Abstract
Considerable progress has been made in understanding the Fas pathway at the molecular and cellular levels, but fundamental questions about the overall biological role of the Fas pathway remain unresolved. A major question is why lymphoproliferation caused by the lpr mutation of Fas and gld mutation of FasL ligand (FasL) is dominated by CD4— and CD8— double negative α T cells (DN T cells) that are otherwise rare components of the peripheral T cell repertoire. A second unresolved question is why inactivation of the Fas pathway prevents organ specific autoimmunity (including as type 1 diabetes and multiple sclerosis) while causing systemic lymphoproliferation? Understanding the mechanisms of these processes, could uncover important aspects of the biological role of the Fas pathway and could have significant therapeutic implications. For example, revealing the basis of how inactivation of the Fas pathway prevents organ specific autoimmunity could lead to new immunotherapeutic strategies to promote self tolerance without causing immunosuppression, as the Fas pathway is not essential for T cell activation. Here we will discuss recent and new findings from my laboratory that address these questions including the nature of DN T cells and role and potential role of the Fas pathway in sequestration of DN T cells within the gut epithelium.
Keywords: Double negative T cells; intraepithelial lymphocytes; lymphoproliferation, apoptosis, immunotherapy; organ specific autoimmunity; type 1 diabetes; Fas; Fas ligand
Introduction
Significant advances have been made in understanding the Fas pathway, the prototypical extrinsic cell death pathway, at the molecular and cellular levels. The expression pattern of Fas and its physiologic ligand (FasL) and signaling cascade by which Fas/FasL interaction induces cell death are well characterized (1). Both Fas and FasL are expressed as homotrimers generated by random preassociation of individual molecules. Fas is constitutively expressed by different cell types, but expression of FasL is tightly regulated and limited to immune privilege sites and activated immune cells. On lymphocytes, FasL is induced after repeated TCR stimulation to trigger apoptosis of effector T cells at the end of the immune response. Fas engagement by FasL induces recruitment of the Fas-associated death domain (FADD) molecule to the intracellular death domain (DD) of Fas, followed by generation of the death inducing signaling complex (DISC) and activation of effector caspases that cleave essential cellular proteins including DNA, resulting in cell apoptosis (2, 3).
At the cellular level, death of TCR activated hybridomas and primary T cells upon Fas/FasL interactions in vitro led to establishment of the paradigm that Fas-mediated activation-induced cell death (AICD) is a major negative regulator of T cell clonal expansion (4–6). The discovery that T cell lymphoproliferation in lpr and gld mice is due to point mutations in Fas and FasL, respectively, confirmed the physiologic role of the Fas pathway in regulating T cell homeostasis (7). Nevertheless, the biological context in which the Fas pathway regulates T cell homeostasis in vivo remains unclear. The basis of the unusual composition of T cells that cause lymphoproliferation in mice bearing homozygous lpr or gld mutations is poorly understood. The lymphoproliferation is predominantly caused by a subset of double negative αβ T cells (hereafter referred to as DN T cells) that lack both CD4 and CD8 coreceptors and that is a rare component of the normal peripheral T cell repertoire. Thymic negative selection proceeds normally in mutant mice ruling out defective T cell development as a major cause of lymphoproliferation (8–11). Furthermore, whereas some early studies indicated a delay or defect in deletion of Fas-deficient T cells in response to stimulation by foreign antigens (12, 13), recent studies reported minor or no disruption of effector T cell clearance in mice with impaired Fas pathway (14–17). Indeed, it is becoming increasingly clear that the proapoptotic molecule Bim (BCL-2 interacting mediator of cell death) is the major regulator of foreign antigen-activated T cell apoptosis in vivo (18). However, deletion of T cells specific for persistent pathogens is reported to be impaired in mutant mice [reviewed in ref. (18)]. Moreover, development of lymphoproliferation is not interrupted in mutant mice kept in a pathogen-free environment, suggesting a primary role for the Fas pathway in controlling homeostasis of chronically activated and autoimmune T cells (19). Why gld and lpr lymphoproliferation, unlike lymphoproliferation caused by, for example lack of CTLA-4 or scurfy mutation of Foxp3 (20, 21), is dominated by DN T cells is poorly understood, however. Understanding the basis of DN T cell lymphoproliferation in lpr and gld mice could provide important insights into the biologic function of the Fas pathway and into the cell type(s) that are the physiological targets of Fas-mediated apoptosis in the steady state.
A second important but poorly understood phenotype associated with the Fas pathway is resistance of mice bearing lpr and gld mutations to spontaneous and antigen-induced organ-specific autoimmune diseases (22, 23) including type 1 diabetes (T1D) and multiple sclerosis even though they develop systemic T cell lymphoproliferation and lupus-like autoimmunity (24). Understanding how inactivation of the Fas pathway prevents organ specific autoimmunity could potentially lead to development of new immunotherapeutic strategies that promote tolerance without causing immunosuppression as the Fas pathway is not essential for T cell activation. Here we discuss current view of the origin of DN T cells in the field and the impact of new and recently published data from our group on this view and in understanding of the precise biologic role of the Fas pathway.
Current view of the origin of double negative T cells that cause lpr and gld lymphoproliferation
CD4 and CD8 T cells are the two main subsets of peripheral T cells in wild type mice. Therefore, it has been puzzling that inactivation of the Fas pathway leads to massive accumulation of TCRαβ DN T cells that lack both CD4 and CD8 coreceptors. The DN T cells have an activated phenotype and express B220, the isoform of CD45 that is normally expressed by B cells. In addition, they are anergic and poor responders to TCR stimulation. Accumulation of DN T cells is dependent on age and genetic-background with the frequency of DN T cells increasing from less than 5% of peripheral T cells in young adult mutant mice (e.g., MRL, C3H/Hej, Balb/c, and NOD-Lt strains) to more than 80% by 16 weeks of age. The increase in DN T cells is not at the expense of SP T cells as there is a global increase in different T cell subsets in mutant mice, but it is particularly dramatic among DN T cells. It is currently widely believed, but with no direct in vivo evidence, that the DN T cells are derived from previously activated single positive CD8 or CD4 T cells that failed to die after re-stimulation through their TCR. Hence DN T cells in lpr and gld mice are generally described as an abnormal or unusual T cell population. However, this view is conflicting with the absence of DN T cell accumulation in mice that develop lymphoproliferation due to deficiency of other proapoptotic molecules. For example, Bim-deficient mice develop T cell lymphoproliferation because of impaired apoptosis but with no significant DN T cell accumulation (25). Thus, resistance of activated CD8 or CD4 T cells to apoptosis per se does not lead to generation of DN T cells. In addition, lpr and gld DN T cells possess suppressor function, suggesting that they are qualitatively different from conventional T cells (26). Thus, the origin of lpr and gld DN T cells remains controversial.
Evidence that Fas-mediated apoptosis restricts tissue localization of DN T cells to the gut epithelium
For several years, we have been using mice carrying the gld mutation to investigate the origin of DN T cells. Analysis of the transcriptional profile of DN T cells, as well as their homeostatic proliferation and apoptosis in the steady state relative to SP T cells, as detailed below, led us to propose that the Fas pathway plays a critical role in compartmentalizing DN T cells into the gut epithelium:
The transcriptional profile of DN T cells provides important clues about their origin.
In the lack of direct support for the notion that gld and lpr DN T cells are SP T cells that have lost their coreceptors, we hypothesized that the gld lymphoproliferation may be due to primary expansion of DN T cells that are normally deleted by Fas-mediated apoptosis. To begin to test this hypothesis, we used a DNA microarray to resolve the gene profile of DN T cells and compared it to that of CD4 and CD8 T cells (27). We combined CD4 and CD8 T cells in one group (hereafter referred to as a single positive (SP) T cells) so that only genes that are differentially expressed by DN T cells relative to both CD4 and CD8 T cell subsets are detected in the analysis. We predicted that if DN T cells are activated SP T cells that merely lost their coreceptors, their gene profile should be more or less similar to that of SP T cells. On the other hand, if DN T cells represent a unique cell type, their gene profile could be substantially different from that of SP T cells. SP and DN T cells were isolated from lymph nodes of the same donor to account for any effect of the in vivo environment on gene expression. In addition, validity of the approach was confirmed by detection of genes encoding proteins known to be expressed by the SP but not DN T cell subset such as CD4 and CD8, among the genes that are downregulated in the DN T cell subset. Results of the analysis showed that DN T cells differentially express molecules that are not expressed by conventional T cells (27). These include syndecan-1 (sdc1), a primary surface marker for intestinal epithelia (28) and epithelial cell adhesion molecule (Ep-CAM; previously called Tacstd1), a regulator of homophilic interactions between epithelia (29). Desmoplakin (DSP) (30, 31); catenin α (32), and galectin 1 [(Lgals1) (33)] that regulate junctional interactions are also differentially expressed by DN T cells. The differentially expressed genes are confirmed by flow cytometry for sdc-1 and by PCR for selected genes. Differential expression of these genes by gld DN T cells relative to SP T cells isolated from the same lymph nodes indicates that there is no global defect in gld T cells. Thus, DN T cells that cause gld lymphoproliferation have an unconventional transcription profile characterized by expression of molecules normally expressed by intestinal epithelia (27).
DN T cells naturally residing in the gut epithelium of wt mice are phenotypically similar to gld DN T cells found in the periphery of mutant mice.
DN T cells constitute a major component of the normal intraepithelial lymphocyte (IEL) repertoire. Expression of sdc1 and other tight junction molecules by peripheral gld DN T cells led us to determine whether peripheral gld DN T cells are related to DN T cells in the gut epithelium. Therefore, we analyzed the gut epithelium for DN T cells bearing gld DN T cell phenotype (i.e., sdc1+, TCRαβ +, B220+, CD4–, and CD8–, CD2– CD5–, CD24A+/−, Thy1+/−) with particular emphasis on sdc1 expression (27). Indeed, there are sdc1-expressing DN T cells in the epithelium of large intestine and to a lesser degree of the small intestine of 6-week-old wt mice. The frequency of sdc1+ DN T cells increases with age in both the small and large intestine until they become the major αβ T cell subpopulation in the large intestine of 21- to 24-week-old wt mice. The spectrum of TCR-Vβ expression by DN T cells is also similar in the periphery and gut epithelium. Similarities of DN in the periphery and gut epithelium of gld mice support the hypothesis that gld DN T cell lymphoproliferation may be due to impaired compartmentalization of intraepithelial DN T cells.
Intense homeostatic proliferation of intraepithelial DN but not SP αβ T cells.
Interestingly, DN T cells in the gut epithelium are dividing rapidly with approximately 50% in the small and large intestines of wt mice incorporating BrdU within a 24-h period compared to less than 5% of their SP T cell counterparts (27). Similar results are obtained after 8 days of continuous BrdU administration. In contrast, DN T cells in the periphery of wt mice are cycling slowly at a rate comparable to that of SP T cells. The gld mutation does not affect the proliferation rate of DN T cells in the gut epithelium, as levels of BrdU uptake by DN T cells in the gut of wt and gld mice were comparable. DN T cells in the periphery of gld mice are not actively proliferating but are cycling at a rate that is slightly but not significantly higher than that of SP T cells (27, 34). Thus, DN T cells, unlike SP T cells, are proliferating at an intense rate in the gut epithelium via a mechanism that is independent of the Fas pathway.
DN T cells are selectively eliminated from the periphery by Fas-mediated apoptosis in the steady state.
High proliferation rates are usually balanced with high rates of apoptosis to maintain normal homeostasis. In accordance with this notion, DN T cells are undergoing apoptosis at higher rate than SP T cells both in the gut epithelium and secondary lymphoid organs (27). Apoptosis of DN T cells in the gut epithelium is Fas-independent, however, as it is not affected by the gld mutation, consistent with previous findings that the Fas pathway plays no major role in apoptosis of IEL (35). Consequently, there is minimal or no impact of the gld mutation on the frequency of DN T cells in the gut epithelium. DN T cells are also dying at a significantly higher rate than SP T cells in the periphery but by a Fas-dependent mechanism. The gld mutation significantly reduces apoptosis of DN T cells to a level similar to that of SP T cells. Blockade of the Fas pathway by using FasL-neutralizing (MFL4) antibody also significantly inhibits DN T cell apoptosis in the periphery of wt mice, confirming that it is mediated by the Fas pathway (27). Furthermore, DN T cells that accumulate in the periphery of gld mice are primed for Fas-mediated apoptosis and die immediately when exposed to recombinant FasL in the absence of concomitant TCR activation (27). These results indicate that Fas-mediated apoptosis plays a critical role in selectively clearing DN T cells from the periphery in the steady state.
New model: Fas-mediated apoptosis enforces gut-compartmentalization of DN T cells
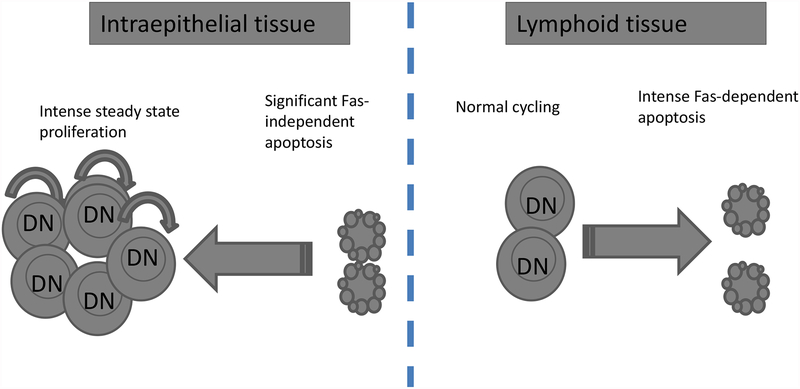
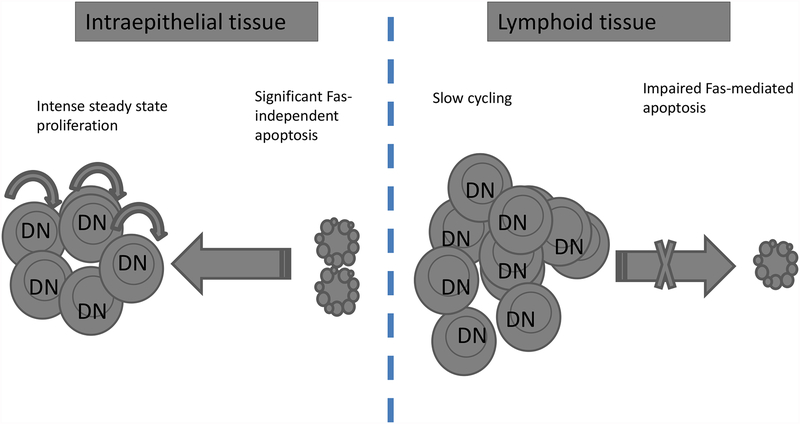
We have shown that DN T cells are uniquely proliferating and dying at high rates but in a tissue-specific manner (27). The high proliferation occurs in the gut epithelium whereas the high apoptosis, which is Fas-mediated, occurs in the secondary lymphoid tissues. DN T cells also die at a high rate in the gut epithelium but by a Fas-independent mechanism. Furthermore, apoptosis of DN T cells in the gut epithelium is balanced with high proliferation and hence DN T cells are able to maintain a significant niche in the gut epithelium. By contrast, DN T cells in the periphery are not dividing at a high rate and are constantly removed by Fas-mediated apoptosis resulting in their paucity in the periphery. We therefore propose that Fas-mediated apoptosis plays a critical role in controlling the tissue distribution of DN T cells in the steady state (Fig. 1). In support of this model, the number and frequency of DN T cells progressively increase but remain confined to the gut epithelium of wt mice. When the Fas pathway is impaired, however, DN T cells progressively accumulate in the periphery leading to DN T cell lymphoproliferation (Fig. 2).
Fig. 1. Scheme of DN αβ T cell proliferation and apoptosis dynamics in the steady state.
DN T cells, unlike SP T cells, proliferate at very high rates in the gut epithelium but not in the periphery by a Fas-independent mechanism. Conversely, DN T cells die at a very high rate both in the gut epithelium and secondary lymphoid tissues. Their death in the gut epithelium is Fas-independent whereas their death in the periphery is Fas-dependent. In the steady state, DN T cells maintain a significant niche in the gut epithelium because of their replenishment through high proliferation but are a minor component of the secondary lymphoid organs as e result of the extremely high apoptosis in the absence of correspondingly high proliferation.
Fig. 2. Impairment of Fas-mediated apoptosis dysregulates tissue compartmentalization of DN αβ T cells.
In the absence of functional Fas pathway in lpr and gld mice, DN T cells slowly accumulate in the secondary lymphoid organs reaching more than 80% of peripheral T cells by the age of 16 weeks in mice with susceptible genetic backgrounds. Homeostasis of DN T cells in the gut epithelium remains steady, however, as both proliferation and apoptosis in the gut epithelium are Fas-independent.
How does inactivation of the Fas pathway restore organ-specific tolerance in autoimmune diabetes-prone NOD mice and its clinical implications?
Many immunotherapeutic options have limited efficacy for treating autoimmune diseases because of their association with nonspecific cytotoxicity and immune suppression; consequently, numerous efforts are being made to identify safer drug targets (36). Our group has been interested in exploring the therapeutic potentials of targeting the Fas pathway because this pathway primarily regulates T cell apoptosis (7), and there is no evidence that inactivation of the Fas pathway compromises host defense against most infections (14, 27). Yet, the lpr and gld mutations potently protect against organ-specific autoimmune diseases, including T1D and multiple sclerosis in mouse models (23, 37). Therefore, development of a new class of therapeutic agents that target this death pathway has the appeal of promoting organ-specific tolerance with minimal or no negative impact on the host defense. In order to achieve this goal, two barriers need to be overcome. The first barrier is the association of homozygous lpr and gld mutations with an age-dependent lymphoproliferation (38). The second barrier is the poor understanding of the underlying mechanism. Here we summarize our efforts towards solving both problems using spontaneous T1D in the widely used the NOD mouse model.
Fas-mediated protection from organ-specific autoimmunity can be achieved without causing immunopathology.
In a step towards realizing the therapeutic potential of the Fas pathway, we have confirmed that protection from T1D and T cell lymphoproliferation caused by gld mutation are dissociable from one another. This was first demonstrated genetically in NOD-gld/+ mice by exploiting the gene dosage effect of the mutation (24, 39). Similar to other members of TNF family, FasL functions as a homotrimer formed by random preassociation of monomers (40, 41). Accordingly, only one-eighth of surface FasL trimers in NOD-gld/+ mice is composed of wild type FasL molecules while the remaining trimers are dysfunctional owing to incorporation of at least one mutated molecule. Interestingly, the NOD-gld/+ mice retained the protective effect of gld mutation but without developing lymphoproliferation (24, 39). These findings rule out DN T cell accumulation as an explanation for how mutant mice are protected from T1D. Furthermore, they show that there is an apparently wide operational window to down-modulate FasL activity to prevent its pathogenic effect without impairing immune homeostasis. More importantly, the dissociation of protection from lymphoproliferation can be achieved pharmacologically. Brief treatment of NOD-wt mice by FasL neutralizing mAb offers indefinite protection from T1D without perturbing immune homeostasis (24). A previous study has also described efficacy of another FasL neutralizing mAb in preventing T1D (42).
Insights into how inhibition of FasL prevents T1D.
It was initially thought that resistance of mutant NOD mice to T1D was due to protection of pancreatic islets from Fas-mediated apoptosis (22). But it soon became apparent that Fas-mediated apoptosis is a dispensable mechanism of beta cell death, as Fas-sufficient and Fas-deficient beta cells are destroyed at similar rates by diabetogenic T cells in vivo (43). In the absence of an alternative plausible mechanistic explanation, protection from organ-specific autoimmune disease by genetic inactivation of the Fas pathway the sense that this is an abnormal phenomenon has pervaded. This perception is reinforced by the massive accumulation of double negative (DN) T cells in NOD mice bearing homozygous gld or lpr mutations. However, as described above, we have found that DN T cell lymphoproliferation and protection from T1D are independent phenomena that can be dissociated from one another in a dose-dependent manner. Currently, we are utilizing NOD-gld/+ and NOD-wt mice and FasL neutralizing mAb to investigate the underlying mechanism of protection and assess it pharmacologically. Our preliminary data indicate that in the absence of Fas-mediated apoptosis, regulatory cells are able to accumulate in pancreatic islets thereby protecting them from diabetogenic T cells (unpublished data).
Concluding remarks
Why loss-of-function mutations of Fas (lpr) and FasL (gld) cause DN T cell lymphoproliferation and protect from organ-specific autoimmunity are importantly but poorly understood questions. Our recent data challenge the long held view that double negative T cells that cause lymphoproliferation are peripheral SP T cells that fail to undergo Fas-mediated apoptosis. Our data indicate that a steady sate role for Fas-mediated apoptosis in removing DN T cells from the periphery and suggest a possible relationship between these DN T cells and intraepithelial DN T cells. Furthermore, we have shown that FasL can be targeted pharmacologically to prevent T1D without causing lymphoproliferation. Understanding how inactivation of the Fas pathway prevents organ-specific autoimmune diseases (a major focus of our group) may lead to development of new therapeutic strategies for T1D and perhaps other autoimmune diseases that are prevented by gld and lpr mutations in animal models such multiple sclerosis. Success of such strategies could mitigate the problem of chronic immunosuppression that has prevented wide application of immunotherapy to treat autoimmune diseases.
Acknowledgments
We thank current and previous members of the lab for their insights roles in these efforts. Our work on T1D is supported by a regular research award from JDRF and the studies on DN T cells were supported by the DK069279 and DK066039 R21 grants.
References
- 1.Siegel RM, Muppidi J, Roberts M, Porter M, and Wu Z. 2003. Death receptor signaling and autoimmunity. Immunol Res 27:499–512. [DOI] [PubMed] [Google Scholar]
- 2.Green DR, Droin N, and Pinkoski M. 2003. Activation-induced cell death in T cells. Immunol Rev 193:70–81. [DOI] [PubMed] [Google Scholar]
- 3.Krueger A, Fas SC, Baumann S, and Krammer PH. 2003. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunol Rev 193:58–69. [DOI] [PubMed] [Google Scholar]
- 4.Brunner T, Mog R, LaFace D, Yoo N, Mahboubl A, Echeverri F, Martin S, Force W, Lynch D, Ware C, and Green D. 1995. Cell autonomous Fas (CD95) / Fas ligand interaction mediates activation induced apoptosis in T cell hybridomas. Nature 373:441–444. [DOI] [PubMed] [Google Scholar]
- 5.Dhein J, Walczack H, Baumler C, Debatin K-M, and Krammer P. 1995. Autocrine T cell suicide mediated by APO-1 (Fas/ CD95). Nature 373:438–441. [DOI] [PubMed] [Google Scholar]
- 6.Ju S, Panks D, Cul H, Ettingor R, Elkhatib M, Sherr D, Stanger B, and Marshak-Rothstein A. 1995. Fas (CD95) / FasL interactions required for prgrammed cell death after T cell activation. Nature 373:444–448. [DOI] [PubMed] [Google Scholar]
- 7.Nagata S, and Suda T. 1995. Fas and Fas ligand:lpr and gld mutations. Immunol. Today 16:39–43. [DOI] [PubMed] [Google Scholar]
- 8.Kotzin BL, Babcock SK, and Herron LR. 1988. Deletion of potentially self-reactive T cell receptor specificities in L3T4-, Lyt-2- T cells of lpr mice. J Exp Med 168:2221–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer PA, Balderas RS, McEvilly RJ, Bobardt M, and Theofilopoulos AN. 1989. Tolerance-related V beta clonal deletions in normal CD4–8-, TCR-alpha/beta + and abnormal lpr and gld cell populations. J Exp Med 170:1869–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mountz JD, Smith TM, and Toth KS. 1990. Altered expression of self-reactive T cell receptor V beta regions in autoimmune mice. J Immunol 144:2159–2166. [PubMed] [Google Scholar]
- 11.Zhou T, Bluethmann H, Zhang J, Edwards CK 3rd, and Mountz JD. 1992. Defective maintenance of T cell tolerance to a superantigen in MRL-lpr/lpr mice. J Exp Med 176:1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillette-Ferguson I, and Sidman CL. 1994. A specific intercellular pathway of apoptotic cell death is defective in the mature peripheral T cells of autoimmune lpr and gld mice. Eur J Immunol 24:1181–1185. [DOI] [PubMed] [Google Scholar]
- 13.Mogil RJ, Radvanyi L, Gonzalez-Quintial R, Miller R, Mills G, Theofilopoulos AN, and Green DR. 1995. Fas (CD95) participates in peripheral T cell deletion and associated apoptosis in vivo. Int Immunol 7:1451–1458. [DOI] [PubMed] [Google Scholar]
- 14.Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, and Marrack P. 2002. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity 16:759–767. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalo JA, Tarazona R, Schuurman HJ, Uytdehaag F, Wick G, Martinez C, and Kroemer G. 1994. A single injection of Staphylococcus aureus enterotoxin B reduces autoimmunity in MRL/lpr mice. Clin Immunol Immunopathol 71:176–182. [DOI] [PubMed] [Google Scholar]
- 16.Lohman BL, Razvi ES, and Welsh RM. 1996. T-lymphocyte downregulation after acute viral infection is not dependent on CD95 (Fas) receptor-ligand interactions. J Virol 70:8199–8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miethke T, Vabulas R, Bittlingmaier R, Heeg K, and Wagner H. 1996. Mechanisms of peripheral T cell deletion: anergized T cells are Fas resistant but undergo proliferation-associated apoptosis. Eur J Immunol 26:1459–1467. [DOI] [PubMed] [Google Scholar]
- 18.Bouillet P, and O’Reilly LA. 2009. CD95, BIM and T cell homeostasis. Nat Rev Immunol 9:514–519. [DOI] [PubMed] [Google Scholar]
- 19.Maldonado MA, Kakkanaiah V, MacDonald GC, Chen F, Reap EA, Balish E, Farkas WR, Jennette JC, Madaio MP, Kotzin BL, Cohen PL, and Eisenberg RA. 1999. The role of environmental antigens in the spontaneous development of autoimmunity in MRL-lpr mice. J Immunol 162:6322–6330. [PubMed] [Google Scholar]
- 20.Chambers CA, Sullivan TJ, and Allison JP. 1997. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 7:885–895. [DOI] [PubMed] [Google Scholar]
- 21.Godfrey VL, Wilkinson JE, Rinchik EM, and Russell LB. 1991. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci U S A 88:5528–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chervonsky AV, Wang Y, Wong FS, Visintin I, Flavell RA, Janeway CA Jr., and Matis LA. 1997. The role of Fas in autoimmune diabetes. Cell 89:17–24. [DOI] [PubMed] [Google Scholar]
- 23.Waldner H, Sobel RA, Howard E, and Kuchroo VK. 1997. Fas- and FasL-deficient mice are resistant to induction of autoimmune encephalomyelitis. J Immunol 159:3100–3103. [PubMed] [Google Scholar]
- 24.Mohamood AS, Guler ML, Xiao Z, Zheng D, Hess A, Wang Y, Yagita H, Schneck JP, and Hamad AR. 2007. Protection from autoimmune diabetes and T-cell lymphoproliferation induced by FasL mutation are differentially regulated and can be uncoupled pharmacologically. Am J Pathol 171:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, and Strasser A. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286:1735–1738. [DOI] [PubMed] [Google Scholar]
- 26.Hamad AR, Mohamood AS, Trujillo CJ, Huang CT, Yuan E, and Schneck JP. 2003. B220+ double-negative T cells suppress polyclonal T cell activation by a Fas-independent mechanism that involves inhibition of IL-2 production. J Immunol 171:2421–2426. [DOI] [PubMed] [Google Scholar]
- 27.Mohamood AS, Bargatze D, Xiao Z, Jie C, Yagita H, Ruben D, Watson J, Chakravarti S, Schneck JP, and Hamad AR. 2008. Fas-Mediated Apoptosis Regulates the Composition of Peripheral alphabeta T Cell Repertoire by Constitutively Purging Out Double Negative T Cells. PLoS ONE 3:e3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inki P, and Jalkanen M. 1996. The role of syndecan-1 in malignancies. Ann Med 28:63–67. [DOI] [PubMed] [Google Scholar]
- 29.Nochi T, Yuki Y, Terahara K, Hino A, Kunisawa J, Kweon MN, Yamaguchi T, and Kiyono H. 2004. Biological role of Ep-CAM in the physical interaction between epithelial cells and lymphocytes in intestinal epithelium. Clin Immunol 113:326–339. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt A, Heid HW, Schafer S, Nuber UA, Zimbelmann R, and Franke WW. 1994. Desmosomes and cytoskeletal architecture in epithelial differentiation: cell type-specific plaque components and intermediate filament anchorage. Eur J Cell Biol 65:229–245. [PubMed] [Google Scholar]
- 31.Borrmann CM, Mertens C, Schmidt A, Langbein L, Kuhn C, and Franke WW. 2000. Molecular diversity of plaques of epithelial-adhering junctions. Ann N Y Acad Sci 915:144–150. [DOI] [PubMed] [Google Scholar]
- 32.Inagaki-Ohara K, Sawaguchi A, Suganuma T, Matsuzaki G, and Nawa Y. 2005. Intraepithelial lymphocytes express junctional molecules in murine small intestine. Biochem Biophys Res Commun 331:977–983. [DOI] [PubMed] [Google Scholar]
- 33.Hughes RC 2001. Galectins as modulators of cell adhesion. Biochimie 83:667–676. [DOI] [PubMed] [Google Scholar]
- 34.Prasad VS, and Sidman CL. 1991. Cell cycle analysis and DNA aneuploidy in autoimmune mice homozygous for the lpr and gld mutations. J Immunol 147:4200–4206. [PubMed] [Google Scholar]
- 35.Tan KH, and Hunziker W. 2003. Compartmentalization of Fas and Fas ligand may prevent auto- or paracrine apoptosis in epithelial cells. Exp Cell Res 284:283–290. [DOI] [PubMed] [Google Scholar]
- 36.Fousteri G, Bresson D, and von Herrath M. 2007. Rational development of antigen-specific therapies for type 1 diabetes. Adv Exp Med Biol 601:313–319. [DOI] [PubMed] [Google Scholar]
- 37.Borges VM, Falcao H, Leite-Junior JH, Alvim L, Teixeira GP, Russo M, Nobrega AF, Lopes MF, Rocco PM, Davidson WF, Linden R, Yagita H, Zin WA, and DosReis GA. 2001. Fas ligand triggers pulmonary silicosis. J Exp Med 194:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen P, and Eisenberg R. 1991. Lpr and gld: Single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol 9:243–269. [DOI] [PubMed] [Google Scholar]
- 39.Su X, Hu Q, Kristan JM, Costa C, Shen Y, Gero D, Matis LA, and Wang Y. 2000. Significant role for Fas in the pathogenesis of autoimmune diabetes. J Immunol 164:2523–2532. [DOI] [PubMed] [Google Scholar]
- 40.Holler N, Tardivel A, Kovacsovics-Bankowski M, Hertig S, Gaide O, Martinon F, Tinel A, Deperthes D, Calderara S, Schulthess T, Engel J, Schneider P, and Tschopp J. 2003. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol 23:1428–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orlinick JR, Vaishnaw AK, and Elkon KB. 1999. Structure and function of Fas/Fas ligand. Int Rev Immunol 18:293–308. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama M, Nagata M, Yasuda H, Arisawa K, Kotani R, Yamada K, Chowdhury SA, Chakrabarty S, Jin ZZ, Yagita H, Yokono K, and Kasuga M. 2002. Fas/Fas ligand interactions play an essential role in the initiation of murine autoimmune diabetes. Diabetes 51:1391–1397. [DOI] [PubMed] [Google Scholar]
- 43.Apostolou I, Hao Z, Rajewsky K, and von Boehmer H. 2003. Effective destruction of Fas-deficient insulin-producing beta cells in type 1 diabetes. J Exp Med 198:1103–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]