Abstract
The Liver Imaging Reporting and Data System (LI-RADS) is composed of four individual algorithms intended to standardize the lexicon, as well as reporting and care, in patients with or at risk for hepatocellular carcinoma in the context of surveillance with US; diagnosis with CT, MRI, or contrast material–enhanced US; and assessment of treatment response with CT or MRI. This report provides a broad overview of LI-RADS, including its historic development, relationship to other imaging guidelines, composition, aims, and future directions. In addition, readers will understand the motivation for and key components of the 2018 update.
© RSNA, 2018
Summary
By updating the criteria for small (size range, 10–19 mm) LR-5 observations and simplifying the definition for threshold growth, Liver Imaging Reporting and Data System v2018 achieved consistency with and integration into the American Association for the Study of Liver Diseases 2018 hepatocellular carcinoma (HCC) clinical practice guidance, a major milestone toward establishing a universal approach to the imaging diagnosis of HCC.
Implications for Patient Care
■ The Liver Imaging Reporting and Data System (LI-RADS) provides algorithms for use in diagnosis, surveillance, and treatment response assessment of hepatocellular carcinoma (HCC).
■ All LI-RADS algorithms are built on the foundation of standardized lexicon, technique, management, and reporting guidelines.
■ LI-RADS version 2018 updated the criteria for small (size range, 10–19 mm) LR-5 lesions and simplified the definition for threshold growth, thereby achieving consistency with and integration into the American Association for the Study of Liver Diseases 2018 HCC clinical practice guidance.
Introduction
The Liver Imaging Reporting and Data System (LI-RADS), supported by the American College of Radiology (ACR), provides standardization for hepatocellular carcinoma (HCC) imaging in the contexts of screening and surveillance, diagnosis, and treatment response assessment. LI-RADS was developed by a multinational consortium of radiologists and other specialists with expertise in liver cancer imaging, and it was integrated into the most recent HCC clinical practice guidance by the American Association for the Study of Liver Diseases (AASLD) (1). This adoption of LI-RADS by the AASLD was motivated by emerging evidence that LI-RADS categories accurately stratify the probability of HCC and overall malignancy (2). While the AASLD is an American institution, it exerts strong influence globally, and its guidance documents aid clinicians worldwide in understanding and applying available evidence in practice. The integration of LI-RADS into the AASLD guidance therefore represents a major milestone toward establishing a universal approach to the imaging diagnosis of HCC and acknowledges the vital role of LI-RADS and radiologists in this process. This report provides an overview of LI-RADS and its application in practice, including changes introduced in the most recent update (version 2018) required for harmonization with the AASLD. For more detailed information on LI-RADS, the interested reader is directed to the ACR website and selected references (3–16).
History of LI-RADS
The first version of LI-RADS was released in 2011 by the ACR after approximately 3 years of arbitration and eventual consensus by a committee of radiologists. Major LI-RADS updates followed in 2013, 2014, and 2017, each of which was informed by user feedback, accrued experience, emerging evidence, and harmonization efforts with clinical organizations, such as the AASLD, United Network for Organ Sharing, European Association for the Study of the Liver, and other international groups. With the changes introduced in the most recent update in 2018 (Fig 1), LI-RADS was integrated into AASLD clinical practice guidance.
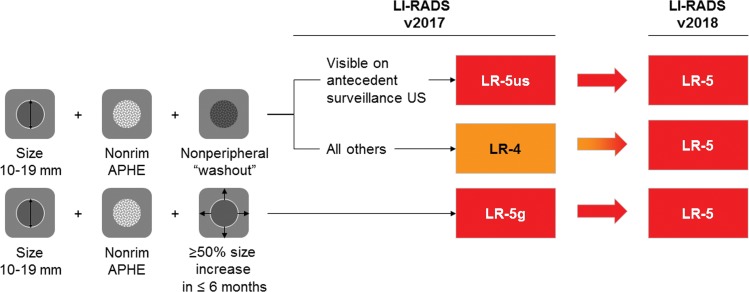
Figure 1a:
Diagram shows changes introduced in Liver Imaging Reporting and Data System (LI-RADS)version 2018. (a) Changes in categorization. Observations 10–19 mm with arterial phase hyperenhancement (APHE) and nonperipheral “washout” are categorized as LR-5 (definite hepatocellular carcinoma), regardless of appearance on antecedent surveillance US images. Qualifiers “-us” and “-g” have been removed. (b) Change in threshold growth definition. Only 50% size increase in 6 months or less qualifies as threshold growth; all other size increases qualify as subthreshold growth.
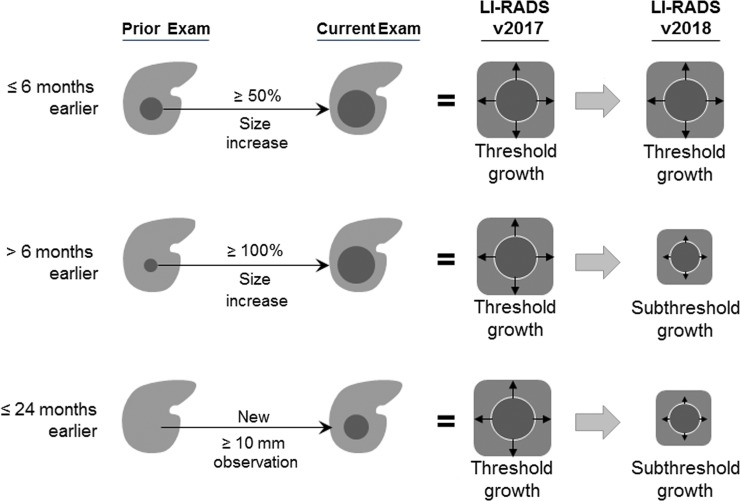
Figure 1b:
Diagram shows changes introduced in Liver Imaging Reporting and Data System (LI-RADS)version 2018. (a) Changes in categorization. Observations 10–19 mm with arterial phase hyperenhancement (APHE) and nonperipheral “washout” are categorized as LR-5 (definite hepatocellular carcinoma), regardless of appearance on antecedent surveillance US images. Qualifiers “-us” and “-g” have been removed. (b) Change in threshold growth definition. Only 50% size increase in 6 months or less qualifies as threshold growth; all other size increases qualify as subthreshold growth.
In parallel with these developments, LI-RADS expanded from one committee of 12 North American radiologists in 2008 to an international multidisciplinary consortium that is presently composed of more than 250 members from more than 100 institutions and more than 30 countries and that is organized into a writing group and 15 working groups overseen by a steering committee. In addition to geographic diversity, the members and contributors come from community and academic settings and include experts in radiology, interventional radiology, hepatology, surgery, and pathology.
LI-RADS is a dynamic system that will continually be refined and updated by these active working groups. Future updates are anticipated to occur in 3–4-year cycles, with the next release scheduled for 2021 (the 10-year anniversary of LI-RADS).
LI-RADS Scope
Terminology
Synthesis of the historic imaging literature on HCC has been challenged by ambiguous and inconsistent terminology. To facilitate progress and construction of an evidence-based diagnostic algorithm, LI-RADS first established a comprehensive lexicon of precisely defined radiologic terms aimed to standardize terminology and enable clear communication for clinical care, education, and research.
International Considerations
Regional HCC Imaging Systems
For nearly 2 decades, numerous scientific organizations and societies have proposed imaging-based criteria and guidelines that can be used to diagnose HCC (14). Three systems originate from Europe; six, from Asia; and four, from North America. One system was produced by a global organization. While these guidelines and imaging-based criteria have advanced the field, incongruities between the systems have introduced regional differences and have prevented global adoption of one guideline for use around the world.
Global Unification of Systems
A long-term goal of LI-RADS is to unify HCC imaging and diagnosis worldwide. The adoption of a unified system would improve clinical care, promote creation of registries, enable multicentric and international studies with common reporting criteria, and facilitate meta-analyses of the published literature. Congruency of LI-RADS with United Network for Organ Sharing/Organ Procurement and Transplantation Network technical and reporting requirements for liver transplantation candidates and the 2018 integration of LI-RADS with AASLD clinical guidance recommendations are important steps toward unification in North America (1). Global unification will require additional effort, and future versions of LI-RADS must account for regional variation in the epidemiology and biology of HCC, as well as financial and technical resource availability (14,17). Importantly, geographic differences in treatment practice affect the desired balance of sensitivity and specificity for diagnosis: While high specificity for HCC is favored for transplantation eligibility in the United States, high sensitivity is favored to maximize early HCC detection for primary resection or local-regional therapy in Asia. For this reason, Western imaging criteria tend to be more stringent, requiring both arterial phase hyperenhancement (APHE) and a size of at least 10 mm in combination with other features (5,6). In comparison, Asian criteria for HCC are more expansive and do not require arterial phase hyperenhancement (14,18–20). Future versions of LI-RADS will need to address and balance these different priorities.
International Membership of LI-RADS and Translation
The LI-RADS international working group was created in 2016 to promote international collaboration; global adoption of LI-RADS for research, education, and clinical care; and worldwide validation of LI-RADS in different patient populations. A key step toward global unification and a major initiative of the international working group is the translation of LI-RADS from English to other languages. Accordingly, LI-RADS is now translated in eight national and supraregional languages (Chinese [both simplified and traditional], French, German, Italian, Japanese, Korean, Portuguese, and Spanish) with other translations (eg, Russian and Arabic) planned (16).
Education
Although standardized interpretation and reporting of liver imaging has many benefits, it represents a new paradigm in many radiology practices, and it requires education. To address this need, the ACR website provides free educational LI-RADS reference materials (16). Additionally, the 40-member LI-RADS Outreach & Education working group develops educational materials and organizes lectures and workshops for radiologists, clinicians, and surgeons involved in the care of patients at risk for or with HCC.
Imaging Contexts and Population
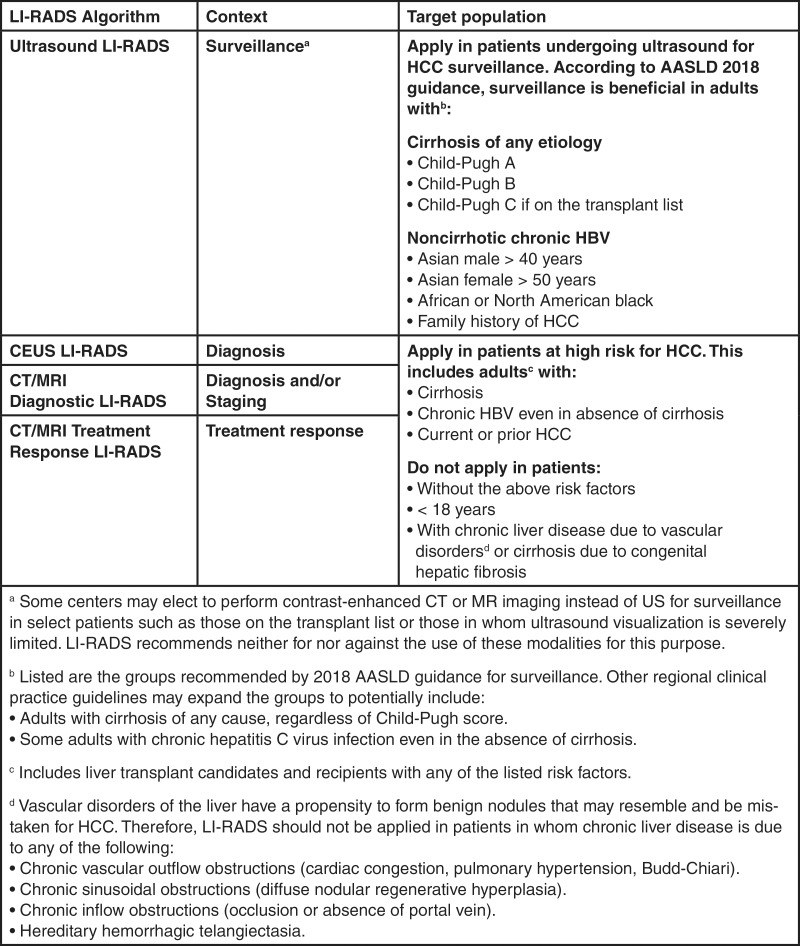
LI-RADS offers four individual imaging algorithms designed for different clinical contexts: (a) US LI-RADS for surveillance, (b) CT/MRI LI-RADS for diagnosis and staging, (c) contrast material–enhanced US LI-RADS for diagnosis, and (d) treatment response LI-RADS to assess response to local-regional therapies (Fig 2). Each LI-RADS core document includes a graphic algorithmic display, a lexicon of relevant terminology, instructions, and supplementary information.
Figure 2:
Chart summarizes Liver Imaging Reporting and Data System (LI-RADS) algorithms and target populations for surveillance, diagnosis, staging, and treatment response. AASLD = American Association for the Study of Liver Diseases, CEUS = contrast-enhanced US, HBV = hepatitis B virus, HCC = hepatocellular carcinoma.
Essential for proper application of an imaging algorithm is a well-defined target population. A sufficiently high pretest probability is required for the algorithm to achieve the desired accuracy. Figure 2 summarizes the target population in each imaging context. Exclusion criteria are provided for the diagnostic, staging, and treatment response algorithms to avoid false-positive diagnosis when the pretest probability is insufficiently high or when there is a propensity for benign nodules that may resemble or be mistaken for HCC.
The LI-RADS Observation
LI-RADS uses the term observation to generically refer to an area with an imaging appearance that is distinctive from the rest of the liver. Such an area may be a true lesion or a pseudolesion; the former has a corresponding pathologic abnormality, and the latter does not. Observations span the spectrum from benign to neoplastic and from to premalignant to malignant, and lesions may be of hepatocellular or nonhepatocellular origin. While HCC is the most common primary hepatic malignancy in LI-RADS target populations, other primary malignancies, including intrahepatic cholangiocarcinoma (iCCA) and combined HCC-iCCA, also may occur. All three types may invade major vessels, manifesting as tumor in vein, or metastasize to extrahepatic locations. Rarely, metastatic disease, sarcomas, or lymphomas may be encountered.
US for Surveillance
Surveillance refers to the repeated application of a diagnostic test at a defined interval in a population at risk for developing a disease (21). All clinical practice guidelines currently recommend US, typically performed every 6 months, as the primary surveillance method for HCC. The goal is to identify HCC at an early stage when it is potentially curable (20–23).
US Technique
As explained in the in US LI-RADS v2017 Core available online (24), US imaging of the liver should be performed with gray-scale and color Doppler US in transverse and longitudinal views, taking care to depict the entire liver.
US Image Interpretation
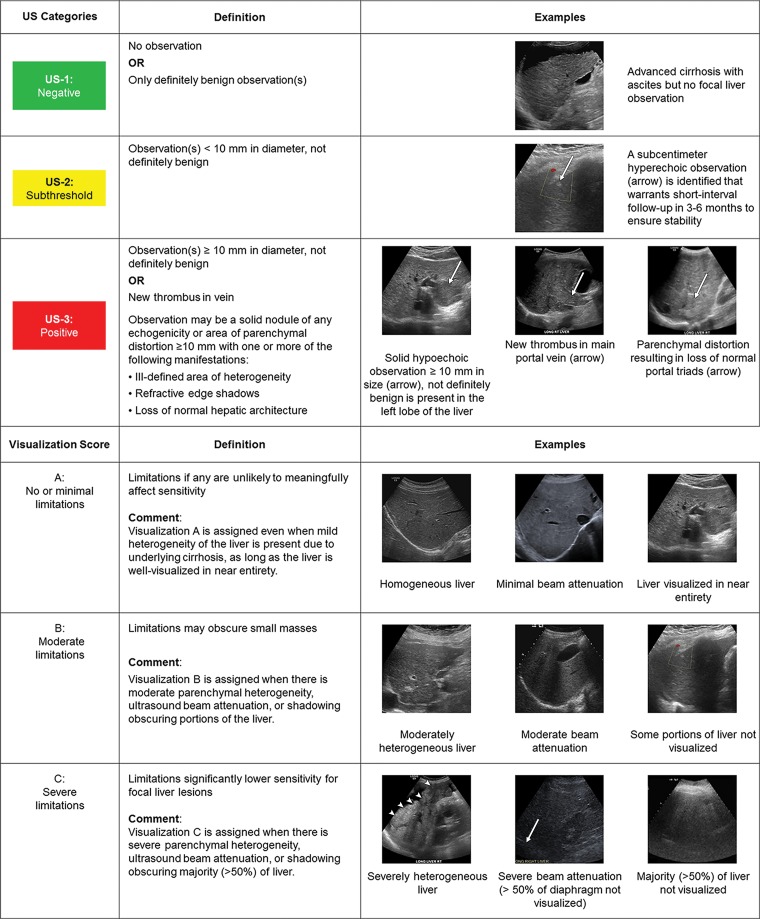
Unlike CT/MRI LI-RADS or contrast-enhanced US LI-RADS, both of which assign categories for each individual observation, US LI-RADS provides scores for the entire examination. Two scores are assigned (Fig 3): (a) the US LI-RADS category, which is used to determine management, and (b) the US LI-RADS visualization score, which is analogous to the breast density score in Breast Imaging Reporting and Data System mammography and conveys the expected level of sensitivity (25,26). Because the effect of visualization scores on outcomes has yet to be studied, the scores are not currently linked directly to management recommendations.
Figure 3:
Summary of US Liver Imaging Reporting and Data System categories and visualization scores.
CT/MRI for Diagnosis
The CT/MRI algorithm permits definitive diagnosis of HCC without pathologic confirmation when applied in patients at high risk (Fig 2) who are undergoing diagnostic CT or MRI.
CT/MRI Technique
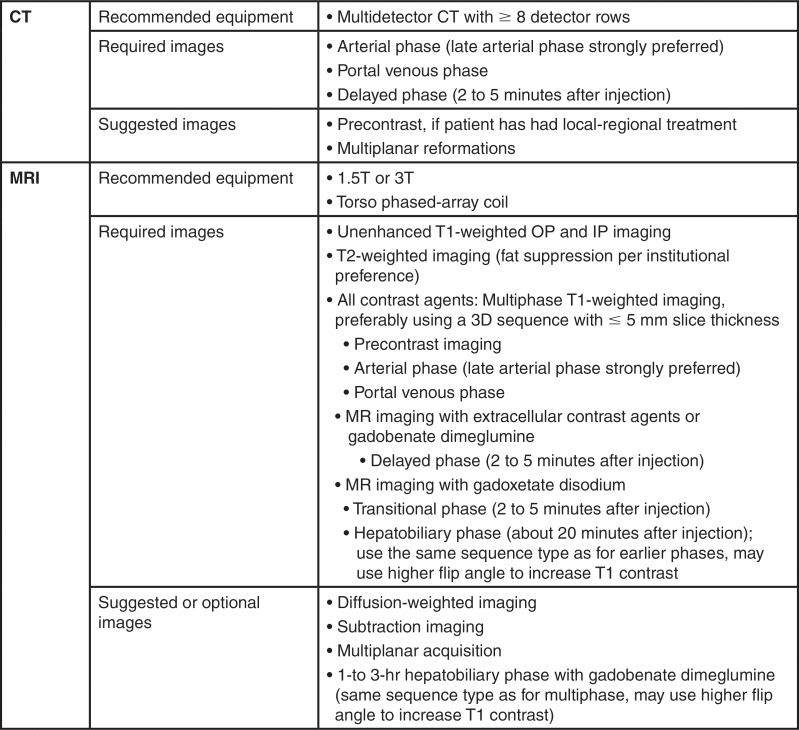
The minimal required and optional images for CT and MRI are listed in Figure 4. Multiphase contrast-enhanced imaging is necessary to capture the features essential to make a diagnosis and assess treatment response. Intravenous extracellular contrast agents are used for CT, while those used for MRI may be extracellular or hepatobiliary; the latter enable both extracellular and hepatobiliary phase imaging. Both gadoxetate disodium and gadobenate dimeglumine may be used in hepatobiliary phase imaging, though gadobenate requires longer delays (1–3 hours for gadobenate vs about 20 minutes for gadoxetate). For treatment-naive patients undergoing CT, unenhanced imaging is optional; however, it is required in the posttreatment setting for CT and all MRI examinations. Late arterial phase imaging is strongly preferred over early arterial phase imaging to maximize the likelihood of depicting APHE, which is a major feature of HCC, as will be discussed later in this article. Guidance on how to achieve optimal arterial phase timing and a more detailed discussion of imaging parameters is provided in the review article by Kambadakone et al (11).
Figure 4:
Chart summarizes Liver Imaging Reporting and Data System version 2018 technical requirements for CT and MRI. IP = in phase, OP = opposed phase, 3D = three dimensional.
When compared with CT or MRI with extracellular contrast agents, MRI with gadoxetate permits detection of not only arterialized cancers in the dynamic phases but also detection of small nonarterialized cancers in the hepatobiliary phase, which increases sensitivity for diagnosis of small HCC (<2 cm) (27). Because of its higher sensitivity, MRI with gadoxetate may be preferable in clinical practice paradigms (such as those in Asia) that emphasize aggressive local-regional treatment or resection for small HCCs. By comparison, MRI with extracellular contrast agents and CT are more specific in the diagnosis of small HCC; thus, they may be preferred in clinical practice paradigms (such as in the United States) that emphasize liver transplantation without biopsy confirmation in the treatment of early stage HCC (27). Recent meta-analyses based on studies at academic centers have suggested that MRI is more sensitive than CT, with a similar specificity; however, the differences are small, and the comparative performance of CT and MRI has not been studied in community settings (27,28). In recognition of the fact that the choice of modality (CT or MRI) and MRI contrast agent (extracelllar or hepatobiliary) depends on patient, institutional, and regional factors, LI-RADS does not endorse any particular imaging method. Rather, it provides guidance on technique, terminology, interpretation, and reporting.
CT/MR Image Interpretation
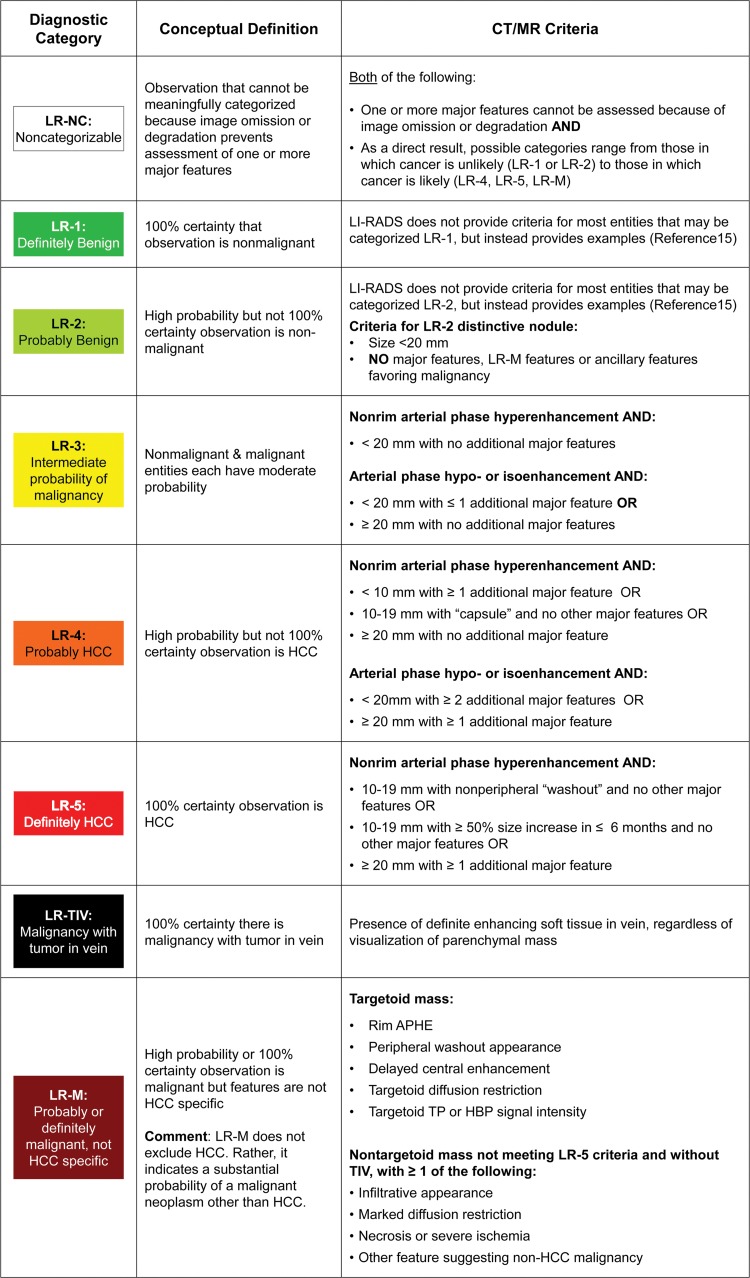
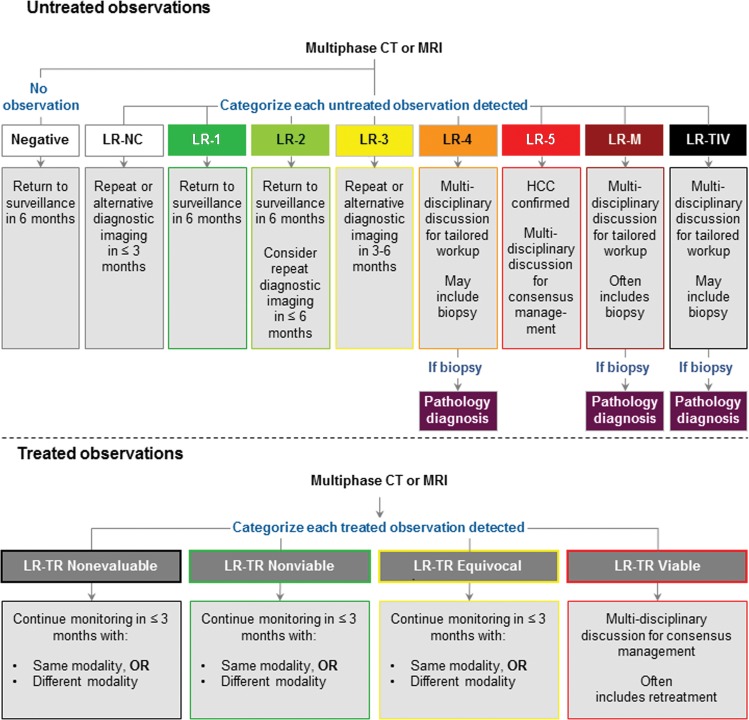
LI-RADS defines eight unique diagnostic categories (Fig 5) based on imaging appearance that reflect the probability of HCC or malignancy with or without tumor in vein (6). The category LR-NC (not categorizable) is applied when image omission or degradation precludes categorization. The categories LR-1 (definitely benign) and LR-2 (probably benign) range from simple cysts to LR-2 distinctive nodules. An LR-2 distinctive nodule is defined by its size (<20 mm) and the absence of any major features of HCC, any features of LR-M, or any ancillary features of malignancy. Examples include otherwise unremarkable T1 hyperintense nodules, T2 hypointense nodules, and hepatobiliary phase hyperintense nodules. LR-3 (intermediate probability of HCC) includes some perfusion alterations that have a nodular shape and true nodules with one or two malignant features. The malignant categories range from probable to definite malignancy and include LR-4 (probably HCC), LR-5 (definitely HCC), LR-M (probably or definitely malignant, not specific for HCC), and LR-TIV (malignancy with tumor in vein). While tumor in vein is often associated with HCC, it can occur in the setting of non-HCC malignancy. Malignant lesions previously confirmed with biopsy (eg, HCC, iCCA) or benign lesions of nonhepatocellular origin (eg, hemangioma) do not require LI-RADS categorization unless there is discordance between imaging and pathology findings or unless there is some other doubt about the diagnosis.
Figure 5:
Summary of CT and MRI diagnostic Liver Imaging Reporting and Data System (LI-RADS) categories. APHE = arterial phase hyperenhancement, HBP = hepatobiliary phase, HCC = hepatocellular carcinoma, TIV = tumor in vein, TP = transitional phase.
The LI-RADS algorithm is a decision tree designed to provide specificity for the diagnosis of HCC through stepwise consideration of other diagnostic categories first (LR-1 or LR-2, LR-TIV, LR-M, or LR-NC) and followed by a diagnostic table to differentiate between LR-3, LR-4, and LR-5 (5,16). A key category in ensuring specificity for HCC is LR-M, which is intended to capture all malignant lesions with a substantial possibility of being something other than HCC. The differential diagnosis of observations categorized as LR-M includes iCCA, combined HCC-CCA, and HCC with atypical or nonspecific imaging features (3,6).
For observations that land in the diagnostic table, the combination of major features is used to determine whether a lesion is categorized as LR-3, LR-4, or LR-5 (5). Importantly, no one feature by itself is enough to categorize a lesion as LR-5 (6). In LI-RADS version 2018, a change in LR-5 criteria was introduced: lesions 10–19 mm in size with APHE and washout are categorized as LR-5 (Fig 1). In prior LI-RADS versions, such observations were categorized as LR-4 or, if visible on antecedent US images, as LR-5us (5). This important change allowed for AASLD integration and consensus in practice guidance (1). In concordance with this change, LI-RADS version 2018 eliminated the “-g” and “-us” qualifiers used previously for subsets of LR-5 observations.
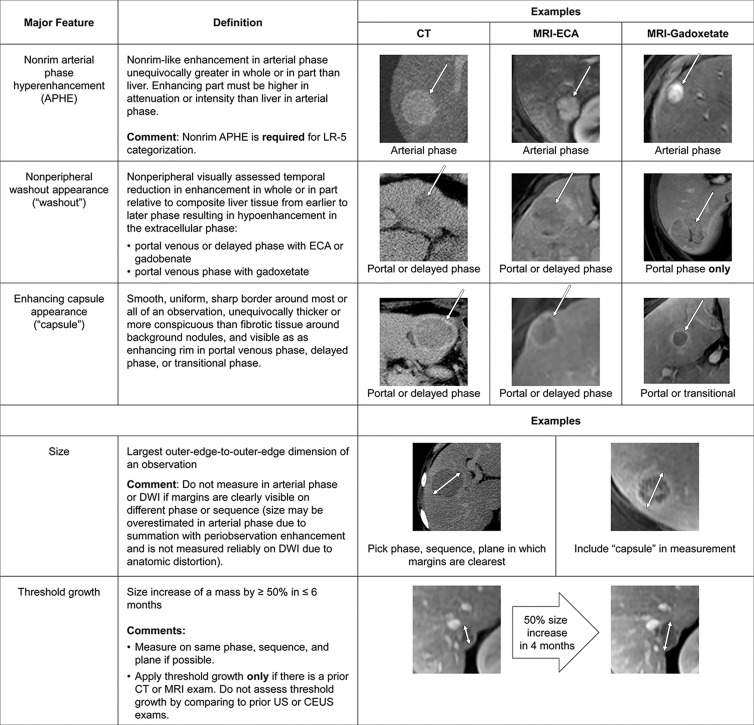
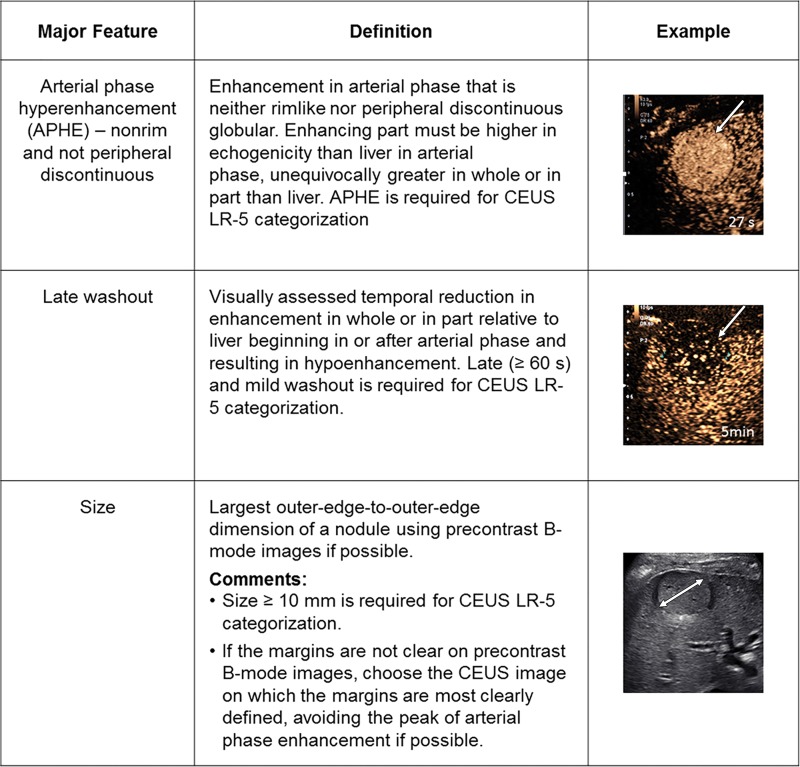
The LI-RADS lexicon divides imaging features into major features, LR-M features, and ancillary features. Only major features contribute to LR-5 categorization (6,7). These features (Fig 6) include nonrim APHE, nonperipheral “washout” appearance, enhancing “capsule” appearance, size, and threshold growth (7). Quotation marks are placed around these terms to signify that these features may be imaging phenomena without definitive pathophysiologic correlates. To ensure specificity, major features should be applied only when their presence is unequivocal. Size should be measured from outer border to outer border (including the “capsule,” if seen) in the phase in which the border is best defined and preferably not on arterial phase images to avoid inclusion of perilesional enhancement. For LR-5 categorization, both APHE and lesion size of at least 10 mm are required. Importantly, LI-RADS version 2018 simplified the threshold growth definition to a size increase of at least 50% in 6 months or less (Fig 1). “Washout” can apply to any enhancing observation, even if there is no APHE. “Washout” assessment is restricted to the portal venous phase with gadoxetate disodium; however, it can be assessed in either the portal venous phase or the delayed phase with extracellular contrast agents or gadobenate dimeglumine (7).
Figure 6:
Summary of CT and MRI diagnostic Liver Imaging Reporting and Data System major features. APHE = arterial phase hyperenhancement, CEUS = contrast-enhanced US, DWI = diffusion-weighted imaging, ECA = extracellular contrast agent.
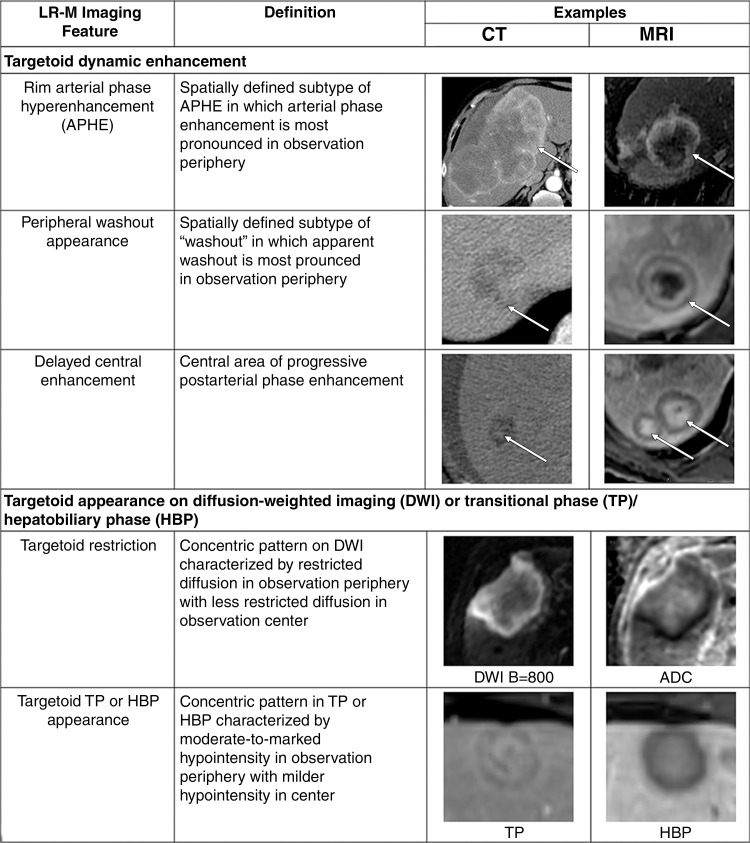
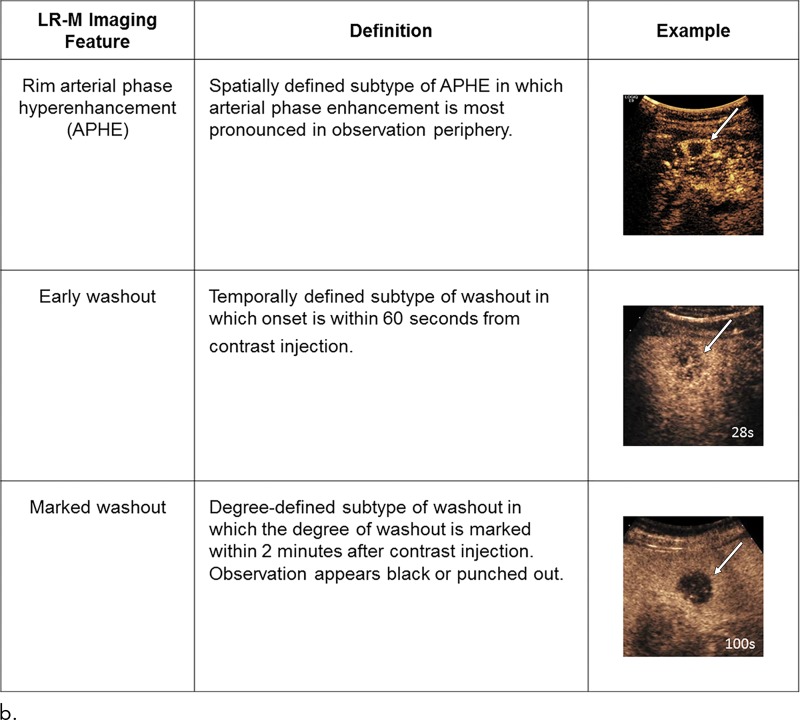
LR-M features (Fig 7) include a targetoid or nontargetoid mass with one or more of the following findings: infiltrative appearance, marked diffusion restriction, necrosis, or other features suggestive of non-HCC malignancy. LR-M features are highly suggestive of malignancy but are not specific for HCC, and the presence of even one such feature prompts categorization as LR-M. There is no requirement that lesions be 10 mm or larger to be categorized as LR-M. Rim APHE is perhaps the most frequently encountered LR-M feature, and differentiation of rim APHE from nonrim APHE is essential for accurate LI-RADS categorization (3,7).
Figure 7:
Summary of CT and MRI LR-M (probably or definitely malignant, not hepatocellular carcinoma specific) features. ADC = apparent diffusion coefficient, APHE = arterial phase hyperenhancement, DWI = diffusion-weighted imaging, HBP = hepatobiliary phase, TP = transitional phase.
Ancillary features, tie-breaking rules, and the final diagnostic check are intended to improve the accuracy of categorization while preserving specificity for HCC and sensitivity for malignancy in general (5,8). Ancillary features are divided into those favoring benignity, those favoring malignancy, and those favoring HCC (8). Their use is optional, and they may be used to up- or downgrade a category by one, but they cannot be used to upgrade to LR-5. Ancillary features also can be used to improve lesion detection or diagnostic confidence. Detailed discussion of ancillary features and tie-breaking rules can be found on the ACR website and in review articles (5,8,29).
Contrast-enhanced US for Diagnosis
Contrast-enhanced US is performed with intravenous injection of a microbubble contrast agent. Contrast-enhanced US is most suitable for problem solving, categorizing individual observations, and differentiating tumor in vein from bland thrombus rather than for staging the entire liver. Contrast-enhanced US requires expertise and specialized equipment designed to maximize sensitivity to microbubbles. Real-time imaging is performed continuously for the 1st minute to capture the arterial phase. This is followed by intermittent scanning every 30–60 seconds for up to about 5 minutes to evaluate washout. Scanning should be kept brief to minimize the destruction of microbubbles by US energy.
The contrast-enhanced US LI-RADS algorithm is similar in concept and application to the CT/MRI LI-RADS algorithm, albeit with some modifications (13). Chief among them is the characterization of washout, which at contrast-enhanced US is considered true washout and therefore requires no quotation marks. Unlike CT and MRI contrast agents, microbubbles are pure blood pool agents that are about the size of red blood cells. They are confined within the blood space and do not leak through endothelial fenestrations into the tumor or parenchymal interstitium. As a result, their distribution on postarterial phase images reflects the relative blood volume. Accordingly, tumors such as HCC, which tend to have only slightly lower blood volume than the liver, typically exhibit mild and late-onset washout. In comparison, iCCAs and other non-HCC malignancies have much lower blood volume than liver, and their washout is early and marked. For these reasons, contrast-enhanced US LI-RADS requires assessment not only for the presence of washout but also for its time of onset after injection and its degree (Fig 8).
Figure 8a:
Summary of contrast-enhanced US Liver Imaging Reporting and Data System (a) major and (b) LR-M (probably or definitely malignant, not hepatocellular carcinoma specific) features. APHE = arterial phase hyperenhancement, CEUS = contrast-enhanced US,
Figure 8b:
Summary of contrast-enhanced US Liver Imaging Reporting and Data System (a) major and (b) LR-M (probably or definitely malignant, not hepatocellular carcinoma specific) features. APHE = arterial phase hyperenhancement, CEUS = contrast-enhanced US,
Another unique feature is that arterioportal shunts, which are a frequent cause of diagnostic confusion at CT and MRI, are not visible on US or contrast-enhanced US images. Since vascular pseudolesions do not occur, any contrast-enhanced US observation that enhances during the arterial phase is a true arterialized nodule. In the setting of cirrhosis, in which benign arterialized lesions such as focal nodular hyperplasias and hepatocellular adenomas are rare, the majority of such nodules are premalignant or malignant and warrant categorization as LR-4 or higher (13). Additionally, threshold growth and enhancing capsule are not major features for contrast-enhanced US, the former because it is not reliably measured on contrast-enhanced US images and the latter because it is not detectable on US images. For similar reasons, the list of ancillary features is shorter for contrast-enhanced US LI-RADS than for CT/MRI LI-RADS.
Treatment Response
The LI-RADS treatment response algorithm applies to multiphase CT or MRI used to assess response after local-regional therapy (15), which includes percutaneous therapy (eg, ethanol and radiofrequency or microwave ablation), transcatheter therapy (eg, transarterial chemoembolization or radioembolization), and external beam radiation therapy. The algorithm also applies to observations at the surgical margin after resection of HCC; observations elsewhere in the liver remnant should be assessed with a diagnostic algorithm. The LI-RADS treatment response algorithm does not apply to systemic chemo-, targeted, or immunologic therapies, nor does it apply to treatment response using contrast-enhanced US. In patients who underwent both systemic therapy and local-regional treatment, the LI-RADS treatment response algorithm can be applied at the discretion of the interpreting radiologist.
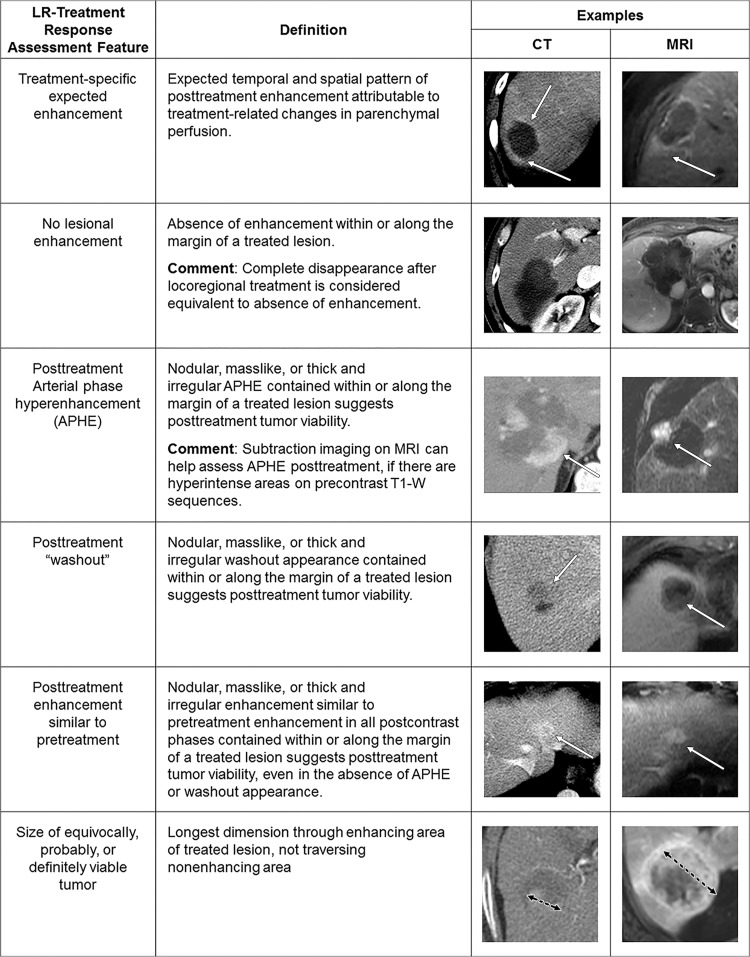
Analogous in concept to the diagnostic algorithms, LI-RADS treatment response category codes reflect the relative probability of tumor viability after local-regional therapy to guide management decisions. Similar to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (30), the LI-RADS algorithm is based on unidimensional measurements of the largest enhancing component of a treated tumor, excluding areas of nonenhancement (Fig 9), an approach that shows high reproducibility in response categories and better prognostication when compared with traditional Response Evaluation Criteria in Solid Tumors, version 1.1, after local-regional therapy (31–35). The LI-RADS algorithm expands on the mRECIST approach not only by defining viable disease but also by providing nonevaluable, equivocal, and nonviable treatment response categories. Unlike mRECIST, the LI-RADS treatment response categories are assigned on a lesion-by-lesion basis and are not assigned to the whole liver or patient. The imaging features used in LI-RADS treatment response categorization are summarized in Figure 9. Unlike diagnostic LI-RADS, where APHE and size of at least 10 mm are required for categorization as LR-5, presence of either APHE or washout or enhancement similar to the pretreatment condition, regardless of size, is sufficient to categorize a treated lesion as viable.
Figure 9:
Summary of CT and MRI treatment response Liver Imaging Reporting and Data System imaging features. APHE = arterial phase hyperenhancement T1-W = T1 weighted.
Reporting
The radiology report should guide management decisions through clear and concise communication of hepatic observations and other findings that may affect treatment decisions. Surveillance reports should include LI-RADS US category and visualization scores. Diagnostic and staging reports should describe individual observations, including their size, major features (and ancillary features if used for category adjustment), and final diagnostic category. Treatment response reports should assign a LI-RADS treatment response category, include the pretreatment LI-RADS category (or histologic diagnosis, if known), and provide a measure of viable or equivocally viable tumor. When the number of untreated or treated observations exceeds five, radiologists may report lesions in aggregate rather than individually to maintain clarity and brevity. Through the ACR website, LI-RADS provides templates, sample reports, and guidance for radiologists in creating comprehensive yet concise reports with relevant acquisition details and features (16).
LI-RADS soon will be integrated into commercial reporting systems to assist the radiologist in applying LI-RADS properly. The dissemination and adoption of these reporting tools will facilitate the use of LI-RADS for clinical care and help ensure that radiology reports are complete and accurate. In addition to imaging features and categorization, reports should also include management recommendations, as will be discussed later in this article.
Management
Management is determined on the patient level, but it is typically strongly influenced by the imaging observation with the highest risk of malignancy (eg, if a patient has multiple LR-3 lesions and an LR-5 lesion, management would be driven by the LR-5 lesion). Figure 10 provides a general management approach for CT- and MRI-based LI-RADS diagnostic categories, which is consistent with the newest AASLD guidance (1). Management for contrast-enhanced US-assigned categories is similar for all categories except contrast-enhanced US LR-3, which is thought to convey slightly higher likelihood of HCC than CT or MRI LR-3 and therefore warrants closer scrutiny (9,13). Figure 10 also provides general management guidance for treatment response categories.
Figure 10:
Summary of management recommendations for CT and MRI diagnostic and treatment response Liver Imaging Reporting and Data System categories.
Imaging findings are one of several elements that may be used to determine diagnosis and management. Although radiologists provide an initial estimate of the relative likelihood of HCC or viable tumor after local-regional treatment by assigning a LI-RADS diagnostic or treatment response category, respectively, this estimate may be refined by clinical history, biomarker levels, and other factors. Similarly, decisions between management options (ie, return to surveillance, repeat diagnostic imaging, alternative diagnostic imaging, biopsy, or treatment as HCC without biopsy) do not follow solely from the estimated probability of HCC but incorporate additional information, including patient comorbidities, preferences, and eligibility for organ transplantation. For these reasons, management decisions are best determined with a multidisciplinary approach, in which physicians consider all potential work-up or treatment options to tailor care to the individual patient. Evidence suggests that such an approach improves accuracy and timeliness of diagnosis, appropriate treatment allocation, and overall survival of patients with or at risk for HCC (36).
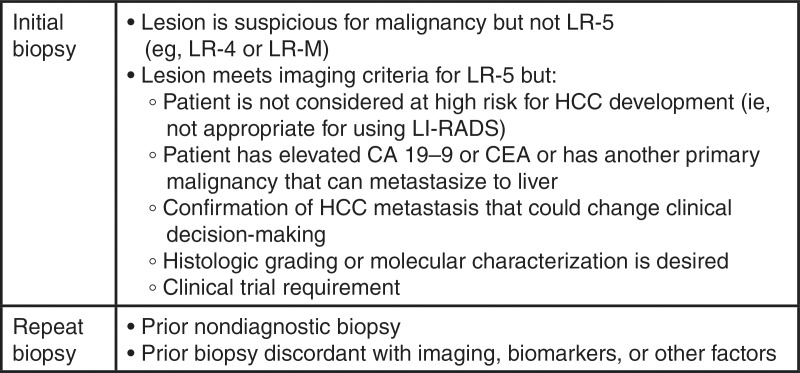
Biopsy Considerations
Judicious use of biopsy in select patients with LR-3, LR-4, or LR-M observations may help tailor management decisions. General indications for biopsy are listed in Fig 11. In general, LR-5 observations do not require pathologic proof of diagnosis, although biopsy may allow for molecular characterization or be needed for a clinical trial.
Figure 11:
Chart summarizes indicators for biopsy considerations. CEA = carcinoembryonic antigen, HCC = hepatocellular carcinoma, LI-RADS = Liver Imaging Reporting and Data System.
Biopsy of LR-3, LR-4, or LR-M observations should be based on a multidisciplinary discussion. A radiology report should not compel a clinician to perform biopsy, as factors outside of the radiologist’s knowledge may affect these decisions.
LI-RADS: Emerging Evidence
By necessity, the creation of LI-RADS preceded clinical validation, and the supporting evidence is just now emerging (8,37–54). Evidence supporting each LI-RADS major feature of HCC is reported in a systematic review and is summarized in this article (39). In patients at risk for disease, nonrim APHE is a sensitive imaging feature for progressed HCC (55–58). The combination of nonrim APHE and nonperipheral washout appearance provides high specificity (39,58–60). Larger observation size increases sensitivity (61–67), specificity (68,69), and probability of HCC (56,61,70,71). Capsule appearance yields high specificity (42,64); however, its incremental value has been questioned (58). Threshold growth helps differentiate HCC from benign entities (72).
The application of ancillary features modifies the LI-RADS category in about 10%–20% of observations (42,73), with upgrades being slightly more common than downgrades (42). Recent data suggest that use of ancillary features increases sensitivity while preserving the specificity of LR-4 or LR-5 for HCC (42).
Interreader agreement on LI-RADS categories has been assessed in several studies (73–79). In a large international multireader study, interreader agreement was excellent (range, 0.84–0.87) for APHE and “washout” (73), and it was unaffected by modality or by readers’ liver imaging expertise, prior familiarity with LI-RADS, or number of years of postresidency practice (73). Interreader agreement for “capsule” was excellent in one study (73) but not in the others (74–79). In studies focused on agreement for LI-RADS categories, interreader agreement was fair for contrast-enhanced US (κ = 0.3–0.39) (80,81), fair to substantial for MRI (κ = 0.35–0.61 [54,74,76,81,82], intraclass correlation coefficient = 0.73 [73]), and substantial for CT (κ = 0.79 [54], intraclass correlation coefficient = 0.67 [73]).
Recent studies show that the likelihood of an observation being an HCC in particular or a malignant neoplasm in general increases with higher ranked LI-RADS categories (Table) (2,40–42,44–53), enabling confirmation that LI-RADS can be used to stratify the probability of HCC and overall malignancy, as intended.
Hepatocellular Carcinoma and Overall Malignancy per LI-RADS Category
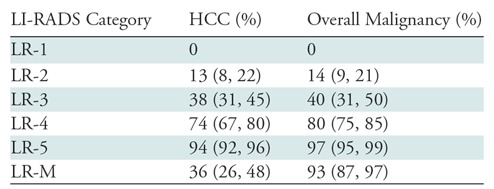
Note.—Adapted from reference 2. Data are based on meta-analysis of 15 published articles and two scientific abstracts using Liver Imaging Reporting and Data System (LI-RADS) version 2014 or LI-RADS version 2017. Data in parentheses are confidence intervals. There are no confidence intervals for the LR-1 estimates, since all studies reported 0% rates of hepatocellular carcinoma (HCC) and overall malignancy. Data from LI-RADS version 2018 are not yet available.
A detailed discussion on the advantages and disadvantages of contrast-enhanced US, CT, and MRI in the diagnosis of HCC is beyond the scope of this report. However, inherent differences in sensitivity and specificity of each modality in the detection of the major features exist, and these result in discordances between the final LI-RADS categories assigned with different modalities (46,54,75–77,81,83–85). Emerging literature indicates that intermodality agreement for LI-RADS category is fair (κ = 0.33–0.39) between CT and MRI and slight to fair between MRI and contrast-enhanced US (κ = 0.22) (54,81). CT has a tendency to result in undercategorization as compared with MRI (84,85).
Gaps and Future Directions
Although each version of LI-RADS has been informed by the best available evidence, there are insufficient high-quality data for many questions in HCC imaging. For example, the interrater reliability is controversial for “capsule” and poorly understood for ancillary features, causing the incremental benefit of these features for LI-RADS categorization to be debated among experts (74–79). Additionally, newer components of LI-RADS, including LI-RADS treatment response and LR-M diagnostic criteria, still require validation. Similarly, the US LI-RADS visualization score was proposed based on expert opinion instead of on data demonstrating an association with surveillance failure. LI-RADS was developed for widespread use; therefore, it purposely avoids the requirements of state-of-the-art imaging technology, such as multiarterial phase MRI and spectral CT. As these cutting-edge technologies become mainstream, research will be needed to determine their effect on LI-RADS categorization and accuracy. Finally, and perhaps most importantly, studies are needed to define the optimal work-up strategy of indeterminate (LR-3) and suspicious (LR-4) observations. Although these lesions carry the risk of being or progressing to HCC, it is unclear how long they require monitoring with diagnostic imaging, if they should undergo biopsy, and whether patients can ever return to the original surveillance schedule. The LI-RADS Research and Development Working Group was formed to help identity, prioritize, and address these gaps to inform future improvements in LI-RADS. Additionally, by linking structured data elements with de-identified databases, the commercial reporting systems for LI-RADS now in development are expected to enable the creation of large multinational observational tumor registries, facilitate big data analytics, and thereby advance understanding of the natural history and outcomes of LI-RADS observations as detected and reported in real-world clinical practice.
Conclusion
By updating the criteria for small (10–19 mm) LR-5 observations and simplifying the definition for threshold growth, LI-RADS version 2018 achieved consistency with and integration into the AASLD 2018 HCC clinical practice guidance, a major milestone toward establishing a universal approach to the imaging diagnosis of HCC. Beyond diagnostic criteria, LI-RADS also provides algorithms for surveillance, treatment response, and contrast-enhanced US–based diagnosis. All LI-RADS algorithms are built on the foundation of standardized lexicon, technique, management, and reporting guidelines. Through education, international collaboration, and continual evaluation of existing and missing evidence, LI-RADS aims to unite and eventually achieve consensus among liver experts around the world on the best practices for caring for patients with or at risk for HCC.
Acknowledgments
Acknowledgments
We thank the following individuals: Adrija Mamidipalli, MD; Alexander Towbin, MD; Amol Shah, MD; Andrej Lyshchik, MD; Avinash Kambadakone, MD; Cynthia Santillan, MD; Demetri Papadatos, MD; Elizabeth Hecht, MD; Rohit Loomba, MD; Eric Ehman, MD; Evan Siegelman, MD; Hero Hussain, MD; Jason Birnbaum, MD; Jay Heiken, MD; Jeff Weinreb, MD; Judy Gichoya, MD, MS; Lauren Hicks; Marc Kohli, MD; Matt McInnes, MD; Maxime Ronot, MD, PhD; Reena Jha, MD; Sasha Roudenko, MD; Soudabeh Fazeli, MD; Tom Hope, MD; and William Hong, MD.
Disclosures of Conflicts of Interest: V.C. disclosed no relevant relationships. K.J.F. disclosed no relevant relationships. A.K. disclosed no relevant relationships. A.Z.K. Activities related to the present article: institution received a grant from General Electric. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. K.M.E. disclosed no relevant relationships. M.R.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for RadMD, institution received grants from Siemens Healthineers, GE Healthcare, NGM Biopharm, TaiwanJ Pharmaceuticals, and Madrigal Pharmaceuticals. Other relationships: disclosed no relevant relationships. Y.K. disclosed no relevant relationships. R.K.D. disclosed no relevant relationships. D.G.M. disclosed no relevant relationships. A.G.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant as is on the speakers bureau for Bayer. Other relationships: disclosed no relevant relationships. A.T. disclosed no relevant relationships. C.B.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is on the advisory boards of AMRA, Guerbet, and VirtualScopics; is a consultant for GE Healthcare, Bayer, Boehringer Ingelheim, AMRA, and Fulcrum; institution received grants from Gilead, GE Healthcare, Siemens, GE MRI, Bayer, GE Digital, GE Ultrasound, ACR Innovation, and Philips; served on the speakers bureau for GE Healthcare; developed educational presentations for Medscape and Resoundant; institution has lab service agreements with Enanta, ICON, Gilead, Shire, VirtualScopics, Intercept, Synageva, Takeda, Genzyme, Janssen, and NuSirt. Other relationships: is the chair of the LI-RADS steering committee.
Abbreviations:
- AASLD
- American Association for the Study of Liver Diseases
- ACR
- American College of Radiology
- APHE
- arterial phase hyperenhancement
- HCC
- hepatocellular carcinoma
- iCCA
- intrahepatic cholangiocarcinoma
- LI-RADS
- Liver Imaging Reporting and Data System
- mRECIST
- modified Response Evaluation Criteria in Solid Tumors
References
- 1.Marrero JA, Kulik LM, Sirlin C, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68(2):723–750. [DOI] [PubMed] [Google Scholar]
- 2.van der Pol CBLC, Bashir MR, Sirlin CB, et al. What is the percentage of hepatocellular carcinoma and overall malignancy within each LI-RADS category? a systematic review. ILCA 2018: 12th Annual Conference of the International Liver Cancer Association, 2018. London, England: International Liver Cancer Association, 2018. [Google Scholar]
- 3.Fowler KJ, Potretzke TA, Hope TA, Costa EA, Wilson SR. LI-RADS M (LR-M.): definite or probable malignancy, not specific for hepatocellular carcinoma. Abdom Radiol (NY) 2018;43(1):149–157. [DOI] [PubMed] [Google Scholar]
- 4.Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY) 2018;43(1):13–25. [DOI] [PubMed] [Google Scholar]
- 5.Chernyak V, Santillan CS, Papadatos D, Sirlin CB. LI-RADS® algorithm: CT and MRI. Abdom Radiol (NY) 2018;43(1):111–126. [DOI] [PubMed] [Google Scholar]
- 6.Santillan C, Chernyak V, Sirlin C. LI-RADS categories: concepts, definitions, and criteria. Abdom Radiol (NY) 2018;43(1):101–110. [DOI] [PubMed] [Google Scholar]
- 7.Santillan C, Fowler K, Kono Y, Chernyak V. LI-RADS major features: CT, MRI with extracellular agents, and MRI with hepatobiliary agents. Abdom Radiol (NY) 2018;43(1):75–81. [DOI] [PubMed] [Google Scholar]
- 8.Chernyak V, Tang A, Flusberg M, et al. LI-RADS® ancillary features on CT and MRI. Abdom Radiol (NY) 2018;43(1):82–100. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell DG, Bashir MR, Sirlin CB. Management implications and outcomes of LI-RADS-2, -3, -4, and -M category observations. Abdom Radiol (NY) 2018;43(1):143–148. [DOI] [PubMed] [Google Scholar]
- 10.Sirlin CB, Kielar AZ, Tang A, Bashir MR. LI-RADS: a glimpse into the future. Abdom Radiol (NY) 2018;43(1):231–236. [DOI] [PubMed] [Google Scholar]
- 11.Kambadakone AR, Fung A, Gupta RT, et al. LI-RADS technical requirements for CT, MRI, and contrast-enhanced ultrasound. Abdom Radiol (NY) 2018;43(1):56–74 [Published correction appears in Abdom Radiol (NY) 2018;43(1):240.]. [DOI] [PubMed] [Google Scholar]
- 12.Morgan TA, Maturen KE, Dahiya N, Sun MRM, Kamaya A; American College of Radiology Ultrasound Liver Imaging and Reporting Data System (US LI-RADS) Working Group. US LI-RADS: ultrasound Liver Imaging Reporting and Data System for screening and surveillance of hepatocellular carcinoma. Abdom Radiol (NY) 2018;43(1):41–55. [DOI] [PubMed] [Google Scholar]
- 13.Wilson SR, Lyshchik A, Piscaglia F, et al. CEUS LI-RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol (NY) 2018;43(1):127–142. [DOI] [PubMed] [Google Scholar]
- 14.Tang A, Cruite I, Mitchell DG, Sirlin CB. Hepatocellular carcinoma imaging systems: why they exist, how they have evolved, and how they differ. Abdom Radiol (NY) 2018;43(1):3–12. [DOI] [PubMed] [Google Scholar]
- 15.Kielar A, Fowler KJ, Lewis S, et al. Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom Radiol (NY) 2018;43(1):218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American College of Radiology . Liver Imaging Reporting and Data System. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS. Accessed September 2, 2018.
- 17.Cruite I, Tang A, Sirlin CB. Imaging-based diagnostic systems for hepatocellular carcinoma. AJR Am J Roentgenol 2013;201(1):41–55. [DOI] [PubMed] [Google Scholar]
- 18.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4(2):439–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudo M, Matsui O, Izumi N, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the Liver Cancer Study Group of Japan. Liver Cancer 2014;3(3-4):458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC) . 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol 2015;16(3):465–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruix J, Sherman M; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology 2011;53(3):1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer . EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56(4):908–943. [DOI] [PubMed] [Google Scholar]
- 23.Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 2011;29(3):339–364. [DOI] [PubMed] [Google Scholar]
- 24.American College of Radiology . US LI-RADS v2017 Core. https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-US-Algorithm-Portrait-2017.pdf?la=en. Accessed 2018.
- 25.Winkler NS, Raza S, Mackesy M, Birdwell RL. Breast density: clinical implications and assessment methods. RadioGraphics 2015;35(2):316–324. [DOI] [PubMed] [Google Scholar]
- 26.Freer PE. Mammographic breast density: impact on breast cancer risk and implications for screening. RadioGraphics 2015;35(2):302–315. [DOI] [PubMed] [Google Scholar]
- 27.Roberts LR, Sirlin CB, Zaiem F, et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology 2018;67(1):401–421. [DOI] [PubMed] [Google Scholar]
- 28.Hanna RF, Miloushev VZ, Tang A, et al. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol (NY) 2016;41(1):71–90. [DOI] [PubMed] [Google Scholar]
- 29.Kielar AZ, Chernyak V, Bashir MR, et al. LI-RADS 2017: an update. J Magn Reson Imaging 2018;47(6):1459–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bargellini I, Bozzi E, Campani D, et al. Modified RECIST to assess tumor response after transarterial chemoembolization of hepatocellular carcinoma: CT-pathologic correlation in 178 liver explants. Eur J Radiol 2013;82(5):e212–e218. [DOI] [PubMed] [Google Scholar]
- 32.Donati OF, Do RK, Hötker AM, et al. Interreader and inter-test agreement in assessing treatment response following transarterial embolization for hepatocelluar carcinoma. Eur Radiol 2015;25(9):2779–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seyal AR, Gonzalez-Guindalini FD, Arslanoglu A, et al. Reproducibility of mRECIST in assessing response to transarterial radioembolization therapy in hepatocellular carcinoma. Hepatology 2015;62(4):1111–1121. [DOI] [PubMed] [Google Scholar]
- 34.Shim JH, Lee HC, Kim SO, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? a validation study of old and new models. Radiology 2012;262(2):708–718. [DOI] [PubMed] [Google Scholar]
- 35.Vincenzi B, Di Maio M, Silletta M, et al. Prognostic relevance of objective response according to EASL criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco-regional therapies: a literature-based meta-analysis. PLoS One 2015;10(7):e0133488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaba RC, Kallwitz ER, Parvinian A, et al. Imaging surveillance and multidisciplinary review improves curative therapy access and survival in HCC patients. Ann Hepatol 2013;12(5):766–773. [PubMed] [Google Scholar]
- 37.Cruite I, Tang A, Mamidipalli A, Shah A, Santillan C, Sirlin CB. Liver Imaging Reporting and Data System: review of major imaging features. Semin Roentgenol 2016;51(4):292–300. [DOI] [PubMed] [Google Scholar]
- 38.Granata V, Fusco R, Avallone A, et al. Critical analysis of the major and ancillary imaging features of LI-RADS on 127 proven HCCs evaluated with functional and morphological MRI: lights and shadows. Oncotarget 2017;8(31):51224–51237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang A, Bashir MR, Corwin MT, et al. Evidence supporting LI-RADS major features for CT- and MR imaging-based diagnosis of hepatocellular carcinoma: a systematic review. Radiology 2018;286(1):29–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fraum TJ, Tsai R, Rohe E, et al. Differentiation of hepatocellular carcinoma from other hepatic malignancies in patients at risk: diagnostic performance of the Liver Imaging Reporting and Data System version 2014. Radiology 2018;286(1):158–172. [DOI] [PubMed] [Google Scholar]
- 41.Horvat N, Nikolovski I, Long N, et al. Imaging features of hepatocellular carcinoma compared to intrahepatic cholangiocarcinoma and combined tumor on MRI using liver imaging and data system (LI-RADS) version 2014. Abdom Radiol (NY) 2018;43(1):169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cerny M, Bergeron C, Billiard JS, et al. LI-RADS for MR imaging diagnosis of hepatocellular carcinoma: performance of major and ancillary features. Radiology 2018;288(1):118–128. [DOI] [PubMed] [Google Scholar]
- 43.Cruite I, Santillan C, Mamidipalli A, Shah A, Tang A, Sirlin CB. Liver imaging reporting and data system: review of ancillary imaging features. Semin Roentgenol 2016;51(4):301–307. [DOI] [PubMed] [Google Scholar]
- 44.Abd Alkhalik Basha M, Abd El Aziz El Sammak D, El Sammak AA. Diagnostic efficacy of the Liver Imaging-Reporting and Data System (LI-RADS) with CT imaging in categorising small nodules (10-20 mm) detected in the cirrhotic liver at screening ultrasound. Clin Radiol 2017;72(10):901.e1–901.e11. [DOI] [PubMed] [Google Scholar]
- 45.An C, Park S, Chung YE, et al. Curative resection of single primary hepatic malignancy: Liver Imaging Reporting and Data System category LR-M portends a worse prognosis. AJR Am J Roentgenol 2017;209(3):576–583. [DOI] [PubMed] [Google Scholar]
- 46.Cha DI, Jang KM, Kim SH, Kang TW, Song KD. Liver Imaging Reporting and Data System on CT and gadoxetic acid-enhanced MRI with diffusion-weighted imaging. Eur Radiol 2017;27(10):4394–4405. [DOI] [PubMed] [Google Scholar]
- 47.Choi SH, Byun JH, Kim SY, et al. Liver Imaging Reporting and Data System v2014 with gadoxetate disodium-enhanced magnetic resonance imaging: validation of LI-RADS category 4 and 5 criteria. Invest Radiol 2016;51(8):483–490. [DOI] [PubMed] [Google Scholar]
- 48.Joo I, Lee JM, Lee DH, Ahn SJ, Lee ES, Han JK. Liver imaging reporting and data system v2014 categorization of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: comparison with multiphasic multidetector computed tomography. J Magn Reson Imaging 2017;45(3):731–740. [DOI] [PubMed] [Google Scholar]
- 49.Kim BR, Lee JM, Lee DH, et al. Diagnostic performance of gadoxetic acid-enhanced liver MR imaging versus multidetector CT in the detection of dysplastic nodules and early hepatocellular carcinoma. Radiology 2017;285(1):134–146. [DOI] [PubMed] [Google Scholar]
- 50.Kim YY, An C, Kim S, Kim MJ. Diagnostic accuracy of prospective application of the Liver Imaging Reporting and Data System (LI-RADS) in gadoxetate-enhanced MRI. Eur Radiol 2018;28(5):2038–2046. [DOI] [PubMed] [Google Scholar]
- 51.Lee SE, An C, Hwang SH, Choi JY, Han K, Kim MJ. Extracellular contrast agent-enhanced MRI: 15-min delayed phase may improve the diagnostic performance for hepatocellular carcinoma in patients with chronic liver disease. Eur Radiol 2018;28(4):1551–1559. [DOI] [PubMed] [Google Scholar]
- 52.Liu W, Qin J, Guo R, et al. Accuracy of the diagnostic evaluation of hepatocellular carcinoma with LI-RADS. Acta Radiol 2018;59(2):140–146. [DOI] [PubMed] [Google Scholar]
- 53.Ronot M, Fouque O, Esvan M, Lebigot J, Aubé C, Vilgrain V. Comparison of the accuracy of AASLD and LI-RADS criteria for the non-invasive diagnosis of HCC smaller than 3 cm. J Hepatol 2017 Dec 21 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 54.Chernyak V, Flusberg M, Law A, Kobi M, Paroder V, Rozenblit AM. Liver Imaging Reporting and Data System: discordance between computed tomography and gadoxetate-enhanced magnetic resonance imaging for detection of hepatocellular carcinoma major features. J Comput Assist Tomogr 2018;42(1):155–161. [DOI] [PubMed] [Google Scholar]
- 55.Lee KH, O’Malley ME, Haider MA, Hanbidge A. Triple-phase MDCT of hepatocellular carcinoma. AJR Am J Roentgenol 2004;182(3):643–649. [DOI] [PubMed] [Google Scholar]
- 56.Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47(1):97–104. [DOI] [PubMed] [Google Scholar]
- 57.Kim TK, Lee KH, Jang HJ, et al. Analysis of gadobenate dimeglumine-enhanced MR findings for characterizing small (1-2-cm) hepatic nodules in patients at high risk for hepatocellular carcinoma. Radiology 2011;259(3):730–738. [DOI] [PubMed] [Google Scholar]
- 58.Rimola J, Forner A, Tremosini S, et al. Non-invasive diagnosis of hepatocellular carcinoma ≤ 2 cm in cirrhosis. diagnostic accuracy assessing fat, capsule and signal intensity at dynamic MRI. J Hepatol 2012;56(6):1317–1323. [DOI] [PubMed] [Google Scholar]
- 59.Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 2010;59(5):638–644. [DOI] [PubMed] [Google Scholar]
- 60.Jang HJ, Kim TK, Khalili K, et al. Characterization of 1-to 2-cm liver nodules detected on hcc surveillance ultrasound according to the criteria of the American Association for the Study of Liver Disease: is quadriphasic CT necessary? AJR Am J Roentgenol 2013;201(2):314–321. [DOI] [PubMed] [Google Scholar]
- 61.Horigome H, Nomura T, Saso K, Itoh M, Joh T, Ohara H. Limitations of imaging diagnosis for small hepatocellular carcinoma: comparison with histological findings. J Gastroenterol Hepatol 1999;14(6):559–565. [DOI] [PubMed] [Google Scholar]
- 62.Yoo HJ, Lee JM, Lee JY, et al. Additional value of SPIO-enhanced MR imaging for the noninvasive imaging diagnosis of hepatocellular carcinoma in cirrhotic liver. Invest Radiol 2009;44(12):800–807. [DOI] [PubMed] [Google Scholar]
- 63.Chen L, Zhang L, Liang M, et al. Magnetic resonance imaging with gadoxetic acid disodium for the detection of hepatocellular carcinoma: a meta-analysis of 18 studies. Acad Radiol 2014;21(12):1603–1613. [DOI] [PubMed] [Google Scholar]
- 64.Khan AS, Hussain HK, Johnson TD, Weadock WJ, Pelletier SJ, Marrero JA. Value of delayed hypointensity and delayed enhancing rim in magnetic resonance imaging diagnosis of small hepatocellular carcinoma in the cirrhotic liver. J Magn Reson Imaging 2010;32(2):360–366. [DOI] [PubMed] [Google Scholar]
- 65.Becker-Weidman DJ, Kalb B, Sharma P, et al. Hepatocellular carcinoma lesion characterization: single-institution clinical performance review of multiphase gadolinium-enhanced MR imaging—comparison to prior same-center results after MR systems improvements. Radiology 2011;261(3):824–833. [DOI] [PubMed] [Google Scholar]
- 66.Liu X, Zou L, Liu F, Zhou Y, Song B. Gadoxetic acid disodium-enhanced magnetic resonance imaging for the detection of hepatocellular carcinoma: a meta-analysis. PLoS One 2013;8(8):e70896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu LM, Xu JR, Gu HY, et al. Is liver-specific gadoxetic acid-enhanced magnetic resonance imaging a reliable tool for detection of hepatocellular carcinoma in patients with chronic liver disease? Dig Dis Sci 2013;58(11):3313–3325. [DOI] [PubMed] [Google Scholar]
- 68.Compagnon P, Grandadam S, Lorho R, et al. Liver transplantation for hepatocellular carcinoma without preoperative tumor biopsy. Transplantation 2008;86(8):1068–1076. [DOI] [PubMed] [Google Scholar]
- 69.Chou R, Cuevas C, Fu R, et al. Imaging techniques for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Ann Intern Med 2015;162(10):697–711. [DOI] [PubMed] [Google Scholar]
- 70.Ohkawa K, Imanaka K, Sakakibara M, et al. Factors related to shift from hepatic borderline lesion to overt HCC diagnosed by CT. Hepatogastroenterology 2014;61(134):1680–1687. [PubMed] [Google Scholar]
- 71.Bolondi L, Gaiani S, Celli N, et al. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology 2005;42(1):27–34. [DOI] [PubMed] [Google Scholar]
- 72.Kudo M, Tochio H. Intranodular blood supply correlates well with biological malignancy grade determined by tumor growth rate in pathologically proven hepatocellular carcinoma. Oncology 2008;75(Suppl 1):55–64. [DOI] [PubMed] [Google Scholar]
- 73.Fowler KJ, Tang A, Santillan C, et al. Interreader reliability of LI-RADS version 2014 algorithm and imaging features for diagnosis of hepatocellular carcinoma: a large international multireader study. Radiology 2018;286(1):173–185. [DOI] [PubMed] [Google Scholar]
- 74.Barth BK, Donati OF, Fischer MA, et al. Reliability, validity, and reader acceptance of LI-RADS: an in-depth analysis. Acad Radiol 2016;23(9):1145–1153. [DOI] [PubMed] [Google Scholar]
- 75.Bashir MR, Huang R, Mayes N, et al. Concordance of hypervascular liver nodule characterization between the organ procurement and transplant network and Liver Imaging Reporting and Data System classifications. J Magn Reson Imaging 2015;42(2):305–314. [DOI] [PubMed] [Google Scholar]
- 76.Davenport MS, Khalatbari S, Liu PS, et al. Repeatability of diagnostic features and scoring systems for hepatocellular carcinoma by using MR imaging. Radiology 2014;272(1):132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ehman EC, Behr SC, Umetsu SE, et al. Rate of observation and inter-observer agreement for LI-RADS major features at CT and MRI in 184 pathology proven hepatocellular carcinomas. Abdom Radiol (NY) 2016;41(5):963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sofue K, Sirlin CB, Allen BC, Nelson RC, Berg CL, Bashir MR. How reader perception of capsule affects interpretation of washout in hypervascular liver nodules in patients at risk for hepatocellular carcinoma. J Magn Reson Imaging 2016;43(6):1337–1345. [DOI] [PubMed] [Google Scholar]
- 79.Zhang YD, Zhu FP, Xu X, et al. Classifying CT/MR findings in patients with suspicion of hepatocellular carcinoma: comparison of Liver Imaging Reporting and Data System and criteria-free Likert scale reporting models. J Magn Reson Imaging 2016;43(2):373–383. [DOI] [PubMed] [Google Scholar]
- 80.Schellhaas B, Pfeifer L, Kielisch C, Goertz RS, Neurath MF, Strobel D. Interobserver agreement for contrast-enhanced ultrasound (CEUS)-based standardized algorithms for the diagnosis of hepatocellular carcinoma in high-risk patients. Ultraschall Med 2018 Jun 7 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 81.Schellhaas B, Hammon M, Strobel D, et al. Interobserver and intermodality agreement of standardized algorithms for non-invasive diagnosis of hepatocellular carcinoma in high-risk patients: CEUS-LI-RADS versus MRI-LI-RADS. Eur Radiol 2018 Apr 19 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 82.Becker AS, Barth BK, Marquez PH, et al. Increased interreader agreement in diagnosis of hepatocellular carcinoma using an adapted LI-RADS algorithm. Eur J Radiol 2017;86:33–40. [DOI] [PubMed] [Google Scholar]
- 83.Allen BC, Ho LM, Jaffe TA, Miller CM, Mazurowski MA, Bashir MR. Comparison of visualization rates of LI-RADS version 2014 major features with IV gadobenate dimeglumine or gadoxetate disodium in patients at risk for hepatocellular carcinoma. AJR Am J Roentgenol 2018;210(6):1266–1272. [DOI] [PubMed] [Google Scholar]
- 84.Hope TA, Aslam R, Weinstein S, et al. Change in Liver Imaging Reporting and Data System characterization of focal liver lesions using gadoxetate disodium magnetic resonance imaging compared with contrast-enhanced computed tomography. J Comput Assist Tomogr 2017;41(3):376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang YD, Zhu FP, Xu X, et al. Liver Imaging Reporting and Data System: substantial discordance between CT and MR for imaging classification of hepatic nodules. Acad Radiol 2016;23(3):344–352. [DOI] [PubMed] [Google Scholar]