Abstract
Pelvic masses can present a diagnostic challenge owing to the difficulty in assessing their origin and the overlap in imaging features. The majority of pelvic tumors arise from gastrointestinal or genitourinary organs, with less common sites of origin including the connective tissues, nerves, and lymphovascular structures. Lesion evaluation usually starts with clinical assessment followed by imaging, or the lesion may be an incidental finding at imaging performed for other clinical indications. Since accurate diagnosis is essential for optimal management, imaging is useful for suggesting the correct diagnosis or narrowing the differential possibilities and distinguishing tumors from their mimics. Some masses may require histologic confirmation of the diagnosis with biopsy and/or up-front surgical resection. In this case, imaging is essential for presurgical planning to assess mass size and location, evaluate the relationship to adjacent pelvic structures, and narrow differential possibilities. Pelvic US is often the first imaging modality performed in women with pelvic symptoms. While US is often useful to detect a pelvic mass, it has significant limitations in assessing masses located deep in the pelvis or near gas-filled organs. CT also has limited value in the pelvis owing to its inferior soft-tissue contrast. MRI is frequently the optimal imaging modality, as it offers both multiplanar capability and excellent soft-tissue contrast. This article highlights the normal anatomy of the pelvic spaces in the female pelvis and focuses on MRI features of common tumors and tumor mimics that arise in these spaces. It provides an interpretative algorithm for approaching an unknown pelvic lesion at MRI. It also discusses surgical management, emphasizing the value of MRI as a road map to surgery and highlighting anatomic locations where surgical resection may present a challenge.
©RSNA, 2019
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ List tumors and tumor mimics that may involve the peritoneal cavity or extraperitoneal pelvic spaces of the female pelvis.
■ Describe the pelvic space and pattern-based approach to characterization of pelvic tumors and tumor mimics with MRI.
■ Discuss the role of MRI as a road map for surgical management.
Introduction
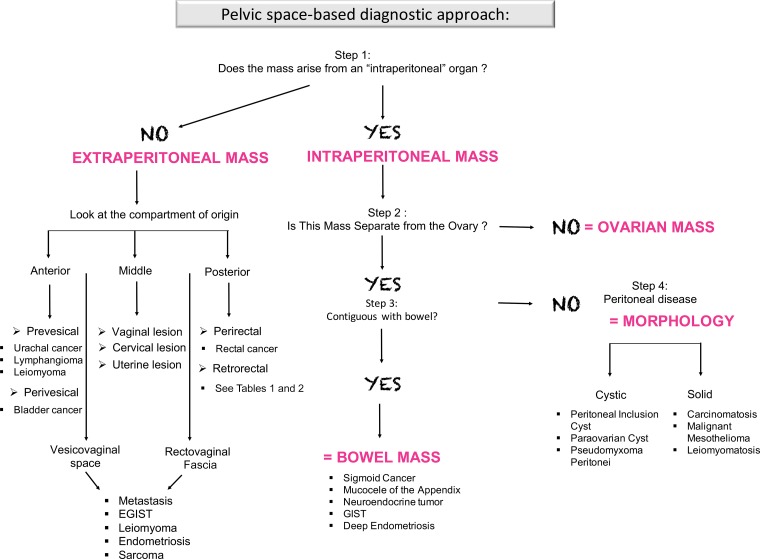
In this article, we describe an interpretation algorithm for evaluating unknown masses in the female pelvis (Fig 1). This approach is rooted in knowledge of the pelvic spaces and key MRI features of common tumors or tumor mimics in the female pelvis. We also discuss surgical management, with emphasis on the anatomic locations where surgical resection may present a challenge.
Figure 1.
Flowchart shows a step-by-step approach to lesion characterization in the female pelvis. First, it is important to determine if a mass is peritoneal or extraperitoneal in location. If the mass arises from one of the intraperitoneal organs or peritoneal cavity, evaluation of the ovaries (in women), the bowel, and tumor morphology is useful to narrow the differential possibilities. If the mass is extraperitoneal in location, it is useful to determine the pelvic space where the mass is situated. EGIST = extragastrointestinal stromal tumor, GIST = gastrointestinal stromal tumor.
The anatomy of the pelvis is complex and may be less well understood than that of the abdomen. As with the abdomen, the pelvis is divided into two main compartments: the peritoneal cavity and subperitoneal space (1). The two spaces are separated by the peritoneum, and each compartment is a continuous space. The subperitoneal space houses all of the abdominal and pelvic organs (and their respective mesenteries), all of the extraperitoneal spaces described herein, as well as ligaments, vessels, nerves, and lymphatics.
The peritoneal cavity is situated between the thin layers of visceral and parietal peritoneum (analogs to the pleura). The visceral peritoneum lines the surfaces of various organs (forming their serosa), and the parietal peritoneum lines the wall of the body cavity. The peritoneal cavity is a potential space devoid of any organs (1,2). However, some organs in the subperitoneal space such as the small bowel, sigmoid colon, and ovaries are nearly enveloped by the visceral peritoneum and suspended by their mesenteries. Thus, the discussion and interpretation algorithm that follow refer to these as “intraperitoneal” organs.
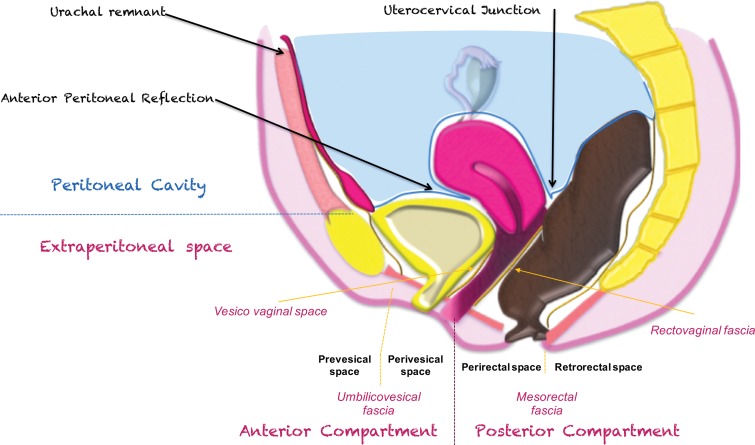
In contrast, other organs in the subperitoneal space are located deep to the peritoneum and are referred to as extraperitoneal organs throughout the article. These include the urinary bladder, ureters, uterine corpus and cervix, vagina, and mid and lower rectum. Some of the extraperitoneal organs such as the urinary bladder and body of the uterus are covered partly but not enclosed by the peritoneum.
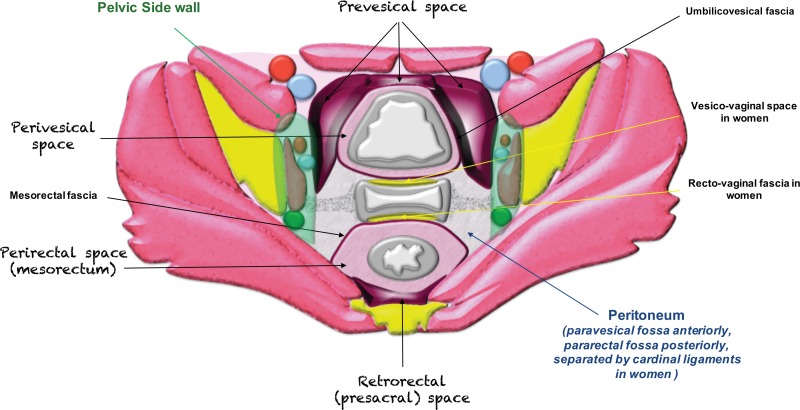
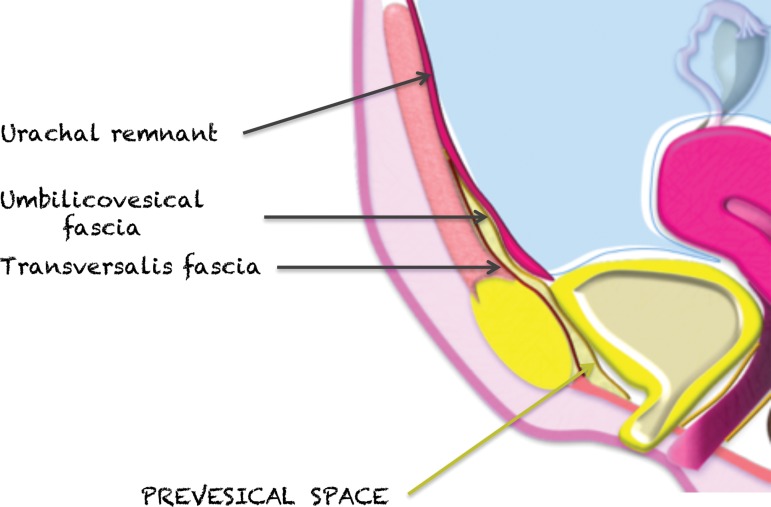
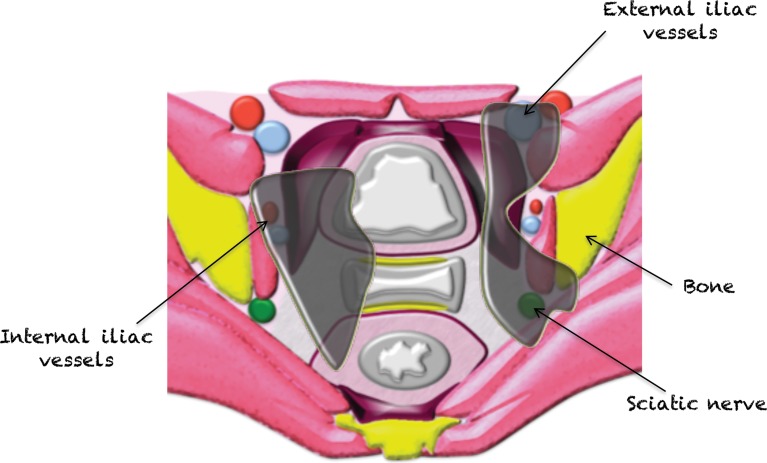
The pelvic extraperitoneum is divided into anterior and posterior portions, with the prevesical and perivesical spaces located anteriorly and the perirectal and retrorectal spaces located posteriorly (Figs 2, 3). In between lies the middle compartment, which includes the vagina, cervix, and uterus. The umbilicovesical fascia encircles the urinary bladder, urachal remnant, and obliterated umbilical arteries into the perivesical space. The umbilicovesical fascia also forms the posterior and lateral margins of the prevesical space.
Figure 2.
Drawing of an axial plane through the female pelvis shows the extraperitoneal spaces.
Figure 3a.
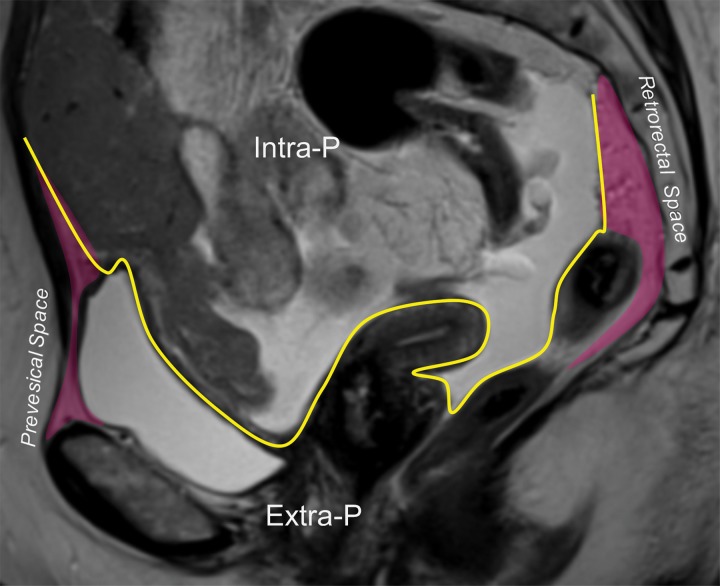
(a) Drawing of a midsagittal plane through the female pelvis shows the peritoneal reflection that divides the pelvis into the peritoneal and extraperitoneal compartments. (b) Sagittal T2-weighted MR image shows the anterior peritoneal reflection (yellow line). The presence of ascites makes it easy to identify the anterior peritoneal reflection. Extra-P = extraperitoneal compartment, Intra-P = intraperitoneal compartment.
Figure 3b.
(a) Drawing of a midsagittal plane through the female pelvis shows the peritoneal reflection that divides the pelvis into the peritoneal and extraperitoneal compartments. (b) Sagittal T2-weighted MR image shows the anterior peritoneal reflection (yellow line). The presence of ascites makes it easy to identify the anterior peritoneal reflection. Extra-P = extraperitoneal compartment, Intra-P = intraperitoneal compartment.
Posteriorly and laterally, the mesorectal fascia surrounds the rectum and defines the perirectal (mesorectal) space. The mesorectal fascia is an aggregate of the rectovaginal fascia anteriorly, the perirectal fascia laterally, and the pelvic fascia posteriorly. The perirectal fascia is also known as the uterosacral ligaments. The most posterior retrorectal (presacral) space is situated posterior to the pelvic fascia (1,2).
Knowledge of the female pelvic anatomy is essential to the step-by-step approach for evaluating an unknown pelvic mass. The first step is assessment of the most likely site of origin of the mass and its location in relation to the peritoneal cavity versus the extraperitoneal spaces. This information is essential to narrow the differential possibilities (or suggest the correct diagnosis) and select the optimal surgical approach.
Stepwise Approach to Lesion Characterization
The stepwise approach to lesion characterization consists of the following considerations: (a) determine the compartment of origin, (b) tumors or tumor mimics arising from intraperitoneal organs, and (c) extraperitoneal spaces and associated tumors or tumor mimics.
Determine the Compartment of Origin
The first step in approaching an unknown pelvic mass is to determine if the lesion originates from one of the intraperitoneal organs (ie, organs enveloped by visceral peritoneum and suspended by their respective mesenteries) or from one of the extraperitoneal organs or spaces (Fig 1), as this decision point is useful in narrowing the diagnostic possibilities.
In the female pelvis, the peritoneum reflects over the dome of the bladder, the uterine corpus, and the rectouterine pouch (of Douglas). Posteriorly, the part of the peritoneum that divides the rectum into its intra- and extraperitoneal portions is also known as the anterior peritoneal reflection (Fig 3). At MRI, the anterior peritoneal reflection is recognized on midsagittal T2-weighted images as a thin hypointense line just posterior to the uterocervical junction (Fig 3) (3). The anterior peritoneal reflection is most readily apparent in the presence of ascites, but may be a challenge to locate in a case of retroverted uterus, in patients with minimal body fat, or after surgery (3).
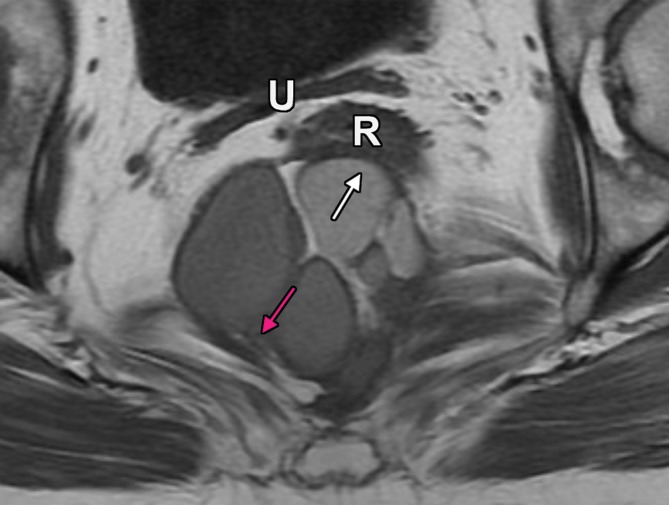
In addition to identifying the anterior peritoneal reflection, it is also useful to look at the displacement of the normal pelvic structures (4). Diagnostic clues that indicate that the mass originates from the extraperitoneum are as follows (Fig 4): (a) anterior or central displacement of extraperitoneal organs (eg, uterus or rectum), (b) anterior or central displacement of the iliac vessels or iliopsoas muscles or encasement of the iliac vessels, and (c) mass effect on or effacement of pelvic sidewall muscles.
Figure 4a.
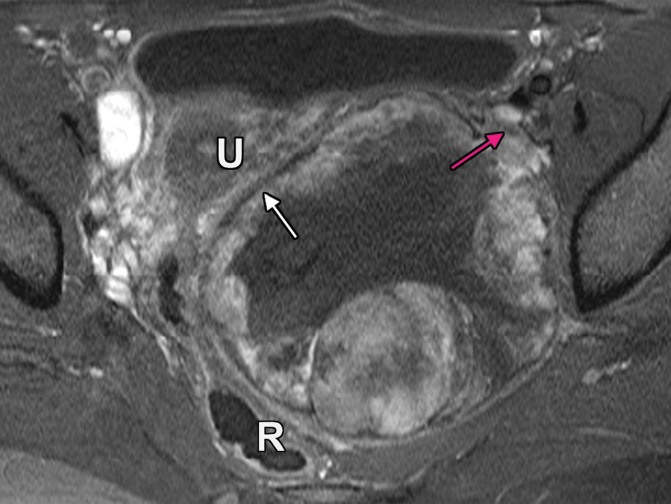
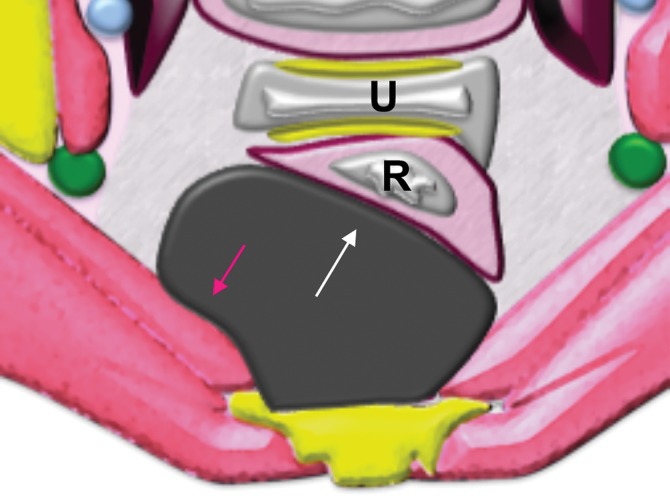
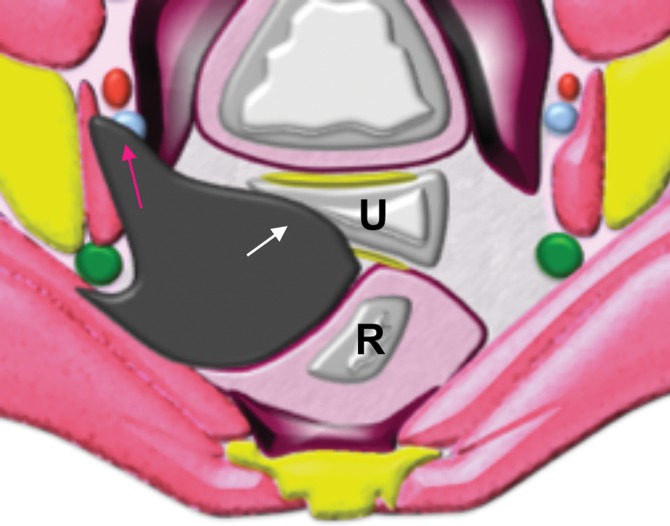
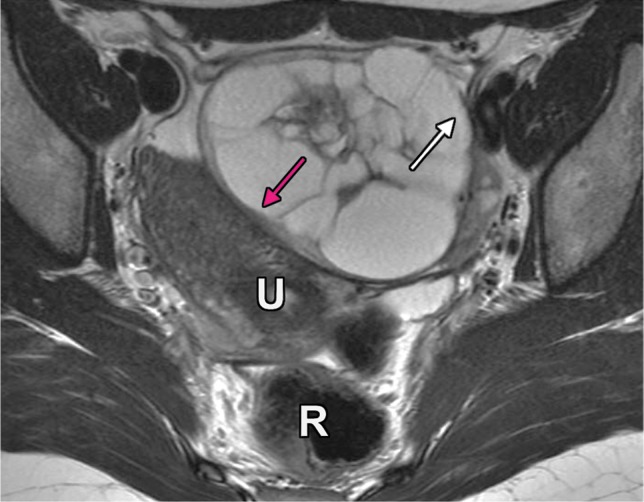
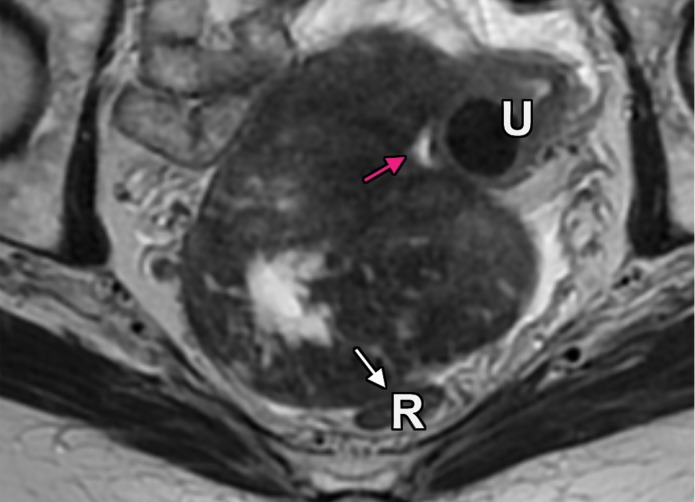
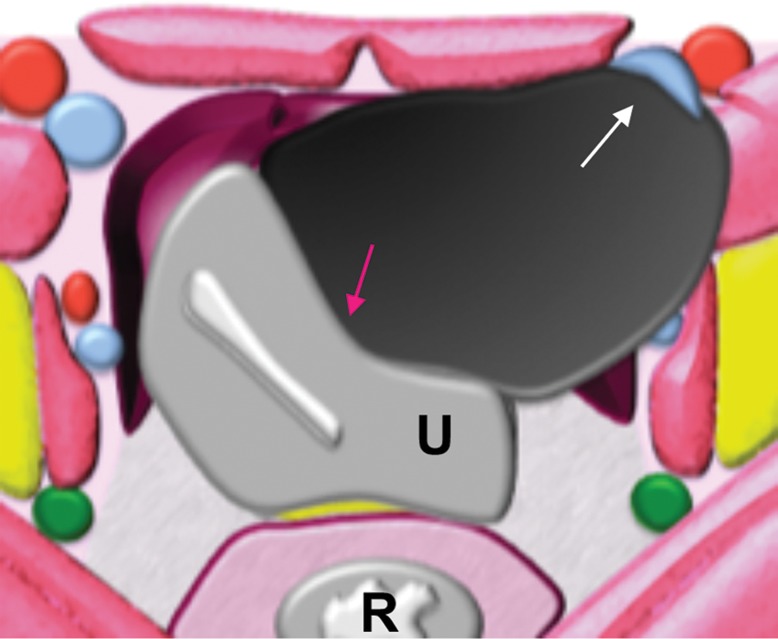
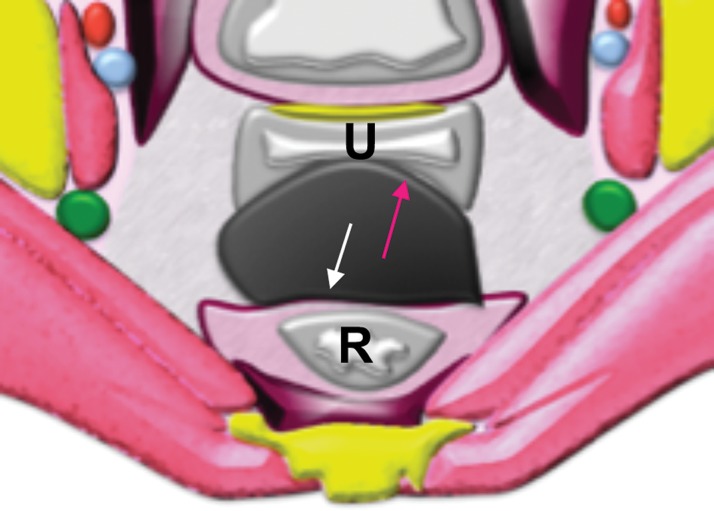
Extraperitoneal pelvic masses. R =rectum, U = uterus. (a) Axial T2-weighted image shows a multicystic retrorectal lesion that displaces the rectum anteriorly (white arrow) and effaces the right iliococcygeal muscle (pink arrow), consistent with an extraperitoneal mass (tailgut cyst). (b) Axial contrast-enhanced MR image shows a chordoma in the retrorectal space, causing anterior and central displacement of the uterus (white arrow) and anterior displacement of the left internal iliac vessels (pink arrow). (c, d) Imaging features of pelvic masses that arise in the extraperitoneal space. (c) Drawing shows anterior and central displacement of the rectum by an extraperitoneal mass (white arrow) with mass effect on the pelvic muscles (pink arrow). (d) Drawing shows central displacement of the uterus (white arrow) and anterior displacement of the right iliac vessels (pink arrow).
Figure 4b.
Extraperitoneal pelvic masses. R =rectum, U = uterus. (a) Axial T2-weighted image shows a multicystic retrorectal lesion that displaces the rectum anteriorly (white arrow) and effaces the right iliococcygeal muscle (pink arrow), consistent with an extraperitoneal mass (tailgut cyst). (b) Axial contrast-enhanced MR image shows a chordoma in the retrorectal space, causing anterior and central displacement of the uterus (white arrow) and anterior displacement of the left internal iliac vessels (pink arrow). (c, d) Imaging features of pelvic masses that arise in the extraperitoneal space. (c) Drawing shows anterior and central displacement of the rectum by an extraperitoneal mass (white arrow) with mass effect on the pelvic muscles (pink arrow). (d) Drawing shows central displacement of the uterus (white arrow) and anterior displacement of the right iliac vessels (pink arrow).
Figure 4c.
Extraperitoneal pelvic masses. R =rectum, U = uterus. (a) Axial T2-weighted image shows a multicystic retrorectal lesion that displaces the rectum anteriorly (white arrow) and effaces the right iliococcygeal muscle (pink arrow), consistent with an extraperitoneal mass (tailgut cyst). (b) Axial contrast-enhanced MR image shows a chordoma in the retrorectal space, causing anterior and central displacement of the uterus (white arrow) and anterior displacement of the left internal iliac vessels (pink arrow). (c, d) Imaging features of pelvic masses that arise in the extraperitoneal space. (c) Drawing shows anterior and central displacement of the rectum by an extraperitoneal mass (white arrow) with mass effect on the pelvic muscles (pink arrow). (d) Drawing shows central displacement of the uterus (white arrow) and anterior displacement of the right iliac vessels (pink arrow).
Figure 4d.
Extraperitoneal pelvic masses. R =rectum, U = uterus. (a) Axial T2-weighted image shows a multicystic retrorectal lesion that displaces the rectum anteriorly (white arrow) and effaces the right iliococcygeal muscle (pink arrow), consistent with an extraperitoneal mass (tailgut cyst). (b) Axial contrast-enhanced MR image shows a chordoma in the retrorectal space, causing anterior and central displacement of the uterus (white arrow) and anterior displacement of the left internal iliac vessels (pink arrow). (c, d) Imaging features of pelvic masses that arise in the extraperitoneal space. (c) Drawing shows anterior and central displacement of the rectum by an extraperitoneal mass (white arrow) with mass effect on the pelvic muscles (pink arrow). (d) Drawing shows central displacement of the uterus (white arrow) and anterior displacement of the right iliac vessels (pink arrow).
Diagnostic clues that indicate that the mass originates from an intraperitoneal organ or within the peritoneal cavity are as follows (Fig 5): (a) posterior or lateral displacement of the uterus, sigmoid colon, or iliac vessels; and (b) anterior displacement of the uterus and posterior displacement of the rectum, indicating that the mass is located in the rectouterine pouch.
Figure 5a.
Masses arising from intraperitoneal organs or within the peritoneal cavity. R =rectum, U = uterus. (a) Axial T2-weighted image shows posterolateral displacement of the uterus (pink arrow) and lateral deviation of the external iliac vessels (white arrow) by a left ovarian mass. (b) Axial T2-weighted image shows an ovarian fibrothecoma in the rectouterine pouch displacing the uterus anteriorly (pink arrow) and rectum posteriorly (white arrow). (c, d) Imaging features of pelvic masses that arise from intraperitoneal organs. (c) Drawing shows posterior displacement of the uterus (pink arrow) by an intraperitoneal mass and lateral deviation of the external iliac vessels (white arrow). (d) Drawing shows a rectouterine mass displacing the uterus anteriorly (pink arrow) and rectum posteriorly (white arrow).
Figure 5b.
Masses arising from intraperitoneal organs or within the peritoneal cavity. R =rectum, U = uterus. (a) Axial T2-weighted image shows posterolateral displacement of the uterus (pink arrow) and lateral deviation of the external iliac vessels (white arrow) by a left ovarian mass. (b) Axial T2-weighted image shows an ovarian fibrothecoma in the rectouterine pouch displacing the uterus anteriorly (pink arrow) and rectum posteriorly (white arrow). (c, d) Imaging features of pelvic masses that arise from intraperitoneal organs. (c) Drawing shows posterior displacement of the uterus (pink arrow) by an intraperitoneal mass and lateral deviation of the external iliac vessels (white arrow). (d) Drawing shows a rectouterine mass displacing the uterus anteriorly (pink arrow) and rectum posteriorly (white arrow).
Figure 5c.
Masses arising from intraperitoneal organs or within the peritoneal cavity. R =rectum, U = uterus. (a) Axial T2-weighted image shows posterolateral displacement of the uterus (pink arrow) and lateral deviation of the external iliac vessels (white arrow) by a left ovarian mass. (b) Axial T2-weighted image shows an ovarian fibrothecoma in the rectouterine pouch displacing the uterus anteriorly (pink arrow) and rectum posteriorly (white arrow). (c, d) Imaging features of pelvic masses that arise from intraperitoneal organs. (c) Drawing shows posterior displacement of the uterus (pink arrow) by an intraperitoneal mass and lateral deviation of the external iliac vessels (white arrow). (d) Drawing shows a rectouterine mass displacing the uterus anteriorly (pink arrow) and rectum posteriorly (white arrow).
Figure 5d.
Masses arising from intraperitoneal organs or within the peritoneal cavity. R =rectum, U = uterus. (a) Axial T2-weighted image shows posterolateral displacement of the uterus (pink arrow) and lateral deviation of the external iliac vessels (white arrow) by a left ovarian mass. (b) Axial T2-weighted image shows an ovarian fibrothecoma in the rectouterine pouch displacing the uterus anteriorly (pink arrow) and rectum posteriorly (white arrow). (c, d) Imaging features of pelvic masses that arise from intraperitoneal organs. (c) Drawing shows posterior displacement of the uterus (pink arrow) by an intraperitoneal mass and lateral deviation of the external iliac vessels (white arrow). (d) Drawing shows a rectouterine mass displacing the uterus anteriorly (pink arrow) and rectum posteriorly (white arrow).
Occasionally, a large pelvic mass may span both peritoneal and extraperitoneal compartments, and it may not be possible to determine the exact site of origin.
Tumors or Tumor Mimics Arising from Intraperitoneal Organs
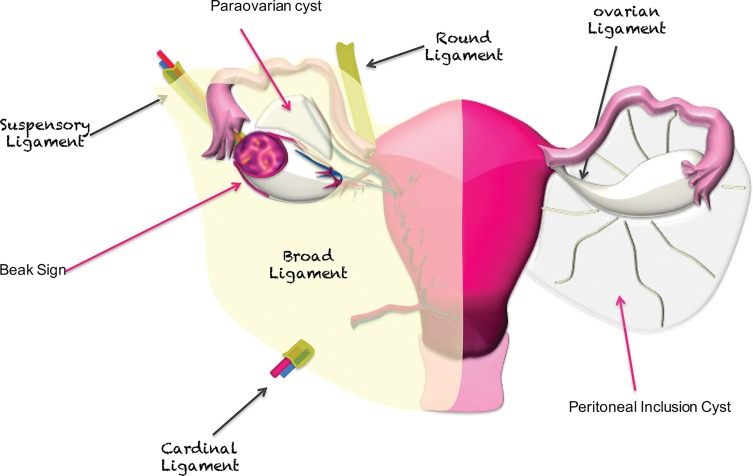
In women, the broad ligaments are paired lateral structures formed by folds of peritoneum and serve as the main ligaments that support pelvic organs (Fig 6) (5). The serosa of the fallopian tubes is continuous with the broad ligament, and the fimbriae of the fallopian tubes open to the peritoneal cavity. The ovaries are surrounded and suspended by the broad ligament (Fig 6). The cardinal ligament (transverse cervical ligament of Mackenrodt) lies at the base of the broad ligament, supporting the cervix and upper vagina and attaching them to the lateral pelvic wall. At the superior aspect of the broad ligament lies the round ligament, which originates at the uterine horns and travels to the inguinal canal (6).
Figure 6.
Main suspensory ligaments of gynecologic organs including the ovaries. The broad ligament is a sheet of peritoneum that folds over and covers the surface of the uterus, fallopian tubes, and ovaries. The ovarian ligament is a fibrous band that lies within the broad ligament and connects each ovary to the uterus. The suspensory ligament is a peritoneal fold that contains ovarian vessels and nerves and extends outward from the ovary to the lateral abdominal wall. The beak sign—sharp angles between the ovary and a mass—is suggestive of ovarian origin. If a lesion is extraovarian, a normal ovary is usually seen adjacent to the mass. Peritoneal inclusion cysts surround the ovary, while paraovarian cysts are located next to but separate from the ovary.
The keywords ovary, bowel, and morphology can remind the radiologist of the key steps for evaluation of an unknown pelvic mass that arises from an intraperitoneal organ or involves the peritoneal cavity (Fig 1).
In women, most pelvic masses are of gynecologic origin and arise from the ovaries or uterus. Thus, it is first useful to distinguish ovarian lesions from extraovarian lesions.
Is the Mass Ovarian or Extraovarian in Origin?
Identify Normal Ovaries.—Identification of a normal ipsilateral ovary excludes ovarian origin of a mass. In contrast, absence of a normal ipsilateral ovary or ovarian follicles around a mass indicate ovarian origin.
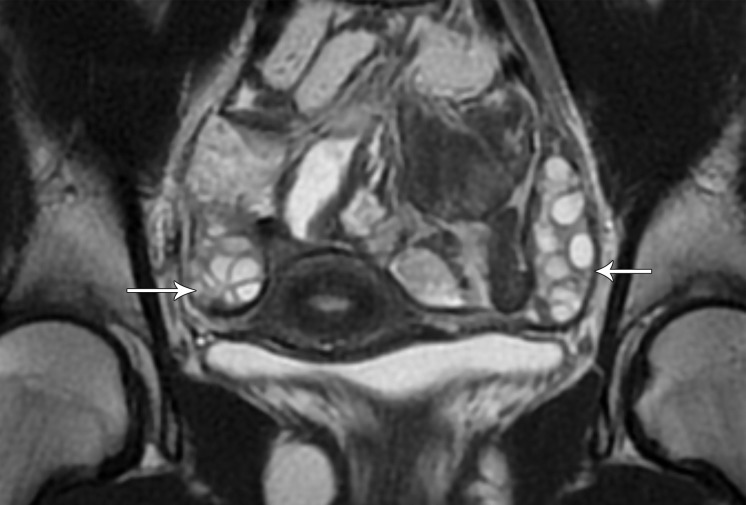
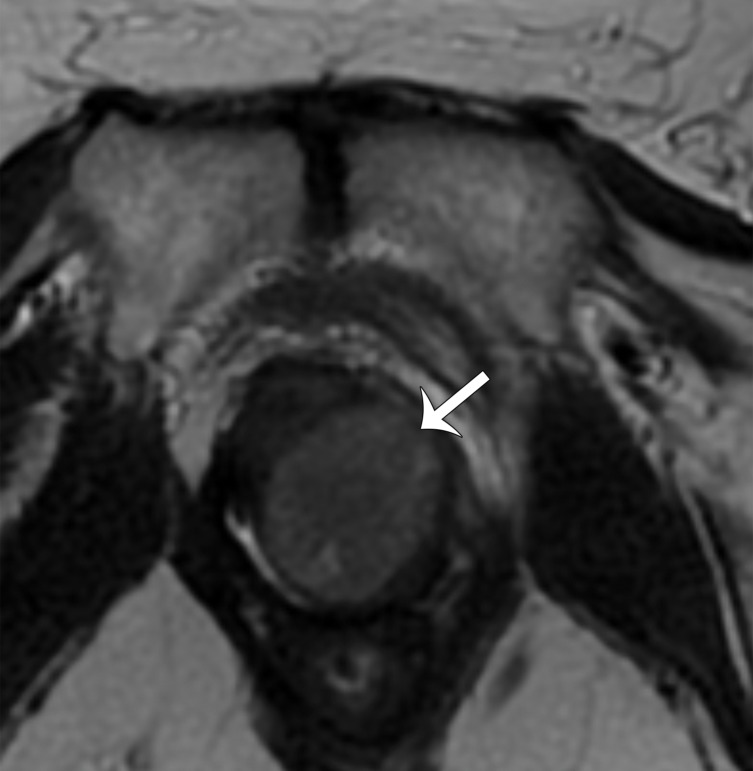
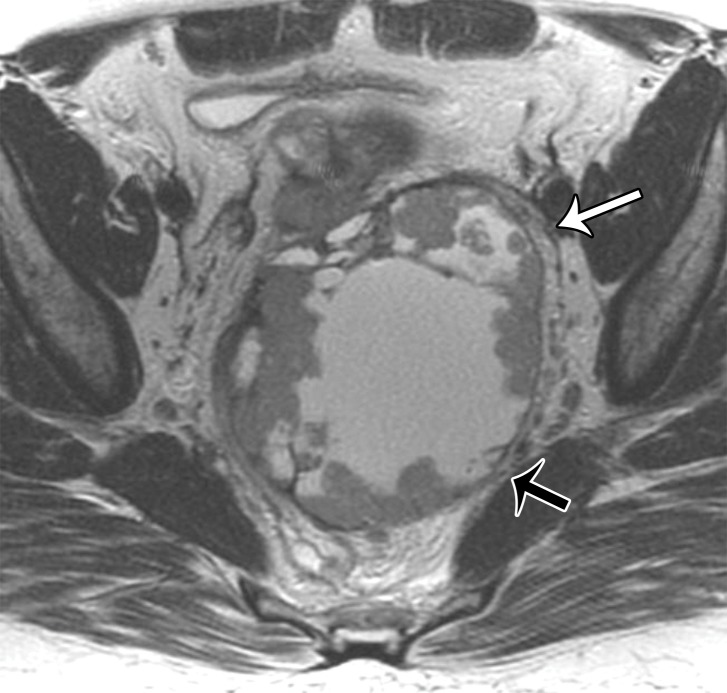
In premenopausal women, the ovaries are usually easily recognized at MRI as ovoid structures with intermediate T2 signal intensity (SI) and with high T2 SI follicles (Fig 7). The ovaries are typically located anterior to the ureters; an ovarian mass may displace the ureters posteriorly (7,8). However, the position of the ovaries is variable between patients and at different times in the same patient owing to ligamentous laxity, especially in parous women.
Figure 7a.
(a) Coronal T2-weighted image in a 15-year-old girl shows multiple ovarian follicles (arrows). (b) Coronal T2-weighted image in a 35-year-old woman shows some ovarian follicles (arrows). (c) Axial T2-weighted image in a 62-year-old woman shows postmenopausal ovaries with homogeneous decreased SI (arrows).
Figure 7b.
(a) Coronal T2-weighted image in a 15-year-old girl shows multiple ovarian follicles (arrows). (b) Coronal T2-weighted image in a 35-year-old woman shows some ovarian follicles (arrows). (c) Axial T2-weighted image in a 62-year-old woman shows postmenopausal ovaries with homogeneous decreased SI (arrows).
Figure 7c.
(a) Coronal T2-weighted image in a 15-year-old girl shows multiple ovarian follicles (arrows). (b) Coronal T2-weighted image in a 35-year-old woman shows some ovarian follicles (arrows). (c) Axial T2-weighted image in a 62-year-old woman shows postmenopausal ovaries with homogeneous decreased SI (arrows).
After menopause, the ovaries may be more difficult to identify because of small size and lack of follicles. At MRI, postmenopausal ovaries demonstrate homogeneous decreased T2 SI compared with the ovaries in premenopausal women (9) (Fig 7).
If a Normal Ipsilateral Ovary Is Not Found, Follow the Gonadal Vessels.—The gonadal (ovarian) vessels are located anterior to the psoas muscles. The right ovarian vein drains into the inferior vena cava; the left ovarian vein drains into the left renal vein. If a gonadal vein joins a pelvic mass, the mass is ovarian in origin (10,11).
If a Mass Abuts the Ovary, Look for Its Relationship with the Ovary.—Several previously described imaging signs help evaluate the relationship of a mass with the adjacent organs (10,12). The beak sign is defined as sharp angles between the ovary and a mass, with the mass deforming the edges of the ovary into a beak shape. The beak sign suggests that the mass is of ovarian origin (Fig 6) (10,12). In contrast, the “bridging vessel” sign and claw sign indicate that the mass originates from the uterus. The bridging vessel sign refers to the vessels extending between the uterus and the mass, as seen with pedunculated uterine leiomyomas (13). The claw sign corresponds to the uterine tissue draping over the mass and is also typically seen with uterine leiomyomas.
In women, uterine leiomyomas (fibroids) are the most common benign gynecologic tumors, with a lifetime prevalence of over 70% (14). At MRI, nondegenerated leiomyomas appear as well-defined low T2 SI masses with homogeneous enhancement similar to that of the outer myometrium. Uterine leiomyomas usually have low SI on high-b-value diffusion-weighted images and apparent diffusion coefficient (ADC) maps, a sign known as the “T2 blackout effect” (15,16). However, the MRI appearance of leiomyomas can vary owing to various types of degeneration or to histologic variants (with cellular leiomyoma being the most common) (17). Extrauterine leiomyomas are rare but can arise anywhere in the pelvis and have MRI features similar to those of their uterine counterparts (18).
In Case of an Ovarian Mass, What Is the Most Likely Diagnosis?—Primary ovarian tumors are divided into epithelial, germ cell, and sex cord–stromal tumors on the basis of their cell of origin. Epithelial ovarian tumors account for 90% of primary ovarian cancers, occur most often in postmenopausal women, and often manifest as advanced-stage disease. In contrast, germ cell tumors and epithelial borderline tumors are more frequent in premenopausal women (19).
An adnexal mass is usually first detected with pelvic US. If a mass cannot be characterized at US as definitely benign or definitely malignant, MRI is the preferred imaging modality for further characterization of a sonographically indeterminate mass (20). Once it is established that the mass arises from the ovary (as described earlier), the diagnostic approach for further lesion characterization with MRI is based on patient age, elevated levels of tumor markers, SI of the lesion on T1-weighted images without and with fat saturation and on T2-weighted images, and lesion morphology (20–22). The details of this topic are beyond the scope of this article; the reader is referred to one of several recent review articles for more in-depth discussion (20,23).
If the Mass Is Extraovarian, Does It Arise from or Is It Associated with the Bowel?
If the ovary is not involved, one should consider if the mass arises from the bowel, with sigmoid cancer being the most common entity.
Sigmoid Cancer.—Adenocarcinoma of the sigmoid colon classically manifests as a circumferential mass that narrows the bowel lumen. Occasionally, a primary colonic tumor can metastasize to the ovaries or directly invade adjacent organs including the ovaries or uterus, making it a challenge to accurately assess its origin. Rarely, acute sigmoid diverticulitis can also involve the ovaries and manifest as a tubo-ovarian abscess.
Padidar et al (24) found that the presence of fluid and engorged vessels in the sigmoid mesentery favors acute diverticulitis. In contrast, enlarged paracolic lymph nodes are an imaging finding of colon cancer (24). In some situations, it may not be possible to reliably distinguish acute diverticulitis from colon cancer at imaging; in this case, histologic sampling is needed after acute inflammation/infection is treated with antibiotics.
At MRI, the classic appearance of tubo-ovarian abscess is an enlarged ovary with a thick-walled fluid-intensity mass with internal gas bubbles, better depicted on T1-weighted images. Ascites and peritoneal fat stranding may be present (24).
Mucocele of the Appendix.—Mucocele of the appendix is a distended appendix filled with mucin and has a reported prevalence of less than 1% among patients who undergo appendectomy. It is more common in middle-aged women (female-to-male ratio, 4:1) and is usually asymptomatic before being discovered incidentally at imaging or surgery (25). There are four histologic subtypes of appendiceal mucocele: retention cyst, mucosal hyperplasia, cystadenoma, and cystadenocarcinoma. Preoperative diagnosis is important to avoid intraoperative rupture and potential subsequent development of pseudomyxoma peritonei (peritoneal seeding by mucinous cystadenocarcinoma with tumor cells producing abundant mucin) (26).
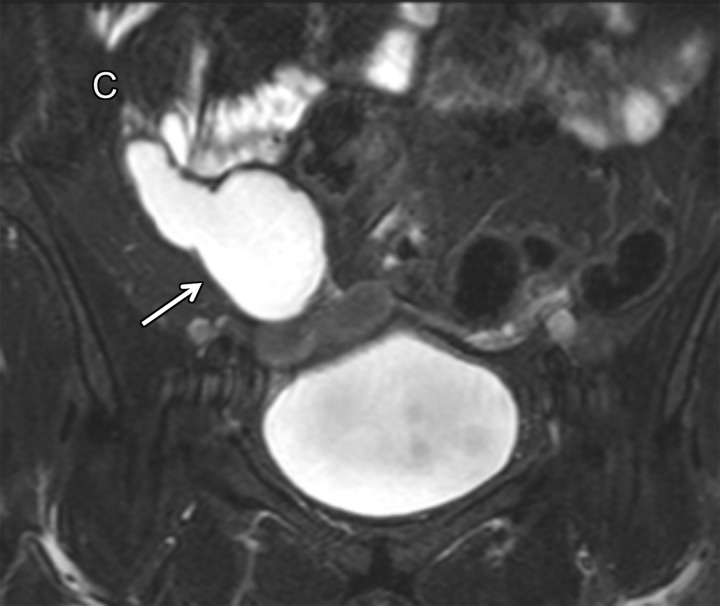
MRI findings are a high T2 SI (fluid SI) tubular structure connected to the base of the cecum (Fig 8) (27). Irregular mural thickening or enhancing nodules in the dilated appendix should raise suspicion for a cystadenocarcinoma. When a mucocele is associated with appendicitis, the clinical manifestation and imaging findings can mimic those of acute appendicitis without mucocele (27).
Figure 8.
Coronal T2-weighted fat-suppressed image shows a high SI tubular cystic lesion (arrow) in the right lower quadrant, consistent with a mucocele of the appendix. C = cecum.
Neuroendocrine Tumor.—Gastrointestinal neuroendocrine tumors, also known as carcinoid tumors, are most frequent in the small intestine, particularly the distal ileum, followed by the colorectum (28). Detection of these tumors is on the rise, possibly owing to the increase in use of endoscopic and imaging studies.
Carcinoid tumor is classically a small avidly enhancing mass in the distal ileum. While the primary tumor is often occult at imaging, mesenteric masses representing nodal metastases or direct tumor extension are often readily apparent. The primary tumor may be seen at MRI as a small, intermediate T2 SI, avidly enhancing mass or eccentric wall thickening. The associated mesenteric masses show avid enhancement and spiculated margins due to desmoplastic reaction (dense fibrosis around the tumor) with or without tethering and/or retraction of adjacent small bowel loops (28). Calcifications are not readily detected at MRI but are present in up to 70% of cases at CT.
Gastrointestinal Stromal Tumor.—Gastrointestinal stromal tumors (GISTs) are the most common nonepithelial tumors of the gastrointestinal tract and arise from the intestinal cells of Cajal. Nearly all GISTs express activating mutations in KIT, a tyrosine kinase growth factor receptor, a useful feature for distinguishing GIST from other mesenchymal tumors (eg, leiomyoma or leiomyosarcoma) (29).
GISTs can occur anywhere along the gastrointestinal tract, most commonly in the stomach and small bowel (29). Approximately 10% of GISTs occur in the colon and rectum (29). Extragastrointestinal stromal tumors (EGISTs) are rare. Prognosis varies on the basis of the location, with tumors located more distally along the gastrointestinal tract or outside the gastrointestinal tract (EGISTs) behaving more aggressively (29).
MRI features of GISTs are variable, with most appearing as large well-defined exophytic masses with intermediate T2 SI and heterogeneous enhancement due to central necrosis and hemorrhage (Fig 9). Small GISTs (<5 cm) often appear as round tumors with early avid homogeneous and persistent enhancement. In contrast, large GISTs (>5 cm) manifest as lobulated tumors with mild gradual heterogeneous enhancement, intratumoral cystic change, and mucosal ulceration (30). While hepatic and peritoneal metastases are common, lymph node involvement is unusual. The MRI appearance of rectal GIST often overlaps with that of other rectal malignancies; however, invasion of adjacent organs, bowel obstruction, and regional lymphadenopathy are uncommon with GIST (31).
Figure 9a.
GIST. (a) Sagittal T2-weighted image shows a mass with intermediate SI (white arrow). Note that the mass is located above the anterior peritoneal reflection (black arrow). (b) Axial T2-weighted image shows that the mass arises from the ileum (arrow). (c) Axial contrast-enhanced fat-suppressed T1-weighted image shows poor enhancement of the mass (white arrow) and communication of the mass with the ileum (black arrow). Results of pathologic analysis were consistent with a GIST arising from the distal ileum.
Figure 9b.
GIST. (a) Sagittal T2-weighted image shows a mass with intermediate SI (white arrow). Note that the mass is located above the anterior peritoneal reflection (black arrow). (b) Axial T2-weighted image shows that the mass arises from the ileum (arrow). (c) Axial contrast-enhanced fat-suppressed T1-weighted image shows poor enhancement of the mass (white arrow) and communication of the mass with the ileum (black arrow). Results of pathologic analysis were consistent with a GIST arising from the distal ileum.
Figure 9c.
GIST. (a) Sagittal T2-weighted image shows a mass with intermediate SI (white arrow). Note that the mass is located above the anterior peritoneal reflection (black arrow). (b) Axial T2-weighted image shows that the mass arises from the ileum (arrow). (c) Axial contrast-enhanced fat-suppressed T1-weighted image shows poor enhancement of the mass (white arrow) and communication of the mass with the ileum (black arrow). Results of pathologic analysis were consistent with a GIST arising from the distal ileum.
Deep Pelvic Endometriosis.—Endometriosis is a common mimic of both cystic and solid pelvic masses. Involvement of the gastrointestinal tract manifests as deep endometriosis or as adhesions causing tethering of bowel loops with or without ovarian involvement. The vast majority of endometriosis cases affect the rectosigmoid junction and are found in the context of retrocervical deep endometriosis (32–34). In such cases, enhancing solid nodular or fan-shaped lesions demonstrate predominantly low SI on T2-weighted images. Small foci of high SI best seen on T1-weighted fat-suppressed images allow specific diagnosis (32–34).
Sites that are less commonly involved by endometriosis include the sigmoid colon, cecum, and ileum. Bowel endometriosis is typically associated with other sites of endometriosis, particularly with endometriomas of the ovaries (32–34). Thus, the combination of MRI findings and childbearing age usually allows correct diagnosis of bowel endometriosis even in cases of circumferential or multisegment involvement.
If the Mass Does Not Involve the Ovary or Bowel, Does It Involve the Peritoneal Cavity?
If the mass does not involve the ovary or bowel but does involve the peritoneal cavity, the next step is to determine whether it is cystic or solid.
Cystic Masses
Cystic masses or tumor mimics of the peritoneum include benign peritoneal inclusion cysts and paraovarian cysts and malignant processes such as pseudomyxoma peritonei. Fallopian tube disease such as hydrosalpinx, pyosalpinx, and hematosalpinx can also manifest as a cystic pelvic mass, but can usually be distinguished from the aforementioned entities with MRI by identifying the tubular nature of the structure (35).
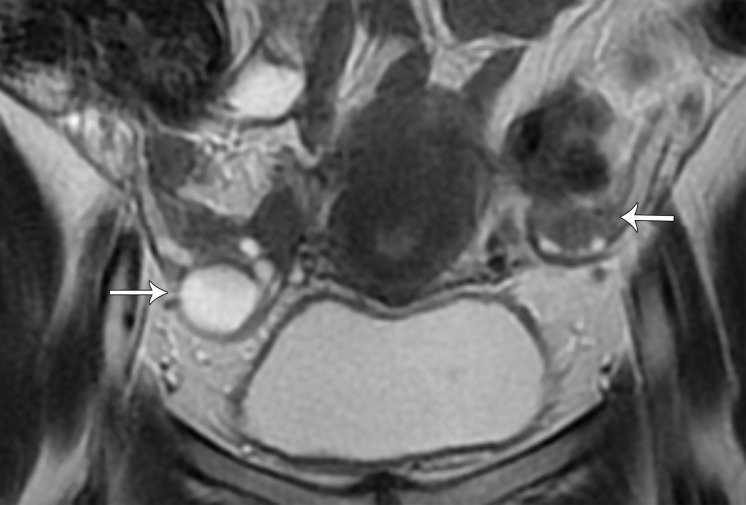
Peritoneal Inclusion Cyst.—Peritoneal inclusion cysts are benign reactive proliferations of mesothelial cells in response to a peritoneal insult (36). Prior surgery, endometriosis, and pelvic inflammatory disease are the most common causes of a peritoneal inclusion cyst. One of the roles of the peritoneum is to reabsorb fluid; however, peritoneal infection or mechanical injury can result in pelvic adhesions and reduced fluid absorption. Peritoneal inclusion cysts are seen largely in premenopausal women with functional ovaries, although they can occasionally be diagnosed after menopause (37). Cysts vary in size and tend to grow slowly as more fluid is secreted by the ovaries and not reabsorbed adequately by the impaired peritoneum (37).
Clues for diagnosis are as follows:
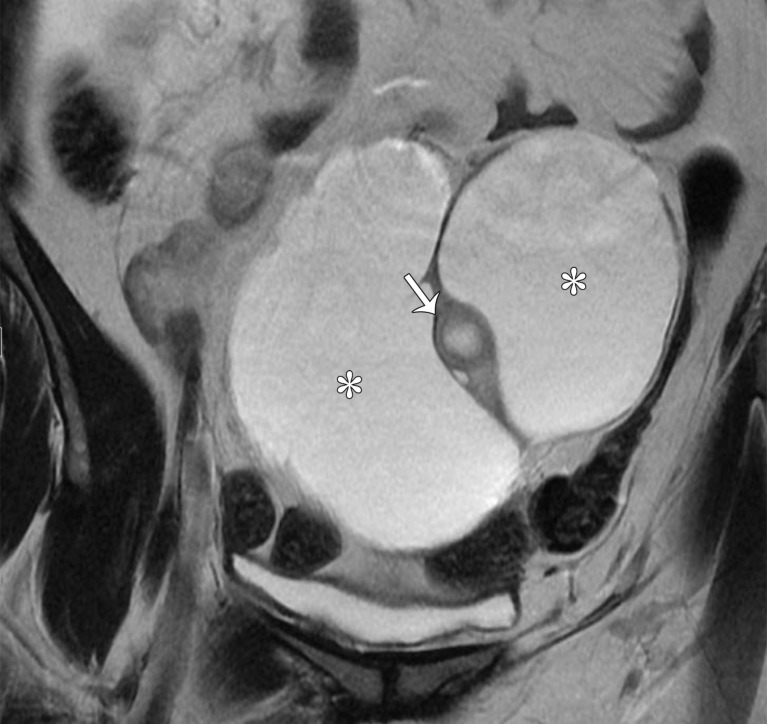
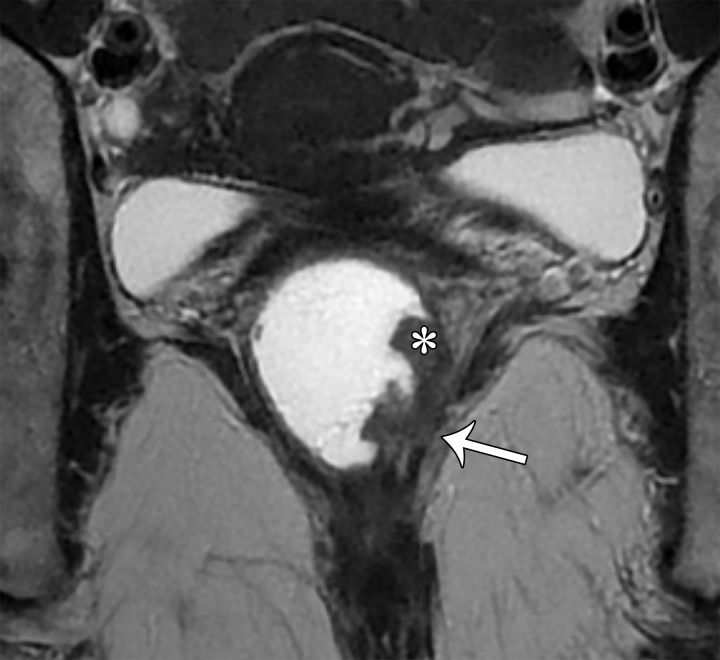
Peritoneal inclusion cyst appears as a uni- or multilocular cyst closely associated with the ovary and demonstrating low T1 SI and high T2 SI at MRI. Septa representing adhesions show mild enhancement. Solid enhancing nodules are absent (Fig 10).
The ovary is entrapped by the peritoneal adhesions and suspended centrally or eccentrically within the cyst, producing a characteristic “spider in a web” appearance (38). The ovary may be distorted but is otherwise normal and should not be confused with a solid enhancing nodule (Figs 6, 10).
Peritoneal inclusion cyst conforms to the contours of the pelvic cavity because the cyst lacks a true wall, with its margins formed by the adhesions and adjacent organs.
Figure 10a.
Peritoneal inclusion cyst. (a) Coronal T2-weighted image shows a large hyperintense lesion (*) surrounding the ovary (arrow). The ovary is entrapped by peritoneal adhesions and suspended centrally, producing a characteristic “spider in a web” appearance. (b) Contrast-enhanced T1-weighted image shows smooth mild enhancement, which represents adhesions. Solid enhancing nodules are absent. Note the normal enhancement of the ovary (arrow). * = cyst.
Figure 10b.
Peritoneal inclusion cyst. (a) Coronal T2-weighted image shows a large hyperintense lesion (*) surrounding the ovary (arrow). The ovary is entrapped by peritoneal adhesions and suspended centrally, producing a characteristic “spider in a web” appearance. (b) Contrast-enhanced T1-weighted image shows smooth mild enhancement, which represents adhesions. Solid enhancing nodules are absent. Note the normal enhancement of the ovary (arrow). * = cyst.
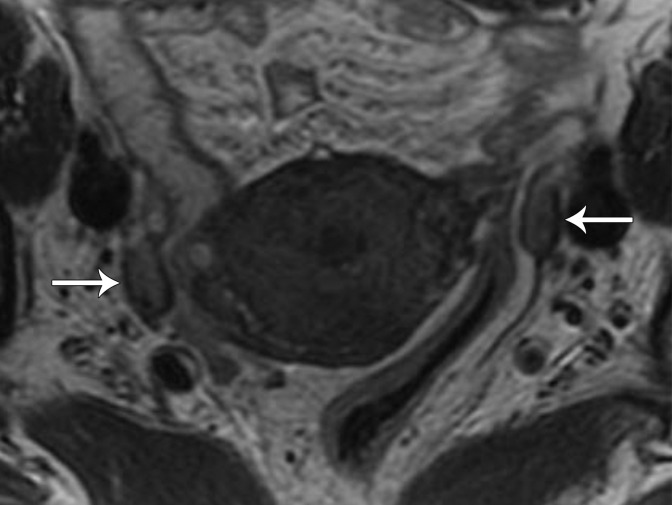
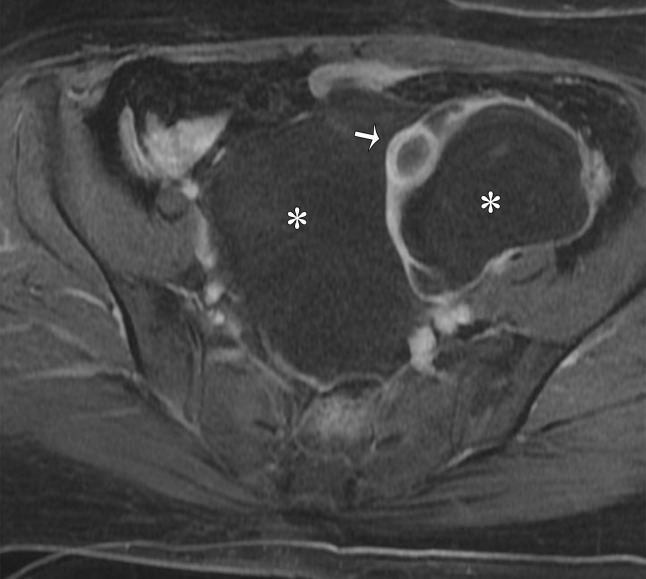
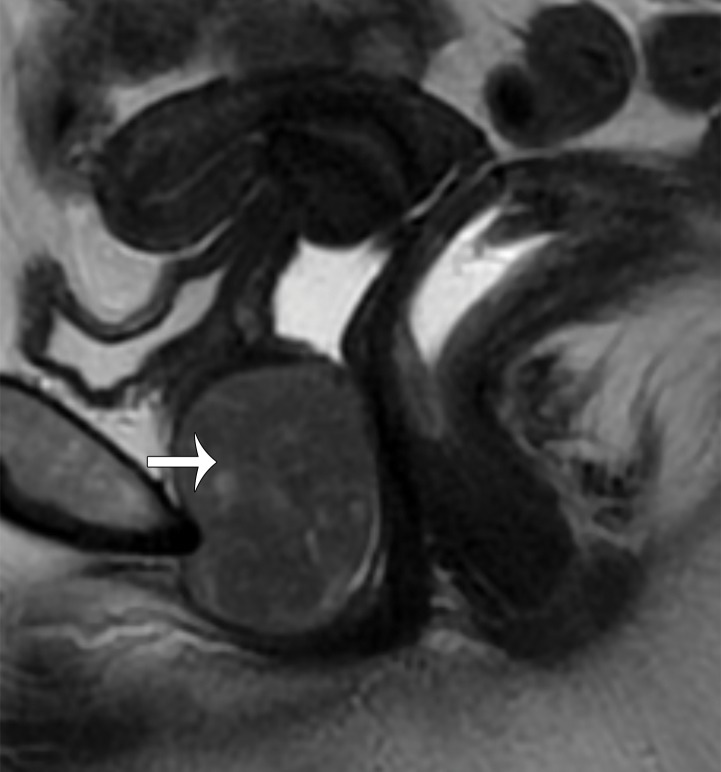
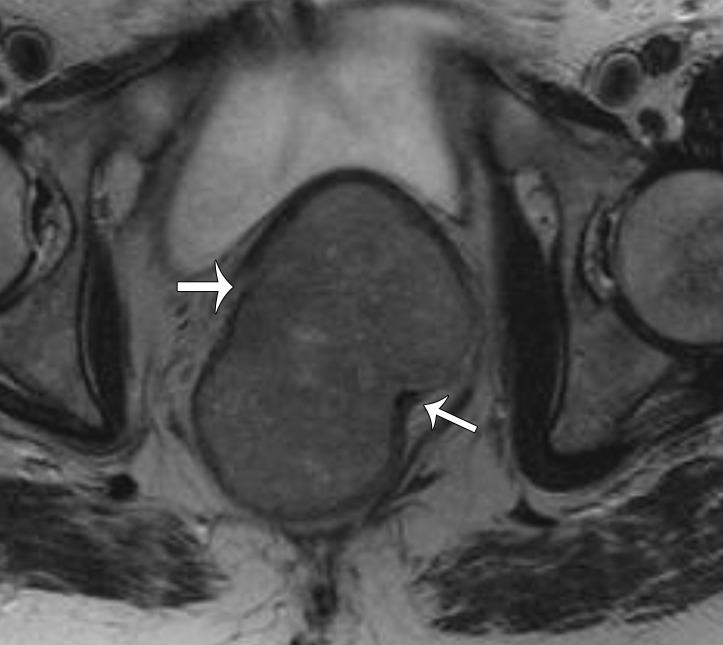
Paraovarian Cyst.—Paraovarian (paratubal) cysts are benign cysts that arise in the broad ligament and account for 10%–20% of all adnexal masses (39). At MRI, paraovarian cyst is typically a simple unilocular cyst with low T1 SI, high T2 SI, and a thin outer wall (<3 mm) adjacent to but separate from the ovary (Fig 11) (40).
Figure 11.
Axial T2-weighted image shows a left paraovarian cyst (white arrow). Note the normal left ovary (black arrow) adjacent to the mass.
Pseudomyxoma Peritonei.—Pseudomyxoma peritonei is defined as the presence of mucinous implants throughout the peritoneal cavity, which develop as a result of ruptured mucinous adenocarcinoma of the appendix and secondary peritoneal seeding (41). At MRI, peritoneal implants from pseudomyxoma peritonei have low T1 SI and high T2 SI because of the mucin. Cystic mucinous implants can encase and narrow the small and large bowel and result in bowel obstruction. Either one or both ovaries may be involved by large frequently complex cystic metastases. Scalloped appearance of the liver and spleen and centrally displaced or narrowed encased bowel loops are useful to distinguish mucinous implants from loculated ascites (41).
Solid Masses
Solid masses in the peritoneal cavity include the more common peritoneal metastases or carcinomatosis and a number of uncommon entities, including primary peritoneal serous papillary carcinoma, primary malignant mesothelioma, primary lymphangiomatosis, disseminated peritoneal leiomyomatosis, and pelvic spleen.
Peritoneal Metastases or Carcinomatosis.—Peritoneal metastases are secondary tumor deposits in the omentum, along the peritoneal surfaces, and occasionally in the mesentery. The most frequent primary tumors that spread to the peritoneum include ovarian and gastrointestinal (gastric, colorectal, pancreatic) carcinomas. Characteristic imaging features include nodules or masses in the omentum and along the peritoneal surfaces with or without ascites, usually on a background of a known primary malignancy. The earliest sites of peritoneal involvement are the rectouterine pouch and peritoneal reflections, which should be carefully evaluated (42).
CT is the primary imaging modality for evaluation of peritoneal carcinomatosis owing to its accessibility and fast acquisition time; however, sensitivity varies widely between studies and is generally low for tumor implants under 1 cm. Use of MRI, particularly with addition of diffusion-weighted imaging (DWI), has allowed sensitivity to surpass that of CT, particularly for tumor implants under 1 cm or those located in anatomically challenging sites such as the mesentery, bowel serosa, and subdiaphragmatic surfaces (42). At MRI, peritoneal metastases appear as intermediate T2 SI lesions with restricted diffusion and variable enhancement after contrast material administration.
Malignant Mesothelioma.—Malignant mesothelioma is a rare malignant tumor that can arise from any serosal surface, with 70% arising from the pleura and 20%–30% from the peritoneum. Asbestos exposure is the strongest risk factor, with an approximately 20–40-year interval between initial exposure and diagnosis (43). It is likely that higher asbestos exposure is required for development of peritoneal mesothelioma compared with pleural mesothelioma. Other risk factors include radiation and exposure to metal fibers. Most cases occur in older patients, and men are affected more frequently than women (43).
Malignant mesothelioma typically manifests as multiple solid enhancing masses throughout the peritoneum with variable amounts of ascites, similar to peritoneal metastases or carcinomatosis. Other manifestations include diffuse infiltrating tumor or multiple small nodules (44). Absence of lymphadenopathy and the presence of calcified pleural plaques at chest CT may serve as useful clues for diagnosis (44).
Disseminated Peritoneal Leiomyomatosis.—Disseminated peritoneal leiomyomatosis is a rare, benign, often asymptomatic condition characterized by multiple smooth muscle tumors dispersed throughout the peritoneal surfaces. Its pathogenesis is a matter of debate, with possible theories including smooth muscle metaplasia or a sequel of surgery (for example, morcellation during laparoscopic myomectomy with secondary seeding of the peritoneum) (18). It mostly affects premenopausal women, since estrogens and progesterone are the main hormonal drivers of tumor growth.
MRI is key to diagnosis, demonstrating well-defined low T2 SI leiomyoma-like masses with homogeneous or heterogeneous enhancement similar to that of the outer myometrium and uterine leiomyomas. Lack of omental involvement, absence of ascites and lymphadenopathy, and a characteristic appearance at MRI are clues to the correct diagnosis. Treatment options include conservative medical treatment with gonadotropin-releasing hormone agonists or bilateral salpingo-oophorectomy (18).
Extraperitoneal Spaces and Associated Tumors or Tumor Mimics
If an unknown mass is located away from the peritoneum, extraperitoneal origin of the mass should be considered. As discussed earlier, the pelvic extraperitoneum is divided into the interconnected prevesical and perivesical spaces anteriorly and perirectal and retrorectal spaces posteriorly (Figs 1, 2). These spaces are most often involved via direct extension of gastrointestinal or genitourinary tumors. However, primary tumors can occur and are mainly of mesenchymal origin, including leiomyoma, sarcoma, or solitary fibrous tumor.
While the focus of this review is the female pelvis, many tumors and tumor mimics in the extraperitoneal pelvis (summarized in Tables 1 and 2) are relevant to both sexes.
Table 1:
Extraperitoneal Tumors That Can Occur Anywhere in the Pelvis
Table 2:
Extraperitoneal Space Tumors That Occur in Specific Locations in the Pelvis
Anterior Compartment
Prevesical Space.—As described earlier, the prevesical space is part of the anterior compartment; its boundaries include the transversalis fascia anteriorly and umbilicovesical fascia posteriorly, the umbilicus superiorly, and the pubovesical ligament inferiorly (45) (Fig 12). The inferior part of the prevesical space posterior to the pubic bones and anterior to the urinary bladder is also known as the retropubic space of Retzius (45). The prevesical space contains loose connective tissue; therefore, tumors and tumor mimics that originate in the prevesical space are extremely rare, with only isolated case reports of primary lesions such as lymphangioma, solitary fibrous tumor, and leiomyoma (45).
Figure 12.
Midsagittal plane through the female pelvis shows the prevesical space, outlined anteriorly by the transversalis fascia and posteriorly and laterally by the umbilicovesical fascia.
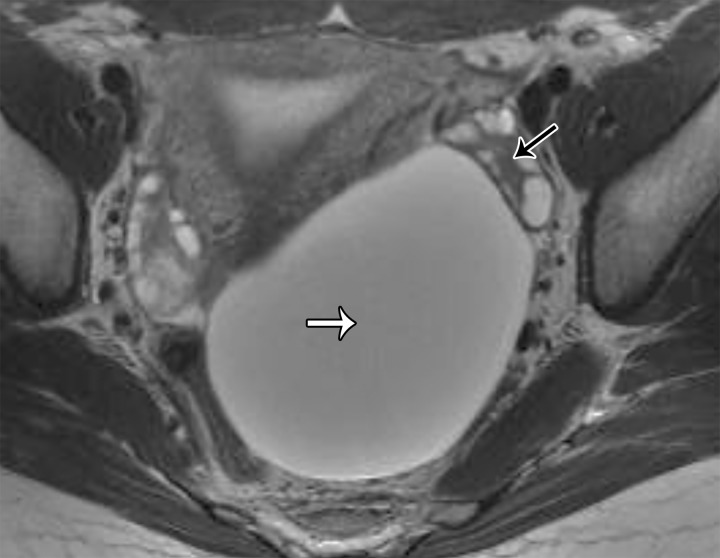
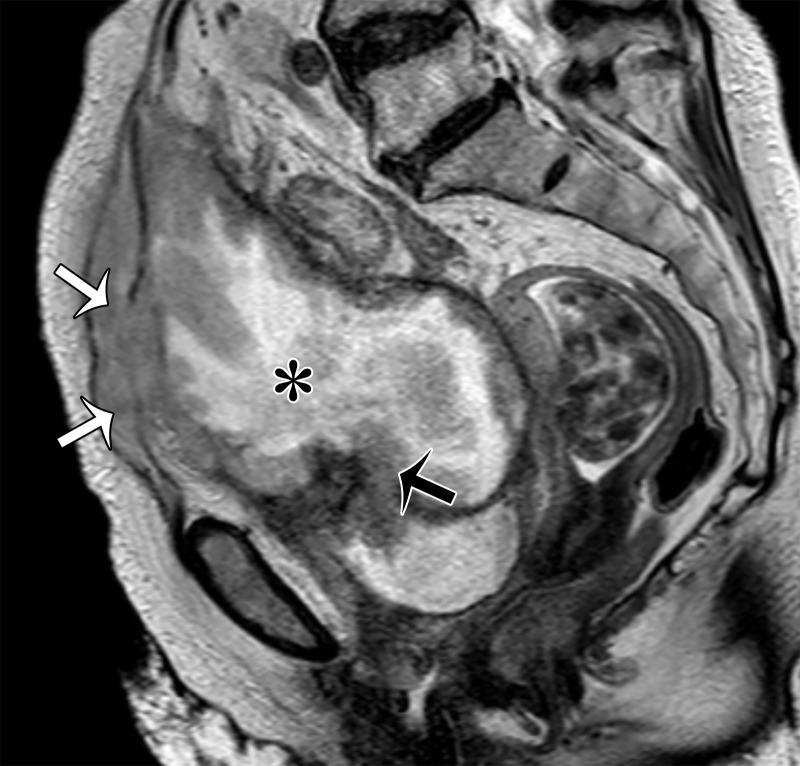
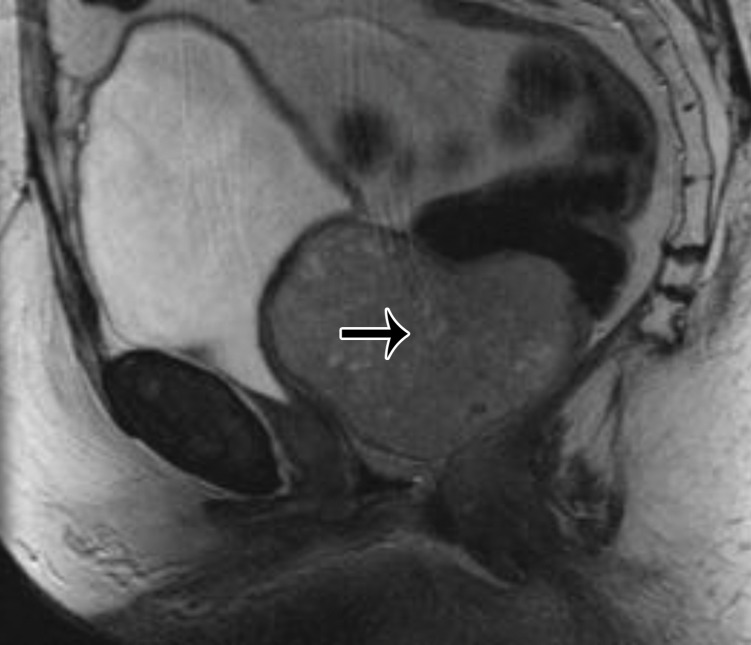
A mass in the prevesical space can be recognized by posterior displacement of the urinary bladder; sagittal images are particularly useful for demonstrating the location of the mass (Fig 13). The most frequent masses that involve the prevesical space include urachal adenocarcinoma and direct spread of other genitourinary tumors. Urachal adenocarcinoma originates from a urachal remnant (located in the perivesical space and described in the following section) and subsequently invades the prevesical space (45,46) (Fig 13).
Figure 13.
Sagittal T2-weighted image shows a midline cystic mass (*) with solid components extending through the bladder dome (black arrow) and into the prevesical space (white arrows), consistent with a urachal adenocarcinoma.
Perivesical Space.—The perivesical space is a small potential space that is enveloped by the umbilicovesical fascia. The umbilicovesical fascia fuses posteriorly with the rectovaginal fascia (1). The perivesical space contains the urachus (median umbilical ligament), obliterated umbilical arteries (medial umbilical folds), and urinary bladder. The perivesical space is continuous posteriorly with the lower uterus. As with the prevesical space, the perivesical space is most commonly involved by direct tumor extension from the bladder or urachus.
Bladder tumors are broadly divided into the common epithelial tumors (95%) and the rare nonepithelial (mesenchymal) tumors. Urothelial lesions (ranging from benign to malignant) are the most common epithelial tumors, with squamous cell carcinoma and adenocarcinoma being the second and third most frequent, respectively. The TNM system is the most widely used staging system, with the local T designation reflecting the depth of tumor invasion (47). According to the recommendations of the American College of Radiology (ACR), dedicated MRI of the pelvis is the best modality for local staging of bladder cancer (48).
Tumors confined to the muscle layer (T2) have a better prognosis than those that extend through the bladder wall into the perivesical fat (T3) and beyond, owing to higher rates of occult metastases at diagnosis with increasing T stage. T3 disease is divided into T3a microscopic perivesical fat invasion, which cannot be resolved at imaging, and T3b macroscopic invasion. Detection of macroscopic invasion with MRI relies on loss of the clear bladder wall–perivesical fat interface and the presence of tumor nodules or fat stranding in the perivesical fat. MRI may be more sensitive than CT in delineating extravesical extension owing to its higher soft-tissue contrast (49). Muscle invasion is seen as interruption of the low T2 SI muscle layer by intermediate T2 SI tumor with restricted diffusion; perivesical extension is seen as intermediate T2 SI tumor with restricted diffusion projecting into high T2 SI perivesical fat.
Urachal lesions can involve the perivesical space as well. The urachus is a tubular structure that extends superiorly from the anterior dome of the bladder to the umbilicus. It is a vestigial remnant of two embryonic structures: the cloaca and allantois. The urachus normally involutes, remaining as a fibrous band with no known function. However, persistence of an embryonic urachal remnant can lead to urachal carcinoma.
Urachal carcinoma is a subtype of adenocarcinoma (one-third of all adenocarcinomas) that is associated with the urachal remnant. The mean age at diagnosis is 50 years; unlike with other bladder cancers (which are more common in men), there is no sex predilection. Patients manifest with large tumors because the disease is clinically silent until advanced stages. The tumor location is best demonstrated on sagittal images.
At MRI, urachal carcinoma is a large enhancing supravesical infraumbilical mass with high T2 SI areas corresponding to mucin (Fig 13) (46). Given the extravesical location, lack of symptoms until a large size is attained, and advanced stage at diagnosis, the pretreatment images should be scrutinized for peritoneal spread and lymphadenopathy. Radical cystectomy with en-bloc resection of the tumor, peritoneum, and abdominal wall is the mainstay of treatment; however, the prognosis is generally poor owing to the advanced stage at presentation (46) (Fig 13).
Middle Compartment
The middle compartment of the female pelvis houses the vagina, uterine cervix, and uterine corpus. A number of benign or malignant lesions can arise from these organs. MRI plays a key role in diagnosis, including assessment of symptomatic uterine leiomyomas before intervention and differentiation of uterine leiomyomas from uterine sarcomas, of which uterine leiomyosarcoma is the most common subtype (15,50). Recently, Lakhman et al (50) demonstrated that the presence of three or more out of four qualitative MRI features—nodular borders, hemorrhage, T2 dark areas, and central nonenhancement—is strongly associated with leiomyosarcoma.
MRI is also central to locoregional staging of cervical, endometrial, and vaginal cancers. In this setting, MRI findings guide patient management. In cervical cancer, MRI allows assessment of tumor extent and helps decide between surgery or chemoradiotherapy (51). Parametrial invasion is diagnosed when tumor disrupts the low SI of the outer stroma. At MRI, a spiculated or nodular tumor-parametrial interface or encasement of parametrial vessels by tumor is highly specific for tumor extension into the parametrium (51).
In endometrial cancer, MRI helps assess the depth of myometrial invasion, a key prognostic factor that correlates with tumor grade, presence of lymph node metastases, and overall survival (52). At MRI, tumor extension of more than 50% of myometrial thickness indicates deep myometrial invasion (52).
Finally, MRI can help clarify the organ of origin when a locally extensive middle compartment tumor invades the anterior or posterior compartment or, occasionally, when results of histopathologic sampling are inconclusive with regard to cervical versus endometrial origin of adenocarcinoma. Detailed discussion of these topics is beyond the scope of this review, but they are discussed in depth in several recent original research and review articles (14,50–52).
Posterior Compartment
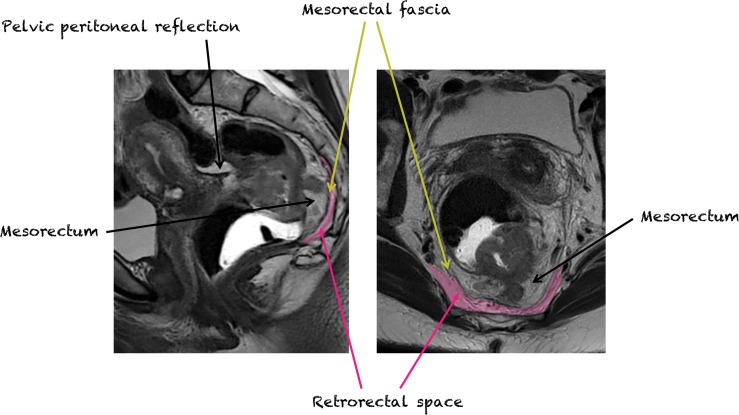
Perirectal Space.—The mesorectal fascia defines the margins of the perirectal space (mesorectum). The mesorectal fascia is an aggregate of the rectovaginal fascia anteriorly and pelvic fascia posteriorly (1). The lateral aspects of the mesorectal fascia are also known as the uterosacral ligaments. The perirectal space surrounds the rectum and contains mesorectal fat with interspersed mesorectal lymph nodes, vessels, nerves, and lymphatic channels.
Involvement of the mesorectum is usually by rectal tumors, with adenocarcinoma of the rectum being the most frequent cause. MRI is a key imaging modality for accurate initial staging and posttreatment assessment of rectal cancer. For initial staging, use of MRI significantly improves detection and evaluation of T3 disease (tumor extension beyond the rectal wall into the mesorectal fat) (53,54). Patients who have T3 tumors with over 5-mm mesorectal invasion have lower survival (around 50% at 5 years) than patients with less than 5-mm tumor invasion (85% at 5 years) (55). The reader is directed to references 53 and 54 for in-depth coverage of this topic.
Other rare primary rectal tumors that may extend into the mesorectal fat include GIST, neuroendocrine tumors, and primary rectal melanoma. The mesorectum can also be involved by lymph node metastases from rectal, prostatic, or gynecologic cancers or by lymphoma.
Retrorectal Space.—The retrorectal space is a virtual space situated between the mesorectal fascia anteriorly and presacral fascia posteriorly. It is bordered superiorly by the peritoneal reflection, inferiorly by the levator ani and coccygeus muscles, and laterally by the ureters and iliac vessels (Fig 14) (56). The retrorectal space may be involved by a number of rare tumors and tumor mimics (Tables 1, 2). Up to two-thirds of tumors in the retrorectal space are congenital, explained by the fact that this space contains embryologic remnants derived from the neuroectoderm, hindgut, and notochord (57).
Figure 14.
Sagittal (left) and axial (right) T2-weighted images show the mesorectum (perirectal space), mesorectal fascia, and retrorectal space.
Retrorectal Tumors and Their Mimics
Retrorectal tumors and their mimics are best evaluated and characterized at MRI using their SI and morphology as a guide (Fig 15) (56). Anterior displacement of the rectum and perirectal space, most easily seen on sagittal images, is the best clue to rectorectal location of a mass.
Figure 15.
Algorithmic approach to retrorectal masses. Conventional T1-weighted imaging (T1) allows retrorectal masses to be subdivided into two types: T1 hyperintense masses and T1 hypointense masses. T1 hyperintense masses require further assessment with fat-saturated (FS) T1-weighted imaging to distinguish fat from blood. T1 hypointense masses require further evaluation with T2-weighted imaging (T2). T1 hypointense masses can be subdivided into T2 cystic masses and T2 solid masses.
The stepwise approach to lesion characterization of the retrorectal space consists of the following considerations: (a) Does a retrorectal lesion have high T1 SI? (b) If the retrorectal lesion has low T1 SI, what is its T2 SI and morphology?
Does the Retrorectal Lesion Have High T1 SI?
Increased SI on T1-weighted images is indicative of a fat-containing or hemorrhagic lesion. Signal loss after fat saturation is consistent with intralesional macroscopic fat, whereas persistent high SI suggests hemorrhagic contents.
Fat-containing Masses
The diagnostic possibilities include dermoid cyst, myelolipoma, lipoma, liposarcoma, and sacrococcygeal teratoma.
Dermoid Cyst.—Dermoid cyst is a type of developmental cyst composed of at least two of the three germ cell layers (ectoderm, mesoderm, and endoderm). At MRI, the presence of signal drop on fat-suppressed images is characteristic (58).
Myelolipoma.—Myelolipoma is a benign mesenchymal tumor composed of fat and hematopoietic elements. Myelolipomas are most frequently found in the adrenal glands. However, presacral myelolipomas account for up to 50% of extra-adrenal occurrences of this entity.
At MRI, high T1 SI with loss of signal on fat-saturated images and intermediate to high T2 SI are typical (59). Distinction between myelolipoma and liposarcoma may be difficult, as both manifest with fat and soft-tissue components. Liposarcoma may demonstrate less well-defined margins and a more invasive and infiltrative pattern than myelolipoma (59).
Lipoma and Liposarcoma.—As with their retroperitoneal abdominal counterparts, distinction between retrorectal lipoma and liposarcoma can be a challenge because their imaging appearances may overlap. Lipomas are usually well-defined homogeneous encapsulated fatty masses. In contrast, the imaging features of liposarcomas vary depending on the tumor grade. Well-differentiated liposarcomas are well-defined predominantly fat-containing lesions with minimal soft tissue. On the other hand, poorly differentiated liposarcomas appear as predominantly soft-tissue masses with minimal fat (60).
Sacrococcygeal Teratoma.—Sacrococcygeal teratoma is a germ cell tumor most commonly seen in infants and children (61). In children, they are mostly found at initial physical examination as a large mass protruding from the sacrococcygeal area. The neonatal form is commonly benign, but those found in the adult population can undergo malignant transformation if left untreated. Their imaging appearance varies from cystic to solid with internal fat and calcification (61). The sacrum and coccyx can be involved.
Hemorrhagic Lesions
Endometriosis is the most common cause of a hemorrhagic lesion in the retrorectal space. Typical MRI features of endometriosis are similar to those in other locations and include a low T1 and T2 SI fibrotic lesion with spicules and retraction due to fibrosis/scarring and with interspersed foci of hemorrhage (high T1 and T2 SI foci that persist on fat-saturated images) (34).
If a Retrorectal Lesion Has Low T1 SI, What Is Its T2 SI and Morphology?
In the case of a low T1 SI presacral lesion, one should distinguish between a cystic mass (T2 hyperintense) versus a solid mass (low to intermediate T2 SI).
T2 Hyperintense Lesion
If the mass is cystic, it is then useful to distinguish between unilocular versus multilocular cystic lesions.
Unilocular Cystic Lesions
Unilocular cystic retrorectal lesions include two type of developmental cysts—namely, rectal duplication cyst and epidermoid cyst—as well as anterior sacral meningocele.
Rectal Duplication Cyst.—Rectal duplication cyst is a sequestration of the hindgut and is usually discovered in children under 2 years of age. It is a spherical or elongated fluid-filled cyst, usually found posterior to the rectum or anus. At MRI, direct communication with the rectal lumen or anus may or may not be present. The cyst wall has its own mucosa and submucosa, while the muscularis propria is shared with the rectum. Surgical removal of a rectal duplication cyst is mandatory, as malignant degeneration to adenocarcinoma or squamous cell carcinoma occurs in 20% of cases (62).
Epidermoid Cyst.—Epidermoid cysts are rare, but when they occur, they are most commonly observed in middle-aged women. They appear as unilocular thin-walled lesions with heterogeneous low T1 SI and high T2 SI with possible small low-SI foci due to the presence of keratin (63). The thin wall is a useful feature to distinguish an epidermoid cyst from a duplication cyst.
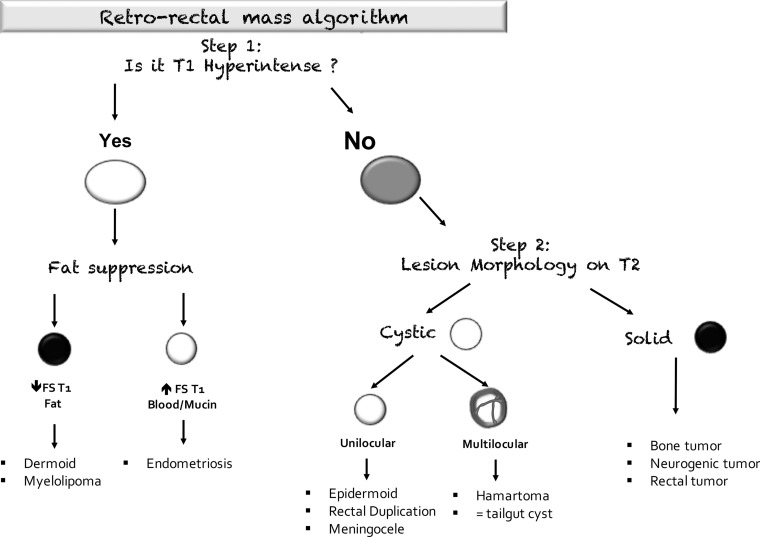
Anterior Sacral Meningocele.—Anterior sacral meningocele is a rare congenital disorder that occurs when the dural sac herniates into the presacral space because of agenesis of a portion of the anterior sacrum. In approximately 50% of patients, it is associated with additional malformations, such as spina bifida, spinal dysraphism, bicornuate uterus, and imperforate anus. At MRI, it is helpful to look for a sacral defect, which is associated with a well-defined unilocular thin-walled fluid-filled lesion communicating with the thecal sac (Fig 16) (64).
Figure 16.
Sagittal T2-weighted image shows a cystic mass in the retrorectal space (black arrow) that communicates with the thecal sac (white arrow), consistent with a meningocele.
Multilocular Cystic Lesions
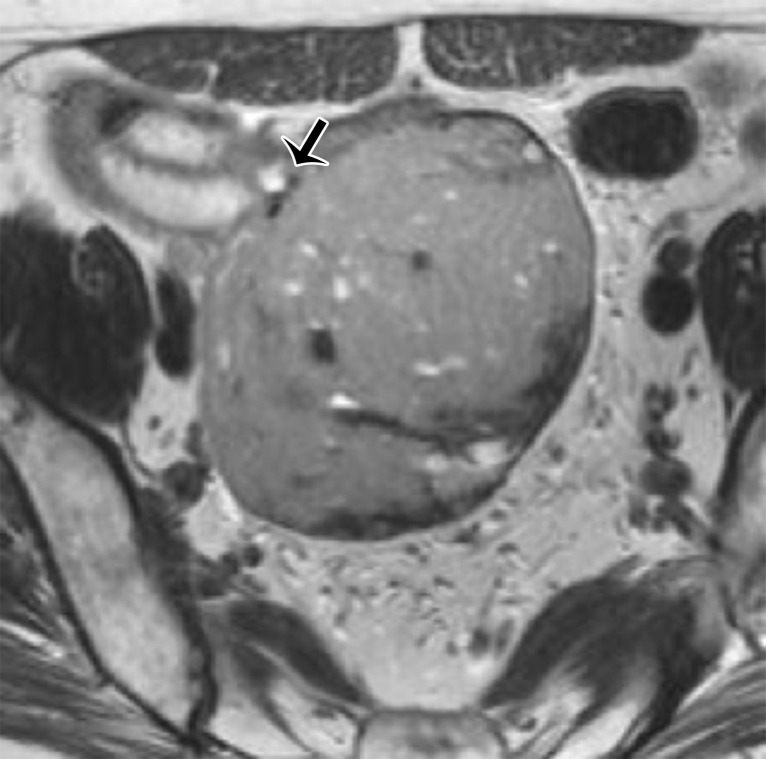
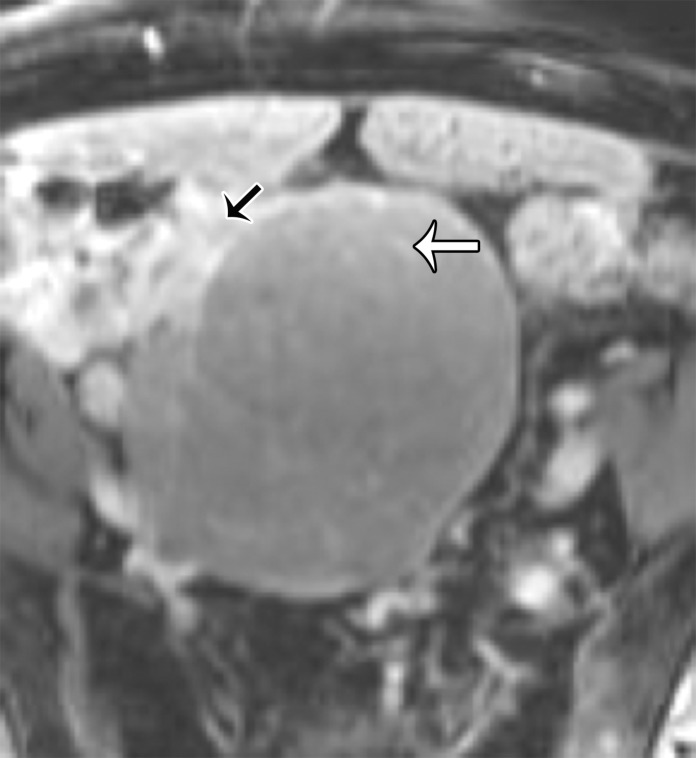
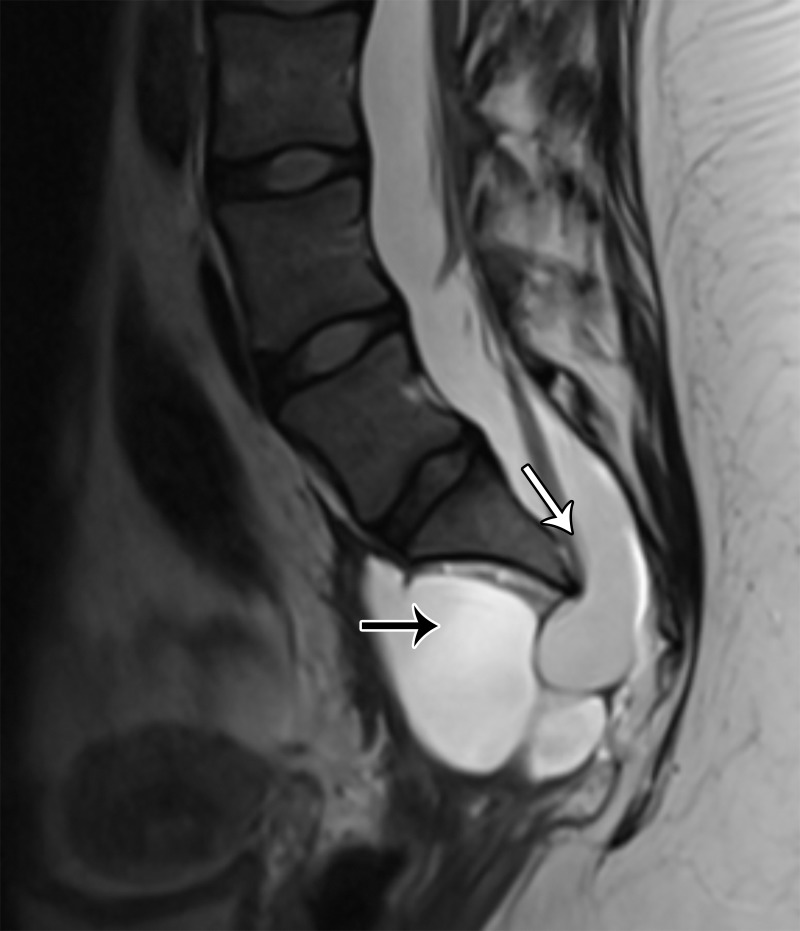
A multilocular cystic retrorectal lesion is suggestive of a tailgut cyst. Tailgut cysts or cystic hamartomas are a type of developmental cyst caused by incomplete regression of the embryonic tailgut. They are most frequent in middle-aged women and are usually discovered incidentally. However, infection may be the first manifestation of a tailgut cyst.
At imaging, they are multicystic lesions with small cysts clustered together and adherent to the main cyst, resulting in a honeycomb pattern (Fig 17) (65). They may adhere to the sacrum or rectum but do not communicate with the rectal lumen. They demonstrate variable SI on T1- and T2-weighted images, depending on the protein and mucin content (63,65). The cyst wall and septa may mildly enhance after contrast material administration.
Figure 17.
Axial T2-weighted image shows a multicystic lesion with a honeycomb pattern (white arrow), consistent with a tailgut cyst. Note the extraperitoneal location of the lesion, displacing the rectum anteriorly (black arrow).
Malignant transformation to adenocarcinoma or carcinoid tumor is suspected when a cystic mass demonstrates irregular margins, wall thickening, and marked enhancement after intravenous contrast material administration or the presence of enhancing internal soft-tissue nodules (65).
T2 Hypointense/Isointense Lesion
T2 hypointense/isointense masses are solid presacral lesions. Most are due to direct tumor extension from the rectum or bones (eg, giant cell tumor, chordoma, Ewing sarcoma, osteosarcoma, chondrosarcoma). Solid masses that arise in the retrorectal space are uncommon and include both benign and malignant neurogenic and mesenchymal tumors. Schwannoma, neurofibroma, paraganglioma (neurogenic tumors), and solitary fibrous tumor (mesenchymal tumor) are described in this section. Myelolipoma, lipoma, and liposarcoma—fat-containing mesenchymal tumors in this location—have already been described in the section on fat-containing retrorectal lesions.
Neurogenic Tumors
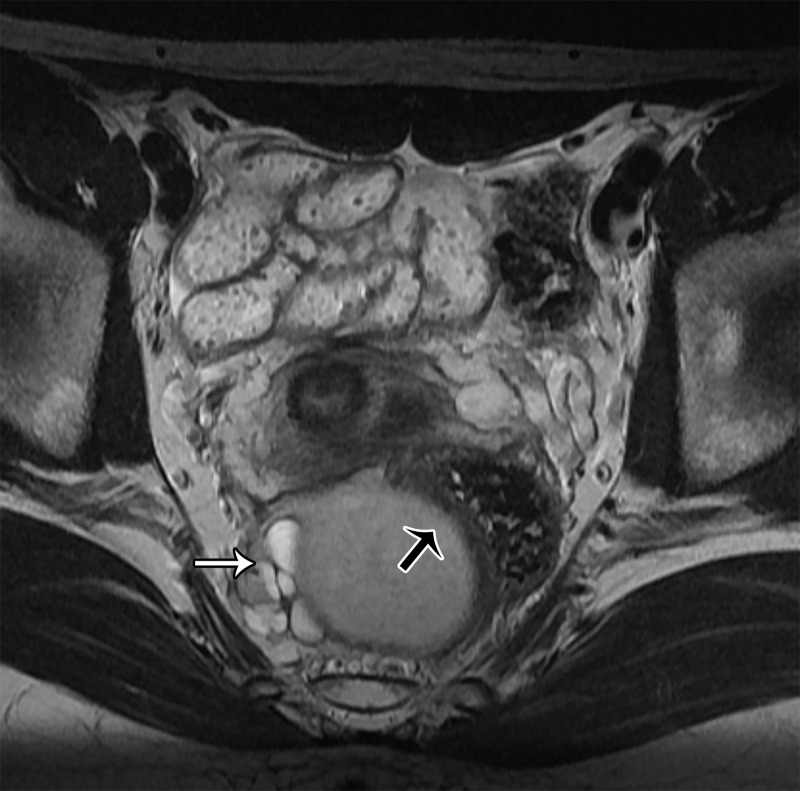
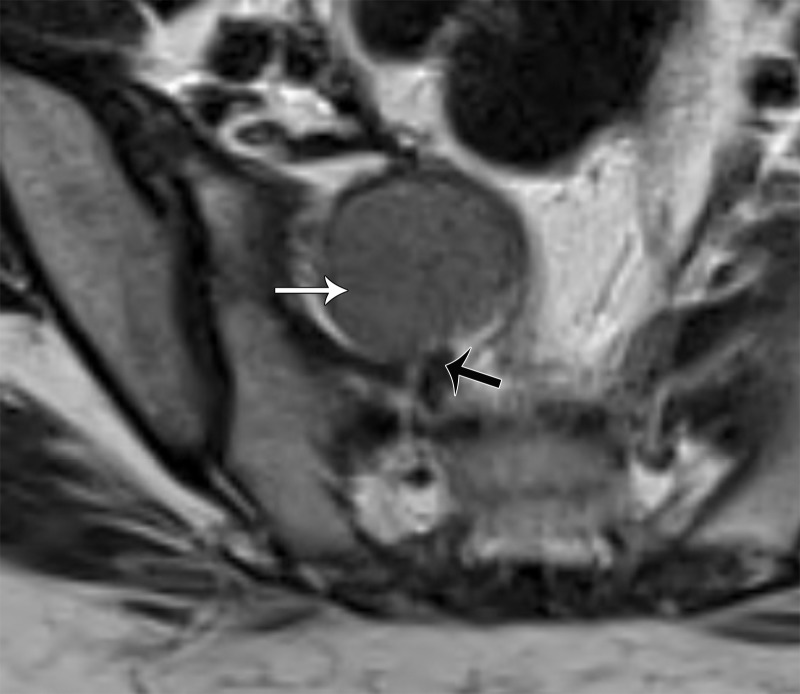
Schwannoma.—Schwannoma is a benign encapsulated neurogenic tumor occurring most commonly in young to middle-aged women (66). At MRI, the mass is usually seen eccentric to the nerve and may demonstrate low T1 SI and high T2 SI (Fig 18). Areas of cystic change, a pseudocapsule, and hemorrhage may also be seen.
Figure 18.
Axial T2-weighted image shows a well-defined moderately hypointense mass (white arrow) located eccentric to the nerve (black arrow), representing a schwannoma.
The fat split, target, and fascicular signs have been described in association with schwannomas (66). The fat split sign is a thin rim of fat around the lesion, seen as high T1 SI. The target sign consists of a peripheral high-SI myxoid component and a central low-SI fibrous component on T2-weighted images. The fascicular sign corresponds to multiple low-SI strands with peripheral high SI on T2-weighted images, representing the fascicular bundles within the nerves (Fig 19).
Figure 19.
Axial T2-weighted image shows an intermediate-SI retrorectal mass (black arrow) that represents a neurofibroma. Note the fascicular sign with multiple low-SI strands (white arrow), which represent the fascicular bundles within the nerves.
Neurofibroma.—Neurofibroma is a benign encapsulated neurogenic tumor arising from nerve sheaths, with plexiform neurofibroma a specific subtype almost always seen in neurofibromatosis type 1. Neurofibromas comprise 5% of all benign soft-tissue neoplasms, with 90% manifesting as solitary masses (67). In the presence of multiple neurofibromas, the diagnosis of neurofibromatosis type 1 should be suspected. On T2-weighted images, neurofibromas demonstrate the target sign more frequently than schwannomas. The fascicular sign can also be seen (67) (Fig 19).
Mesenchymal Tumors
Solitary Fibrous Tumor.—Solitary fibrous tumor is a mesenchymal tumor of fibroblastic or myofibroblastic origin (68). Solitary fibrous tumors are most common in the pleura but can occur in other anatomic locations, including the pelvic peritoneum and extraperitoneal organs/spaces. These tumors manifest in middle-aged patients, have no sex predilection, and often come to medical attention owing to pressure symptoms. The majority are benign slow-growing masses; malignant masses may arise de novo or by way of malignant degeneration.
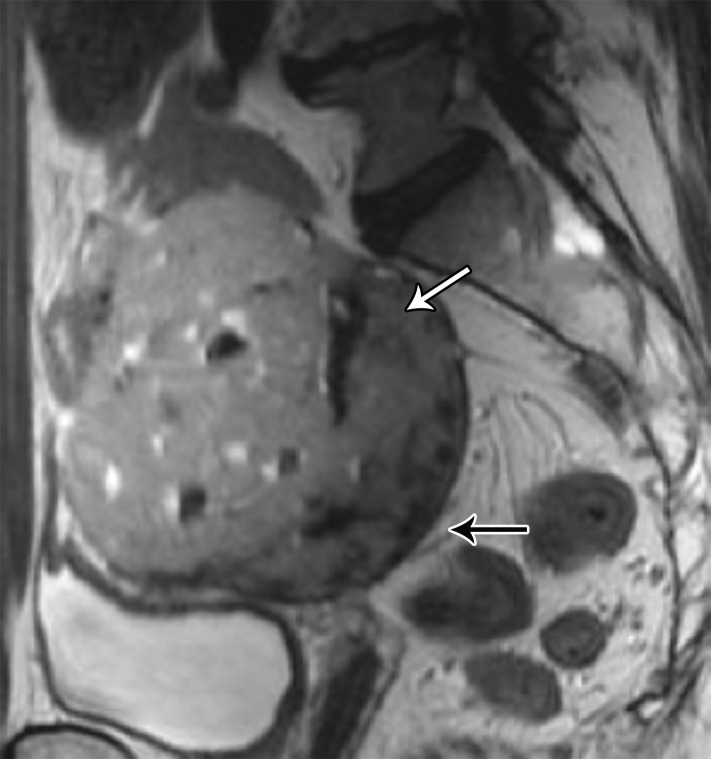
MRI features suggestive of solitary fibrous tumor include a well-defined lobulated mass with heterogeneous low SI and flow voids on T2-weighted images and avid and persistent enhancement after contrast material administration due to high vascularity and fibrous stroma (Fig 20) (68).
Figure 20a.
(a) Sagittal T2-weighted image shows a large retrorectal mass with low-to-intermediate SI due to fibrous tissue (black arrow) and areas of high SI consistent with cystic areas (pink arrow). (b) Axial CT image shows intense enhancement (arrow). Results of pathologic analysis were consistent with solitary fibrous tumor.
Figure 20b.
(a) Sagittal T2-weighted image shows a large retrorectal mass with low-to-intermediate SI due to fibrous tissue (black arrow) and areas of high SI consistent with cystic areas (pink arrow). (b) Axial CT image shows intense enhancement (arrow). Results of pathologic analysis were consistent with solitary fibrous tumor.
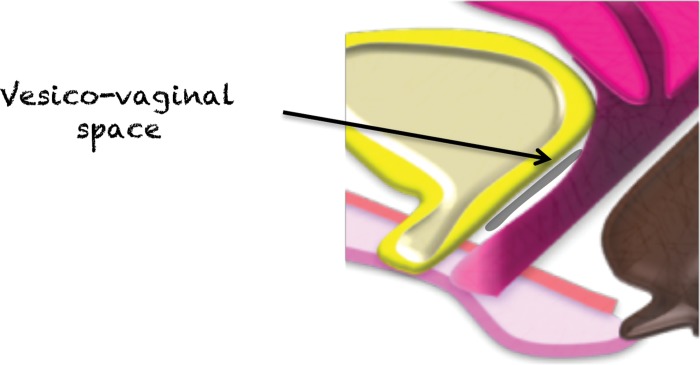
Vesicovaginal Space and Rectovaginal Fascia
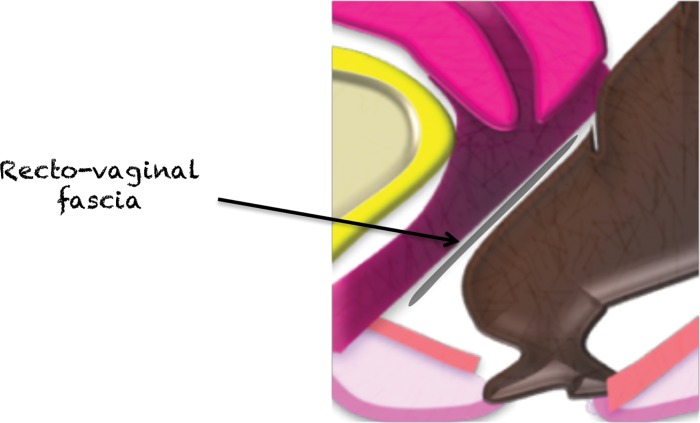
The vesicovaginal space (Fig 21) and rectovaginal fascia (often referred to as the rectovaginal septum) (Fig 22) represent virtual extraperitoneal spaces. They consist of a meshwork of areolar connective tissue, collagen fibers, and smooth muscles. Apart from separation of the urogenital organs and the rectum, they serve as anchors of the pelvic floor and conduits of nerves (69).
Figure 21a.
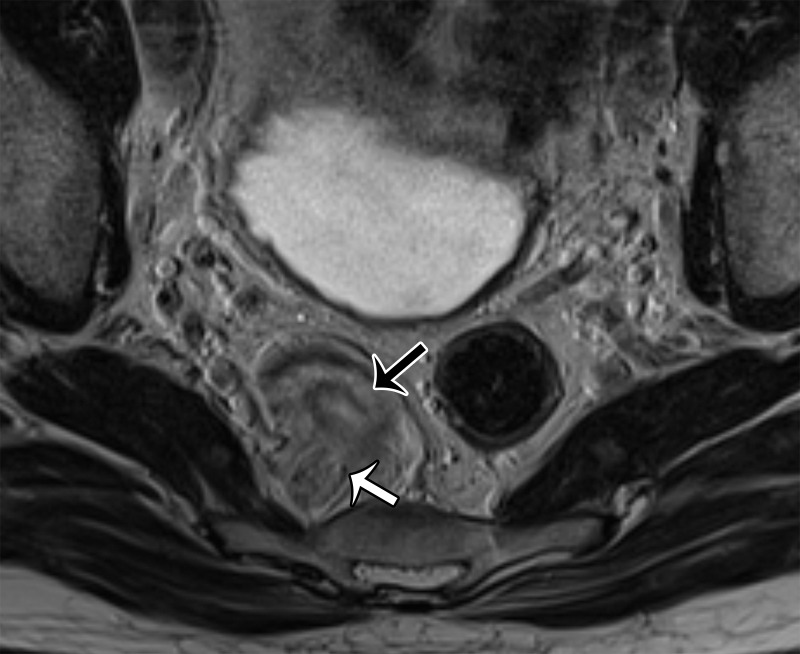
(a) Midsagittal plane through the female pelvis shows the vesicovaginal fascia. (b, c) Sagittal (b) and axial (c) T2-weighted images show an intermediate-SI lesion (arrow) in the vesicovaginal fascia. Note the mass effect on the urethra anteriorly and vagina posteriorly. At biopsy, the lesion represented vaginal sarcoma.
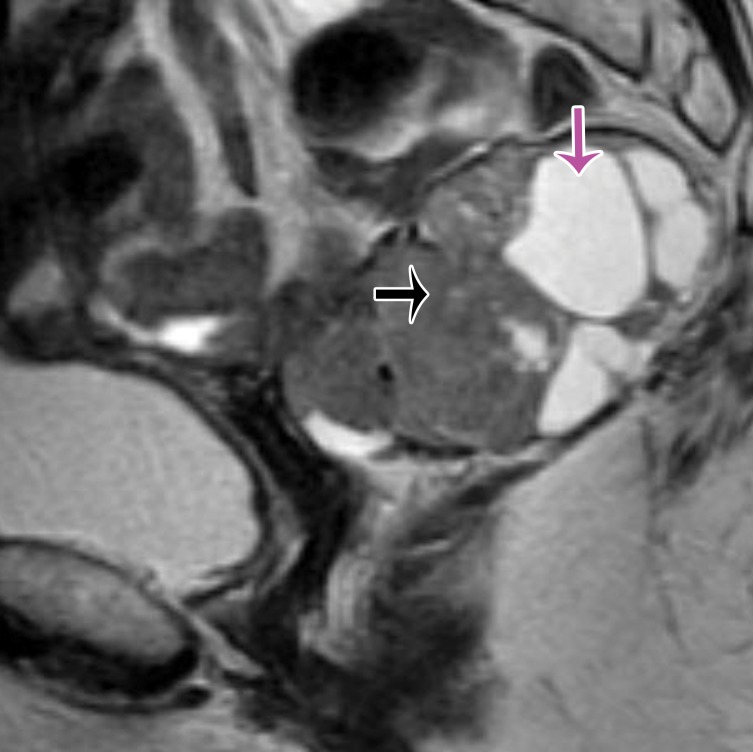
Figure 22a.
(a) Midsagittal plane through the female pelvis shows the rectovaginal fascia. (b, c) Sagittal (b) and axial (c) T2-weighted images show a large mass in the rectovaginal fascia (arrow in b), which communicates with the rectum (arrows in c). At biopsy, the mass was consistent with a GIST.
Figure 21b.
(a) Midsagittal plane through the female pelvis shows the vesicovaginal fascia. (b, c) Sagittal (b) and axial (c) T2-weighted images show an intermediate-SI lesion (arrow) in the vesicovaginal fascia. Note the mass effect on the urethra anteriorly and vagina posteriorly. At biopsy, the lesion represented vaginal sarcoma.
Figure 21c.
(a) Midsagittal plane through the female pelvis shows the vesicovaginal fascia. (b, c) Sagittal (b) and axial (c) T2-weighted images show an intermediate-SI lesion (arrow) in the vesicovaginal fascia. Note the mass effect on the urethra anteriorly and vagina posteriorly. At biopsy, the lesion represented vaginal sarcoma.
Figure 22b.
(a) Midsagittal plane through the female pelvis shows the rectovaginal fascia. (b, c) Sagittal (b) and axial (c) T2-weighted images show a large mass in the rectovaginal fascia (arrow in b), which communicates with the rectum (arrows in c). At biopsy, the mass was consistent with a GIST.
Figure 22c.
(a) Midsagittal plane through the female pelvis shows the rectovaginal fascia. (b, c) Sagittal (b) and axial (c) T2-weighted images show a large mass in the rectovaginal fascia (arrow in b), which communicates with the rectum (arrows in c). At biopsy, the mass was consistent with a GIST.
Masses in these locations are rare; the majority result from direct tumor growth from the rectum, uterus, cervix, or vagina or less commonly from the bladder. Metastases may derive from ovarian cancer or rarely hematogenous spread from extragenital cancers (eg, of the gastrointestinal tract, pancreas, or breast).
Primary tumors are rare with limited information in the literature and include EGIST, adenocarcinoma, sarcomas, and leiomyomas. In contrast to GISTs, EGISTs are associated with younger age at presentation, poorer prognosis, and high risk of local recurrence (70). At MRI, they manifest as exophytic heterogeneously enhancing masses compressing the rectum or vagina. No continuity with the rectum is found, in contrast to GIST.
Adenocarcinomas arising in the rectovaginal septum are rare and most commonly are associated with endometriosis, thus likely representing malignant degeneration of deep pelvic endometriosis (71). Involvement of the rectovaginal septum has been reported in 10% of cases of deep pelvic endometriosis (33). Imaging features include a solid intermediate T2 SI mass with variable enhancement and small foci of hemorrhage, depicted as high-SI foci on fat-saturated T1-weighted images, consistent with a background of endometriosis.
Imaging as a Road Map for Surgical Management
Pelvic tumor management depends on results of tumor histologic analysis, peritoneal versus extraperitoneal location, size, which structures are involved, and whether the tumor is primary or recurrent. Pelvic tumor recurrence is beyond the scope of this discussion, as we focus on considerations for primary tumor management.
The role of the radiologist, as a consultant, is mainly to provide some clues as to the diagnosis of the mass, its location, and its extent to guide treatment selection. When disease is limited, surgical resection is often the method of choice for treating primary pelvic tumors. It allows alleviation of symptoms, a final diagnosis, and a chance for a cure. However, up-front surgery may not be possible or may be challenging. When surgery is not possible, patients are referred for chemotherapy, radiation therapy, or hormonal therapy, depending on tumor type and lesion size.
The radiology report can be summarized by three keywords: tumor, location, and extent.
First, evaluation of the tumor, including its size and MRI SI characteristics, needs to be described. These features, in combination with the location (which is addressed next), will assist in deciding what are the most likely diagnosis and most likely differential possibilities, which should be given in the conclusion of the report. Indeed, benign or probably benign tumors at imaging will be managed differently from possibly malignant tumors.
Benign pelvic lesions are usually treated with circumferential excision, in contrast to malignant or potentially malignant tumors, which are treated with en bloc resection (ie, removal of tumor by incising through normal surrounding tissues, preventing contact with the tumor and resulting in a wide surgical margin). Lesion measurements in the three planes are important, as large lesions require wider excision and, in some cases, a combined surgical approach (ie, opening of both the peritoneal cavity and extraperitoneal sites).
Second, careful evaluation of the location of the mass is mandatory, and as stated previously, is important in narrowing the differential diagnosis. Specifically, determining whether a mass involves the intraperitoneal or extraperitoneal spaces (or both) can dictate the surgical approach. A peritoneal approach (ie, opening the peritoneal cavity to access the intraperitoneal organs) is proposed for intraperitoneal tumors, such as tumors originating from the bowel or ovaries.
An extraperitoneal approach (ie, limited surgery without opening the peritoneal cavity) can be used for extraperitoneal masses, depending on their size, diagnosis, and location. For example, in the case of a small suspected benign or developmental retrorectal tumor, a transsacral approach (known as the Kraske procedure) is favored. It consists of posterior transection of the coccyx and a portion of the sacrum (72).
Third, tumor extent in relation to surrounding organs and neurovascular and musculoskeletal structures needs to be carefully evaluated to determine whether complete surgical resection is possible. Absolute and relative contraindications to surgical resection can vary widely between institutions and are influenced by available local surgical expertise. Consequently, the role of the radiologist is not to describe disease as resectable or unresectable, but rather to alert the surgeon to tumor features that may complicate or preclude surgery.
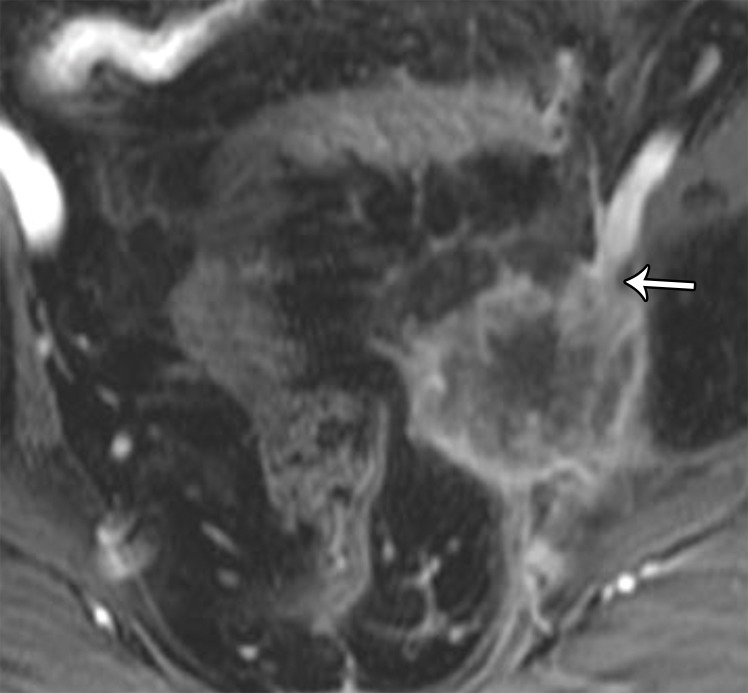
Key features that should be addressed in the imaging report include the presence of bladder, urethral, or rectal invasion, including invasion of the anal canal (Fig 23). In addition, the likelihood of vascular (common and external iliac), neural (lumbosacral plexus and sciatic nerve), muscle, or bone involvement and the presence of lymphadenopathy, peritoneal implants, or distant metastases need to be carefully assessed.
Figure 23.
Coronal T2-weighted image shows a hemicircumferential left rectal tumor (*) extending into the puborectalis muscle. Note the intermediate SI of the tumor (arrow) disrupting the low SI of the left puborectalis muscle.
Rectal or bladder involvement is best evaluated on T2-weighted images. Serosal involvement is suspected when the tumor abuts the bladder/rectum with loss of the high-SI fat plane between the tumor and the organ. In deeper involvement of the bladder/rectal wall, tumor interrupts the low SI of the muscular layer (muscle involvement). In contrast, a well-defined fat plane between the tumor and the bladder/rectum excludes involvement.
Tumor extension to the iliac vessels (common and external iliac) should be scrutinized for abutment (tumor surrounding <50% of the vessel circumference), encasement (tumor surrounding >50% of the vessel circumference), and/or luminal invasion (tumor seen within the vessel) (Fig 24). At MRI, a well-defined and uninterrupted fat plane around vessels excludes involvement. Encasement of the common iliac and external iliac vessels may be a relative surgical contraindication, depending on available surgical expertise and tumor type. Vascular reconstruction with grafts may be appropriate in treatment of some tumors, such as primary soft-tissue sarcomas (23). Involvement of the internal iliac vessels is not an obstacle because, if involved, these vessels can be sacrificed owing to existing collaterals (73).
Figure 24a.
(a) Axial plane through the female pelvis shows key features important for resection. Involvement of the internal iliac vessels or pelvic organs is not a contraindication to resection. Involvement of the external iliac vessels, bones, or proximal nerves (lumbosacral plexus and sciatic nerve) is a relative contraindication to resection. (b) Axial T2-weighted image shows a recurrence of endometrial cancer with muscular abutment but no invasion (black arrow) and anterior abutment of the external iliac vessels without encasement (white arrow). (c) Contrast-enhanced T1-weighted image shows a recurrence of cervical cancer with encasement of the external iliac vessels (arrow).
Figure 24b.
(a) Axial plane through the female pelvis shows key features important for resection. Involvement of the internal iliac vessels or pelvic organs is not a contraindication to resection. Involvement of the external iliac vessels, bones, or proximal nerves (lumbosacral plexus and sciatic nerve) is a relative contraindication to resection. (b) Axial T2-weighted image shows a recurrence of endometrial cancer with muscular abutment but no invasion (black arrow) and anterior abutment of the external iliac vessels without encasement (white arrow). (c) Contrast-enhanced T1-weighted image shows a recurrence of cervical cancer with encasement of the external iliac vessels (arrow).
Figure 24c.
(a) Axial plane through the female pelvis shows key features important for resection. Involvement of the internal iliac vessels or pelvic organs is not a contraindication to resection. Involvement of the external iliac vessels, bones, or proximal nerves (lumbosacral plexus and sciatic nerve) is a relative contraindication to resection. (b) Axial T2-weighted image shows a recurrence of endometrial cancer with muscular abutment but no invasion (black arrow) and anterior abutment of the external iliac vessels without encasement (white arrow). (c) Contrast-enhanced T1-weighted image shows a recurrence of cervical cancer with encasement of the external iliac vessels (arrow).
Tumor extent in relation to important neural structures requires evaluation. While small nerves can be sacrificed, larger structures such as the lumbosacral plexus or proximal sciatic nerve are a surgical challenge with high morbidity risk, and their involvement may be a contraindication to resection. The following features raise suspicion for nerve invasion: nerve enlargement (larger than the adjacent artery), increased T2 SI of the nerve (similar to that of the adjacent blood vessels), loss of fascicular pattern, and enhancement of the nerve (74).
Involvement of the pelvic sidewall muscles (obturator internus and piriformis) or abdominal wall muscles may not be an absolute contraindication to resection but requires pretreatment documentation to adequately plan resection and any required reconstructive surgery. Similarly, tumor extension within the pelvic floor (eg, puborectal muscle, perineum, ischioanal fossa) must be carefully scrutinized, as it changes surgical management. For example, puborectal extension precludes sphincter-sparing surgery (Fig 23). Features of muscle invasion at MRI include intermediate T2 SI within the muscle, muscle enlargement and enhancement, or discrete enhancing tumor nodules within the muscle.
Similarly, bone involvement needs to be defined to plan for involvement of an orthopedic surgeon during resection. Bone invasion is suspected at MRI when tumor abuts the bone and contiguous low T1 SI replaces the normal high T1 SI of bone marrow. Cortical bone destruction is seen as loss of well-defined low T1 SI cortex superficial to the bone marrow and is also well visualized at CT. In general, it is possible to resect the pelvic bones, sacrum, and coccyx; however, this can lead to increased morbidity, positive margins, and perioperative bleeding risk.
Conclusion
Pelvic tumors and their mimics in the female pelvis can present a diagnostic challenge. The reasons are complex anatomy of the pelvis, a wide variety of potential causes, difficulty in assessing the organ of origin, and overlap in imaging appearances between the lesions. We hope that the description of the anatomy and the systematic approach to MRI interpretation presented in this article can help the reader navigate the pelvic spaces and commonly encountered lesions in these spaces with greater confidence.
I.N., V.P., H.A.V., and Y.L. supported by Cancer Center Support Grant P30 CA008748 from the National Cancer Institute.
Presented as an education exhibit at the 2017 RSNA Annual Meeting.
For this journal-based SA-CME activity, the authors, editor, and reviewers have disclosed no relevant relationships.
Abbreviations:
- EGIST
- extragastrointestinal stromal tumor
- GIST
- gastrointestinal stromal tumor
- SI
- signal intensity
References
- 1.Meyers M, Charnsangvej C, Oliphant M. Meyers’ dynamic radiology of the abdomen. New York, NY: Springer, 2011. [Google Scholar]
- 2.Pannu HK, Oliphant M. The subperitoneal space and peritoneal cavity: basic concepts. Abdom Imaging 2015;40(7):2710–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gollub MJ, Maas M, Weiser M, et al. Recognition of the anterior peritoneal reflection at rectal MRI. AJR Am J Roentgenol 2013;200(1):97–101. [DOI] [PubMed] [Google Scholar]
- 4.Janvier A, Rousset P, Cazejust J, Bouché O, Soyer P, Hoeffel C. MR imaging of pelvic extraperitoneal masses: a diagnostic approach. Diagn Interv Imaging 2016;97(2):159–170. [DOI] [PubMed] [Google Scholar]
- 5.Bazot M, Deligne L, Boudghène F, Buy JN, Lassau JP, Bigot JM. Correlation between computed tomography and gross anatomy of the suspensory ligament of the ovary. Surg Radiol Anat 1999;21(5):341–346. [DOI] [PubMed] [Google Scholar]
- 6.Oktem O, Oktay K. The ovary: anatomy and function throughout human life. Ann N Y Acad Sci 2008;1127(1):1–9. [DOI] [PubMed] [Google Scholar]
- 7.Saksouk FA, Johnson SC. Recognition of the ovaries and ovarian origin of pelvic masses with CT. RadioGraphics 2004;24(suppl 1):S133–S146. [DOI] [PubMed] [Google Scholar]
- 8.Foshager MC, Hood LL, Walsh JW. Masses simulating gynecologic diseases at CT and MR imaging. RadioGraphics 1996;16(5):1085–1099. [DOI] [PubMed] [Google Scholar]
- 9.Outwater EK, Mitchell DG. Normal ovaries and functional cysts: MR appearance. Radiology 1996;198(2):397–402. [DOI] [PubMed] [Google Scholar]
- 10.Karaosmanoglu D, Karcaaltincaba M, Karcaaltincaba D, Akata D, Ozmen M. MDCT of the ovarian vein: normal anatomy and pathology. AJR Am J Roentgenol 2009;192(1):295–299. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Jeong YK, Park JK, Hwang JC. “Ovarian vascular pedicle” sign revealing organ of origin of a pelvic mass lesion on helical CT. AJR Am J Roentgenol 2003;181(1):131–137. [DOI] [PubMed] [Google Scholar]
- 12.Arikawa S, Uchida M, Shinagawa M, Tohnan T, Hayabuchi N. Significance of the “beak sign” in the differential diagnosis of uterine lipoleiomyoma from ovarian dermoid cyst. Kurume Med J 2006;53(1-2):37–40. [DOI] [PubMed] [Google Scholar]
- 13.Kim JC, Kim SS, Park JY. “Bridging vascular sign” in the MR diagnosis of exophytic uterine leiomyoma. J Comput Assist Tomogr 2000;24(1):57–60. [DOI] [PubMed] [Google Scholar]
- 14.Stewart EA. Clinical practice: uterine fibroids. N Engl J Med 2015;372(17):1646–1655. [DOI] [PubMed] [Google Scholar]
- 15.Kubik-Huch RA, Weston M, Nougaret S, et al. European Society of Urogenital Radiology (ESUR) guidelines: MR imaging of leiomyomas. Eur Radiol 2018;28(8): 3125–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamashita Y, Torashima M, Takahashi M, et al. Hyperintense uterine leiomyoma at T2-weighted MR imaging: differentiation with dynamic enhanced MR imaging and clinical implications. Radiology 1993;189(3):721–725. [DOI] [PubMed] [Google Scholar]
- 17.Bolan C, Caserta MP. MR imaging of atypical fibroids. Abdom Radiol (NY) 2016;41(12):2332–2349. [DOI] [PubMed] [Google Scholar]
- 18.Fasih N, Prasad Shanbhogue AK, Macdonald DB, et al. Leiomyomas beyond the uterus: unusual locations, rare manifestations. RadioGraphics 2008;28(7):1931–1948. [DOI] [PubMed] [Google Scholar]
- 19.Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med 2017;14(1):9–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forstner R, Thomassin-Naggara I, Cunha TM, et al. ESUR recommendations for MR imaging of the sonographically indeterminate adnexal mass: an update. Eur Radiol 2017;27(6):2248–2257. [Published correction appears in Eur Radiol 2017;27(6):2258.] 10.1007/s00330-016-4600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohaghegh P, Rockall AG. Imaging strategy for early ovarian cancer: characterization of adnexal masses with conventional and advanced imaging techniques. RadioGraphics 2012;32(6):1751–1773. [DOI] [PubMed] [Google Scholar]
- 22.Imaoka I, Wada A, Kaji Y, et al. Developing an MR imaging strategy for diagnosis of ovarian masses. RadioGraphics 2006;26(5):1431–1448. [DOI] [PubMed] [Google Scholar]
- 23.McEvoy SH, Nougaret S, Abu-Rustum NR, et al. Erratum to: Fertility-sparing for young patients with gynecologic cancer: how MRI can guide patient selection prior to conservative management. Abdom Radiol (NY) 2017;42 (12):2966–2973. [DOI] [PubMed] [Google Scholar]
- 24.Padidar AM, Jeffrey RB, Jr, Mindelzun RE, Dolph JF. Differentiating sigmoid diverticulitis from carcinoma on CT scans: mesenteric inflammation suggests diverticulitis. AJR Am J Roentgenol 1994;163(1):81–83. [DOI] [PubMed] [Google Scholar]
- 25.Dhage-Ivatury S, Sugarbaker PH. Update on the surgical approach to mucocele of the appendix. J Am Coll Surg 2006;202(4):680–684. [DOI] [PubMed] [Google Scholar]
- 26.Carr NJ, Cecil TD, Mohamed F, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) modified Delphi process. Am J Surg Pathol 2016;40(1):14–26. [DOI] [PubMed] [Google Scholar]
- 27.Koga H, Aoyagi K, Honda H, Fujishima M. Appendiceal mucocele: sonographic and MR imaging findings. AJR Am J Roentgenol 1995;165(6):1552. [DOI] [PubMed] [Google Scholar]
- 28.Baxi AJ, Chintapalli K, Katkar A, Restrepo CS, Betancourt SL, Sunnapwar A. Multimodality imaging findings in carcinoid tumors: a head-to-toe spectrum. RadioGraphics 2017;37(2):516–536. [DOI] [PubMed] [Google Scholar]
- 29.Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol 2016;40:39–46. [DOI] [PubMed] [Google Scholar]
- 30.Yu MH, Lee JM, Baek JH, Han JK, Choi BI. MRI features of gastrointestinal stromal tumors. AJR Am J Roentgenol 2014;203(5):980–991. [DOI] [PubMed] [Google Scholar]
- 31.Jiang ZX, Zhang SJ, Peng WJ, Yu BH. Rectal gastrointestinal stromal tumors: imaging features with clinical and pathological correlation. World J Gastroenterol 2013;19(20):3108–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bazot M, Darai E, Hourani R, et al. Deep pelvic endometriosis: MR imaging for diagnosis and prediction of extension of disease. Radiology 2004;232(2):379–389. [DOI] [PubMed] [Google Scholar]
- 33.Del Frate C, Girometti R, Pittino M, Del Frate G, Bazzocchi M, Zuiani C. Deep retroperitoneal pelvic endometriosis: MR imaging appearance with laparoscopic correlation. RadioGraphics 2006;26(6):1705–1718. [DOI] [PubMed] [Google Scholar]
- 34.Bazot M, Bharwani N, Huchon C, et al. European Society of Urogenital Radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur Radiol 2017;27(7):2765–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rezvani M, Shaaban AM. Fallopian tube disease in the nonpregnant patient. RadioGraphics 2011;31(2):527–548. [DOI] [PubMed] [Google Scholar]
- 36.van Ruth S, Bronkhorst MW, van Coevorden F, Zoetmulder FA. Peritoneal benign cystic mesothelioma: a case report and review of the literature. Eur J Surg Oncol 2002;28(2):192–195. [DOI] [PubMed] [Google Scholar]
- 37.Jain KA. Imaging of peritoneal inclusion cysts. AJR Am J Roentgenol 2000;174(6):1559–1563. [DOI] [PubMed] [Google Scholar]
- 38.Dillman JR, DiPietro MA. Hemorrhagic “spider-in-web”: atypical appearance of a peritoneal inclusion cyst. Pediatr Radiol 2009;39(11):1252. [DOI] [PubMed] [Google Scholar]
- 39.Barloon TJ, Brown BP, Abu-Yousef MM, Warnock NG. Paraovarian and paratubal cysts: preoperative diagnosis using transabdominal and transvaginal sonography. J Clin Ultrasound 1996;24(3):117–122. [DOI] [PubMed] [Google Scholar]
- 40.Kishimoto K, Ito K, Awaya H, Matsunaga N, Outwater EK, Siegelman ES. Paraovarian cyst: MR imaging features. Abdom Imaging 2002;27(6):685–689. [DOI] [PubMed] [Google Scholar]
- 41.Chira RI, Nistor-Ciurba CC, Mociran A, Mircea PA. Appendicular mucinous adenocarcinoma associated with pseudomyxoma peritonei, a rare and difficult imaging diagnosis. Med Ultrason 2016;18(2):257–259. [DOI] [PubMed] [Google Scholar]
- 42.Nougaret S, Addley HC, Colombo PE, et al. Ovarian carcinomatosis: how the radiologist can help plan the surgical approach. RadioGraphics 2012;32(6):1775–1800; discussion 1800–1803. [DOI] [PubMed] [Google Scholar]
- 43.Kim J, Bhagwandin S, Labow DM. Malignant peritoneal mesothelioma: a review. Ann Transl Med 2017;5(11):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Busch JM, Kruskal JB, Wu B. Best cases from the AFIP: malignant peritoneal mesothelioma. RadioGraphics 2002;22(6):1511–1515. [DOI] [PubMed] [Google Scholar]
- 45.Kim SW, Kim HC, Yang DM, Min GE. The prevesical space: anatomical review and pathological conditions. Clin Radiol 2013;68(7):733–740. [DOI] [PubMed] [Google Scholar]
- 46.Monteiro V, Cunha TM. Urachal carcinoma: imaging findings. Acta Radiol Short Rep 2012;1(1):arsr.2011.110018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spiess PE, Agarwal N, Bangs R, et al. Bladder cancer, version 5.2017: NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15(10):1240–1267. [DOI] [PubMed] [Google Scholar]
- 48.Expert Panel on Urologic Imaging , van der Pol CB, Sahni VA, et al. ACR Appropriateness Criteria® pretreatment staging of muscle-invasive bladder cancer. J Am Coll Radiol 2018;15(5S):S150–S159. [DOI] [PubMed] [Google Scholar]
- 49.Panebianco V, De Berardinis E, Barchetti G, et al. An evaluation of morphological and functional multi-parametric MRI sequences in classifying non-muscle and muscle invasive bladder cancer. Eur Radiol 2017;27(9):3759–3766. [DOI] [PubMed] [Google Scholar]
- 50.Lakhman Y, Veeraraghavan H, Chaim J, et al. Differentiation of uterine leiomyosarcoma from atypical leiomyoma: diagnostic accuracy of qualitative MR imaging features and feasibility of texture analysis. Eur Radiol 2017;27(7): 2903–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balleyguier C, Sala E, Da Cunha T, et al. Staging of uterine cervical cancer with MRI: guidelines of the European Society of Urogenital Radiology. Eur Radiol 2011;21(5):1102–1110. [DOI] [PubMed] [Google Scholar]
- 52.Nougaret S, Horta M, Sala E, et al. Endometrial cancer MRI staging: updated guidelines of the European Society of Urogenital Radiology. Eur Radiol 2018 Jul 11 [Epub ahead of print] 10.1007/s00330-018-5515-y. [DOI] [PubMed] [Google Scholar]
- 53.Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2018;28(4):1465–1475. [Published correction appears in Eur Radiol 2018;28(6):2711.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gollub MJ, Arya S, Beets-Tan RG, et al. Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017. Abdom Radiol (NY) 2018 May 21 [Epub ahead of print]. [DOI] [PubMed]
- 55.Brown WE, Koh CE, Badgery-Parker T, Solomon MJ. Validation of MRI and surgical decision making to predict a complete resection in pelvic exenteration for recurrent rectal cancer. Dis Colon Rectum 2017;60(2):144–151. [DOI] [PubMed] [Google Scholar]
- 56.Dahan H, Arrivé L, Wendum D, Docou le Pointe H, Djouhri H, Tubiana JM. Retrorectal developmental cysts in adults: clinical and radiologic-histopathologic review, differential diagnosis, and treatment. RadioGraphics 2001;21(3):575–584. [DOI] [PubMed] [Google Scholar]
- 57.Reiter MJ, Schwope RB, Bui-Mansfield LT, Lisanti CJ, Glasgow SC. Surgical management of retrorectal lesions: what the radiologist needs to know. AJR Am J Roentgenol 2015;204(2):386–395. [DOI] [PubMed] [Google Scholar]
- 58.Jones M, Khosa J. Presacral tumours: a rare case of a dermoid cyst in a paediatric patient. BMJ Case Rep 2013 May 15;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JJ, Dickson BC, Sreeharsha B, Gladdy RA, Thipphavong S. Presacral myelolipoma: diagnosis on imaging with pathologic and clinical correlation. AJR Am J Roentgenol 2016;207(3):470–481. [DOI] [PubMed] [Google Scholar]
- 60.Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology 2002;224(1):99–104. [DOI] [PubMed] [Google Scholar]
- 61.Wishnia SC, Rosen JE, Hamid MA, Haas S, Moreno-Ruiz N. Management of a presacral teratoma in an adult. J Clin Oncol 2008;26(15):2586–2589. [DOI] [PubMed] [Google Scholar]
- 62.Beattie CH, Garvey CJ, Hershman MJ. Endorectal magnetic resonance imaging of a rectal duplication cyst. Br J Radiol 1999;72(861):896–898. [DOI] [PubMed] [Google Scholar]
- 63.Dwarkasing RS, Verschuuren SI, van Leenders GJLH, Braun LMM, Krestin GP, Schouten WR. Primary cystic lesions of the retrorectal space: MRI evaluation and clinical assessment. AJR Am J Roentgenol 2017;209(4):790–796. [DOI] [PubMed] [Google Scholar]
- 64.Lee KS, Gower DJ, McWhorter JM, Albertson DA. The role of MR imaging in the diagnosis and treatment of anterior sacral meningocele: report of two cases. J Neurosurg 1988;69(4):628–631. [DOI] [PubMed] [Google Scholar]
- 65.Shetty AS, Loch R, Yoo N, Mellnick V, Fowler K, Narra V. Imaging of tailgut cysts. Abdom Imaging 2015;40(7):2783–2795. [DOI] [PubMed] [Google Scholar]
- 66.Hoarau N, Slim K, Da Ines D. CT and MR imaging of retroperitoneal schwannoma. Diagn Interv Imaging 2013;94(11):1133–1139. [DOI] [PubMed] [Google Scholar]
- 67.Sehgal VN, Srivastava G, Aggarwal AK, Oberoi R, Sharma S. Solitary plexiform neurofibroma(s): role of magnetic resonance imaging. Skinmed 2007;6(2):99–100. [DOI] [PubMed] [Google Scholar]
- 68.Yan J, Mann F, Lewis D, Eary JF. Solitary fibrous tumor: WHO classification, histopathological correlation contribution to the radiology-pathology conference, FDG PET-CT imaging, and biological behaviors. Clin Imaging 2013;37(5):977–978. [DOI] [PubMed] [Google Scholar]
- 69.Aigner F, Zbar AP, Ludwikowski B, Kreczy A, Kovacs P, Fritsch H. The rectogenital septum: morphology, function, and clinical relevance. Dis Colon Rectum 2004;47 (2):131–140. [DOI] [PubMed] [Google Scholar]
- 70.Sawaki A. Rare gastrointestinal stromal tumors (GIST): omentum and retroperitoneum. Transl Gastroenterol Hepatol 2017;2(12):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mabrouk M, Vicenzi C, Ferrini G, et al. Mixed adenocarcinoma of the rectovaginal septum associated with endometriosis and endometrial carcinoma: a case report. Case Rep Oncol 2011;4(1):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Messick CA, Hull T, Rosselli G, Kiran RP. Lesions originating within the retrorectal space: a diverse group requiring individualized evaluation and surgery. J Gastrointest Surg 2013;17(12):2143–2152. [DOI] [PubMed] [Google Scholar]
- 73.Song TK, Harris EJ, Jr, Raghavan S, Norton JA. Major blood vessel reconstruction during sarcoma surgery. Arch Surg 2009;144(9):817–822. [DOI] [PubMed] [Google Scholar]
- 74.Capek S, Howe BM, Amrami KK, Spinner RJ. Perineural spread of pelvic malignancies to the lumbosacral plexus and beyond: clinical and imaging patterns. Neurosurg Focus 2015;39(3):E14. [DOI] [PubMed] [Google Scholar]