Abstract
Background
In adults with primary focal segmental glomerulosclerosis (FSGS), daily prednisone may induce complete remissions (CR) and partial remissions (PR), but relapses are frequent and adverse events are common.
Methods
We carried out two open-label, uncontrolled trials to explore the efficacy and tolerability of pulse oral dexamethasone as an alternative to daily prednisone. We enrolled adult patients with proteinuria >3.5 g/d despite the use of renin-angiotensin-aldosterone blockade. In the first trial, we enrolled 14 subjects with FSGS and administered 4 dexamethasone doses (25 mg/m2) daily for four days, repeated every 28 days over 32 weeks. The second trial involved a more intensive regimen. Eight subjects received 4 dexamethasone doses of 50 mg/m2 every 4 weeks for 12 weeks, followed by four doses of 25 mg/m2 every 4 weeks for 36 weeks; subjects were randomized to 2 doses every 2 weeks or 4 doses every 4 weeks
Results
In the first trial, we enrolled 14 subjects with FSGS and 1 with minimal change disease and found a combined CR and PR rate of 36%. In the second trial, we enrolled 8 subjects. The combined CR and PR rate was 29%. Analysis combining both trials showed a combined CR and PR rate of 33%. Adverse events were observed in 32% of subjects, with mood symptoms being most common. There were no serious adverse events related to the study.
Conclusion
We conclude that high dose oral dexamethasone is well tolerated by adults with idiopathic nephrotic syndrome and may have some efficacy.
Keywords: steroids, glucocorticoids, proteinuria, remission
Introduction
Focal segmental glomerulosclerosis (FSGS), including collapsing glomerulopathy, is the most common cause of primary nephrotic syndrome in adults. FSGS can be classified into six forms: primary, adaptive, APOL1-associated, high-penetrance genetic variant-associated, viral infection-associated, and medication-associated forms 1. Conventional treatment for primary FSGS typically begins with daily prednisone or less commonly alternate day prednisone.
Among FSGS patients who receive glucocorticoids as the initial treatment, 61% enter complete remission following a prolonged initial treatment compared to 15% who received treatment for a short treatment of 16 weeks 2. Such prolonged high dose steroid course has substantial toxicity and significant morbidity. Nevertheless, a recent retrospective case series from the University of North Carolina suggested more favorable outcomes, with a hazard ratio for renal survival of 0.49 (95% confidence interval 0.28 – 0.86 in subjects with FSGS who received high-dose glucocorticoid therapy for a median of 3.0 months (interquartile range, 1.5–5. 9months) 3.
The mechanism by which glucocorticoid therapy induces remission has long remained unknown. There at least two possibilities: these agents modulate immune functions and/ord have a direct effect on podocytes. A recent report suggested that in FSGS and MC), glucocorticoid sensitivity is due to an expansion of the Cd11b+ HLA-DR−-CD14− CD15+ cell population; these cells are immature myeloid-derived stem cells (MDSC) that can suppress T cell responses 4. This would add glucocorticoid-sensitive FSGS and MCD to the list of inflammatory and autoimmune diseases in which MDSC are believed to play a role, including type 1 diabetes, multiple sclerosis, and inflammatory bowel disease 5.
Recent work suggests that dexamethasone has direct effects on cultured human podocytes, which express an intact glucocorticoid signaling pathway 6. Saleem and colleagues showed that dexamethasone activates glucorticoid signaling in human podocytes. working at both transcriptional and post-transcriptional levels 6, Mathieson and colleagues demonstrated that dexamethasone increases expression of nephrin and down-regulates expression of vascular endothelial growth factor, the latter being known to be up-regulated in minimal change disease 7.
Pulse oral dexamethasone has been used in several conditions. As initial therapy for acute and chronic immune thrombocytopenia, high dose dexamethasone (40 mg/d) administered to patients as a single dose daily for four consecutive days, every 14 days for four courses, has shown efficacy equivalent to daily prednisone 8. As therapy for multiple myeloma, oral pulse dexamethasone was administered at 20 mg/m2/day for 4 days of each 35-day cycle. Downward dose adjustment was required in 8% of patients because of adverse events. Complications severe enough to require hospitalization, including pancreatitis, perforated viscus, or pneumonia were seen in 4 of 112 subjects. Candidiasis, herpetic lesions, exacerbation of diabetes or proximal muscle weakness each occurred in <5% of patients 9. Current therapy for multiple myeloma involves lower doses of dexamethasone in combination with a second medication. In a study involving patients with chronic inflammatory demyelinating polyradiculoneuropathy, pulse oral dexamethasone was found to have fewer adverse events compared to standard prednisolone treatment 10. These studies led us to examine whether dexamethasone, used for FSGS, might have similar efficacy and reduce toxicity compared to the standard approach to daily prednisone use.
In this report, we present the results of two trials using pulse oral dexamethasone administered over 32 weeks (study 1) and 48 weeks (study 2) as initial immunosuppressive therapy for adult patients with primary FSGS. These trials were open- label, uncontrolled studies designed to develop preliminary evidence of efficacy and safety that would justify future controlled studies of this approach.
Methods
Human subjects review.
The protocols for study 1 and study 2 were approved in advance by the appropriate institutional review boards at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH and at Mayo Clinic. All subjects gave written informed consent. The studies were listed at the US Federal ClinicalTrials.gov website as NCT00004990.
Entry Criteria
We enrolled patients with primary FSGS (including the variants tip lesion, collapsing variant, and not otherwise specified variant) and MCD. Adaptive FSGS was excluded based on the presence of characteristic features, including normal or near normal serum albumin levels despite nephrotic-range proteinuria, enlarged glomeruli, perihilar sclerosis and hyalinosis, and limited podocyte foot process effacement. Patients with possible medication-associated FSGS, such as those who had received lithium or interferon alpha, were excluded.
Entry criteria also included nephrotic range proteinuria, defined as urine protein ≥3.5 g/1.73m2/d, and Chronic Kidney Disease Epidemiology Collaboration (CKE-EPI) GFR ≥40ml/min/1.73m2 at the time of entry while receiving maximally tolerated doses of angiotensin enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) for at least 4 weeks prior to study entry and prior steroid use was <8 weeks of daily or alternate day steroids at >0.5 mg/kg. Exclusion criteria included patients with poorly controlled diabetes mellitus, poorly controlled hypertension (>25% of values >125/75 mmHg), HIV-1 infection, hepatitis C infection, hepatitis B infection, and untreated Mycobacterial infection. Pregnant or nursing women and those unwilling or unable to use effective contraception were excluded.
Kidney biopsies
After study conclusion, kidney biopsies performed prior to study enrollment from 21 subjects were reviewed and classified according to histologic variant. The slide sets were reviewed by either of two nephropathologists. Two slide sets had been destroyed and one slide set was unavailable for review; the original biopsy interpretations were collapsing glomerulopathy; FSGS, NOS; and MCD, and these interpretations were retained. Diagnostic categories of FSGS and collapsing glomerulopathy were applied following the Columbia FSGS classification11.
Intervention
In study 1 (32 weeks duration), subjects were given oral dexamethasone 25 mg/m2/d, on days 1–4 of each 28-day cycle, for a total of 8 cycles. The minimum daily dose was established as 40 mg and the maximum dose as 128 mg. The doses were selected as being similar to doses used for thrombocytopenia and multiple myeloma described above. The study drug supplies were maintained at the NIH Clinical Center pharmacy, and patients received a supply of dexamethasone for the next treatment cycle at each monthly visit.
Blood pressure medications were adjusted to maintain baseline blood pressure. ACE inhibitor, ARB or non-dihydropyridine calcium channel blockers were not started during the study, as these medications modify proteinuria. All patients were given 1500 mg elemental calcium and 800 units of vitamin D daily while taking dexamethasone to minimize glucocorticoid-related bone loss.
In order to examine whether length and dose of treatment had an effect on proteinuria, the dose and duration of dexamethasone were increased in study 2 (48-week duration). Subjects received doses of 50 mg/m2 for 12 weeks (minimum dose 88 mg/d and maximum dose 128 mg/d), and then doses of 25 mg/m2 for the remaining 36 weeks (minimum dose 44 mg and maximum dose 60 mg). Subjects were randomized (1:1) using a stratified block design, to 2 doses every 2 weeks versus 4 doses every 4 weeks for both periods. The increase in doses and duration from the first to the second study was based on estimate of how much the dexamethasone dose could be increased while preserving a tolerable safety profile. Treatment regimens are summarized in Table 1.
Table 1.
Study protocols
| Dexamethasone dose | Minimum and maximum daily doses given | Cycles | Total treatment duration | |
|---|---|---|---|---|
| Study 1 Duration 32 weeks | 25 mg/m2 D1, 2, 3, 4 |
Minimum dose 40 mg Maximum dose 128 mg |
Eight 4 week cycles | 32 weeks |
| Study 2 Duration 36 weeks | 50 mg/m2 D1, 2, 3, 4 |
Minimum dose 88 mg Maximum dose 128 mg |
Three 4 week cycles | 12 weeks |
| 25 mg/m2 D1, 2, 3, 4 |
Minimum dose 44 mg Maximum dose 60 mg |
Six 4 week cycles | 24 weeks |
Efficacy outcomes
The primary outcome was pre-specified as the combination complete remission (CR) and partial remission rate (PR) at end of study (EOS). Baseline proteinuria was determined as the mean value obtained from three urine collections collected within eight weeks of commencing glucocorticoid therapy. In addition, long-term remission status was evaluated 24 months after EOS and again after a mean of 5.4 years after study initiation.
CR was defined as proteinuria <0.3 g/d. PR was defined as a 50% fall in proteinuria compared to baseline, proteinuria < 3.5 g/d, and a preserved estimated glomerular filtration rate (eGFR), specified as >60% of baseline value. Importantly, this definition of PR has been shown to correlate with improved long-term renal survival in adults with FSGS, compared to those who fail to experience a PR 12. Limited response (LR), was defined as a 50% fall in proteinuria compared to baseline proteinuria; our rationale for defining this exploratory outcome was that we believed it was worth noting substantial reductions in proteinuria that did not meet full criteria for a PR. Preserved eGFR during treatment was defined as an eGFR of at least a 75% of baseline value, it was calculated using the CKD-EPI calculation. Preserved eGFR at last evaluation was defined as eGFR of at least a 60% of baseline value using the CKD-EPI calculation. The definitions of LR and preserved eGFR for long-term follow up have not been validated and were intended for exploratory purposes only. All other outcomes were described as non-response (NR).
Only urine collections that had an adequate creatinine excretion were used to determine baseline proteinuria and proteinuria outcomes; adequate creatinine excretion was defined as a urine collection that had between 75% and 125% of the average creatinine excretion of all urine collections for that subject, evaluated retrospectively. During follow-up, proteinuria was assessed using either a 24 hour urine collection or a urine protein/creatinine ratio, multiplied by the average creatinine excretion in that subject to generate an estimated daily protein excretion.
Safety Outcomes
Adverse events associated with glucocorticoid therapy were evaluated in the following five domains. Following the definition of the US Food and Drug Administration, serious adverse events were defined those that involve death. are life-threatening, require hospitalization, result in disability or permanent damage, involve a congenital anomaly, or require intervention to prevent permanent damage or impairment.
Adrenal Suppression
An adrenocorticotrophic hormone (ACTH) stimulation test at baseline, follow-up visits and EOS were performed. Serum cortisol levels were determined 30 and 60 minutes after administration of ACTH 0.25 mcg intravenously. A plasma cortisol of >18μg/dL and an increase from baseline of >7μg/dL at either 30 or 60 minutes respectively were considered a normal response.
Cushingoid appearance
Photographs were taken of the patient’s face and body from both the front and the side. These pictures were taken in their underwear, shorts and tank tops. The photographs were assessed by the investigators masked as to the status of the photograph (baseline versus EOS) for steroid treatment changes in fat distribution.
Ophthalmologic complications
Subjects had a slit lamp evaluation done by an ophthalmologist for the presence of cataracts and elevated intraocular pressure at baseline, 10 weeks, 22 weeks and at end of study.
General health and psychological survey instruments
Four instruments, SF-36, Young mania scale (YMRS), Delirium Rating Scale-R-98 (DRS-R-98) and Beck depression inventory-II (BDI-II), were used to assess general health (quality of life) and psychiatric symptoms at baseline and EOS. Baseline evaluation included a psychiatric history to elicit past or present symptoms suggestive of depression or psychosis. All questionnaires were administered to all subjects by research personnel, with the exception of the BDI-II which was self-administered and completed at either NIH or at Mayo Clinic.
The SF-36 consists of an 8-scale profile of functional health and well-being; scores range from 0 to 100, with 0 representing self-reported poorest-health and 100 representing best health. The YMRS was used to measure patient’s mood state, it consists of an eight point global rating which includes eleven questions each with defined grades of severity13. A YMRS score of 0–1 indicates euthymic; 2–3 indicates hypomanic; 4–5 indicates manic and a score of 6–7 indicates severely manic. A BDI-II was used to assess for the presence and severity of depression; a score of 0–12 suggests non-depressed, a score of 13–19 suggests dysphoria and a score of 20–63 suggests dysphoric-depressed9. The DRS-R-98 was used to evaluate delirium symptom severity. The DRS-R-98 scores fall in three clusters: a score of 0–7 suggests no delirium; a score of 8–13 suggests subclinical/prodromal delirium, and score > 14: suggests delirium14.
Bone health
Bone density was assessed by dual photon densitometry (DEXA) of lumbar spine using anterior-posterior (AP) view and femur (total hip view) at baseline, at midpoint (16 or 24 weeks), and at end of study. Osteopenia was defined as bone mass score on DEXA scan of the lumbar (L) 1-L4 spine (AP view) reduced by ≥2 SD units and osteoporosis was defined as bone mass T score on baseline DEXA scan at L1-L4 spine reduced by ≥ 2.5 SD units. Magnetic resonance (MR) imaging of the hips were performed at baseline and at EOS to assess for avascular necrosis.
Statistical analysis
Data are presented as median with interquartile range. Statistical analysis included paired t test and ANOVA was performed using Prism software (GraphPad, San Diego, CA).
Results
Study population
14 subjects with FSGS enrolled in and completed study 1; 7 subjects with FSGS and 1 with MCD were enrolled in and completed study 2. In study 2, 4 subjects were randomized to every 2-week dosing and 4 subjects were randomized to every 4-week dosing. One subject in study 2 voluntarily withdrew from the study without receiving study medication and Is not included in this report. Summary baseline demographic and clinical characteristic are summarized in Supplemental Table 1. Of 17 cases for which data were available, 13 had reduced serum albumin levels (Supplemental Table 3). Kidney biopsy FSGS histologic variant data provided in Supplemental Table 4.While children were eligible for participation, no children were enrolled; the ages range from 24 to 71 years. The median age of patients in study 1 (46 years) was numerically greater than in study 2 (34 years). For all 14 subjects taken together, the median (inter-quartile range) for proteinuria was 66 (54, 82). The median proteinuria levels at baseline were similar, 9.7 (7.9, 12.3) g/d in study 1 and 9.6 (8.1, 12.2) g/d in study 2. The eGFR levels were similar at baseline. Baseline and follow-up eGFR are presented as CKD-EPI eGFR, as this equation more accurately assesses GFR values over 60 ml/min/1.73m2 15. There were no notable differences in race, ethnicity and gender between the two groups.
Proteinuria outcomes
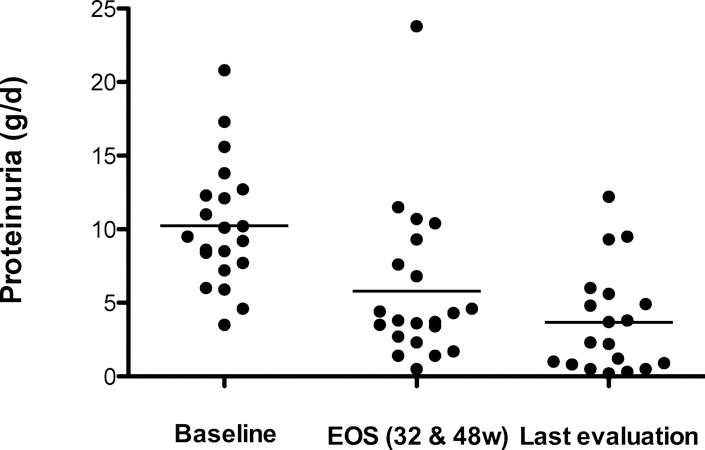
The primary study outcome was remission (CR plus PR) at EOS. PR occurred in 5 of 14 (36%) of the subjects in study 1 and 2 of 7 (29%) of subjects with FSGS in study 2; the overall study remission rate was 33%. The remaining subjects had LR or NR. When the subject with MCD is included (she has only an LR, somewhat surprisingly), the response rates for the subjects in study 2 was 25%). In the pooled study group, proteinuria decreased from 9.5 (7.7, 12.3) g/d at base to 4.3 (3.4, 7.6) g/d at EOS (P = <0.05) (Figure 1 and Supplemental Figure 2). There were no CR at EOS. The median eGFR at EOS was unchanged from baseline (66 mL/min/1.73m2).
Figure 1. Proteinuria at baseline, EOS, and Last evaluation.
Shown are the mean 24 hour protein excretion rates at baseline, at end-of-study and at last evaluation. Baseline and EOS outcomes for 21 subjects, 1 subject was withdrawn from study. At last follow-up evaluation 1 subject was lost to follow-up and 1 died from pancreatitis 8 years after completion of study.
Short-term follow up data, defined as the 24 months after EOS, was available for 18 subjects. Data were not obtained during this interval in 3 subjects, all of whom were classified as NR at EOS. Two of these 18 subjects began alternate immunosuppressive therapy soon after EOS; none of the changes in remission status were associated with increased ACE inhibitor or ARB therapy during the treatment period or the 24-month follow-up period. Remission status during this time was defined as the lowest proteinuria during any 6-month period. We used the average of at least two proteinuria measurements from 14 subjects, with the exception of single measurement in 4 subjects (three with persistent NR at EOS and during follow-up, and one with PR that persisted for 6 years after EOS). As shown in Supplemental Table 3, protein excretion fell during this time in 6 subjects, including 1 subject entering CR and 5 subjects entering PR. Thus, the remission status assessed at EOS or within 24 months after completing dexamethasone therapy included CR in 1 subject and PR in 9 subjects, for a combined response rate of 48%. Data are censored after additional immunosuppressive therapy began.
Long-term outcomes were assessed after a median of 5.4 years following EOS, with a range of 1 to 10 years. Additional immunosuppression was used in 5 subjects, as shown in Supplemental Table 3. At final evaluation, 2 subjects were in CR, 6 subjects in PR and 5 subjects had LR or NR, and 6 subjects had CKD stage 4 and 5, with 2 subjects lost to follow-up. Subject 1 transitioned from PR to CR late in his CKD course, with no additional immunosuppressive therapy and adequate eGFR. Subject 13 relapsed months after completing treatment and received additional immunosuppressive therapy before transitioning to CR from NR at EOS. All CKD stage 5 subjects have begun renal replacement therapy, including 3 undergoing kidney transplantation. Subject 7 died of pancreatic cancer; the other subjects were alive at last evaluation. No subject progressed to ESKD during treatment period or follow up.
Adverse events
Adverse events are summarized in Table 2. The most common side effects were mood symptoms followed by sleep disturbances (particularly insomnia). Mood symptoms occurred in 3 of 14 (21%) subjects in study 1 and 3 of 8 (38%) subjects in study 2. Mood symptoms included the following: irritability, aggressive behavior, short temper and there was one episode of road rage that was found not to be related to the study. One subject had an episode of a mild increase intraocular pressure that was treated with brimonidine eye drops. The episode of road rage, herpes simplex recurrent infection, and increased intraocular pressure, all occurred to the same subject in study 2. The subject remained in the study after evaluation by dermatology, ophthalmology and psychiatry. Herpes simplex was the only infection that occurred during the study. Acne was not observed.
Table 2.
Adverse events.
| Adverse event | Study 1 | Study 2 (Every 2 week dosing) | Study 2 (Every 4 week dosing) | Total study group |
|---|---|---|---|---|
| N | 14 | 4 | 4 | 22 |
| Headache | 1 (7%) | 1 (25%) | 0 | 2 (9%) |
| Sleep disturbances | 3 (21%) | 1 (25%) | 1 (25%) | 5 (23%) |
| Mood swings | 3 (21%) | 2 (50%) | 1 (25%) | 6 (27%) |
| Changes in appetite | 0 | 0 | 1 (25%) | 1 (4%) |
| Weight gain | 1 (7%) | 0 | 1 (25%) | 2 (9%) |
| Impaired glucose tolerance | 1 (7%) | 0 | 1 (25%) | 2 (9%) |
| Cushingoid Appearance | 1 (7%) | 0 | 1 (25%) | 2 (9%) |
| Muscle pains | 1 (7%) | 1 (25%) | 0 | 2 (9%) |
| Infections | 0 | 1 (25%) Herpes Zoster |
0 | 0 |
| Serious adverse event | 0 | 1 (25%) Tendon rupture |
2 (50%) Acute appendicitis Increased intraocular pressure |
3 (14%) |
| Subjects with any adverse event | 3 (21%) | 3 (75%) | 1 (25%) | 7 (32%) |
The number and percentage of subjects with common adverse events are shown. Mood swings included irritability, anxiety, aggressive behavior, short temper and one episode of road rage. No one develop overt diabetes
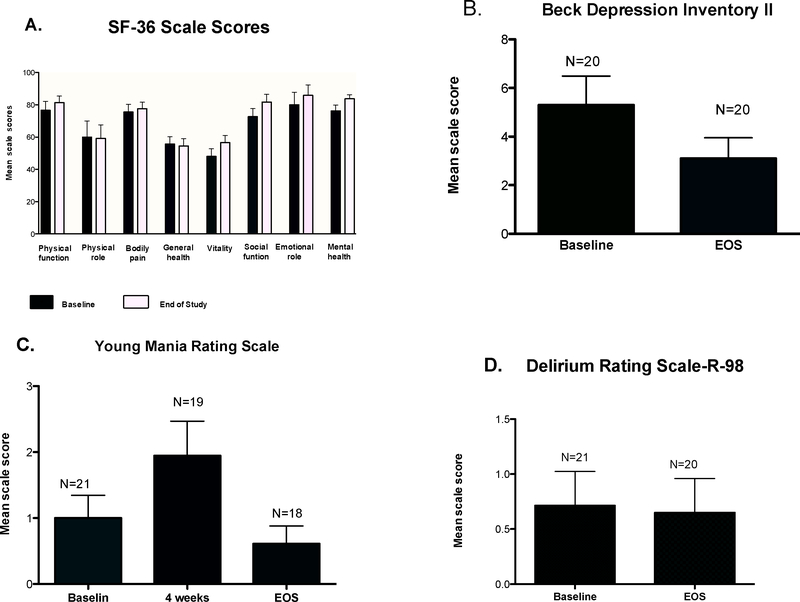
There were two serious adverse events, both of which were judged to be unrelated to study medication: acute onset of appendicitis requiring appendectomy and a biceps tendon rupture. The appendicitis occurred 3 months after the start of the study. The tendon rupture occurred in an avid exercise enthusiast 3 months after EOS. One subject developed increased fasting glucose, Cushingoid appearance and weight gain and was withdrawn after 24 weeks of treatment on the every-4 week arm in study 2; the subject had not developed overt diabetes at 5 year follow up. All subjects had a normal adrenal response to ACTH stimulation test at baseline and EOS. No subject developed cataract during the study. Overall perceptions of health were evaluated with an SF-36 survey, completed at baseline and EOS, in which higher scores denote higher function and the maximal score in each domain is 100 (Figure 2 and Supplemental table 5). Data are limited to 19 subjects who completed two questionnaires; 3 subjects are not represented, including the subject who withdrew. The only domains that reached or approached statistical significance at the EOS were improvements in mental health (P<0.01) and vitality/energy (P<0.06).
Figure 2. Results of Survey instruments for general health and psychological health.
Presented are the scores, as mean ± SD. A. Short Form Health Survey (SF-36), B. Beck Depression Inventory II (BDI-II), C. Young Mania Rating Scale, and D. Delirium rating scale at baseline, 4 weeks (for the mania scale) and end-of-study (EOS).
Psychological function
Mental health was specifically evaluated with three survey instruments (Figure 2). On the BDI-II, mean score was 5.76 ± 5.5 vs. 2.43 ± 2.6 at baseline and EOS respectively (P<0.05) Scores from 0 to 9 represent no depressive symptoms; six subjects exceeded these values (10, 12, 13 and 15, both obtained at baseline). The mean YMRS score was 1.0 ± 1.6 at baseline, increased to 1.95 ± 2.3 at 4 weeks and decreased to 0.6 ± 1.1 at end of study (P values by ANOVA, with comparison of baseline to 4 weeks and baseline to EOS were P = 0.10, P = 0.96 respectively). The DRS-R-98 showed similar results at baseline and EOS, all subjects having no signs or symptoms of delirium.
Bone health
Bone mass was assessed by DEXA scan at hip and spine at three points: baseline, study mid-point (16 weeks for study 1 and 24 weeks for study 2) and EOS. Three individuals had missing baseline DEXA scans and six individuals had missing EOS DEXA scans; therefore, we limited our analysis to 13 subjects with both baseline and EOS scans. No subject had osteopenia at any time point and the changes from baseline to either midpoint or EOS were not significant (Supplemental Figure 2). One subject in study 2 had a bone mass reduction of 1.3 SD units at L1-L4 site at EOS. All subjects underwent hip MRI at the start and at the end of the study; no subject developed avascular necrosis. One subject exceeded the weight limit for the scan; all others were studied at EOS.
Discussion
Idiopathic nephrotic syndrome, and primary FSGS in particular, represents a challenging entity for nephrologists, with most therapies having limited success and substantial toxicity. Initial treatment for adults involves daily prednisone administered for 2–4 months, at a dose of 1mg/kg per day 16. In the present report, we have evaluated an alternative regimen using pulse oral dexamethasone in a study group comprised of 21 FSGS subjects and 1 MCD subject. Our results suggest that intermittent oral dexamethasone induces PR rate of 33% at EOS. Induction of CR plus PR following dexamethasone therapy was not predictive of long term follow up outcomes. Therapy was generally well-tolerated. Our study has several strengths. First, this is the first study to explore oral pulse glucocorticoids for primary FSGS. Second, we determined short-term outcomes (up to 24 months after completing therapy) in nearly all subjects and as well as long term follow-up outcomes (median of 5.4 years) in most subjects. Third, we explored several potential adverse events, involving a range of testing.
Our study has several important limitations. First, this was an uncontrolled study, as have been most published studies using prednisone. Second, the sample size was small, and was divided into three different treatment regimens; it is not possible to conclude whether any single regimen had an advantage over any other. Third, three patients were lost to follow up at long-term evaluation.
The efficacy results demonstrated in the trials reported here should be placed in the context of the previously-published trials. Achieving a partial reduction in proteinuria and its maintenance have evidence of improving renal survival and slowing the rate of renal function decline 12. One retrospective study suggested that FSGS patients with nephrotic range proteinuria may benefit from a longer length course of therapy with steroids 17. Heavier proteinuria, particularly >10 g/d, is associated with a higher risk of progression. In the present study, 10 (48%) subjects presented with proteinuria in excess of 10 g/d. Four subjects attained PR rates at EOS and three sustained PR rates at 24 months follow-up after EOS; 1 of 4 reached CR with no additional immunosuppressive therapy; 2 of 4 remained PR at 7 year follow up and one progressed to end stage kidney disease.
Korbet suggested using four months of daily prednisone followed by a 4 month taper; this would provide an 80 kg adult with 13.5 g prednisone 18Ponticelli and colleagues suggested beginning with prednisone at 1 mg/kg for 16 weeks2 (assuming there is some response after 8 weeks, to justify continuing therapy), followed by a slow taper (possibly over 20 weeks); in an 80 kg subject, this would total 9.4 g of prednisone. In a clinical study, they administered doses of 1 mg/kg for 8 weeks, followed taper, with a mean duration of 24 weeks, and reported a combined response rate of 68% (52% CR, 16% PR) 19 Crook and colleagues reported a series of African Americans with FSGS, treated with daily prednisone for a mean of 12 months, with cumulative prednisone dose of 10.8 g 20. In this study, 60% achieved a urine PCR < 2g/g, suggesting CR or PR. Of note, only 63% had entry uPCR of >3 g/d, suggesting that many subjects were not nephrotic. Thus, two studies with daily prednisone have reported higher response rates compared to those in the present study. The decision to use daily prednisone versus pulse dexamethasone will require a balancing of the risks versus the benefits of each therapy.
How does the glucocorticoid dose employed here compare to comparable doses associated with conventional prednisone regimens recommended for FSGS in adults? The treatment protocol in study 1 equates to 1.6 g of dexamethasone in an 80 kg subject. (6.6 mg of prednisone is comparable to 1 mg of dexamethasone), which is equivalent in anti-inflammatory potency to 10.6 g of prednisone. For the same idealized subject in study 2, the treatment protocol involved administering 2.8 g of dexamethasone, which is equivalent to 18.4 g of prednisone. Thus, glucocorticoid doses administered in study 1, when adjusted for relative anti-inflammatory potency, are comparable to those used in conventional nephrology practice, while the doses used in study 2 are substantially higher. Our study, while not powered to show a difference between study 1 and study 2, shows no trend toward improved outcomes with the higher dose regimen. We conclude that the dose used in study 1 is may be helpful in designing for further trials of pulse oral dexamethasone.
We defined a limited response (LR) as an exploratory outcome to see whether a 50% fall in proteinuria without reaching a sub-nephrotic level proteinuria. Our rationale was to identify subject with substantial reduction in proteinuria who do not achieve a PR. The follow up duration was too short to determine whether this will prove to be a useful approach for future studies.
The mechanisms by which glucocorticoids induce remission in FSGS are unclear. Glucocorticoids were introduced into therapy for the podocytopathies largely based on empiric evidence of success, particularly for MCD. The responses to this therapy have been taken as evidence of support for an immune role in pathogenesis and there is some evidence for this 4. Also, dexamethasone has direct effects to podocyte phenotype in vitro, including increasing nephrin mRNA expression 21.
There are relatively few predictors of a favorable response to glucocorticoid therapy. One study reported the degree of tubulointerstitial changes and mean glomerular diameter were two significant predictors found to correlate with poor response to glucocorticoid therapy thus poor renal outcomes 22. Alexopoulos and colleagues studied the factors influencing the course and the response to treatment in 33 adults with primary FSGS, of whom 28% had a CR, 54% PR and 18% did not respond, showed that only age and plasma creatinine at biopsy had an independent predictive value for renal survival in nephrotic patients and only the severity of interstitial fibrosis predicted response to treatment 23.
In general. the regimens we report here were well tolerated, with relatively few adverse events and mood symptoms being the most common complaint. Seven of the twenty-two subjects had at least one adverse event, two of which were considered serious but not related to therapy. Our results demonstrate that high dose of dexamethasone is well tolerated in adults with FSGS. Prednisone is the most widely accepted glucocorticoid used as initial therapy for adult FSGS, with reported 41% of subjects reporting serious adverse events: diabetes mellitus, hypertension exacerbation, cellulitis, and severe myopathy24. High dose dexamethasone has been used as therapy for multiple myeloma, both accompanied with and without additional chemotherapeutic agents. The most common side effects are Cushingoid features and/or insomnia. Other effects such as: oral candidiasis, herpes lesions, aggravation of diabetes, peptic ulcer, hiccoughs or quadriceps weakness have been noted 9.
In conclusion, these two open-label trials involving 22 subjects suggest that oral pulse may have modest efficacy in adults with idiopathic nephrotic syndrome (mostly primary FSGS) and is generally well-tolerated with an acceptable safety profile. The study size was too small to reach definitive conclusions about efficacy but the preliminary data are encouraging in this regard. This study justifies further studies on the use of pulse oral dexamethasone for idiopathic nephrotic syndrome, which could offer a more tolerable approach to a prolonged course of daily glucocorticoid therapy. We propose that a randomized trial should compare daily or alternate day prednisone versus intermittent oral dexamethasone
Supplementary Material
Acknowledgement
This work was supported by the NIDDK Intramural Research Program under ZO1-DK04312. M. We gratefully acknowledge the assistance of the NIH Clinical Center Pharmacy with drug assignment, the ophthalmology and radiology physicians who were involved in patient care, Dr. Meryl Waldman for critical review of the manuscript.
Footnotes
Competing interests. The authors have no competing interests to declare.
References
- 1.Deegens JK, Steenbergen EJ, Wetzels JF. Review on diagnosis and treatment of focal segmental glomerulosclerosis. Neth JMed. 2008;66(1): 3–12. [PubMed] [Google Scholar]
- 2.Ponticelli C, Villa M, Banfi G, et al. Can prolonged treatment improve the prognosis in adults with focal segmental glomerulosclerosis? American Journal of Kidney Diseases. 1999;34(4): 618–625. [DOI] [PubMed] [Google Scholar]
- 3.Laurin LP, Gasim AM, Poulton CJ, et al. Treatment with Glucocorticoids or Calcineurin Inhibitors in Primary FSGS. Clinical journal of the American Society of Nephrology : CJASN. 2016;11(3): 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L, Zhang T, Diao W, et al. Role of Myeloid-Derived Suppressor Cells in Glucocorticoid-Mediated Amelioration of FSGS. J Am Soc Nephrol. 2015;26(9): 2183–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boros P, Ochando J, Zeher M. Myeloid derived suppressor cells and autoimmunity. Hum Immunol. 2016;77(8): 631–636. [DOI] [PubMed] [Google Scholar]
- 6.Guess A, Agrawal S, Wei CC, Ransom RF, Benndorf R, Smoyer WE. Dose- and time-dependent glucocorticoid receptor signaling in podocytes. American journal of physiology. Renal physiology. 2010;299(4): F845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW. Direct effects of dexamethasone on human podocytes. Kidney Int. 2006;70(6): 1038–1045. [DOI] [PubMed] [Google Scholar]
- 8.Andersen JC. Response of resistant idiopathic thrombocytopenic purpura to pulsed high-dose dexamethasone therapy. N Engl J Med. 1994;330(22): 1560–1564. [DOI] [PubMed] [Google Scholar]
- 9.Alexandria R, Dimopoulos MA, Delasalle K, B. B. Primary dexamethasone treatment of multiple myeloma. Blood. 1992;80: 887–890. [PubMed] [Google Scholar]
- 10.van Schaik IN, Eftimov F, van Doorn PA, et al. Pulsed high-dose dexamethasone versus standard prednisolone treatment for chronic inflammatory demyelinating polyradiculoneuropathy (PREDICT study): a double-blind, randomised, controlled trial. The Lancet Neurology. 2010;9(3): 245–253. [DOI] [PubMed] [Google Scholar]
- 11.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. American Journal of Kidney Diseases. 2004;43(2): 368–382. [DOI] [PubMed] [Google Scholar]
- 12.Troyanov Sp, Wall CA, Miller JA, Scholey JW, Cattran DC, Group ftTGR. Focal and Segmental Glomerulosclerosis: Definition and Relevance of a Partial Remission. Journal of the American Society of Nephrology. 2005;16(4): 1061–1068. [DOI] [PubMed] [Google Scholar]
- 13.Young RC, Biggs JT, Zielger VE, Meyer DA. A Rating Scale for Mania: Reliability, Validity and Sensitivity. British Journal of Psychiatry. 1978;133: 429–435. [DOI] [PubMed] [Google Scholar]
- 14.Franco JG, Trzepacz PT, Mejia MA, Ochoa SB. Factor Analysis of The Colombian Translation of The Delirium Rating Scale (DRS), Revised-98. Psychosomatics. 2009;50(3): 255–262. [DOI] [PubMed] [Google Scholar]
- 15.Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56(3): 486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korbet SM. Angiotensin antagonists and steroids in the treatment of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23(2): 229–233. [DOI] [PubMed] [Google Scholar]
- 17.Rydel JJ, Korbet SM, Borok RZ, Schwartz MM. Focal segmental glomerular sclerosis in adults: Presentation, course, and response to treatment. American Journal of Kidney Diseases. 1995;25(4): 534–542. [DOI] [PubMed] [Google Scholar]
- 18.Korbet SM. Treatment of primary FSGS in adults. JAm Soc Nephrol. 2012;23(11): 17691776. [DOI] [PubMed] [Google Scholar]
- 19.Ponticelli C, Villa M, Banfi G, et al. Can prolonged treatment improve the prognosis in adults with focal segmental glomerulosclerosis? Am J Kidney Dis. 1999;34(4): 618–625. [DOI] [PubMed] [Google Scholar]
- 20.Crook ED, Habeeb D, Gowdy O, Nimmagadda S, Salem M. Effects of steroids in focal segmental glomerulosclerosis in a predominantly African-American population. Am J MedSci. 2005;330(1): 19–24. [DOI] [PubMed] [Google Scholar]
- 21.Takano Y, Yamauchi K, Hiramatsu N, et al. Recovery and maintenance of nephrin expression in cultured podocytes and identification of HGF as a repressor of nephrin. American Journal Physiology- Renal Physiology. 2007;292(5): 1573–1582. [DOI] [PubMed] [Google Scholar]
- 22.Shiiki H, Nishino T, Uyama H Clinical and morphological predictors of renal outcome in adult patients with focal and segmental glomerulosclerosis (FSGS). Clin Nephrol. 1996;46: 362. [PubMed] [Google Scholar]
- 23.Alexopoulos E, Stangou M, Papagianni A, Pantzaki A, Papadimitriou M Factors influencing the course and the response to treatment in primary focal segmental glomerulosclerosis. Nephrology Dialysis Transplantation. 2000;15(9): 1348–1356. [DOI] [PubMed] [Google Scholar]
- 24.Uzu T, Harada T, Sakaguchi M, et al. Glucocorticoid-Induced Diabetes Mellitus: Prevalence and Risk Factors in Primary Renal Diseases. Nephron Clinical Practice. 2007;105(2): c54–c57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.