Abstract
The accurate diagnosis and seroprevalence investigations of Zika virus (ZKV) infections remain complex due to cross reactivity with other flaviviruses. Two assay formats, both using labelled Zika virus NS1 antigen as a revealing agent (a double antigen binding assay, DABA, and an immunoglobulin Ig capture assay, G capture) were initially developed and compared with the indirect EuroimmunZ assay for the detection of anti-Zika antibody. Of 147 pre-Zika period serum samples, 39 (27%) were reactive in the EuroimmunZ or the DABA assays, 28 sera concordantly so. Such false reactivity was influenced by the serotype of Dengue virus (DV) to which individuals had been exposed to. Thus, of sera from patients undergoing secondary Dengue virus infection of known serotype, 91%, 45% and 28% of Dengue virus serotype 2, 3 and 4 respectively were reactive in one or more of the three assays. A novel method of quenching false sero-reactivity was therefore developed for the DABA and G capture assays. Initial addition of a single homologous Dengue virus serotype 3 NS1Ag quench significantly ablated false reactivities in the pre-Zika period sera. An equipotent quadrivalent quench comprising homologous Dengue virus serotypes 1 to 4 NS1Ag was shown to be optimum yet retained sensitivity for the detection of specific anti-Zika antibody. Comparing DABA and G capture assays using quenched and unquenched conjugates in comparison with EuroimmunZ early in the course of PCR-confirmed infection indicated that a significant component of the apparent early anti-ZIKA antibody response is likely to be due to a Zika virus-driven anamnestic anti-Dengue virus response. The increased specificity provided by homologous antigen quenching is likely to provide a significant improvement in sero-diagnostics and to be of clinical value.
Introduction
Zika virus (ZKV), first described in a nonhuman primate in 1947, is named after a forested area in Uganda [1]. Its ability to infect humans was first demonstrated in 1952 as reviewed by Kindhauser and colleagues [2] with antibody to ZKV detected both by neutralisation and by animal protection experiments [3]. Zika virus is one of a number of closely related Flaviviruses, previously termed Arboviruses, principally transmitted through the bite of an infected Aedes mosquito, most commonly Aedes aegypti but which may also be transmitted directly between humans through sexual contact or vertically, from mother to the unborn baby. Although possible, no cases of transmission through transplantation of cells, tissues or organs have been reported to date; probable transmission through blood products have been reported [4–6].
Similar to other related flaviruses, ZKV infection is believed to be asymptomatic in up to 80% of cases, with a minority manifesting often mild symptomatology [7]. The clinical illness is of short duration and detectable viraemia seldom exceeds seven days from the illness onset [8]. The infection may persist for longer in a number of sanctuary sites [9] including the male genital tract [4,10].
It is likely that ZKV may have caused outbreaks of disease but that these may well have been unrecognised because the clinical illness is similar to that caused by other known pathogens including Dengue (DV) and Chikungunya viruses. Where ZKV differs is that its neuro-tropism and ability to infect the unborn child transplacentally leads to serious neurological birth defects [11]. The significant morbidity caused by Congenital Zika Syndrome, the societal impact and costs of this in both Polynesia and more recently in countries in South and Central America have added to the requirement for both sensitive and specific tests for the detection of antibody to ZKV (anti-ZKV). However, extensive serological cross-reactivity between flaviviruses and their co-circulation has rendered this difficult to achieve using the conventional indirect immunoassay format.
In collaboration with Corti and his colleagues we previously investigated a novel competitive immunoassay for the detection of anti-ZKV [12]. Whilst it conferred considerably improved accuracy in relation to the limited assay options available, we have further investigated two other test formats for the detection of anti-ZKV including reverse capture (G capture) and double antigen binding assay (DABA) principles. Both use a common revealing conjugate of enzyme labelled ZKV NS1 antigen, which together with the addition of un-labelled competitor homologue DV NS1 proteins provides a unique and novel approach to the specific quenching of false reactions due principally to coexisting antibody to other related flaviviruses. Here we describe the performance of these assays and the novel approach to quenching non-specific reactivity in the face of antibody to Dengue viruses as this was the closely-related flavivirus of most relevance in the South American cohorts used in this study, nevertheless recognising that in a global context, other related viruses may induce false serological reactivity. We compared the performance of these two assays with the widely used Euroimmune ZKV antibody Elisa (EuroimmunZ) for the detection of antibody to ZKV in the presence of antibody to Dengue virus (anti-DV) of various serotypes.
Materials and methods
Patient samples
Overall, 422 samples were available for analysis (Table 1)
Table 1. Sample and donor characteristics.
| Sample characteristic | Year | n | Source | PCR Δ | Donor characteristic |
|---|---|---|---|---|---|
| Pre-ZIKA (N = 147) | 2013 | 29 | SP | N/A | Blood donors |
| 2014 | 118 | RdeJ | N/A | Hepatology Outpatients | |
| Dengue Virus (n = 134) | Pre 2014 | 28 | SP | DV1 | Acute Dengue Fever case |
| 32 | SP | DV2 | |||
| 34 | SP | DV3 | |||
| 40 | SP | DV4 | |||
| ZIKA Virus (n-138) | Post 2014 | 95 | SP | ZKV | Acute ZIKA Fever case |
| 31 | RdeJ | ||||
| 12 | NHSBT |
Table listing sample used in the analysis. PCR Δ diagnostic PCR where available; N/A: PCR not available. SP: Sao Paolo; RdeJ: Rio de Janeiro. NHSBT: UK National Health Blood Transfusion Service. DV1- DV4; serotype specificity of the diagnostic PCR.
Known Dengue types seropositive samples
Sera were available in São Paulo from 134 patients previously infected with DV of PCR confirmed serotype; most samples were taken before 2014, when ZKV is believed to have been introduced into the Americas, including 28 patients with Dengue virus serotype 1 (DV1) primary infection sampled in 2015; 32 patients with Dengue virus serotype 2 (DV2) secondary infection sampled in 2010; 34 patients with Dengue virus serotype 3 (DV3) proven or presumed secondary infection sampled in 2002 and 2005; 40 patients with proven or presumed Dengue virus serotype 4 (DV4) secondary infection sampled in 2013. The DV serotype of the initial infection is not known but will be a reflection of the viruses circulating in the community at the time.
Pre-Zika period samples
One hundred and forty-seven sera drawn prior to the recognized introduction of ZKV into Brazil were available for testing. One hundred and eighteen were available in Rio de Janeiro from outpatient clinic attenders at Fiocruz during 2014 (outpatient panel) and 29 samples were available in São Paulo from blood donors taken in 2013; 23 of these samples were positive for DV IgG.
Zika samples
One hundred and thirty eight sera taken from patients between 1 day and > 1 year after the onset of arboviral-like symptoms of PCR-confirmed Zika infection (95 from São Paulo and 31 from Rio de Janeiro, 12 from UK returnees sampled by the English blood transfusion service, NHSBT) were available for testing.
Ethics
In Rio, the procedures applied in this study were performed in accordance with the ethical standards of the Instituto Nacional de Infectologia Evandro Chagas. The study protocol was approved by its Comitê de Ética em Pesquisa (reference CAAE 0026.0.009.000–07). In São Paulo, procedures conformed with terms agreed by the Institutional Review Board from Hospital das Clínicas, University of São Paulo (CAPPesq- Research Projects Ethics Committee). Specimens were anonymised to ensure patient confidentiality.
Flavivirus antigens
Recombinant ZKV NS1 (rZKVNS1Ag) antigen expressed by baculovirus in insect cells (MyBioSource Inc, San Diego, USA) was used both to coat the solid-phase where appropriate and, when directly conjugated with horseradish peroxidase, to provide a revealing agent for captured antibodies.
Recombinant DV NS1 antigens (rDVNS1Ag) from the four serotypes (DV 1–4 inclusive) expressed in mammalian cells (The Native Antigen Company, Kidlington, Oxfordshire, OX5 1LH, UK) were used in molar excess as components of the conjugate diluent. The addition of these un-labelled antigens served to quench reactivity to epitopes on Dengue related antigens.
Assay formats
Indirect immunoassay. The EuroimmunZ kit for the detection of G anti-ZKV antibody (EUROIMMUN AG, Luebeck, Germany) was used in accordance with the manufacturer’s instructions and defined kit cut-off.
G capture assay (G capture). Solid-phase wells (NUNC Immunomodule, U8 Maxisorp wells) were coated with 100μl volumes of Affinipure rabbit anti-human ɣ (Jackson ImmunoResearch, Ely, Cambridgeshire UK) at 5μg/ml in MicroImmune Coating Buffer for ELISA with preservative; (ClinTech, Guildford, UK). Coating was overnight at 2–8°C, followed by 3 hours at 35–37°C. Wells were then washed with PBS Tween 20 and quenched with MicroImmune Blocking Solution (ClinTech, Guildford, UK) for 3–4 hours at 37°C. Wells were aspirated and stored dry at 4°C in sealed pouches with desiccant until use. Prior to testing, serum samples were diluted to 1 in 200 in Transport Medium (TM: Phosphate buffered saline supplemented with Amphotericin B 0.5ug/ml, Gentamicin 0.25mg/ml, 10% v/v heat inactivated fetal calf serum, Tween 20 0.05% v/v, Red Dye 0.05% v/v). One hundred microlitres of diluted serum were added to the wells, incubated for 60 ±2 minutes at 37°C prior to washing and the addition of the conjugate. After a further incubation for 30 ±2 minutes at 37°C the solid-phase was again washed and 100 μl of substrate added, incubated for 30 ±2 minutes at 37°C, the reaction then stopped and measured at 450/630nm. Full details are provided as information for use (IFU) leaflets in supplementary information.
Double antigen binding assay (DABA). Solid-phase wells (NUNC Immunomodule, U8 Maxisorp wells) were coated with 100μl volumes of rZKVNS1Ag at 2μg/ml in MicroImmune Coating Buffer, overnight at 2–8°C, followed by 3 hours at 35–37°C. Wells were then washed with PBS Tween 20 and quenched with MicroImmune Blocking Solution for 3–4 hours at 37°C. Wells were aspirated and stored dry at 4°C in sealed pouches with desiccant until use. Prior to testing 70 μl of sample diluent (MicroImmune Sample Diluent; ClinTech, Guildford, UK) were added to each well. Thirty microlitres of control or test sera were added singly to each well and incubated for 60 ± 2 minutes at 37°C prior to washing and addition of conjugate. After a further incubation for 120 ±5 minutes at 37°C the solid phase was again washed and 100 μl of substrate added, incubated for 30 ±2 minutes at 37°C, the reaction then stopped and measured at 450/630nm. Full details are provided as information for use (IFU) leaflets in supplementary information.
Data from three-way comparisons between EuroimmunZ, the G capture and the DABA data generated by individual sera are displayed as Venn diagrams. Two way comparisons are displayed as X by Y plots. Exploration of the efficacy of conjugate performance is also displayed as X by Y plots, comparing conjugate with (Quenched) and without (Unquenched) the addition of DV NS1 antigens to the conjugate diluent.
Conjugation of rZKVNS1Ag
One hundred microlitres of recombinant ZIKV NS1 antigen (ensuring at an optimal protein concentration range of 0.5–5.0mg/ml) were coupled to 100μg of lyophilized HRPO mix using the LYNX Rapid HRP Conjugation kit (Bio-Rad Laboratories Ltd, Watford, UK) in accordance with the manufacturer’s instructions Once conjugated the product was diluted 1 in 10 in HRP Stabilising Buffer (ClinTech, Guildford, UK) and stored un-fractionated in 50μl volumes at -20°C.
Conjugates for capture and DABA
HRPO-conjugated rZKVNS1Ag was appropriately diluted in conjugate diluent (Initially product kit GE 34/36, (gifted by DiaSorin UK, Dartford, Kent, UK) and then subsequently Clintech Conjugate diluent, (ClinTech, Guildford, UK) and either used as such (unquenched) or diluted in the same conjugate diluent to which had been added a molar excess of rDVNS1Ag of defined serotype (quenched). The performance of the conjugate either quenched or unquenched was compared across the range of dengue sero-positive samples and samples likely to contain anti-ZKV from patients with confirmed ZKV infection.
A schematic illustrating the DABA and G Capture assay formats and the various conjugate options is shown in Fig 1.
Fig 1. Illustration detailing the immunoglobulin G capture assay (G capture) and the double antigen binding assay (DABA).
The G Capture solid phase (cross hatched) is coated with rabbit anti-human gamma FC to capture G from the clinical sample in the first step of the assay. After washing, conjugate is applied and specific antibody (Y) revealed by ZIKA virus NS1 antigen (ZKVNS1Ag) labelled with horseradish peroxidase (HRPO; Z *, standard). The DABA solid-phase is coated with ZKVNS1Ag to capture antibody to NS1Ag of any class. After washing, conjugate is applied and bound antibody (Y) revealed by ZKV NS1Ag labelled with HRPO as above.
Several options for additions to the conjugate to quench non-specific reactivity have been investigated. The conjugate diluent has been used with (i) no additional unlabelled viral added antigen (Standard); (ii) with added unlabelled Dengue virus 3 NS1 antigen alone (D, DV 3 Quench); (iii) with added unlabelled equi-potent mix of four Dengue virus 1 to 4 NS1 antigens (Q, Quad Quench); and (iv) with added unlabelled ZKV NS1Ag (Z, ZIKA Quench) as described in text.
Serological controls for capture and DABA
Human G1 anti-ZKV virus NS1 supplied by the Native Antigen company (Kidlington, Oxford, UK) was used as a positive control. Used at 10ug/ml in G capture assay as the positive quality control it was required to give an optical density (OD) between 0.8 and 1.5. Used at 2ug/ml in the DABA assay it was required to give an OD between 2.5 and 3.5.
Pooled normal UK human plasma (NHP) previously screened for blood borne viruses constituted the unreactive control. Test samples reacting greater than the mean negative control +0.1 OD where considered to be reactive for anti-ZKV.
Detection of Dengue antibody
Samples from UK returning travellers were tested for Dengue antibody using the PanBio Dengue IgG Indirect ELISA (Alere/Abbott Diagnostics, Abingdon, UK).
Results
Demonstration of cross-reactivity
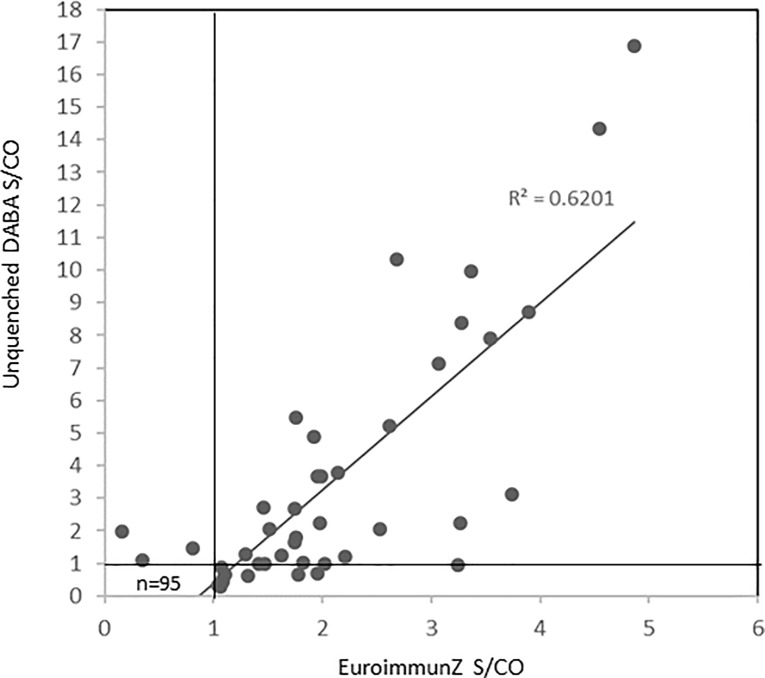
The antibody reactivity of 147 samples (118 samples from the 2014 outpatient panel and 29 blood donor samples from 2013) archived before the recognised introduction of ZKV into Brazil was determined in the EuroimmunZ indirect Elisa and in the unquenched DABA (Fig 2). Thirty five samples (24%) were reactive on the EuroimmunZ whilst 32 samples (22%) were reactive on the unquenched DABA, 28 (19%) were concordantly reactive in both assays. Fourteen of the 147 (10%) were reactive in the indeterminate zone in EuroimmunZ, one of which was reactive in the unquenched DABA. Ninety eight samples (67%) were un-reactive in the EuroimmunZ, three were reactive in the unquenched DABA.
Fig 2. EuroimmunZ vs Unquenched DABA reactivity in pre-Zika era panels.
Plot of sample to cut off (S/CO) ratios of 147 pre-Zika era samples tested in un-quenched DABA and EuroimmunZ. Fourteen samples with EuroimmunZ ratios <1.1 were considered indeterminate by EuroimmunZ criteria. Three samples were reactive by DABA alone. Solid lines represent the cut off value for each assay. Trend line is displayed.
The reactivity given by sera containing anti-DV antibody following known infection by Dengue serotypes 1 through 4 was determined in each of the three assays, EuroimmunZ, DABA and G capture, both using un-quenched conjugates. The proportion of samples reactive for anti-ZKV in one or more assays ranged from 29 of 32 (91%) of samples from patients with secondary DV2 infections, 9 of 20 (45%) of samples from patients with secondary DV3 to 11 of 40 (28%) of samples from patients undergoing secondary DV4 samples. Seven of 28 (25%) samples from patients with primary DV1 reacted in one or more assays (data not shown).
Sensitivity of different assay formats for detecting a ZKV serological response
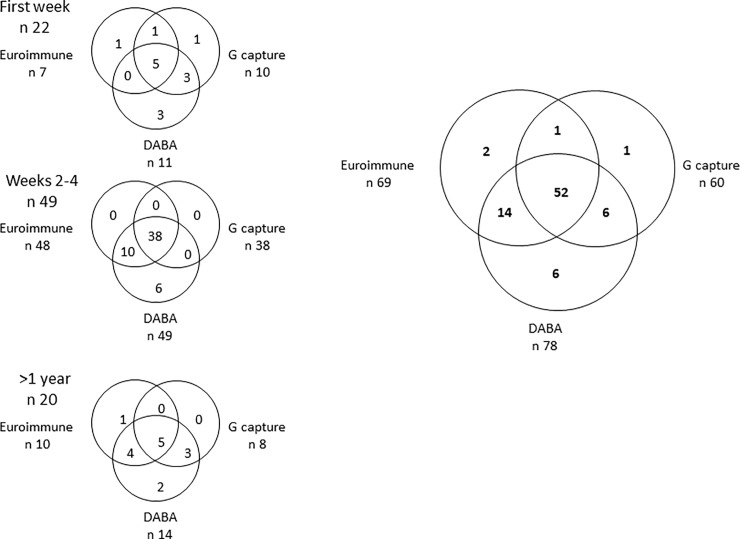
The evolution of detectability of antibody to ZKV in EuroimmunZ, in DABA and in G capture using unquenched conjugates, was investigated using a panel of samples from 56 patients undergoing a confirmed acute ZKV infection. Ninety one sera were tested, 69 were reactive in EuroimmunZ, 78 were reactive in DABA and 60 in G capture (Fig 3). The majority of the samples (52/91, 57%) were concordantly reactive in all three assays. Ranking the samples by time after the onset of symptoms indicated that of the 21 samples that were taken in the first week of symptoms, seven were reactive in EuroimmunZ, 11 were reactive in DABA and ten in G capture. Of the 49 samples taken between two and four weeks after the onset of symptoms, all but one were reactive in both EuroimmunZ and DABA, only 38 were reactive in G capture. Of the 20 samples taken more than one year after the acute illness only 15 were reactive in any assay, ten in EuroimmunZ, 14 in the DABA and eight in the G capture.
Fig 3. Antibody reactivity across three assays in samples from PCR confirmed Zika cases.
Venn plots for 91 samples from patients with PCR confirmed ZKV infection. Samples giving sample to cut off ratios >1.0 were defined as reactive. The right hand plot shows reactivity for the panel of 91 samples tested in the three different assays. The three panels on the left in vertical order show the reactivity broken down into the first week, second to fourth week and one or more year after onset of symptoms. The individual reactivity of samples in each test is shown and the overall reactivity for each assay displayed.
Conjugate quenching to reduce cross-reactivity
The addition of quenching amounts of unlabelled recombinant DV NS1 antigen of a single serotype was initially investigated to reduce the signal of apparent Dengue serum antibody reactivity in the two assays, G capture and DABA, both using HRPO-conjugated rZKVNS1Ag as a detector. A panel comprising 32 DV antibody-containing sera samples including those that were previously falsely reactive in the unquenched ZKV G capture and/or DABA assays was selected. The panel was then retested in both assays using a modified conjugate diluent containing unlabelled rDV3NS1Ag at a concentration of 25μg/ml. A single serum remained reactive in the DABA, all other samples the presumed non-specific reactivities were quenched by the use of conjugate diluent containing rDV3NS1Ag (data not shown).
Sensitivity, specificity and optimisation of quenched conjugates for detecting anti-ZKV
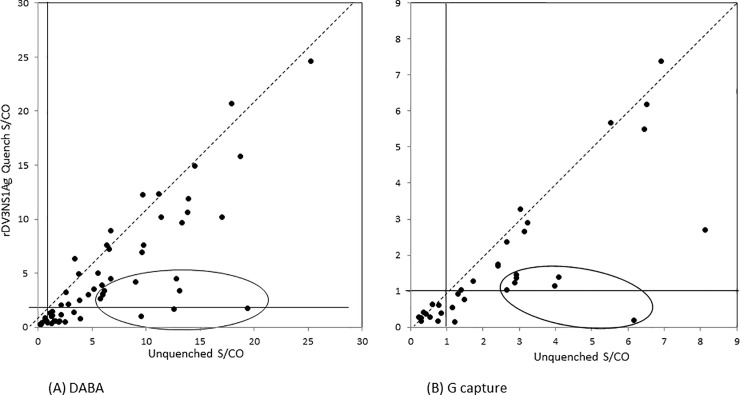
A subset of the panel of 95 sera from ZKV patients were re-tested initially using the rDV3NS1Ag as the sole constituent of a quenched buffer. In the majority of samples, the level of anti-ZKV reactivity was not reduced in both G capture and DABA with by the addition of DV1 antigen to the conjugates. It is noticeable however that a proportion of samples (six from 59 in DABA and eight from 41 in G capture) showed significant reduction of reactivity with the quenched conjugate (these are circled, Fig 4A and 4B respectively).
Fig 4.
4A and 4B. G capture and DABA data on Zika confirmed panels comparing DV3NS1Ag quenched and un-quenched conjugates. X by Y plots of sample reactivity from patients known to have been infected with ZKV. Reactivity is expressed as sample to cut off ratios when tested using un-quenched conjugate and rDV3NS1Ag-quenched conjugate. Left hand panel (A) displays the results with 59 ZKV convalescent sera (São Paulo and Rio de Janeiro) tested in the DABA using either unquenched or quenched conjugates. Dotted line is a line of interpolated equivalence assuming no difference in reactivity. Solid lines represent the assay cut off values. Right hand panel (B) similarly displays the reactivity of 41 samples from patients with confirmed ZKV infection tested in the G capture using unquenched and quenched conjugate diluents. Samples from patients with proven ZKV showing a reduced reactivity resulting from the quenched conjugate are circled in both panels.
To investigate in more detail the serotype specificity of the quenching effect of rDVNS1 proteins, a panel of 20 sera previously displaying strong reactivity in the unquenched G capture assay was run a second time in the G capture and the DABA assay with two different conjugate diluents. A normal conjugate diluent was used for the unquenched version as referent in comparison with the quenched version of the assay using conjugates containing quenching amounts of rDVNS1Ag, present as rNS1Ag of each DV serotype individually. The degree of residual activity displayed by the different quenching rDVNS1Ags was greatly influenced by the rDVNS1Ag serotype (Table 2). We also investigated quenching as a quadrivalent equipotent rNS1Ag-blend of all 4 serotypes at concentrations from 25, 12.5 to 6.25μg/ml. The quadrivalent mix ablated DV-related cross reactivity in the DABA, two samples remained consistently reactive in the G capture assay. Specific anti-ZKV control reactivity remained detectable under all quenching mixtures (Table 3). There was some evidence for a titration of the quenching function over the range from 25 to 6.25μg/ml of competitor DVNS1 homologue in the G capture conjugate, with mean quenched sample reactivity ranging from 0.63 through 0.74 to 0.87 across the two fold titration of quenching NS1 antigen. A similar trend is seen for DABA with mean reactivities of 0.56, 0.61 and 0.63. A single stochastic low level reaction was seen in the 6.25μg/ml quench DABA assay but the sample was not retested. Based on the relative effectiveness at 6.25μg/ml, this homologue antigen quench concentration was adopted for further studies.
Table 2. Quenching of non-specific antibody reactivity using a panel of Dengue serotype 1 to 4 antigens.
| COHORT | G Capture | DABA | ||||||
|---|---|---|---|---|---|---|---|---|
| Unquenched | Dengue 1 | Dengue 2 | Dengue 3 | Dengue 4 | Quad mix | Unquenched | Quad mix | |
| Dengue 2 | ||||||||
| Dengue 2 | ||||||||
| Dengue 2 | ||||||||
| Dengue 2 | ||||||||
| Dengue 2 | ||||||||
| Dengue 2 | ||||||||
| Dengue 2 | ||||||||
| Dengue 2 | ||||||||
| Dengue 2 | ||||||||
| Dengue 2 | ||||||||
| Dengue 2 | ||||||||
| Dengue 2 | ||||||||
| Dengue 2 | ||||||||
| Dengue 2 | ||||||||
| Dengue 3 | ||||||||
| Dengue 3 | ||||||||
| Dengue 3 | ||||||||
| Dengue 4 | ||||||||
| Dengue 4 | ||||||||
| Dengue 4 | ||||||||
| ZIKA | 3.925 | 3.583 | 3.185 | 3.42 | 3.354 | 2.882 | 5.794 | 4.196 |
| ZIKA | 9.237 | 4.109 | 6.276 | 6.735 | 6.626 | 4.617 | 6.212 | 2.088 |
| ZIKA | 16.109 | 4.050 | 10.291 | 13.186 | 12.467 | 6.621 | 8.607 | 2.113 |
| ZIKA | 16.661 | 6.812 | 10.976 | 14.22 | 13.439 | 8.062 | 5.713 | 1.387 |
The reactivity of a panel of 20 samples from patients with previous dengue virus infection of known serotype when tested with unquenched and quenched (rDVNS1Ag 12.5μg/ml) conjugate diluents in the G capture and the DABA assay. The quenching component in a quenched conjugate is of either a single DV serotype as shown for the G capture or of a quadrivalent preparation containing equipotent quantities of all four serotypes as shown for both the G and the DABA. Shaded cells represent sample/cut off reactivity >1. Reactivity of the control ZIKA-positive samples, arbitrarily chosen from patients in Sao Paolo, was maintained (bottom four rows) though clearly reduced in magnitude.
Table 3. Titration of un-labelled competitor homologue quadrivalent DV NS1 quench.
| COHORT | G Capture | DABA | ||||||
|---|---|---|---|---|---|---|---|---|
| Unquenched | Quad Mix 25ug/ml S/CO |
Quad Mix 12.5ug/ml S/CO |
Quad Mix 6.25ug/ml S/CO |
Unquenched S/CO |
Quad Mix 25ug/ml S/CO |
Quad Mix 12.5ug/ml S/CO |
Quad Mix 6.25ug/ml S/CO |
|
| S/CO | ||||||||
| Dengue 2 | 2.184 | 0.384 | 0.493 | 0.623 | 12.278 | 0.513 | 0.536 | 0.632 |
| Dengue 2 | 8.421 | 0.439 | 0.6 | 0.778 | 6.57 | 0.528 | 0.572 | 0.641 |
| Dengue 2 | 4.379 | 0.544 | 0.754 | 1.472 | 11.858 | 0.491 | 0.557 | 0.632 |
| Dengue 2 | 6.926 | 0.485 | 0.571 | 0.759 | 9.968 | 0.543 | 0.68 | 0.784 |
| Dengue 2 | 8.574 | 0.453 | 0.799 | 0.858 | 6.091 | 0.468 | 0.557 | 0.637 |
| Dengue 2 | 3.011 | 0.475 | 0.526 | 0.594 | 4.608 | 0.445 | 0.546 | 0.582 |
| Dengue 2 | 1.426 | 0.485 | 0.654 | 0.67 | 5.301 | 0.687 | 0.722 | 0.559 |
| Dengue 2 | 4.337 | 0.686 | 0.857 | 1.075 | 0.77 | 0.468 | 0.5 | 0.486 |
| Dengue 2 | 2.279 | 0.457 | 0.447 | 0.467 | 4.421 | 0.347 | 0.433 | 0.426 |
| Dengue 2 | 6.405 | 0.517 | 0.547 | 0.665 | 0.977 | 0.589 | 0.577 | 0.591 |
| Dengue 2 | 4.268 | 0.37 | 0.576 | 0.585 | 8.252 | 0.558 | 0.588 | 1.072 |
| Dengue 2 | 2.642 | 1.573 | 1.723 | 2.024 | 2.214 | 0.408 | 0.536 | 0.431 |
| Dengue 2 | 5.453 | 0.562 | 0.675 | 0.783 | 1.864 | 0.543 | 0.619 | 0.646 |
| Dengue 2 | 6.611 | 0.539 | 0.671 | 0.807 | 9.221 | 0.445 | 0.531 | 0.486 |
| Dengue 3 | 2.021 | 0.59 | 0.621 | 0.693 | 1.95 | 1.245 | 0.938 | 0.948 |
| Dengue 3 | 9.416 | 0.777 | 0.915 | 0.91 | 14.375 | 0.725 | 0.768 | 0.756 |
| Dengue 3 | 3.337 | 0.709 | 0.634 | 0.679 | 2.229 | 0.702 | 0.711 | 0.591 |
| Dengue 4 | 2.226 | 1.207 | 1.379 | 1.429 | 12.66 | 0.506 | 0.588 | 0.49 |
| Dengue 4 | 2.553 | 0.736 | 0.762 | 0.717 | 25.864 | 0.528 | 0.608 | 0.564 |
| Dengue 4 | 9.947 | 0.594 | 0.683 | 0.788 | 1.663 | 0.521 | 0.572 | 0.577 |
| Mean S/CO | 4.821 | 0.629 | 0.744 | 0.869 | 7.157 | 0.563 | 0.607 | 0.627 |
| ZIKA | 3.505 | 3.237 | 2.882 | 3.09 | 5.794 | 5.894 | 4.196 | 3.216 |
| ZIKA | 6.221 | 4.649 | 4.617 | 4.915 | 6.212 | 2.702 | 2.088 | 1.865 |
| ZIKA | 12.621 | 5.888 | 6.621 | 7.887 | 8.607 | 2.694 | 2.113 | 1.746 |
| ZIKA | 12.937 | 7.991 | 8.062 | 9.123 | 5.713 | 4.325 | 1.387 | 2.465 |
Optimisation of the quadrivalent un-labelled competitor homologue DV NS1quench. Sample /cut off ratios are shown for both the G capture assay and the DABA. The sample reactivity unquenched and quenched at the three levels of 25, 12.5 and 6.25μg/ml is shown for each assay, samples still reactive in the presence of conjugate quenching are displayed in bold. Reactivity of the control ZIKA-positive samples is displayed (bottom four rows).
To explore further the quenching effect of using conjugate quenched with the optimised quadrivalent mixture of rDVNS1Ag, 87 samples from the 2014 outpatient cohort were retested using quadrivalent quenched conjugate at 6.25ug/ml. Twenty three samples reactive in the unquenched DABA and 27 reactive in EuroimmunZ were all unreactive in the quadrivalent quenched DABA (Fig 5A and 5B). Nine samples giving indeterminate reactivity on the EuroimmunZ were unreactive in the quadrivalent quenched DABA. A further 19 anti-DV reactive samples were subsequently included to give a panel total of 106 samples which were all unreactive in the DABA indicating a high specificity.
Fig 5.
5A and 5B. Antibody reactivity in Dengue quadrivalent antigen quenched assay: comparison with unquenched DABA and EuroimmunZ. X by Y plots of reactivity displayed by 87 selected samples (São Paulo and Rio de Janeiro) drawn from the pre ZIKA-era panel expressed as sample to cut off ratios. Left hand panel (A) displays the results with sera tested in DABA using both quad quenched and unquenched conjugates. Right hand panel (B) similarly displays the reactivity of the same samples tested in quad quenched DABA and EuroimmunZ.
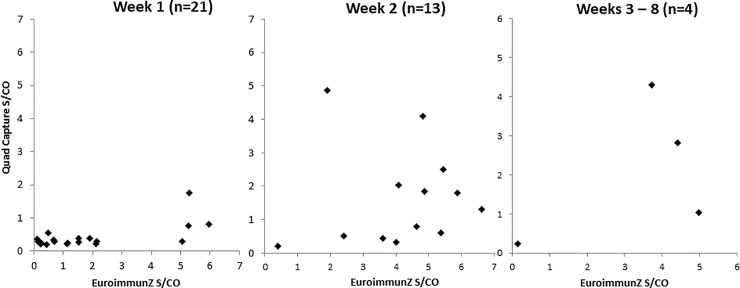
A further panel of 38 samples from 19 ZKV-infected patients taken at various times after the onset of disease was again re-tested in the G capture assay only (to conserve sample volumes) using the optimised quadrivalent quench and the resulting reactivity compared with the reactivity in the EuroimmunZ assay. This demonstrated a considerable divergence between reactivity in the two assays, most prominently observed early in the illness time course with the anti-ZKV reactivity in the GG capture assay with a quenched conjugate often found to be very much reduced, particularly in the first week after diagnosis (Fig 6).
Fig 6. Zika seroconversion panels: EuroimmunZ vs Quadrivalent antigen quenched G capture by time from infection.
X by Y plots of sample reactivity, expressed as sample to cut off ratios in G capture assay using quad quenched conjugate and in EuroimmunZ, of 38 samples grouped by time since onset of symptoms.
To investigate the specific reactivity of sera from patients not domiciled in a Dengue endemic country, a panel of samples from twelve UK returning travellers diagnosed with acute Zika infection were examined. The samples comprised paired specimens taken at the time of diagnosis and at the time of donating in the late recovery period.
The samples were tested by the DABA using either (a) the standard conjugate with no additional additive; (b) with added Zika NS1 quench; or (c) with the Dengue quadrivalent quench. The reduction of the Zika signal reactivity given by the Dengue quadrivalent quench was relatively small (Table 4) comparing the S/CO ratios between the no quench addition with that seen in the Dengue quadrivalent quench. The most marked reduction was seen in a patient whose index sample also contained IgM reactivity to Dengue (UK11). The least marked reduction was evidenced in the two patients whose samples were unreactive for antibody to Dengue both at the time of Zika diagnosis and at the time of donation (UK1 and 2). It is notable that the addition of the Zika quench effectively ablated all reactivity in all samples and the positive control.
Table 4. Zika and Dengue antibody reactivity in samples from UK returning travellers.
| Sample | Zika DABA | Dengue antibody at | |||
|---|---|---|---|---|---|
| Donor ID | Unquenched S/CO | ZIKA NS1 Quench S/CO | Dengue QUAD Quench S/CO | Diagnosis (S/CO) | Donation (S/CO) |
| UK15 | 20.71 | 0.13 | 18.12 | 4.84 | 2.95 |
| UK16 | 5.77 | 0.12 | 5.32 | 1.33 | 3.71 |
| UK6 | 7.96 | 0.09 | 8.02 | 0.56 | 2.78 |
| UK7 | 13.32 | 0.13 | 12.60 | 0.84 | 1.68 |
| UK12 | 3.95 | 0.10 | 2.92 | 1.33 | 3.30 |
| UK10 | 24.56 | 0.13 | 22.24 | 3.72 | 4.22 |
| UK11* | 7.42 | 0.13 | 4.48 | 4.00 | 4.69 |
| UK1 | 9.80 | 0.12 | 10.07 | 0.05 | 0.35 |
| UK2 | 2.54 | 0.07 | 2.39 | 0.02 | 0.58 |
| UK13 | 8.12 | 0.09 | 6.45 | 3.42 | 2.71 |
| UK3 | 17.74 | 0.11 | 12.38 | 0.19 | 1.89 |
| UK4 | 15.16 | 0.10 | 15.06 | 0.20 | 3.01 |
| Positive | 25.79 | 0.12 | 25.74 | N/A | N/A |
| Cutoff | 0.15 | 0.11 | 0.12 | N/A | N/A |
The reactivity of 12 samples from UK returning travellers with confirmed Zika infection when tested in DABA with unquenched, Zika NS1 antigen quench and Dengue quadrivalent antigen quench conjugate diluents. Dengue antibody has been determined on a commercial assay.
Discussion
After the first description of ZKV virus infection in Africa nearly 80 years ago and the sporadic numbers of human infections identified subsequently associated with mild erythematous illness, the first significant cluster of cases since then was reported from Yap Island in Micronesia in 2007 [2]. By the early part of 2016, the WHO declared that ZKV infection, associated as it was with severe neurological disorders in the new-born, was a public health emergency of international concern. A particular aspect arising from this decision was the requirement for specific and sensitive serological assays for the detection of anti-ZKV antibody. This was always going to be challenging because of the potential exposure to multiple co-circulating Flavi-viruses in endemic areas, with the known potential to elicit serological cross reactivity between these infections. Particular assay formats have individual attributes, an observation which underlines the value of taking a broader view when setting out to develop serological tests for infectious agents. This approach is exemplified by our recent experience with Ebola virus serology [13] and echoed by Balmaseda and colleagues [12]. Recognising that antibody to envelope proteins is often cross-reactive within subgroups of the Flavi-viridae family, Stettler and her colleagues [14] moved to use the non-structural protein NS1 in view of data suggesting that this protein was likely to provide a more species-specific antigen moiety for use as the solid phase in immunoassays. A similar conceptual approach is employed in the benchmark and widely used EuroimmunZ assay which uses rZKVNS1Ag in an indirect assay format. These observations informed our choice of the NS1 target and our decision to explore assay formats other than the indirect immunoassay (Fig 1).
It is clear that the use of the NS1 protein in the EuroimmunZ indirect immunoassay format is still associated with significant cross-reactivity likely to be generated by prior Flavi-virus co-circulation and repeated exposure in the community prior to the introduction of ZKV into new geographical areas (Fig 2). In examining the different assay formats of G capture and DABA we find a similar lack of high specificity from patient and blood donor samples collected in two different areas of South East Brazil before the introduction of ZKV into the Americas. These observations subsequently led us to explore quenching non-specific reactivity displayed by the labelled conjugate.
However before doing so we wanted to explore the comparative sensitivity of the three assay formats using 91 samples from Zika fever patients sampled at different times after the onset of disease symptoms (Fig 3) using unquenched conjugates in the G capture and DABA formats. In overall ranking it is likely that the DABA format, in essence a total antibody test, was marginally more proficient at detecting early and late serological markers than the other two formats. The DABA format will recognise antibody of any class and does indicate the potential of being a total antibody assay of high sensitivity. It is however questionable exactly what reactivity was being detected in the unquenched assay, as false reactivity was also displayed by all three formats in samples from persons who had previous DV infection. This was most notable after secondary DV2 infection and all three test formats were variously susceptible including those using a labelled antigen conjugate. We therefore investigated a novel approach of including unlabelled Dengue virus antigens in the conjugate diluent. In the first instance one antigen serotype rDV3NS1Ag at 25μg/ml was used in a quenched conjugate. This was effective in quenching nonspecific reactivity in both formats but reduced the reactivity of ZKV convalescent samples (Fig 4). In many cases there was a concordant reduction of reactivity in both assay formats, individual samples displayed within the circles.
Our data showed that extinguishing cross reactivity was best achieved by using an equipotent four component mix of rDVNS1Ags (Table 2). Optimisation indicated that and this was used in all subsequent analyses at 6.25μg/ml. It was effective in quenching false reactivity of the pre-ZKV period samples (Fig 5). To investigate further the nature of the apparent reduction of the Zika specific reaction in the response to ZKV infection using a panel of 35 samples from patients taken at various times following confirmed ZKV diagnosis, a significant and notable reduction in the magnitude of the ZKV-specific signal was seen again using the quenched conjugate, particularly early in the course of the antibody response (Fig 6). In the small panel of samples taken from UK returning travellers (Table 4), the signal suppression by the Dengue quench was relatively minor but was nevertheless seen clearly in those patients whose both diagnostic samples and those whose later donation samples were reactive for anti-Dengue. Reinforcing the cross reactivity between these two viruses, ten donation samples were reactive for anti-Dengue as a result of the primary Zika infection and variously showed a degree of signal quenching. Only two donors (UK1 and 2) remained sero-negative for anti-Dengue throughout and showed no quenching. Taken together our data ndicated that a considerable component of the early antibody reactivity in Zika seroconversion especially in persons living in a Dengue-endemic environment, irrespective of assay format, may be related to prior cross reacting dengue or other related flavi-virus antibody. This is not altogether surprising since not only does ZKV infection boost dengue antibody [15] but the concept of “original sin” can also be applied to flavi-virus reinfection [16]. Taken together these data sets generated by using quenched conjugates suggest that ZKV infection may induce an anamnestic boost to DV antibody from previous Dengue virus exposure and that the apparent early strong serological response following ZKV infection is not actually Zika specific antibody. As might be anticipated the addition of cold unlabelled antigen of the same specificity of both the solid phases and the label in the DABA totally ablated any signal, presumably including both the Zika and the Dengue component reactivities.
Such observations again underline the need for a serological strategy to minimise cross reactivity and reveal ZKV-specific responses which can easily be masked by cross reaction [17]. Approaches to absorb out interfering reactivity prior to analysis provide one option, so too does the use of residue deletion to remove cross reacting epitopes in recombinant proteins. It is likely that the attribute of using assay formats that allow quenching of the conjugate as described here will deliver specificity whilst retaining sensitivity. It also allows the inherent sensitivity of the DABA format to be used without the usual trade off between sensitivity and specificity. The additional advantage of this for reverse immunoglobulin capture assays is considerable as it opens up not only a potential increase in specificity for IgM detection but also the application of serology to non-blood analytes especially oral fluid, a long established methodology [18], which remains of considerable value in field epidemiology as we have found with other emerging infections including Ebola [19].
In summary there remains a clear need for better ZKV serological tools for diagnostic and epidemiological purposes; the data presented here support the utility of the assay formats developed and it is now important to generate more comprehensive field data in order fully to validate the application of the DABA and the Capture assay as described and provide more substantive sensitivity and specificity data in the first instance. Whilst active transmission has declined substantially across the Americas, much remains to be elucidated about the natural history of ZKV virus infection. Cases are being sporadically described elsewhere, with potential for rapid spread where environmental and population conditions are favourable. Efforts to develop a ZKV vaccine also continue. In this context, affordable, highly specific and sensitive antibody tests are needed to expand the testing capacity in areas that have been or may become affected by ZKV virus.
Supporting information
(XLSX)
Acknowledgments
We would like to thank colleagues in Diasorin for the kind gift of the conjugate diluent. DB is partially funded by European Union’s horizon 2020 research and innovation programme (grant agreement no. 734857), Zikaction and Zikaplan consortia (grant agreement 734584). RMC is affiliated to the NIHR HPRU in Emerging and Zoonotic Infections at University of Liverpool in partnership with PHE, in collaboration with LSHTM.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by Medical Research Council Zika Rapid Response award (MC_PC_15093) to RST, SI, DWGB, IUL, https://mrc.ukri.org/; Public Health England Pipeline fund (PLF 1819-115/ RA) to RST, SI, https://www.gov.uk/government/organisations/public-health-england. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dick GW, Kitchen SF, Hadow AJ. ZKV virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952; 46(5): 509–20. 10.1016/0035-9203(52)90042-4 [DOI] [PubMed] [Google Scholar]
- 2.Kindhauser MK, Allen T, Frank V, Santhana RS and Dye C. Zika the origin and spread of a mosquito-borne virus. Bull World Health Organ. 2016; 94(9): 675–686C. 10.2471/BLT.16.171082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore DL, Causey OR, Carey DE, Reddy S, Cooke AR, Akinkugbe FM et al. Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann Trop Med Pasrasitol. 1975; 69(1): 49–64. [DOI] [PubMed] [Google Scholar]
- 4.Musso D, Richard V, Teissier A, Stone M, Lanteri MC, Latoni G, et al. Detection of Zika virus RNA in semen of asymptomatic blood donors. Clin Microbiol Infect. 2017; 23(12): 1001.e1–1001.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barjas-Castro ML, Angerami RN, Cunha MS, Suzuki A, Nogueira JS Rocco IM, et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion 2016; 56(7): 1684–1688. 10.1111/trf.13681 [DOI] [PubMed] [Google Scholar]
- 6.Motta IJ, Spencer BR, Cordeiro da Silva SG, Arruda MB, Dobbin JA, Gonzaga YB et al. Evidence for Transmission of Zika Virus by Platelet Transfusion. N Engl J Med. 2016; 375: 1101–1103. 10.1056/NEJMc1607262 [DOI] [PubMed] [Google Scholar]
- 7.Moghadas SM, Shoukat A, Espindola AL, Pereira RS, Abdirizak F, Laskowski M, et al. Asymptomatic transmission and the dynamics of Zika infection. Scientific reports. 2017; 7: 5829 10.1038/s41598-017-05013-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bingham AM, Cone M, Mock V, Heberlein-Larson L, Stanek C, Blackmore C et al. Comparison of test results for Zika virus RNA in urine, serum, and saliva specimens from persons with travel-associated Zika virus disease—Florida, 2016. Morb Mortal Wkly Rep. 2016; 65(18): 475–478 [DOI] [PubMed] [Google Scholar]
- 9.Mead PS, Duggal NK, Hook SA, Delorey M, Fischer M, Olzenak McGuire D et al. Zika virus shedding in semen of symptomatic infected men. N Engl J Med. 2016; 378(15): 1377–1385. [DOI] [PubMed] [Google Scholar]
- 10.Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—Continental United States, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65: 215–216. 10.15585/mmwr.mm6508e2 [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016; 374:1981–1987. 10.1056/NEJMsr1604338 [DOI] [PubMed] [Google Scholar]
- 12.Balmaseda A, Stettler K, Medialdea-Carrera R, Collado D, Jin X, Zambrana JV et al. , Antibody-based assay discriminates ZKV virus infection from other flaviviruses. Proc Natl Acad Sci USA: 2017; 114(31): 8384–8389. 10.1073/pnas.1704984114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tedder RS, Samuel D, Dicks S, Scott JT, Ijaz S, Smith CC, et al. Detection, characterization, and enrolment of donors of Ebola convalescent plasma in Sierra Leone. Transfusion. 2018; 58(5):1289–1298. 10.1111/trf.14580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S et al. Specificity, cross-reactivity, and function of antibodies elicited by ZKV virus infection. Science. 2016. August 19;353(6301):823–826. 10.1126/science.aaf8505 [DOI] [PubMed] [Google Scholar]
- 15.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AL et al. Genetic and Serologic Properties of ZKV Virus Associated with an Epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008; 14(8): 1232–1239. 10.3201/eid1408.080287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halstead SB, Rojanasuphot S, Sangkawibha N. Original antigenic sin in dengue. Am J Trop Med Hyg. 1983; 32: 154–156. 10.4269/ajtmh.1983.32.154 [DOI] [PubMed] [Google Scholar]
- 17.Priyamvada L, Hudson W, Ahmed R, Wrammert J. Humoral cross-reactivity between Zika and dengue viruses: implications for protection and pathology. Emerg Microbes Infect. 2017; 6(5): e33 10.1038/emi.2017.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown DW, Ramsay ME, Richards AF, Miller E. Salivary diagnosis of measles: a study of notified cases in the United Kingdom, 1991–3. BMJ. 1994; 308(6935):1015–1017. 10.1136/bmj.308.6935.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glynn JR, Bower H, Johnson S, Houlihan CF, Montesano C, Scott JT et al. Asymptomatic infection and unrecognised Ebola virus disease in Ebola-affected households in Sierra Leone: a cross-sectional study using a new non-invasive assay for antibodies to Ebola virus. Lancet Infect Dis. 2017; 17(6): 645–653. 10.1016/S1473-3099(17)30111-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.