity cysts at T1-weighted imaging (* in e) and enhancement (arrow in f). (g) Photomicrograph shows multiple cysts (*) filled with mucin, corresponding to the cysts seen at imaging. (H-E stain; original magnification, ×2.)
Figure 10f.
Mucinous borderline tumor in a 55-year-old woman with increasing abdominal bloating and early satiety. (a, b) Sagittal US images show a cystic mass (white *) in the right adnexa. Note the size of the mass compared with the uterus (black * in a), (c–f) Sagittal (c) and axial (d) T2-weighted, axial fat-saturated T1-weighted (e), and axial contrast-enhanced fat-saturated T1-weighted (f) images show a multiloculated cystic mass (* in c and d) with high-signal-intensity cysts at T1-weighted imaging (* in e) and enhancement (arrow in f). (g) Photomicrograph shows multiple cysts (*) filled with mucin, corresponding to the cysts seen at imaging. (H-E stain; original magnification, ×2.)
Figure 10g.
Mucinous borderline tumor in a 55-year-old woman with increasing abdominal bloating and early satiety. (a, b) Sagittal US images show a cystic mass (white *) in the right adnexa. Note the size of the mass compared with the uterus (black * in a), (c–f) Sagittal (c) and axial (d) T2-weighted, axial fat-saturated T1-weighted (e), and axial contrast-enhanced fat-saturated T1-weighted (f) images show a multiloculated cystic mass (* in c and d) with high-signal-intensity cysts at T1-weighted imaging (* in e) and enhancement (arrow in f). (g) Photomicrograph shows multiple cysts (*) filled with mucin, corresponding to the cysts seen at imaging. (H-E stain; original magnification, ×2.)
The echogenicity of the intralocular fluid varies on the basis of the mucinous elements of the cyst contents. Cysts filled with thick proteinaceous mucin often demonstrate low-level echoes (34). Calcifications are rare but when present tend to be linear. Papillary projections into the cyst lumen are a rare finding (32). In the setting of cyst rupture, mucinous fluid may be seen in the abdomen and/or pelvis (32).
MRI may be used to further characterize a cystic mass detected at US. The main reason for performing MRI of a known cystic ovarian lesion is to fully image and evaluate a mass that is too large to be adequately imaged with US (34). MRI may also be useful in determining the site of origin (ie, ovarian vs uterine vs metastasis) and for further characterization of masses with indeterminate features at US (33). MRI has sensitivity and specificity greater than or equal to 94% for distinguishing benign from malignant disease in the setting of an indeterminate adnexal mass (35).
In the setting of a mucinous cystic neoplasm, MRI demonstrates a large, unilateral, multiloculated cystic mass of varying complexity (31) (Figs 2, 5). The thickness of the cyst wall and septa and the presence of internal solid components or nodules are nicely demonstrated at postcontrast imaging, typically with T1-weighted fat-saturated sequences (Figs 3, 4). The signal intensity of the contents of the cyst loculi varies on T1- and T2-weighted images, depending on their internal composition (34). Thick mucinous material may increase the T1 signal intensity of the cyst contents and decrease the T2 signal intensity (31,32) (Fig 10).
CT is not recommended in initial evaluation of a suspected adnexal mass (26). In the setting of an indeterminate complex cystic mass, CT may be useful in confirming the diagnosis of a mature cystic teratoma by demonstrating internal macroscopic fat and coarse calcifications/teeth (36). When a mucinous cystic neoplasm is imaged using CT, the imaging findings again include a large, unilateral, complex cystic mass (Figs 3, 9). The attenuation of the fluid within the cyst loculi is variable (34). The rare internal linear calcifications are well seen at CT (32).
Imaging findings have been reported that suggest malignancy over benignity in the setting of an ovarian epithelial neoplasm. Features suggestive of malignancy include a thick irregular wall, thick septa, papillary projections, and a large soft-tissue component with necrosis (32,34). Ancillary findings of pelvic organ invasion; spread to the peritoneum, omentum, or mesentery; ascites; and adenopathy are also suggestive of malignancy (32). These findings apply to epithelial neoplasms as a whole and are not specific to the subset of epithelial neoplasms that are mucinous neoplasms. In fact, the large majority of mucinous ovarian neoplasms are benign (80%) or borderline (16%–17%) at pathologic analysis (1).
Specifically, papillary projections are a feature more strongly associated with serous tumors rather than mucinous tumors. Ascites in the setting of a mucinous tumor may represent pseudomyxoma peritonei rather than malignant ascites related to carcinomatosis (31,32). In the setting of pseudomyxoma peritonei and an ovarian mass, an appendiceal-origin mucinous tumor should be considered, even if the appendiceal tumor is not visible at imaging (24).
Staging/Treatment Planning
Staging of ovarian cancer, including mucinous ovarian neoplasms, is done using the FIGO system (37). FIGO staging is a surgical staging system, but the prognosis of ovarian cancer is based on a combination of histologic type, radiographic findings, and operative extent of the disease (37). Precise histopathologic diagnosis is a prerequisite for categorization and treatment of ovarian cancers, as different histologic types respond differently to treatment.
FIGO stage I disease includes tumor confined to the ovaries or fallopian tubes. Stage II disease includes tumors that involve one or both ovaries with pelvic extension that remains below the pelvic brim. Stage III disease includes tumors that have spread to the peritoneum outside the pelvis and/or to retroperitoneal lymph nodes. Stage IV disease includes the presence of distant metastases other than to the peritoneum or a malignant pleural effusion (37).
Although advanced cross-sectional imaging, such as CT, is not specifically recommended by the FIGO staging system, it is a common practice in resource-rich countries such as the United States (Table 2) (38). Imaging with CT, fluorine 18 (18F) fluorodeoxyglucose (FDG) PET/CT, and/or MRI may assist in initial treatment planning by allowing assessment of the resectability of tumors, the candidacy of patients for effective cytoreductive surgery, the need for neoadjuvant or postoperative chemotherapy if debulking is suboptimal, and the need for referral to a gynecologic oncologist (38).
Table 2:
CT, MRI, and Fluorine 18 (18F) PET/CT in the Setting of Ovarian Mucinous Carcinoma
Note.—FDG = fluorodeoxyglucose, FOV = field of view.
CT of the abdomen and pelvis with intravenous and oral contrast material is the imaging modality of choice in initial staging of pretreatment ovarian cancer (38) (Fig 7). CT is useful for detecting metastases to the peritoneum, omentum, mesentery, liver, spleen, lymph nodes, and lungs (39,40). CT has been reported to be 94% accurate in staging of ovarian cancer (38,41). CT is limited in detection of small (<5 mm) metastases, especially to the bowel surface, mesentery, and peritoneum (38). CT of the chest is useful for detecting pleural and pulmonary metastases at initial staging (38). Although MRI has been shown to have equivalent accuracy to CT for staging ovarian cancer, MRI remains primarily a problem-solving technique (38).
18F FDG PET/CT is a valuable tool for staging advanced ovarian cancer, with higher reported accuracy than CT alone (38). Specifically, 18F PET/CT may increase the rate of detection of small metastases, particularly to the peritoneum or lymph nodes. These metastases may be too small to detect at CT but become apparent at PET/CT when metabolically active. Unfortunately, false negatives have been reported with mucinous carcinoma (42).
Follow-up
The role of surveillance imaging in patients in clinical remission is unclear (38). The National Comprehensive Cancer Network (NCCN) guidelines for follow-up of patients with complete response to primary chemotherapy include imaging as needed, using CT, 18F FDG PET/CT, MRI, or chest radiography (38,43).
In the setting of clinically suspected tumor recurrence, CT with oral and intravenous contrast material is the imaging modality of choice (38). CT has reported sensitivity and specificity for tumor recurrence of 58%–84% and 59%–100%, respectively (38). The major limitation of CT is limited sensitivity for small (<5 mm) metastases to the mesentery, peritoneum, and bowel surface.
MRI is less accurate than CT or PET/CT for detecting recurrent ovarian cancer (38). MRI is mainly used to further assess inconclusive CT findings or when iodinated intravenous contrast material cannot be administered. 18F FDG PET/CT shows at least comparable accuracy to that of CT for detection of ovarian cancer recurrence (44). Importantly, false-negative results may occur with PET/CT in the setting of mucinous carcinoma (38,42).
Conclusion
Mucinous ovarian neoplasms are an important subset of ovarian epithelial neoplasms. Mucinous ovarian neoplasms may be benign, borderline, or malignant at pathologic analysis, but the vast majority are benign or borderline. The imaging findings parallel the gross pathologic findings, which include a large unilateral cystic mass that is usually multilocular, with variable appearance of the cyst fluid owing to differences in the mucin content. Mucinous ovarian neoplasms are staged and treated surgically and have a favorable prognosis.
Supported by the American Institute for Radiologic Pathology, the Joint Pathology Center, and Uniformed Services University of the Health Sciences. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Defense or the U.S. Government.
For this journal-based SA-CME activity, the author B.A.C. has provided disclosures all other authors, the editor, and the reviewers have disclosed no relevant relationships.
Disclosures of Conflicts of Interest.—: B.A.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: board member for American Society of Cytopathology and College of American Pathologists. Other activities: disclosed no relevant relationships.
Abbreviations:
- FIGO
- International Federation of Gynecology and Obstetrics
- H-E
- hematoxylin-eosin
- WHO
- World Health Organization
References
- 1.Kurman RJ, Carcangiu ML, Herrington S, Young RH. World Health Organization classification of tumours of the female reproductive organs. 4th ed Lyon, France: IARC, 2014. [Google Scholar]
- 2.Prat J, D’Angelo E, Espinosa I. Ovarian carcinomas: at least five different diseases with distinct histological features and molecular genetics. Hum Pathol 2018;80:11–27. [DOI] [PubMed] [Google Scholar]
- 3.Lee KR, Young RH. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. Am J Surg Pathol 2003;27(3):281–292. [DOI] [PubMed] [Google Scholar]
- 4.Berek JS, Hacker NF. Berek & Hacker’s gynecologic oncology. Philadelphia, Pa: Lippincott Williams & Wilkins, 2015. [Google Scholar]
- 5.Riopel MA, Ronnett BM, Kurman RJ. Evaluation of diagnostic criteria and behavior of ovarian intestinal-type mucinous tumors: atypical proliferative (borderline) tumors and intraepithelial, microinvasive, invasive, and metastatic carcinomas. Am J Surg Pathol 1999;23(6):617–635. [DOI] [PubMed] [Google Scholar]
- 6.Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol 2003;27(7):985–993. [DOI] [PubMed] [Google Scholar]
- 7.Tuxen MK, Sölétormos G, Dombernowsky P. Tumor markers in the management of patients with ovarian cancer. Cancer Treat Rev 1995;21(3):215–245. [DOI] [PubMed] [Google Scholar]
- 8.Engelen MJ, de Bruijn HW, Hollema H, et al. Serum CA 125, carcinoembryonic antigen, and CA 19-9 as tumor markers in borderline ovarian tumors. Gynecol Oncol 2000;78(1):16–20. [DOI] [PubMed] [Google Scholar]
- 9.Stenchever MA, Droegemueller W, Herbst AL, Mishell D. Comprehensive gynecology. 4th ed. St Louis, Mo: Mosby, 2001. [Google Scholar]
- 10.Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol 2004;23(1):41–44. [DOI] [PubMed] [Google Scholar]
- 11.Lee KR, Scully RE. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with “pseudomyxoma peritonei.” Am J Surg Pathol 2000;24(11):1447–1464. [DOI] [PubMed] [Google Scholar]
- 12.Schmeler KM, Tao X, Frumovitz M, et al. Prevalence of lymph node metastasis in primary mucinous carcinoma of the ovary. Obstet Gynecol 2010;116(2 Pt 1):269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JE, Seo S, Kushner DM, Rose SL. The role of appendectomy for mucinous ovarian neoplasms. Am J Obstet Gynecol 2013;208(1):46.e1–46.e4. [DOI] [PubMed] [Google Scholar]
- 14.Barnhill DR, Kurman RJ, Brady MF, et al. Preliminary analysis of the behavior of stage I ovarian serous tumors of low malignant potential: a Gynecologic Oncology Group study. J Clin Oncol 1995;13(11):2752–2756. [DOI] [PubMed] [Google Scholar]
- 15.Hart WR, Norris HJ. Borderline and malignant mucinous tumors of the ovary: histologic criteria and clinical behavior. Cancer 1973;31(5):1031–1045. [DOI] [PubMed] [Google Scholar]
- 16.Kurman RJ, Shih IM. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol 2016;186(4):733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess V, A’Hern R, Nasiri N, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol 2004;22(6):1040–1044. [DOI] [PubMed] [Google Scholar]
- 18.Winter WE, 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 2007;25(24):3621–3627. [DOI] [PubMed] [Google Scholar]
- 19.Frumovitz M, Schmeler KM, Malpica A, Sood AK, Gershenson DM. Unmasking the complexities of mucinous ovarian carcinoma. Gynecol Oncol 2010;117(3):491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuatrecasas M, Villanueva A, Matias-Guiu X, Prat J. K-ras mutations in mucinous ovarian tumors: a clinicopathologic and molecular study of 95 cases. Cancer 1997;79 (8):1581–1586. [DOI] [PubMed] [Google Scholar]
- 21.Yemelyanova AV, Vang R, Judson K, Wu LS, Ronnett BM. Distinction of primary and metastatic mucinous tumors involving the ovary: analysis of size and laterality data by primary site with reevaluation of an algorithm for tumor classification. Am J Surg Pathol 2008;32(1):128–138. [DOI] [PubMed] [Google Scholar]
- 22.Ronnett BM, Kajdacsy-Balla A, Gilks CB, et al. Mucinous borderline ovarian tumors: points of general agreement and persistent controversies regarding nomenclature, diagnostic criteria, and behavior. Hum Pathol 2004;35 (8):949–960. [DOI] [PubMed] [Google Scholar]
- 23.Bagué S, Rodríguez IM, Prat J. Sarcoma-like mural nodules in mucinous cystic tumors of the ovary revisited: a clinicopathologic analysis of 10 additional cases. Am J Surg Pathol 2002;26(11):1467–1476. [DOI] [PubMed] [Google Scholar]
- 24.Rouzbahman M, Chetty R. Mucinous tumours of appendix and ovary: an overview and evaluation of current practice. J Clin Pathol 2014;67(3):193–197. [DOI] [PubMed] [Google Scholar]
- 25.Anglesio MS, Kommoss S, Tolcher MC, et al. Molecular characterization of mucinous ovarian tumours supports a stratified treatment approach with HER2 targeting in 19% of carcinomas. J Pathol 2013;229(1):111–120. [DOI] [PubMed] [Google Scholar]
- 26.Atri M, Alabousi A, Reinhold C, et al. ACR appropriateness criteria: clinically suspected adnexal mass, no acute symptoms. https://acsearch.acr.org/docs/69466/Narrative/. Accessed November 12, 2018. [DOI] [PubMed]
- 27.Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q 2010;26(3):121–131. [DOI] [PubMed] [Google Scholar]
- 28.Amor F, Vaccaro H, Alcázar JL, León M, Craig JM, Martinez J. Gynecologic Imaging Reporting and Data System: a new proposal for classifying adnexal masses on the basis of sonographic findings. J Ultrasound Med 2009;28(3):285–291. [DOI] [PubMed] [Google Scholar]
- 29.Lucidarme O, Akakpo JP, Granberg S, et al. A new computer-aided diagnostic tool for non-invasive characterisation of malignant ovarian masses: results of a multicentre validation study. Eur Radiol 2010;20(8):1822–1830. [DOI] [PubMed] [Google Scholar]
- 30.Amor F, Alcázar JL, Vaccaro H, León M, Iturra A. GI-RADS reporting system for ultrasound evaluation of adnexal masses in clinical practice: a prospective multicenter study. Ultrasound Obstet Gynecol 2011;38(4):450–455. [DOI] [PubMed] [Google Scholar]
- 31.Jeong YY, Outwater EK, Kang HK. Imaging evaluation of ovarian masses. RadioGraphics 2000;20(5):1445–1470. [DOI] [PubMed] [Google Scholar]
- 32.Jung SE, Lee JM, Rha SE, Byun JY, Jung JI, Hahn ST. CT and MR imaging of ovarian tumors with emphasis on differential diagnosis. RadioGraphics 2002;22(6):1305–1325. [DOI] [PubMed] [Google Scholar]
- 33.Togashi K. Ovarian cancer: the clinical role of US, CT, and MRI. Eur Radiol 2003;13(suppl 4):L87–L104. [DOI] [PubMed] [Google Scholar]
- 34.Wagner BJ, Buck JL, Seidman JD, McCabe KM. From the archives of the AFIP: ovarian epithelial neoplasms—radiologic-pathologic correlation. RadioGraphics 1994;14(6):1351–1374; quiz 1375–1376. [DOI] [PubMed] [Google Scholar]
- 35.Adusumilli S, Hussain HK, Caoili EM, et al. MRI of sonographically indeterminate adnexal masses. AJR Am J Roentgenol 2006;187(3):732–740. [DOI] [PubMed] [Google Scholar]
- 36.Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. RadioGraphics 2001;21(2):475–490. [DOI] [PubMed] [Google Scholar]
- 37.Mutch DG, Prat J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol 2014;133(3):401–404. [DOI] [PubMed] [Google Scholar]
- 38.Kang SK, Reinhold C, Atri M, et al. ACR appropriateness criteria: staging and follow-up of ovarian cancer. https://acsearch.acr.org/docs/69378/Narrative/. Accessed November 15, 2018. [DOI] [PubMed]
- 39.Chandrashekhara SH, Thulkar S, Srivastava DN, et al. Pre-operative evaluation of peritoneal deposits using multidetector computed tomography in ovarian cancer. Br J Radiol 2011;84(997):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akin O, Sala E, Moskowitz CS, et al. Perihepatic metastases from ovarian cancer: sensitivity and specificity of CT for the detection of metastases with and those without liver parenchymal invasion. Radiology 2008;248(2):511–517. [DOI] [PubMed] [Google Scholar]
- 41.Forstner R, Hricak H, Occhipinti KA, Powell CB, Frankel SD, Stern JL. Ovarian cancer: staging with CT and MR imaging. Radiology 1995;197(3):619–626. [DOI] [PubMed] [Google Scholar]
- 42.Alessi A, Martinelli F, Padovano B, et al. FDG-PET/CT to predict optimal primary cytoreductive surgery in patients with advanced ovarian cancer: preliminary results. Tumori 2016;102(1):103–107. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong DK, Alvarez RD, et al. National Comprehensive Cancer Network guidelines version 2.2018: ovarian cancer. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Published 2018. Accessed November 12, 2018.
- 44.Tawakol A, Abdelhafez YG, Osama A, Hamada E, El Refaei S. Diagnostic performance of 18F-FDG PET/contrast-enhanced CT versus contrast-enhanced CT alone for post-treatment detection of ovarian malignancy. Nucl Med Commun 2016;37(5):453–460. [DOI] [PubMed] [Google Scholar]