Abstract
Background:
Substance use can reduce care engagement for individuals with HIV. However, little is known as to whether heavy drinkers differ from drug users. This study compares heavy drinkers, drug users, and those drinking heavily and using drugs on their HIV care engagement.
Methods:
HIV-infected adult inpatients (n=801; 67% male; 78% Black) from 11 urban hospitals across the United States participated in a multisite clinical trial to improve patient engagement in HIV care and virologic outcomes. All participants drank heavily and/or used drugs, and had poorly controlled HIV. Participants reported care history at baseline. We compared heavy drinkers, drug users, and those both drinking heavily and using drugs (reference group) on their engagement in care.
Results:
Heavy drinkers reported lowest rates of lifetime HIV care, AOR = 0.59 (95% CI = 0.36, 0.97). Groups did not differ in recent care, prescription of HIV medication, medical mistrust, or patient-provider relationship. Drug users evidenced the best medication adherence, AOR = 2.38 (95% CI = 1.33, 4.23). Exploratory analyses indicated that drinkers had lower initial care engagement, but that it increased more rapidly with duration of known HIV infection, with similar rates of recent care. Drinkers had the lowest CD4 counts (B=−0.28, p<0.0001), but no difference in viral load.
Conclusions:
Heavy drinkers were least likely to have ever been in HIV care. More research is needed to determine why heavy drinkers evidence the lowest initial care engagement and current CD4 counts, and whether drinking intervention early in infection may increase HIV care engagement.
Keywords: Alcohol, Drug, HIV, Treatment, Care Engagement
1. Introduction
Given availability of highly effective treatments for HIV infection, increasing attention has been devoted to enhancing engagement in HIV care. The highly publicized “90–90–90” goals aim to ensure that 90% of HIV-infected individuals are diagnosed, 90% of diagnosed persons are treated, and 90% of treated persons are virally suppressed by the year 2020 (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2014). However, despite efforts to ensure treatment engagement and viral suppression, many HIV-infected individuals remain virally unsuppressed, even in high-resource countries such as the United States (Castel et al., 2016). This is alarming, as improving engagement in care and treatment is essential to increasing survival for HIV and decreasing HIV forward transmission.
Substance use is a risk factor for acquiring HIV (U.S. Department of Health and Human Services, 2017), is prevalent among those infected (Mimiaga et al., 2013; Pacek et al., 2014), and interferes with engagement in HIV care (Amirkhanian et al., 2016; Cavaleri et al., 2010; Gwadz et al., 2016 ; Kuchinad et al., 2016; Lancaster et al., 2017; Nicholas et al., 2014; Pecoraro et al., 2015). Although illicit drug use has been studied and treated in conjunction with HIV since the epidemic began, it took longer for alcohol to be recognized as relevant to care (Fritz et al., 2010; Williams et al., 2016). In response to the documented lack of attention to alcohol in HIV primary care (Metsch et al., 2008), alcohol interventions have now been developed for this setting (e.g., (Hasin et al., 2013)). Yet, one study suggests that drinkers may still experience shorter session times, and less satisfaction with their HIV care and communication from providers, while drug users do not (Korthuis et al., 2011). Given the importance of care engagement to the health and survival of individuals with HIV, particularly those with poorly controlled infection, it is imperative that we ensure that key groups are not being left behind. More research is needed on whether HIV-infected heavy drinkers are less likely to engage in care activities (i.e., attend care, take medication) and whether they have worse experiences in their care (i.e., relationships with and mistrust toward providers) than other substance users. Recognizing inequities in stigmatized groups is important, as it can allow us to enhance care access and quality among those who may have been neglected.
We aimed to determine the relative contribution of heavy drinking, drug use, and heavy drinking plus drug use to engagement with and quality of HIV medical care among individuals with poorly controlled HIV infection. To address this issue, we used baseline data from a large multisite clinical trial of persons with poorly controlled HIV, conducted across the United States.
2. Material and Methods
2.1. Participants and Procedures
This study is a secondary analysis of data from Project HOPE -- Hospital Visit as Opportunity for Prevention and Engagement for HIV-Infected Drug Users (Metsch, 2016; Metsch et al., 2016), a multi-site clinical trial sponsored by the National Drug Abuse Treatment Clinical Trials Network (CTN 0049). Patients were recruited from 2012 to 2014, from 11 hospitals in major urban areas with high HIV prevalence across the United States: Boston, New York, Philadelphia, Baltimore, Pittsburgh, Chicago, Atlanta, Miami, Birmingham, Dallas, and Los Angeles. A total of 2291 patients were assessed for eligibility. Clinical eligibility criteria required individuals to (a) be an HIV-infected inpatient at a participating hospital, (b) be an adult (18 years and older), (c) have poorly controlled HIV infection (have a current AIDS-defining illness or meet CD4 and viral load cutoffs [<350 cells/uL and >200 copies/mL, respectively; or <500 cells/uL and >200 copies/mL or unknown but inadequately medicated]), and (d) have self-reported or documented opioid, stimulant, or heavy alcohol use (as defined below in Measures). Other eligibility criteria required provision of informed consent, locator information, and medical record release; ability to communicate in English and return for follow-up visits; and a Karnofsky Performance Score ≥60 (global assessment of functioning scale, this omits those severely disabled). A total of 801 eligible patients completed baseline assessment, and were then assigned to one of three conditions (Patient Navigation, Patient Navigation with financial incentives, Treatment as Usual). Interventions were designed to help link and retain patients in HIV and substance use care, and to help them initiate/maintain HIV antiretroviral medication, with the goal of attaining virologic suppression. Primary trial results have been published previously (Metsch et al., 2016). Current analyses use baseline data (prior to intervention) of all 801 patients.
2.2. Measures
2.2.1. Substance Use Category.
Participants were included in the trial on the basis of heavy drinking, illicit drug use, or both, which was used to define substance use category in the current study. Specifically, heavy drinking was determined using the Alcohol Use Disorders Identification Test (AUDIT)-C (positive: scores of >3 for women, >4 for men), referencing the past 12 months (Frank et al., 2008). Illicit drug use was indicated by any report or medical record documentation of ever using opioids or stimulants (cocaine, ecstasy, or amphetamine). A separate category included those who met both heavy drinking and drug use criteria.
For exploratory analyses, we also considered the associations between higher levels of heavy drinking and of drug use with engagement in HIV care. Specifically, using the full AUDIT (Babor et al.) (a reliable/valid (Reinert and Allen, 2007) measure of past-year hazardous and harmful drinking), we examined the association between AUDIT score (continuous) with engagement in care. Similarly, using the Drug Abuse Screening Test 10-item scale [DAST] (Yudko et al., 2007) (a reliable/valid (Maisto et al., 2000; Yudko et al., 2007) measure of drug abuse problems—timeframe not specified), we examined the association between the DAST score with engagement in care. We also conducted analyses using dichotomizations of these variables (AUDIT >8; DAST >6). These exploratory analyses (using the full sample) compared those with varying levels of problem use on engagement in care. Therefore, although the inclusion criteria for the study were broad (ever using drugs and/or surpassing modest AUDIT-C cutoffs in the past year), meant to include a wide range of substance users who may benefit from the interventions to enhance engagement in care, these exploratory analyses explore whether the same patterns hold with particularly heavy users of alcohol, drugs, or both alcohol and drugs.
2.2.2. Engagement in HIV Care and Treatment.
Lifetime and recent HIV care were both assessed in order to determine individuals at risk for (a) never having been connected with HIV primary care, and (b) not being connected with HIV primary care at the time of survey (i.e., with poorly controlled HIV). Specifically, participants reported whether they ever had HIV primary care (yes or no). In a separate section on recent care utilization, they reported whether they had had any medical care within the prior six months (yes or no), and if yes, whether they had had an HIV primary care visit in this timeframe (yes or no). They reported whether they had been prescribed HIV medication (“Have you been prescribed any anti-HIV medication?”, yes or no), and if prescribed, their percentage of self-reported adherence in the past month (allowing us to define those with and without excellent [≥95%] adherence over this timeframe). Finally, patients reported their date of HIV diagnosis (month and year), which was used to calculate duration of known HIV infection, to test whether substance use group interacted with duration of known HIV infection in predicting lifetime care engagement in exploratory analyses.
2.2.3. Patient-provider relationship.
Participants who reported having a provider who cares for their HIV reported on their relationship with their HIV provider using a 30-item scale (Schneider et al., 2004). All items were rated using Likert type response options, with the specific response options differing slightly among items (e.g., frequency for items rating provider behaviors, quality for items rating the nature of the interactions). This scale has been previously used with HIV patients, where it was shown to be reliable and to relate to antiretroviral adherence (Schneider et al., 2004). Although the scale had previously been scored as a series of seven subscales, our exploratory factor analysis found better support for three factors (communication: α=0.97; participatory decision-making: α=0.91, satisfaction/trust: α=0.78) or one overall scale (α=0.96). The overall scale was used in the current study because general satisfaction with the patient-provider relationship was of primary interest, and due to statistical indications that subscales were similar (e.g., high correlations among subscales: r-squared values = 0.58–0.72, p-values < 0.0001; some cross-loading of items to multiple subscales). Possible scores ranged from 0–160, with higher scores indicating a better patient-provider relationship.
2.2.4. Medical Mistrust.
The Medical Mistrust Scale assesses patient mistrust of health care professionals and systems. Participants rated twelve statements regarding the medical treatment of their racial or ethnic group, using response options ranging from Strongly Agree to Strongly Disagree (Thompson et al., 2004). This scale has demonstrated reliability and validity in prior research (Thompson et al., 2004). Cronbach’s alpha for the full scale was 0.86 in this sample. Possible scores ranged from 0–60, with higher scores indicating more medical mistrust.
2.2.5. Physical Health (CD4 cell count and HIV viral load).
As objective measures of health, patients’ CD4 cell count and HIV-1 plasma viral load were assessed at local laboratories.
2.3. Analysis Plan
First, descriptive statistics are presented, along with uncontrolled comparisons of demographics and care engagement between heavy drinkers, drug users, and heavily drinking drug users. Uncontrolled analyses used chi-square difference tests for dichotomous variables and generalized linear models for continuous variables. Second, a series of controlled models compared the three substance use groups on engagement in care. Controlled models used logistic regressions for dichotomous outcomes (ever had HIV primary care, had an HIV primary care visit within the past six months [recent care], prescribed HIV medication, excellent medication adherence), and generalized linear models for continuous outcomes (patient-provider relationship, medical mistrust). Model specifications accounted for the distribution shapes of the continuous outcome variables (patient-provider relationship: a negative binomial distribution and log link were used after the scale was reversed-scored to account for the left-skewed distribution of the data; medical mistrust: a normal distribution and identity link were used). These models used heavily drinking drug users as the reference group, and controlled for age, gender, race/ethnicity, education, and study site. Analyses for the patient-provider relationship were restricted to those who reported having a provider who cares for their HIV. Analyses for medication adherence were restricted to those who reported being prescribed medication.
Three sets of exploratory analyses were also conducted. First, to understand the correlates of high levels of substance use, univariate logistic regressions were conducted to determine whether higher levels of heavy drinking (AUDIT) and of drug use (DAST) were associated with engagement in care. This was done using both continuous and dichotomized (AUDIT>8, DAST>6) versions of these variables. Second, to better understand a significant difference between substance use groups in likelihood of lifetime HIV care, an interaction term was added to this model to investigate whether duration of known HIV infection interacted with substance use group in predicting lifetime HIV care. Third, to consider potential differences in objective health among groups, we considered differences between substance use groups in CD4 cell count (both as a continuous variable using poisson regression, and as a dichotomous variable CD4>350 cells/μL using univariate logistic regression) and HIV viral load (both as a continuous variable using linear regression after a log10 transformation, and as a dichotomous variable VL>200 copies/ml using univariate logistic regression).
All analyses were conducted in SAS Version 9.4.
3. Results
3.1. Descriptive Information and Unadjusted Comparisons
Of the full sample of 801 participants, 188 (23.5%) were heavy drinkers, 330 (41.2%) were drug users, and 283 (35.3%) were both heavy drinkers and drug users (Table 1). Patients were on average 44.15 years of age (SD = 9.98), mostly male (67.4%), and primarily Black (74.65%; 12.55% White, 11.04% Hispanic/Latino, 1.38% Other). Most (66.17%) were never married, and most (60.2%) had completed at least a high school education (Metsch et al., 2016). Heavy drinkers were the youngest (drinkers: Mean [M]=42 years; drug users: M=44 years; both: M=46 years; p<0.01), the most likely to be employed (drinkers: 24%; drug users: 5%; both: 11%; p<0.0001), the least likely to have been incarcerated (drinkers: 63%; drug users: 82%; both: 82%; p<0.0001), unstably housed (drinkers: 23%; drug users: 38%; both: 47%; p<0.0001), or in substance use treatment (drinkers: 33%; drug users: 59%; both: 66%; p<0.0001), and had the shortest duration of known HIV infection (drinkers: M=120 months; drug users: 157 months; both: 155 months; p<0.001). Those both drinking heavily and using drugs were most likely to meet cutoffs for severe substance use (AUDIT>8; DAST>6) (drinkers: 68%; drug users: 49%; both: 84%; p<0.0001).
Table 1.
Sample characteristics for (mutually exclusive) substance use groups.
| Characteristic1 | Heavy Drinker2 | Drug user3 | Both | Comparison p-value |
All (N=801) |
||||
|---|---|---|---|---|---|---|---|---|---|
| (n=188) | (n=330) | (n=283) | |||||||
| DESCRIPTIVE CHARACTERISTICS | |||||||||
| Gender | 0.06 | ||||||||
| Men | 139/188 | 73.94% | 221/330 | 66.97% | 180/283 | 63.60% | 540/801 | 67.42% | |
| Women | 49/188 | 26.06% | 109/330 | 33.03% | 103/283 | 36.40% | 261/801 | 32.58% | |
| Age4 | 42.47 | 10.54 | 43.86 | 10.11 | 45.61 | 9.24 | 0.003 | 44.15 | SD=9.98 |
| Race/ethnicity | 0.44 | ||||||||
| Black/African American | 146/187 | 78.07% | 236/328 | 71.95% | 216/282 | 76.60% | 598/797 | 74.65% | |
| Hispanic/Latino | 15/187 | 8.02% | 43/328 | 13.11% | 30/282 | 10.64% | 88/797 | 11.04% | |
| White | 22/187 | 11.76% | 46/328 | 14.02% | 32/282 | 11.35% | 100/797 | 12.55% | |
| Other | 4/187 | 2.14% | 3/328 | 0.91% | 4/282 | 1.42% | 11/797 | 1.38% | |
| Marital status | 0.69 | ||||||||
| Married/Cohabiting | 26/188 | 13.83% | 35/330 | 10.61% | 29/283 | 10.25% | 90/801 | 11.24% | |
| Widowed/Divorced/Separated | 29/188 | 15.43% | 77/330 | 23.33% | 75/283 | 26.50% | 181/801 | 22.60% | |
| Never Married | 133/188 | 70.74% | 218/330 | 66.06% | 179/283 | 63.25% | 530/801 | 66.17% | |
| Education | 0.32 | ||||||||
| Less than High School | 64/188 | 34.04% | 135/330 | 40.91% | 120/283 | 42.40% | 319/801 | 39.83% | |
| Completed High School | 71/188 | 37.77% | 104/330 | 31.52% | 96/283 | 33.92% | 271/801 | 33.83% | |
| Greater than High School | 53/188 | 28.19% | 91/330 | 27.58% | 67/283 | 23.67% | 211/801 | 26.34% | |
| Employment status | <0.0001 | ||||||||
| Working | 45/188 | 23.94% | 18/330 | 5.45% | 30/283 | 10.60% | 93/801 | 11.61% | |
| Unemployed | 61/188 | 32.45% | 115/330 | 34.85% | 105/283 | 37.10% | 281/801 | 35.08% | |
| Disabled | 74/188 | 39.36% | 187/330 | 56.67% | 138/283 | 48.76% | 399/801 | 49.81% | |
| Other | 8/188 | 4.26% | 10/330 | 3.03% | 10/283 | 3.53% | 28/801 | 3.50% | |
| Personal annual income5 | 9020 | 9600 | 8367 | 7540 | 8400 | 7232 | 0.21 | 8400 | 7000 |
| Health insurance | 119/187 | 63.64% | 227/328 | 69.21% | 188/280 | 67.14% | 0.43 | 534/795 | 67.17% |
| Ever incarcerated | 118/188 | 62.77% | 271/330 | 82.12% | 231/281 | 82.21% | <0.0001 | 620/799 | 77.60% |
| Unstably housed in previous 6 months | 42/185 | 22.70% | 125/326 | 38.34% | 131/276 | 47.46% | <0.0001 | 298/787 | 37.87% |
| Time since HIV diagnosis in months4 | 120.2 | 97.83 | 157.3 | 103.2 | 155.3 | 103.3 | 0.0002 | 147.84 | 103.04 |
| Meets severe substance use cutoffs6,7 | 127/188 | 67.55% | 162/330 | 49.09% | 239/283 | 84.45% | <.0001 | 528/801 | 65.92% |
| Ever previous substance use treatment | 62/188 | 32.98% | 194/330 | 58.79% | 187/282 | 66.31% | <0.0001 | 443/800 | 55.38% |
| ENGAGEMENT IN CARE | |||||||||
| Prescribed HIV medication | 91/188 | 48.40% | 163/329 | 49.54% | 150/283 | 53.00% | 0.56 | 404/800 | 50.50% |
| Excellent (≥95%) adherence | 21/90 | 23.33% | 49/161 | 30.43% | 25/148 | 16.89% | 0.02 | 95/399 | 23.81% |
| Ever engaged in HIV care | 143/188 | 76.06% | 280/329 | 85.11% | 241/282 | 85.46% | 0.01 | 664/799 | 83.10% |
| HIV care in previous 6 months | 73/188 | 38.83% | 160/330 | 48.48% | 132/283 | 46.64% | 0.10 | 365/801 | 45.57% |
| Physician-patient relationship4 | 108.9 | 25.53 | 108.8 | 25.75 | 106.7 | 27.33 | 0.70 | 108.08 | SD=26.24 |
| Medical mistrust4 | 28.54 | 7.52 | 28.48 | 7.79 | 28.94 | 7.95 | 0.75 | 28.66 | SD=7.78 |
Denominators vary due to missing data.
Alcohol use disorders identification test (AUDIT)-C score greater than 4 for men, 3 for women.
Report or medical record documentation of any opioid or stimulant (cocaine, ecstasy, or amphetamine) use
Mean and Standard Deviation are presented.
Median and (IQR), $1000s, are presented.
AUDIT score greater than or equal to 8.
DAST score greater than or equal to 6.
Of the 799 participants who provided data on lifetime care status, 664 (83.10%) reported ever having had HIV care. Of the 801 providing data on recent HIV care status, 365 (45.57%) reported HIV care in the past six months. Of the 800 providing data on prescription of HIV medication, 404 (50.50%) reported being prescribed HIV medication, and of the 399 prescribed who reported on adherence, 95 (23.81%) reported excellent (≥95%) adherence in the past month. The average score on the patient-provider relationship scale was 108.08 (SD = 26.24; range: 17.00 – 140.00), indicating generally positive ratings of the relationships. The average score on the medical mistrust scale was 28.66 (SD = 7.78; range: 3.00 – 60.00), indicating moderate levels of medical mistrust. In unadjusted comparisons of these outcomes, heavy drinkers were least likely to have ever been engaged in HIV care (drinkers: 76%; drug users: 85%; both: 85%; X2 (2, N=799) = 8.69, p=0.01). Drug users were most likely to report excellent adherence (drinkers: 23%; drug users: 30%; both: 17%; X2 (2) = 7.81, p=0.02). No other outcomes differed between groups.
3.2. Adjusted Comparisons: Engagement In Care And Treatment
Results for adjusted logistic regression models are presented in Table 2. Compared with heavily drinking drug users, individuals solely reporting heavy drinking were less likely to report ever having HIV care, AOR = 0.59 (95% CI = 0.36, 0.97). The groups did not differ in recent HIV care or in prescription of HIV medication. Drug users were more likely to report excellent (≥95%) adherence compared to heavily drinking drug users, AOR = 2.38 (95% CI = 1.33, 4.23).
Table 2.
Differences in HIV care engagement among heavy drinking, drug using, and heavy drinking / drug using groups.
| Heavy drinkers (vs. Heavily drinking drug users) |
Drug users (vs. Heavily drinking drug users) |
|
|---|---|---|
| Engagement in HIV care and treatment | ||
| Ever had HIV care | AOR = 0.59 (95% CI = 0.36, 0.97)* | AOR = 1.04 (95% CI = 0.65, 1.66) |
| HIV care in past six months | AOR = 0.76 (95% CI = 0.51, 1.14) | AOR = 1.06 (95% CI= 0.76, 1.50) |
| Prescribed HIV medication | AOR = 0.92 (95% CI = 0.61, 1.38) | AOR = 0.96 (95% CI= 0.68, 1.36) |
| Excellent (95%) adherence | AOR = 1.37 (95% CI= 0.68, 2.73) | AOR = 2.38 (95% CI= 1.33, 4.23)* |
| Attitudes toward medical providers | ||
| Patient-provider relationshipa | b = 0.02, p = 0.47 | b = 0.02, p = 0.45 |
| Medical mistrust | b = 0.15, p = 0.84 | b = −0.02, p = 0.98 |
Note. AOR = Adjusted Odds Ratio (controlled for age, gender, race/ethnicity, education, and study site). 95% CI = 95% Confidence Interval.
All three subscales of the patient-provider relationship scale were similarly nonsignificant. In these analyses the patient-provider scales are reversed scored and estimated with a negative binomial distribution such that higher scores are associated with a worse relationship.
3.3. Adjusted Comparisons: Attitudes Toward Medical Providers
Results for adjusted generalized linear models are also presented in Table 2. The groups did not differ in reported satisfaction in their relationship with their physician, p-values > 0.40, nor did they differ in their medical mistrust, p-values > 0.80.
3.4. Exploratory Analyses: Substance Use Severity
Continuous analyses indicated that those with greater drug use severity were more likely to have been prescribed HIV medication, B=0.06, p=0.01, to have had recent HIV care, B=0.06, p=0.02, and to have ever had HIV primary care, B=0.11, p<0.001. Drug use severity did not relate to medical mistrust or physician satisfaction (p-values>0.70). Dichotomous analyses also showed that those that met or exceeded higher cutoffs of drug use severity were more likely to have been prescribed HIV medication, OR = 1.37 (95% CI = 1.03, 1.81), to have had recent HIV care, OR = 1.35 (95% CI = 1.02, 1.79), and to have ever had HIV primary care, OR = 1.80 (95% CI = 1.21, 2.69). Again, those exceeding cutoffs did not differ on medical mistrust or physician satisfaction (p-values>0.50).
Similar analyses for alcohol indicated no relation between higher levels of heavy drinking with any outcomes (p-values>0.05).
3.5. Exploratory Analyses: Duration of Known HIV Infection as Moderator of the Association Between Substance Use Category and Ever-Care
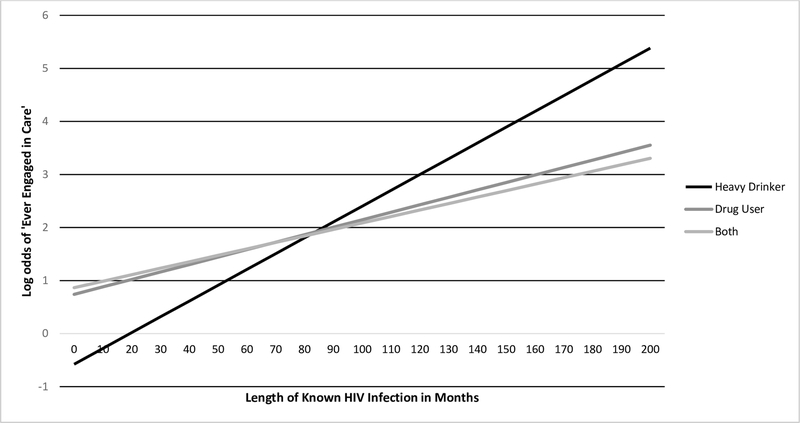
In a model that included substance use category, duration of known HIV infection, and their interaction, the interaction term was a significant predictor of lifetime engagement in care (B=0.02, p=0.002). The interaction indicated that drinkers were less likely to have been in care early in their known infection, but their care engagement changed more quickly by year of known HIV infection than drug users, such that rates of recent care were similar (Figure 1).
Figure 1.
Differences between substance use groups in engagement in HIV care by duration of known HIV infection.
3.6. Exploratory Analyses: Differences in Objective Health (CD4 Cell Count and HIV Viral Load)
Heavy drinkers evidenced lower CD4 values than drug users, B=−0.25, p<0.0001, and heavily drinking drug users, B=−0.28, p<0.0001. In dichotomous analyses, heavy drinkers were significantly less likely to have high CD4 counts (CD4≥350) than drug users, OR = 0.47 (95% CI = 0.26, 0.87), but did not differ significantly from heavily drinking drug users, OR = 0.56 (95% CI = 0.30, 1.05). There were no significant differences found for viral load in continuous or dichotomous analyses (likely to due to viral load eligibility requirements).
4. Discussion
In the current era of effective HIV treatment, engagement in care can be lifesaving. In a sample of substance users with poorly controlled HIV infection, we found that those who drank heavily but did not use drugs were less likely to report lifetime engagement in HIV care (with a rate of 76%), even when compared with those who both drank heavily and used drugs (rates were 85% in both drug users and heavily drinking drug users). Those drinking heavily also evidenced a lower CD4 count than drug users and heavily drinking drug users in exploratory analyses, suggesting poorer immune health. However, heavy drinkers and drug users evidenced similar rates of recent care (i.e., recent HIV appointment, current medication). Exploratory analyses clarified that heavy drinkers had lower engagement in care soon after diagnosis but had a faster rate of engagement by duration of known HIV infection, with similar levels of recent care. That heavy drinkers were less connected to care early in their infection may be surprising (particularly for providers who continue to recognize drug users but not drinkers as a high-risk group), but in fact, exploratory analyses indicated that individuals with more extensive drug use were more engaged in HIV care. Individuals who use drugs thus appear more likely to find their way into HIV treatment (possibly through referrals from drug treatment services), whereas fewer heavy drinkers connect to care soon after learning of their infection. This is important to know, as early linkage to care can help ensure treatment when HIV is easiest to control and damage to the immune system is at its minimum. Low levels of early care engagement may relate to the lower CD4 counts among drinkers, although causality cannot be concluded using these cross-sectional variables. The different groups of substance users did not differ in attitudes toward providers (i.e., relationship with their physician, medical mistrust), suggesting that different types of substance users may feel similarly about their providers once the connection to care is made.
Disruption in HIV care by substance use in general has been well documented (Amirkhanian et al., 2016; Cavaleri et al., 2010; Gwadz et al., 2016 ; Kuchinad et al., 2016; Lancaster et al., 2017; Nicholas et al., 2014; Pecoraro et al., 2015). Although fewer studies have documented discrepancies in the experiences of heavy drinkers and drug users, a previous study did find poorer provider engagement with heavy drinkers than drug users (Korthuis et al., 2011). However, that study showed deficits in session time and patient provider communication in particular (Korthuis et al., 2011), not reflected in the nonsignificant findings for the patient-provider relationship of those who engaged in care in this study. In the current study, we found fewer heavy drinkers to have entered into HIV care, particularly early in their (known) infection. How alcohol interferes with this early engagement (and how this can be addressed) requires study. For example, it may be due to the longstanding neglect of the connection between HIV and drinking (Fritz et al., 2010), leading fewer HIV-infected drinkers to have been identified and linked to HIV care. Or, alcohol may interfere with motivation or skills needed to engage in care (e.g., planning, memory), or may be associated with psychiatric comorbidities (depression, personality disorders) that interfere with care. Although one might speculate that alcohol causes practical barriers to appointment attendance (unstable housing, incarceration), many of these socioeconomic barriers were actually lowest in the heavy drinking group in our sample. Recent care was not significantly different among the different substance using groups, although drinkers still reported the lowest rate (39%) in comparison with drug users (48%) and heavily drinking drug users (47%). It is possible that symptoms stemming from currently poorly controlled HIV may account for the more similar rates of recent care across groups. However, drinkers’ lower level of early engagement in care indicates that more work needs to be done to connect heavy drinkers with services in the first place, before they feel ill or lose control over their infection. Although the time between initial infection and onset of poorly-controlled infection in our sample is not known, all patients in the current sample evidenced poorly controlled infection (per inclusion criteria), and less time had elapsed for heavy drinkers between HIV diagnosis and study onset (120 months for drinkers versus 157 for drug users and 155 for heavily drinking drug users). Future research should investigate whether drinkers’ lower early engagement in care is associated with quicker disease progression (i.e., less time to poorly controlled infection) when compared with drug users.
The current study is subject to certain limitations. Results are cross-sectional, so we cannot determine temporal effects of involvement with alcohol or drugs. It is therefore possible that individuals initiated substance use more recently, in response to HIV diagnosis or related illnesses. Also related to timeframe, it is possible that drinkers fared worse due to study inclusion criteria specifying past-year alcohol use but ever drug use. Although we do not have a past-year drug use measure to confirm that most drug users used in this timeframe, we can confirm that comparable proportions (approximately two thirds) of our alcohol and drug use groups used the respective substances in the past 30 days (alcohol: 67.6% reported past 30 day alcohol use; drug users: 60.6% reported past 30 day drug use; heavily drinking drug users: 71.3% and 64.9% reported past 30 day alcohol and drug use, respectively). Our sample consisted of inpatients with poorly controlled HIV infection, so results may not apply to non-hospitalized individuals or those with well-controlled infection. Our study lacks a comparison group with no substance use, limiting our ability to form conclusions about the effects of any substance use compared with none. However, many studies over the years have shown that those who abuse alcohol and drugs evidence worse engagement in care than those who do not (Lucas, 2011; Vagenas et al., 2015), with fewer studies comparing problem drinkers and drug users with one another. Also, our study was conducted in English only, limiting generalizability to the HIV infected population as a whole, which includes a large Hispanic population, some of whom are monolingual Spanish speakers. Although it would have been helpful to better understand specific patterns of care (e.g., continuity/gaps in care since diagnosis, whether care recommendations were followed, variation in specific providers seen) and substance use (e.g., increases or decreases over time), this detailed information was not available in the current study. We also did not have information on time since the moment of HIV infection (only time since diagnosis), time on medication, or delay from diagnosis to medication, potentially useful variables in understanding our results. Finally, our study relies on self-report, which may be subject to under-reporting or reporting error. However, much substance use research utilizes self-report. Prospective research, research with different HIV populations, and use of additional sources of information (e.g., biological testing) would be informative in addressing these gaps. However, strengths of this study include attention to the important but understudied population of substance users with poorly controlled HIV, the large sample, the multi-site sampling of patients across the United States, and control for demographic covariates.
4.1. Conclusions
In this group of substance users with poorly controlled HIV, those who drank heavily were less likely to have engaged in HIV care, particularly early in their known infection. Alcohol therefore may interfere with one’s ability to link to HIV care, for reasons that require further study, perhaps using qualitative methods to better understand barriers to treatment. Yet, heavy drinkers do not appear to differ in their relationship with their provider, trust in the medical system, or prescription of medication, possibly suggesting that once they are in care, it can be successful. Yet, drug users evidence greater adherence than drinkers, possibly reflecting well-documented adherence difficulties related to problem drinking (Azar et al., 2010). Future research should investigate ways to engage heavy drinkers into consistent, quality HIV care and treatment early on, before they develop poor control over their infection.
Highlights.
Individuals with HIV who use substances evidence less engagement in care.
It is unclear whether alcohol or drugs are more disruptive to engagement in HIV care.
We compared heavy drinkers, drug users, and those using both on HIV care engagement.
Heavy drinkers were least likely to report lifetime HIV care engagement.
Drinkers and drug users reported similar recent care and relationship with providers.
Role of Funding Source
This work was supported by the National Institutes of Health [grants: U10-DA01372011 (Project HOPE -- Hospital Visit as Opportunity for Prevention and Engagement for HIV-Infected Drug Users; Metsch), K23AA023753 (Elliott), R01AA023163 (Hasin)], and the New York State Psychiatric Institute (Hasin). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Conflicts of Interest
No conflict declared.
References
- Amirkhanian Y, Kelly J, DiFranceisco W, Kuznetsova A, Tarima S, Yakovlev A, Musatov V, 2016. Predictors of HIV Care Engagement, Antiretroviral Medication Adherence, and Viral Suppression Among People Living with HIV Infection in St. Petersburg, Russia. AIDS Behav. 3, 791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar MM, Springer SA, Meyer JP, Altice FL, 2010. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 112, 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG, 2001. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care, second ed World Health Organization; http://www.who.int/iris/handle/10665/67205 [Google Scholar]
- Castel AD, Kalmin MM, Hart RL, Young HA, Hays H, Benator D, Kumar P, Elion R, Parenti D, Ruiz ME, Wood A, D’Angelo L, Rakhmanina N, Rana S, Bryant M, Hebou A, Fernandez R, Abbott S, Peterson J, Wood K, Subramanian T, Binkley J, Happ LP, Kharfen M, Masur H, Greenberg AE, 2016. Disparities in achieving and sustaining viral suppression among a large cohort of HIV-infected persons in care - Washington, DC. AIDS Care. 10.1080/09540121.2016.1189496, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaleri MA, Kalogerogiannis K, Mckay MM, Vitale L, Levi E, Jones S, Wallach F, Flynn E, 2010. Barriers to HIV care: an exploration of the complexities that influence engagement in and utilization of treatment. Soc. Work Health Care. 49, 934–945. [DOI] [PubMed] [Google Scholar]
- Frank D, DeBenedetti AF, Volk RJ, Williams EC, Kivlahan DR, Bradley KA, 2008. Effectiveness of the AUDIT-C as a screening test for alcohol misuse in three race/ethnic groups. J. Gen. Intern. Med 23, 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz K, Morojele N, Kalichman S, 2010. Alcohol: the forgotten drug in HIV/AIDS. Lancet. 376, 398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwadz M, de Guzman R, Freeman R, Kutnick A, Silverman E, Leonard N, Ritchie A, Muñoz-Plaza C, Salomon N, Wolfe H, Hilliard C, Cleland C, Honig S, 2016. Exploring how substance use impedes engagement along the HIV care continuum: a qualitative study. Front. Public Health 8, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Aharonovich E, O’Leary A, Greenstein E, Pavlicova M, Arunajadai S, Waxman R, Wainberg M, Helzer J, Johnston B, 2013. Reducing heavy drinking in HIV primary care: a randomized trial of brief intervention, with and without technological enhancement. Addiction 108, 1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS), 2014. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf.
- Korthuis PT, Saha S, Chander G, McCarty D, Moore RD, Cohn JA, Sharp VL, Beach MC, 2011. Substance use and the quality of patient-provider communication in HIV clinics. AIDS Behav. 15, 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinad K, Hutton H, Monroe A, Anderson G, Moore R, Chander G, 2016. A qualitative study of barriers to and facilitators of optimal engagement in care among PLWH and substance use/misuse. BMC Res. Notes 22, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster KE, Lungu T, Mmodzi P, Hosseinipour MC, Chadwick K, Powers KA, Pence BW, Go VF, Hoffman IF, Miller WC, 2017. The association between substance use and sub-optimal HIV treatment engagement among HIV-infected female sex workers in Lilongwe, Malawi. AIDS Care 29, 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, 2011. Substance abuse, adherence with antiretroviral therapy, and clinical outcomes among HIV-infected individuals. Life Sci. 88, 948–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Carey MP, Carey KB, Gordon CM, Gleason JR, 2000. Use of the AUDIT and the DAST-10 to identify alcohol and drug use disorders among adults with a severe and persistent mental illness. Psychol. Assess 12, 186–192. [DOI] [PubMed] [Google Scholar]
- Metsch LR, Feaster DJ, Gooden L, Matheson T, Stitzer M, Das M, Jain MK, Rodriguez AE, Armstrong WS, Lucas GM, Nijhawan AE, Drainoni ML, Herrera P, Vergara-Rodriguez P, Jacobson JM, Mugavero MJ, Sullivan M, Daar ES, McMahon DK, Ferris DC, Lindblad R, VanVeldhuisen P, Oden N, Castellon PC, Tross S, Haynes LF, Douaihy A, Sorensen JL, Metzger DS, Mandler RN, Colfax GN, del Rio C, 2016. Effect of patient navigation with or without financial incentives on viral suppression among hospitalized patients with HIV infection and substance use: a randomized clinical trial. JAMA 316, 156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsch LR, Pereyra M, Colfax G, Dawson-Rose C, Cardenas G, McKirnan D, Eroglu D, 2008. HIV-positive patients’ discussion of alcohol use with their HIV primary care providers. Drug Alcohol Depend. 95, 37–44. [DOI] [PubMed] [Google Scholar]
- Mimiaga MJ, Reisner SL, Grasso C, Crane HM, Safren SA, Kitahata MM, Schumacher JE, Mathews WC, Mayer KH, 2013. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems Cohort. Am. J. Public Health 103, 1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas P, Willard S, Thompson C, Dawson-Rose C, Corless I, Wantland D, Sefcik E, Nokes K, Kirksey K, Hamilton M, Holzemer W, Portillo C, Rivero Mendez M, Robinson L, Rosa M, Human S, Cuca Y, Huang E, Maryland M, Arudo J, Eller L, Stanton M, Driscoll M, Voss J, Moezzi S, 2014. Engagement with care, substance use, and adherence to therapy in HIV/AIDS. AIDS Res. Treat 10.1155/2014/675739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Harrell PT, Martins SS, 2014. Cigarette smoking and drug use among a nationally representative sample of HIV-positive individuals. Am. J. Addict 23, 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro A, Mimiaga M, O’Cleirigh C, Safren S, Blokhina E, Verbitskaya E, Yaroslavtseva T, Ustinov A, Lioznov D, Zvartau E, Krupitsky E, Woody G, 2015. Depression, substance use, viral load, and CD4+ count among patients who continued or left antiretroviral therapy for HIV in St. Petersburg, Russian Federation. AIDS Care 27, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert DF, Allen JP, 2007. The alcohol use disorders identification test: an update of research findings. Alcohol. Clin. Exp. Res 31, 185–199. [DOI] [PubMed] [Google Scholar]
- Schneider J, Kaplan SH, Greenfield S, Li W, Wilson IB, 2004. Better physician-patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. J. Gen. Intern. Med 19, 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HS, Valdimarsdottir HB, Winkel G, Jandorf L, Redd W, 2004. The Group-Based Medical Mistrust Scale: psychometric properties and association with breast cancer screening. Prev. Med 38, 209–218. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, 2017. Substance Abuse/Use. https://www.aids.gov/hiv-aids-basics/prevention/reduce-your-risk/substance-abuse-use/.
- Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, Altice FL, 2015. The impact of alcohol use and related disorders on the HIV continuum of care: a systematic review. Curr. HIV/AIDS Rep. 12, 421–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH, 2016. Alcohol use and human immunodeficiency virus (HIV) infection: current knowledge, implications, and future directions. Alcohol. Clin. Exp. Res 40, 2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudko E, Lozhkina O, Fouts A, 2007. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J. Subst. Abuse Treat 32, 189–198. [DOI] [PubMed] [Google Scholar]