Abstract
Pathological vascular remodeling is observed in various cardiovascular diseases including pulmonary hypertension (PH), a disease of unknown etiology that has been characterized by pulmonary artery vasoconstriction, right ventricular hypertrophy, vascular inflammation, and abnormal angiogenesis in pulmonary circulation. G protein-coupled receptors (GPCRs) are the largest family in the genome and widely expressed in cardiovascular system. They regulate all aspects of PH pathophysiology and represent therapeutic targets. We overview GPCRs function in vasoconstriction, vasodilation, vascular inflammation-driven remodeling and describe signaling cross talk between GPCR, inflammatory cytokines, and growth factors. Overall, the goal of this review is to emphasize the importance of GPCRs as critical signal transducers and targets for drug development in PH.
Keywords: Pulmonary hypertension, vascular remodeling, vasoconstriction, vascular inflammation, GPCR, intracellular signaling
INTRODUCTION
Pulmonary hypertension (PH) is a complex disease of unknown etiology. The pulmonary circulation responds to hypoxia by vasoconstriction, thereby diverting blood to oxygen rich regions. However, prolonged hypoxic vasoconstriction leads to remodeling of pulmonary arteries (PAs) and increased PA pressure. Increased pressure initially results in compensatory cardiac hypertrophy, but eventually causes de-compensatory cardiac remodeling and death by heart failure. Recent research indicates that PH in all its forms, especially associated with left heart disease, is more common than previously thought[1]. Current PH therapies that include endothelin-1 (ET1) receptor antagonists, prostacyclin analogs, cGMP- phosphodiesterase (PDE) inhibitors, and Ca2+ channel blockers impede, but do not stop the disease process, emphasizing the need for a finding of alternate treatments[2]. Over the years, preclinical research in PH has identified many protein targets, but very few have translated to the bench side. By contrast, G-protein-coupled receptors (GPCRs), the largest superfamily in the genome, play an important role in the development of PH and can be easily targeted by drugs[3]. The heart, a prime target for development of new PH therapies, expresses 200 GPCRs[4]. GPCR signaling cascades are critical for cardiovascular function and are targeted for the treatment of hypertension and heart failure by agonist and antagonist strategies. Here we reviewed the current knowledge on GPCR signaling in cardiac, vascular, and blood cells and highlighted some critical outcomes in PH, such as vasoconstriction/vasodilation responses, vascular inflammation, vascular and cardiac remodeling, and endothelial dysfunction (ED).
GPCR-MEDIATED SIGNALING
GPCRs are a family of 7-transmembrane domain proteins, forming a deep binding pocket for the extracellular ligand, agonist, which activates the receptor. Intracellular loops make contact to heterotrimeric G-proteins of 4 different classes (Gαs, Gαi, Gαq, Gα12) [Table 1][5]. Agonist binding to GPCRs stimulates GDP/GTP exchange on Gα subunits, converting them into the active state and promote dissociation of Gβγ subunits. G proteins interact with multiple effectors, leading to generation of second messengers, including cAMP, 1,2-diacylglycerol, phosphatidylinositol-3, 4, 5-trisphosphate (PIP3), and Ca2+. These signaling events are translated into complex hierarchy of kinase network [PKA, PKC, Akt, Ca2+/calmodulin-dependent protein kinase (CAMK)] leading to the regulation of gene expression and cellular functions. There are four families of Gα subunits with multiple members. αs exists as multiple transcripts 42 short and 44kD long forms. αI subfamily has αi1, αi2, αi3, αz, αO1, αO2; the αq subfamily has α11, α14, and α16; and the α12/13 family. The β subunits are β1–5; and the γ subunits are γ1–5,7,8,10,11,13. The βγ subunits, like Gα subunits, activate intracellular effector pathways including MAPK cascades, Rac1, phospholipase C-β (PLC-β, phosphoinositide 3 kinase γ (PI3K- γ, and ion channels and show variation as to the GPCR-Gα -complexes they interact with. Termination of G protein activation cycle occurs by the transition of Gα subunits to GDP-bound state, that is catalyzed by GTPase activating proteins (GAPs), known as regulators of G-protein signaling (RGS proteins). There are 31 proteins, containing the RGS domain that function as GTPase enzymes, terminating G-protein signaling[6,7].
Table 1.
G protein-coupled receptor physiology and pathology in pulmonary hypertension
| Physiology | Ligand-receptor-reference | Cell | G-protein | Important pathways | PH pathology |
|---|---|---|---|---|---|
| Vasodilation | Adenosine-A2A-AR; PGI2-IP[110–112] | VSMC | Gs | PKA | + |
| EC-eNOS-NO dependent vasodilation | Adenosine-A2A-AR; ApelinAPJ; Relaxin-RXFP; Opioid-KOR[50,51,66,110–112,178,179,182,245,246] | EC | Gi | PKG | + |
| Vasoconstriction | ET1/ETA; Ang II-AT1; TXA2-TP; PAF/ PAFR; Shingosine-1-P/S1P1–5; Ca2+-CaSR[12,21,42,47,54–56,58,69,249,250] | VSMC | Gq/Gi | Ca2+ | − |
| Anti-inflammatory | Adenosine-A2A-AR; PGI2-IP[110] | VSMC | Gs | PKA | + |
| PGI2-IP; adenosine-A2AAR[232,239] | Macrophage | Gs | PKA | + | |
| PGI2-IP; adenosine-A2AAR[110] | Fibroblast | Gs | PKA | + | |
| PGI2-IP; Adenosine-A2A-AR[110] | EC | Gs | PKA | + | |
| Pro-inflammatory | ET1-ETA; MCP1-CCR2; RANTES-CCR5; TXA2-TP[69,163] | VSMC | Gq/Gi | Ca2+ | − |
| LTB4-LTB4R; MCP1-CCR2[163,164] | Macrophage | Gq/Gi | Ca2+ | − | |
| PAF-PAFR; TXA2-TP[46,167,169] | EC | Gq/Gi | Ca2+ | − | |
| Cardiac myocyte hypertrophy | AngII-AT1; succinate-GPR91; thrombin-PAR[205,206] | Cardiac myocyte | Gq/Gi | Ca2+ | − |
| Cardiac fibrosis | Thrombin-PAR1–4[223,225] | Cardiac fibroblast | Gq/Gi/G12/13 | Ca2+/RhoA | − |
+: PH-protective; −: PH-pathogenic; VSMC: vascular smooth muscle cells; EC: endothelial cell
GPCR SIGNALING IN VASOCONSTRICTION AND VASCULAR REMODELING
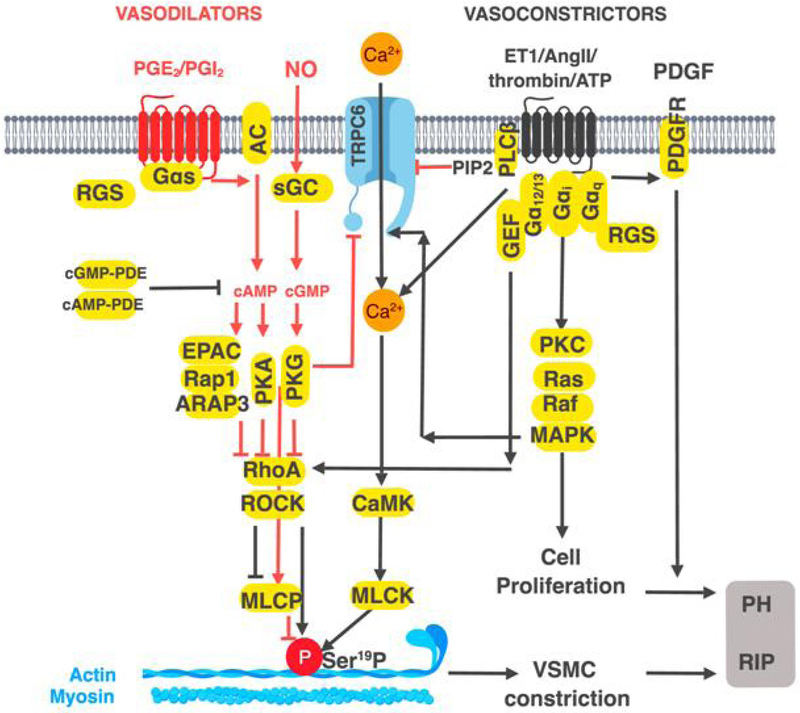
Vasoconstriction is driven by Ca2+-dependent phosphorylation of myosin light chain (MLC) on Ser19-MLC, whereas vasodilators oppose this event[8–10] [Figure 1, Tables 1 and 2]. In vascular smooth muscle cells (VSMC), the vasoconstrictor response is mediated by Gi-, Gq-, or G12/13-coupled GPCRs for ET1, angiotensin II (Ang II), serotonin, and thrombin[11–16]. Gi and Gq activate PLC pathways, increasing Ca2+ and receptor operated calcium entry (ROCE) via transient receptor potential cation channel subfamily C member 6 (TRPC6) channels. TRPC6-activation occurs by several mechanisms, including direct ERK1/2-mediated phosphorylation of TRPC6. Secondly, phosphoinositide-4, 5-bisphosphate (PIP2), the substrate for PLC, is an inhibitor of TRPC6[17,18]. Activation of G12/13 by vasoconstrictor GPCRs stimulates G12/13-dependent RhoA GEFs to increase the activity of, RhoA. In turn, RhoA activates Rho associated kinase (ROCK), which leads to increased Ser19-MLC and thereby, vasoconstriction[19,20]. Vasodilators, such as prostaglandin I2 (PGI2), acting via Gs-coupled (IP) receptor on VSMC, activate PKA and decrease intracellular Ca2+, leading to reduced MLC phosphorylation on Ser19 [Figure 1, Table 1].
Figure 1.
Schematic presentation of the mechanisms by which G protein-coupled receptors (GPCRs) regulate vascular tone and vascular smooth muscle cells (VSMC) proliferation. Vasoconstrictors like Ang II, ET1, thrombin, activate Gαi, Gαq, or G12/13-coupled GPCRs, increase Ca2+ via PLCβ activity, and receptor operated calcium channels such as TRPC6. Increase in PLCβ activity decreases PIP2 relieving tonic inhibition of TRPC6. Increase in Erk1/2 activity by Gi/Gq-coupled GPCRs activates TRPC6 by phosphorylation leading to increased Ca2+ entry and calmodulin-dependent protein kinase (CAMK) activation. CAMK increases MLCK activity by phosphorylation, which in turn phosphorylates MLC phosphorylation causing vasoconstriction. GPCRs coupled to G12/13 increase RhoA activity and the downstream kinase ROCK. ROCK increases MLC phosphorylation by inhibiting MLCP, or by direct phosphorylation. Vasodilators, such as PGI2 acting via Gs-coupled receptors activate PKA thereby inhibit Ca2+ increase by PKA-mediated phosphorylation of PLCβ and TRPC6. In ECs, Gi, or Gq-coupled GPCRs, increase, PI3K-Akt signaling and activate eNOS by phosphorylation at Ser1177. NO diffuses to nearby VSMC, activating soluble guanylate cyclase, increasing cGMP, activating PKG, and inhibiting TRPC6 by phosphorylation. PKG also activates the GAPs for Gq, RGS2 and RGS4 to inhibit PLCβ activity thereby attenuating Ca2+ entry. Both PKG- and PKA inhibit RhoA by direct phosphorylation and promote vasodilation
Table 2.
Current G protein-coupled receptor clinical trials in pulmonary hypertension
| Clinical trials name | Sponsor | Drug | Target |
|---|---|---|---|
| Tomorrow | Acetilon | Macitentan | ETA/ETB antagonist |
| ADAPT | United therapeutics | IP agonist | |
| Orenitram | IP agonist | ||
| Lung biotechnology | BPS-314d oral treprostanil | IP agonist | |
| Arena pharmaceuticals | APD-811 | IP agonist | |
| INSPIRE | Liquidia technologies | Inhaled treprostanil | IP agonist |
Vasodilators decrease intracellular Ca2+ by inhibiting PLCβ and TRPC6. The mechanism involves PKA/PKG-mediated phosphorylation of PLCβ and TRPC6 (on Ser28) and by phosphorylation of RGS4, which inhibits Gq-dependent activation of PLCβ[21–23]. Vasodilator GPCRs that increase cAMP may also activate cAMP-binding domain in exchange factor EPAC1, a GEF for the small molecular weight G-protein Rap1, a member of Ras superfamily. Rap1 activates ARAP3, a Rho GAP, which in turn, inhibits RhoA, leading to reduced MLC phosphorylation and vasodilation[24,25]. Vasodilation also occurs via endothelial cell (EC)-dependent production of nitric oxide (NO) by endothelial nitric oxide synthase (eNOS), which is activated by Akt or ERK1/2 by phosphorylation on Ser1177 residue[26]. Highly permeable NO readily enters VSMC, stimulates soluble guanylate cyclase (sGC) and activates cGMP-PKG, antagonizing Ca2+ action on phosphoSer19-MLC and promoting vasodilation. More specifically, NO-sGC-cGMP-PKG-axis inhibits Ca2+ increase by stimulating TRPC6 phosphorylation at Thr69, decreasing ROCE and increasing vasodilation[27]. PKG phosphorylates and activates RGS2, and RGS4, that leads to the inhibition of Gi/Gq,-rergulated PLC activity and termination of the vasoconstrictor Ca2+ signal[23]. Both PKG and PKA phosphorylate and inhibit RhoA and increase the activity of myosin light chain phosphatase (MLCP), thereby decreasing MLC contraction[28,29]. MLCP is also activated by vasodilators by PKG-mediated phosphorylation of a MLCP inhibitory subunit[20]. In addition, PKG and PKA reduce the ability of RhoA to inhibit the delayed rectifier potassium channel (KDR), which attenuates extracellular Ca2+ entry[30]. The enzyme PDE5A, a target of sildenafil therapy in PH, hydrolyzes cGMP to counter the effects of NO-cGMP-PKG signaling. However, other PDEs, including cAMP PDEs, play important roles[31]. Vasoconstrictors activate PDE5A to reduce cGMP in VSMC by RhoA/PKC-mediated inhibition of protein phosphatase 1 (PP1), thereby increasing phosphorylation of PDE5A and activating it[32]. GPCRs, including those for adenosine, ATP, adiponectin, apelin, prostaglandin E2 (PGE2,), PGI2 generally increase NO from EC, which diffuses to VSMC, or directly increase cAMP in VSMCs[33–39].
As a final summation statement, all current PH therapies intersect GPCR actions by modulating critical signaling effects. Firstly they, ultimately inhibit intracellular Ca2+ signaling and vasoconstriction. This includes the cGMP-PDE inhibitors, soluble guanylate cyclase (sGC) activators, PGI2 analogs, Ca2+-channel blockers, and ET-1 receptor antagonists. Secondly, they exert anti-inflammatory effects on vascular cells, as all of these therapeutics are known to do[2,40,41].
GPCR ligand-dependent vasoconstrictor response
Vasoconstrictor ligands, including ET-1, TxA2, and serotonin are increased in serum of PH patients; for serotonin a 4–5 fold increase has been reported, (8.8 ± 0.6 nmol/L) vs. (38.8 ± 7.3 nmol/L)[42–47]. Serotonin, acting via 5-HT1B-Gi coupled and 5-HT2A/2B-Gq coupled GPCRs, stimulates VSMC proliferation via the activation of the transcription factor GATA-4 and increase of cytokine generation from leukocytes, such as dendritic cells[48]. TxA2 level in PH is elevated due to up-regulation of thromboxane-A synthase[46]. Increased presence of inflammatory cytokines, such as TNFα and IFNγ, stimulates ET1 release from VSMC, believed to be an important source of the vasoconstrictor ET-1 in PH. This effect of cytokines and ET1 is antagonized by the PGI2-IP axis[49].
GPCR ligand-dependent vasodilator response
In contrast to vasoconstrictors, several vasodilators are decreased in PH, promoting vasoconstriction in pulmonary vascular system. Apelin, the ligand for CVD protective GPCR (APJ), modestly falls in PH patients (1.25 ng/mL vs. 0.89 ng/mL, P = 0.037)[50–52]. Decreased PGI2 synthase (PGIS) in ECs also plays a role in vasodilation and inflammation[45,46].
Increased activity of vasoconstrictor GPCRs
GPCR activity is frequently altered in diseases via internalization, phosphorylation, and expression levels. In lung, increased activity of TxA2 and its Gq-coupled GPCR (TP) occurs via palmitoylation of TP and increasing the proportion of the active receptor at the plasma membrane, consistent with pathophysiological action of TP in PH[53–56]. Similarly, increased expression of other GPCRs involved in PH pathogenesis has been noted for ET1 (ETA) and serotonin receptors, 5-HT1BR and 5-HT2BR in COPD-PH patients[54,55,57,58].
Decreased activity of vasodilator GPCRs
In PH, decreased serum concentrations of PGI2 is accompanied by decrease in levels of the receptor IP, reducing the effectiveness of PGI2 therapy[59]. Similarly, chronic stimulation of PGI2-IP axis, occurring with prostacyclin therapy in PH patients, is likely to even further down regulate the PGI2-IP axis via heterologous desensitization, compounding a pathogenic situation[60–62]. GPCRs such as IP, which increase cAMP-PKA, frequently exert anti-inflammatory effects, inhibiting key pro-inflammatory/pro-proliferative transcription factors, including NF-κB[63,64], Hippo pathway transcription factors Yaz-Taz (co-factors for the pro-proliferative transcription factor TEAD1) and, no doubt, many others[65]. Induction of anti-inflammatory/anti-proliferative PPARγ is also another mechanism, by which PGI2 acts[66]. PPARγ, along with sibling, transcription factors PPARβ/δ all are protective in PH and other cardiovascular diseases[34,66–71]. The induction of PPARγ activity by PGI2 was once thought to be a direct binding event to the PPARγ, but it now appears to occur by indirect mechanism. Activation of PKA or p38MAPK by PGI2-IP stimulates the cAMP response element-binding protein (CREB) by phosphorylation. Activated CREB turns on the transcriptional co-activator, peroxisome proliferator-activated receptor gamma co-activator 1α (PGC1α) gene, increases PGC1a activity and stimulates PPARγ, leading to protective anti-inflammatory effects[71] Molecular targets of PPARγ include inhibition of NF-κB and hypoxic activation of HIF-1α[72]. HIF-1α is clearly important in VSMC proliferation occurring in PH, as it helps the cell switch to a glycolytic/Warburg metabolic phenotype and has been connected to the increased expression of Ca2+ entry channel, TRPC6, both aiding VSMC proliferation[73–76]. Targeted KO of HIF-1α inhibitor protein, prolyl-hydroxylase domain containing protein 2 (PHD2), reduced O2-driven proteolysis of HIF-1α, thereby increasing HIF-1α -dependent proliferation of VSMC[76]. There are 3 PHD (PHD1–3) enzymes, which in presence of O2 hydroxylate proline residues, 402 and 564, ultimately resulting in the proteolysis of HIF-1α. A small molecule drug, R59949, a PDH inhibitor, has shown potential to combat PH in the hypoxic mouse model[76].
Post-receptor mechanisms leading to increased vasoconstrictor GPCR response
In VSMC, Angiotensin II (Ang II) up regulates Gi expression, thereby increasing the activation of PLCβ and mobilization of Ca2+, further enhancing vasoconstriction and proliferation by a post-receptor mechanism[77]. Of the PH pre-clinical therapeutics, RhoA-ROCK inhibitor, fasudil and statins both act at post GPCR level[78,79]. Statins, such as simvastatin, can work in combination with sildenafil, the cGMP-PDE inhibitor, likely an important feature of any new therapy. Although some studies reported no drug combination yet tested, the combination could be more effective for patients’ survival than any monotherapy[2,80,81]. Statins may work in PH models by inhibition of isoprenoid intermediates, farnesyl pyrophosphate and geranyl-geranyl pyrophosphate, essential for the post-translational isoprenylation, membrane localization, and activation of Ras and Rho small GTP-binding protein families, respectively, thus inhibiting RhoA-ROCK[82].
Post-receptor mechanisms leading to decreased vasodilator GPCR responses
Post-receptor mechanisms also operate to limit vasodilator response in PH, such as the several hits to the critical NO-cGMP-PKG vasodilation system. Firstly, inflammatory cytokines down regulate eNOS and upregulate reactive oxygen species (ROS), including superoxide[83–85]. Secondly, due to peroxynitrite formation, NO level is depleted[86]. Thirdly, vasodilator response can be limited due to increased PDE5A expression[87,88]. Up regulation of both cAMP-PDEs, and cGMP-PDE is an important pathological event, which decreases effectiveness of vasodilator GPCRs and needs further investigation[89]. The PDEs are a complex family of enzymes with 21 genes, and 11 subfamilies, and some share little sequence identity[31]. Due to a combination of post-receptor mechanisms, increased expression of cAMP- and cGMP-PDEs, inhibition of eNOS activity, and decreased NO availability (as a result of ROS production), the effects of vasodilators in PH are diminished.
HOW GPCRS FUNCTION IN VASCULAR INFLAMMATION-DRIVEN REMODELING
GPCRs induce cytokine/chemokine production from leukocytes, VSMC, ECs, fibroblasts, and cardiac myocytes and are pathogenic in PH. Up regulation of SDF-1 in activated T cell results to the expression and secretion of RANTES and Monocyte Chemo-attractant protein 1 (MCP-1). These chemokines promote proliferation of VSMC, matrix remodeling, and ROS production[90–92]. Additionally, GPCRs like serotonin receptor and purinergic P2Y14R, promote migration of bone marrow derived blood cells, essential to the development of PH[93,94].
DAMAGE MOLECULAR PATTERNS AS A POTENTIAL CONTRIBUTOR TO VASCULAR INFLAMMATION IN PH
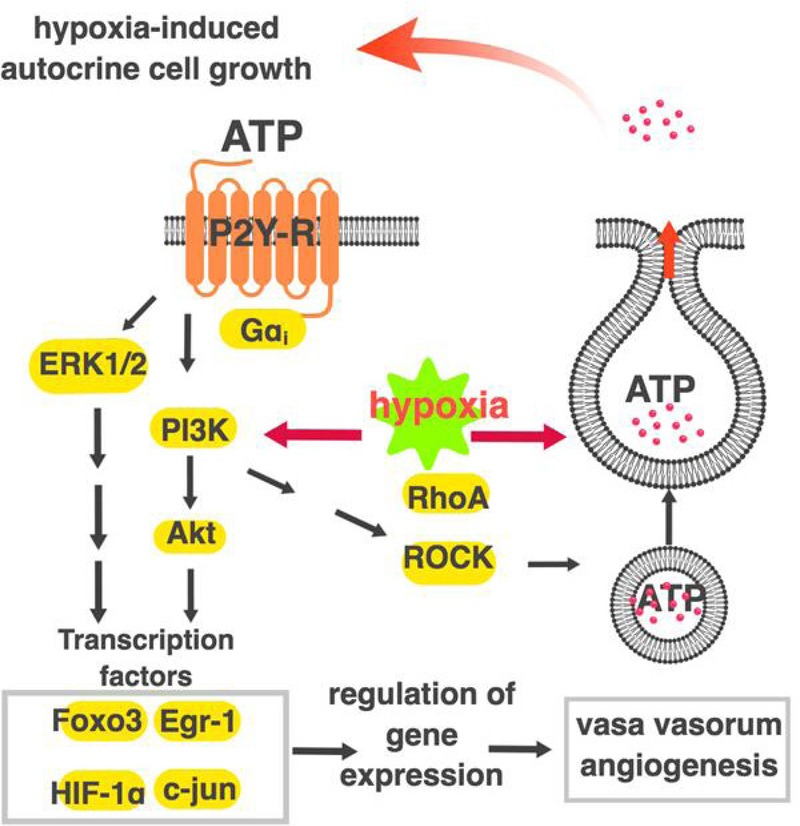
The driving forces behind vascular inflammation in PH are unclear, but it is likely that sterile inflammation-damage molecular pattern (DAMP) systems play a role. Purinergic receptors are also critical in DAMP responses. ATP, ADP, or adenosine are released from extracellular stimuli-activated, hypoxic, or damaged cells and play prominent roles in inflammatory and secretory responses associated with tissue repair. Of the 19 purinergic receptors, 12 are GPCRs nucleotide P2YR1, 2, 4, 6, 11–14 and adenosine A1, A2A, A2B A3, and the remaining 7 purinergic receptors P2X1–7, are ligand gated cation channels[95–100]. Macrophage activation in PH is potentiated by the P2Y6[101–103]. Some data suggest antagonizing the ATP-activated P2X1 purinergic receptor could be beneficial in PH[104]. Both P2Y1 and P2Y12 purinergic receptors have been shown to be partially responsible for PA pressure increase due to hypoxia[105]. Hypoxia-induced ATP release from PA adventitial fibroblasts and vasa vasorum endothelial cells (VVEC) induces mitogenic and angiogenic responses in VVEC in autocrine/paracrine manner[95,96,106] [Figure 2]. Released ATP and ADP are degraded rapidly to adenosine. Activation of the A2A adenosine receptor has been reported to be protective against PH, but the activation of A2B-AR results in pathogenic effects[107–112]. The involvement of DAMPS-GPCRs in PH is understudied, and therapeutic possibilities remain to be explored.
Figure 2.
Schematic diagram illustrating a role of PI3K, Rho and ROCK pathways in hypoxia-induced ATP release and ATP-mediated angiogenic effects in vasa vasorum endothelial cells. Activation of PI3K/Rho/ROCK pathway in response to hypoxia results in regulated ATP release from VVEC. In turn, extracellular ATP triggers/initiates P2YR-dependent activation of PI3K/Rho/ROCK pathway leading to angiogenic responses in vasa vasorum endothelial cells. VVEC: vasa vasorum endothelial cells
PATHOGENIC CHEMOKINE GPCRS
Small G-proteins in chemokine receptor-stimulated VSMC proliferation
In VSMC, MCP-1 acting via Gi-coupled CCR2, stimulates Gi-dependent proliferation, that also involves activation of the small G proteins[113]. One of the mechanisms includes p115RhoGEF-dependent activation of the Rac and Nuclear factor of activated T-cells (NFAT1)-dependent up-regulation of cyclin D1 expression in VSMC[113].
Involvement of ROS in chemokine receptor-stimulated responses
ROS is a pathogenic factor in PH by mechanisms, which include reducing NO; promoting VSMC proliferation; initiating sterile inflammation-DAMP response; and promoting vasoconstriction via increased membrane depolarization[74,114]. Gi-coupled GPCRs, such as MCP-1, SDF-1, thrombin, PAF, and purinergic receptors, stimulate ROS production[115–117]. ROS are produced as bactericidal compounds in large amounts in phagocytes (neutrophils, monocytes, macrophages) and, in a lesser amounts, in vascular cells. In phagocytes, chemokines, such as N-Formylmethionyl-leucyl-phenylalanine, PAF, complement C5a (C5a), LTB4, and MCP-1 are Gi-coupled-GPCRs and activate Rac1-NAD(P)H oxidase-superoxide system. NOX2 is a neutrophil NADPH oxidase responsible for producing increased amounts of superoxide. There are 7 NOX like oxidases, NOX1–5 DUOX1, 2 of which are expressed in vascular cells, and their activation involves Rac1 stimulation by the GEFs, such as engulfment and cell motility protein 1 (ELMO1)[115,117,118]. The superoxide generated by NOX enzymes in the extracellular space, is converted to H2O2, some of which enters the cell to stimulate proliferation. H2O2 induces proliferation by changing the balance in protein kinase-protein phosphatase networks by inhibiting key protein phosphatases via the oxidation of labile sensitive cysteine in the active site[119].
The involvement of HIF-1α in chemokine/GPCR action with respect to PH
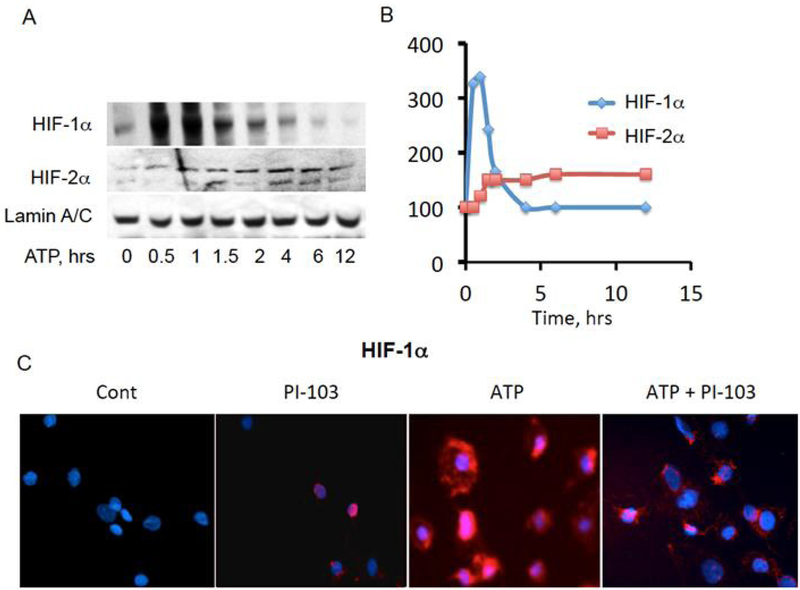
HIF-1α and HIF-2α may play a pathophysiological role in PH, and the action of GPCRs overlaps with that of HIFs[76,120,121]. Firstly, some GPCRs, such as those for estrogen G-protein coupled estrogen recetor-1 (GPER), ET1 (ETA), PGE1 (EP1), and PGI2 (IP), can activate HIF-1α even under normoxic conditions[122–131]. Secondly, ROS increased by GPCRs signaling, inhibit PHD proteins by oxidative inactivation, which in turn promotes HIF1α activation and its pathological action in PH[132–135]. Thirdly, hypoxic activation of HIF1α up regulates Gi-coupled receptor for SDF-1, CXCR4, implicated in PH by promoting VSMC proliferation[136–139]. Moreover, hypoxia can stimulate ATP release from vasa vasorum endothelial cells (VVEC) by PI3K-dependent mechanism to promote angiogenesis in an autocrine manner [Figure 2]. This mechanism implicates purinergic GPCR-dependent activation of HIF-1α and HIF-2α that may amplify hypoxia-induced vasa vasorum expansion [Figure 3].
Figure 3.
Extracellular ATP up regulates HIF-1α and HIF-2α transcription factors in pulmonary artery vasa vasorum endothelial cells. A, B: ATP (10 μmol/L), applied to VVEC, results in activation of both HIF-1α and HIF-2α with distinct time courses. VVEC were serum starved for 18 h and stimulated for indicated times. Nuclear fractions were subjected for Western blot analysis for HIF-1α, HIF-2α, and lamin A/C expression; C: cells were stained for HIF-1α at 1 h post stimulation with ATP (10 μmol/L), with or without PI3K inhibitor, PI-103 pretreatment (0.5 μmol/L, 15 min). VVEC: vasa vasorum endothelial cells
INTERACTION OF INFLAMMATORY CYTOKINES AND GROWTH FACTORS WITH GPCRS SIGNALING IN PH
PDGF-induced proliferation of VSMC is believed to be a major factor in PH. It is known to be dependent on Akt activation that can occur in co-operation with some GPCRs, termed trans-activation[140]. Ang II receptor works in concert with PDGF-receptor tyrosine kinase, promoting Akt-dependent VSMC proliferation[77,141–143]. Thrombin-PAR trans-activates the TGF-β receptor to promote VSMC proteoglycan synthesis[144]. It is of some interest that PGI2 has been described as unable to significantly inhibit PDGF-induced VSMC proliferation, suggesting that other PDGF-neutralizing strategies are needed in PH[145]. MCP-1 and IL-6 also work together to induce VSMC proliferation[146]. Activation of inflammatory TXA2-TP inhibits FGF-2- or VEGF-stimulated angiogenesis, which could relate to vascular pruning in cardiac and pulmonary vessels, and is an example of GPCR-cytokine interaction[41,147–149]. Protective interactions of GPCRs with cytokines and growth factors could include the ability of PGI2-IP to inhibit the IFNγ-induced inflammation, dependent upon induction of suppressor of cytokine signaling 3 (SOCS3)[150]. The GPCR GPR4 expressed on ECs, promotes angiogenesis in a Notch-dependent manner[151]. Vessel architecture is maintained by the ligand-receptor pair jagged expression on EC and Notch expression on VSMC, keeping VSMC in a differentiated non-proliferating state[152–156]. Both HIF-1a-induced VEGF for reparative angiogenesis and hypoxia-induced epithelial to mesenchymal transition require Ras family member, RhoE, which activation involves SDF-1 GPCR, CXCR4 signaling[157]. RhoE aids in HIF-1α maintenance and is induced by cAMP via Gs-coupled GPCRs[158]. Cardiac angiogenesis is believed to be critically protective in heart disease and potentially links SDF-1, cAMP, RhoE, HIF-1α, and VEGF into signaling networks[159].
INTERSECTIONS OF EICOSANOIDS AND GPCRS IN VASCULAR INFLAMMATION
Many eicosanoids induced by vascular inflammation, have short half-lives and must therefore be produced at the site of action either by monocyte/macrophages, ECs, fibroblasts, cardiac myocytes, or fibroblasts[160,161]. Injection of the GPCR-Gq/Gi-coupled ligand, PAF into rat lung causes rapid increase in PA pressure, linked to LTB4 production. LTB4-LTB4R, and PAF-PAFR coupled Gq/Gi are macrophage activators and plays a pathological role in PH[162–169]. PGE2, an important eicosanoid, which activates several GPCRs, such as Gq-coupled EP1, Gs-coupled EP2/4 and Gαi/Gα13-coupled EP3. EP3 promotes PH by increasing Rho/TGF-β1 signaling[170]. Protective eicosanoids, like PGI2, exert anti-inflammatory effects following LPS-induced lung injury and PH-induced cardiac inflammation and is active against T cells and macro-phages[41,171–174].
INFLAMMATION-DRIVEN ENDOTHELIAL DYSFUNCTION (ED) AS A MECHANISM OF VASCULAR REMODELING: INVOLVEMENT OF GPCRS
Inflammatory stimuli, IL-1 or TNFα down-regulate eNOS, attenuate reparative angiogenesis, promote EC apoptosis, and increase endothelial to mesenchymal transition (EMT) - all of which contribute to ED[46,83,175,176]. TxA2, acting on both ECs and VSMCs, is pathological in PH and inhibits VEGF- or FGF-2-promoted angiogenesis[46,147–149,165]. By contrast, many PH protective GPCR agonists (apelin, PGI2) increase eNOS activity by phosphorylation of Ser1177 or by increasing eNOS expression[50–52,177–179]. Some PH therapeutics, apelin and sildenafil, increase recruitment of endothelial cell progenitors, thereby counteracting ED[180–184].
THROMBOSIS AND PLATELET ACTIVITY CROSS TALK WITH VASCULAR INFLAMMATION AND GPCR ACTION
Platelets from patients with the sub-form of PAH, due to thromboembolic PAH, exhibit increased reactivity to thrombin, which stimulates the Gq/Gi-coupled protease activated receptor 1 (PAR1), promoting VSMC proliferation[185,186]. Thrombin receptors exist on EC and have been reported to inhibit angiogenesis.
RV REMODELING AND FAILURE
Cardiac myocytes (CMs) are terminally differentiated cells. The compensatory cardiac hypertrophy is entirely due to increased CM cell size, rather than proliferation. The adult heart is 56% CM, 27% fibroblasts, 10% VSMC, and 7% ECs, and these ratios change little between the four chambers[187]. During PH, the ratios of fibroblasts increases, and the ratio of ECs/CMs decreases[188]. The transition to heart failure has been linked to endothelial dysfunction due to insufficient reparative angiogenesis - a loss of capillaries supplying cardiac myocytes with O2, leading to capillary pruning, inflammation, and ROS production[147–149,188–193].
Pathological role of GPCRs in cardiac myocyte with respect to RV failure
The hypertrophy response is engaged when increased Ca2+- and cAMP-dependent contractile signals lead to activation of NFAT, MEF2, and GATA4. These signals are driven by GPCR agonists, such as Ang II, thrombin, ET1, PGF2α, β-AR[194–197]. Typical gene expression changes include decreased expression of sarcoplasmic reticulum Ca2+ re-uptake channel (SERCA2), increased expression of slow twitch contractile protein myosin heavy chain β9 (β-MHC, a.k.a. MyH7), and decreased expression of the fast twitch a-MHC/MyH6, amongst others[198,199]. The transcription factor, Egr-1 has been linked to the down regulation of cardiac SERCA2 in hypertrophy and was found to be overexpressed in PAs of PH patients[200–202]. GPCR-induced increase in intracellular Ca2+ stimulates PKD activity, promoting nuclear export of histone deacetylase 5 (HDAC5), thereby activating MEF2 to initiate hypertrophic gene program[203,204]. GPR91, a receptor for succinate expressed in CMs, promotes cardiac hypertrophy by coupling to Gi/Gq-PI3K-Akt signaling[205,206]. Succinate may be accumulated during cardiac remodeling due to changes in metabolism, and when released from the cells, promotes positive feedback loop by activating GPR91 leading to hypertrophy, or as also reported, to CM apoptosis via caspase3[188].
Protective role of GPCRs in cardiac myocyte with respect to RV failure
The estrogen-activated GPER, found in CM, has been considered cardio-protective in a PI3K-Akt-dependent mechanism[207,208]. RGS proteins 2, 4, 10, 14 modulate cardiac hypertrophy by inhibiting the Gi/GqPLCβ-Ca2+ signaling axis. PKG activates RGS2 by phosphorylation, inhibiting Gs, Gq, and Gi signaling, which in turn, attenuates β-AR-induced hypertrophy and that of other GPCRs[209–212]. Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) exert CV protective actions by the activation of cGMP-dependent PKG, which phosphorylates and activates RGS4, aiding its inhibition of GPCR-Gq-PLCβ-Ca2+ axis[213]. RGS6 promotes cardiac myocyte apoptosis associated with decompensation due to its capacity to increase ROS[214]. RGS10 inhibits the cardiac hypertrophy induced by Ang II[215]. RGS14 protects against aortic banding-induced cardiac hypertrophy and fibrosis, decreasing ERK1/2 hypertrophy signals[216].
ACTION OF GPCRS ON ENDOTHELIAL CELLS WITH RESPECT TO RV FAILURE
ED, occurring in failing RV, interconnects with fibrosis, as this appears to be a factor in the decreased capillary density-ED observed in hypertrophy and with the altered metabolism of CM, critical towards HF[217,218]. ED can result in potentially uncontrolled inflammation of local RV tissue and in turn can lead to EC apoptosis, down regulation of eNOS and PGIS. TGF-β, which is pathologic in PH, is induced by inflammation, promotes lung and heart fibrosis, but also promotes ED by inhibiting differentiation of endothelial progenitor cells (EPCs) into ECs to repopulate damaged endothelium, counteracting the effects of endothelium protective GPCR ligand, apelin[219,220]. Cardiovascular protective GPER is found in ECs, promotes angiogenesis, and could be significant in defending against endothelial dysfunction[207,221,222].
VASCULAR FIBROBLASTS AND CARDIAC FIBROSIS
Cardiac fibrosis, seen in animal models of PH, involves expansion of fibroblast populations, their differentiation to myofibroblast, and the stiffening of the extracellular matrix by synthesis of collagens[198]. Fibroblasts also can derive from EMT via conversion of EC to fibroblasts[175]. GPCRs promoting cardiac fibrosis include Gq-PLC-Ca2+- coupled 5-HT2B, Ang II, and endothelin CPCRs. The thrombin receptor, PAR1 is the most highly expressed GPCR in cardiac fibroblasts, therefore is a potentially important profibrotic GPCR[223–225]. P2Y6-purinergic receptors are reported to enhance pressure overload-induced fibrosis by increasing TGF-β1 and CTGF release[226]. The p38a MAPK, activated by Ang-II or non-GPCR stimuli, such as TGF- β1, or cyclic stretch, has been identified as a master switch, common to many different receptors stimulating fibrosis[198]. The ligand relaxin and its GPCR, RFXP1–4, are Gs-coupled and exert anti-hypertrophic and anti-fibrotic effects[227]. In cardiac fibroblasts, PGI2-IP-PKA axis activates CREB to inhibit Ang II-induced SMAD2 activation, attenuating proliferation[228].
ROLE OF GPCRS IN MONOCYTE/MACROPHAGE WITH RESPECT TO RV FAILURE
Macrophage features in the inflammation associated with heart failure, with resident macrophages being described as protective, while recruited being pathogenic[191]. Increasing activity of the transcription factor KLF4 in resident macrophages to aid their survival or inhibiting MCP-1-CCR2 activity of recruited monocytes, has been suggested as a potential therapy[191]. Macrophage polarization in PH is thought to contribute to cardiac and pulmonary inflammation-induced damage and remodeling. M1 macrophage phenotype is considered pro-inflammatory (versus the M2 phenotype), is involved in resolving inflammation, but implicated in tissue fibrosis[229]. Some studies in PH suggest that M2 macrophages are more damaging than M1. Antagonizing the CX3CR1 chemokine receptor reduces pathogenic M2 in favor of less damaging M1 phenotype[90,230]. Most chemokine receptors activate Gαi1/Gαi3, which have been linked to promotion of polarization to M1 macrophage via increased LPS-TLR4-NF-κB, in contrast to CX3CR1 signaling[76]. An interesting development in macrophage polarization/anti-inflammatory responses are the 6 atypical chemokine receptors, ACKR1–6, which are “duds” unable to activate G-proteins, and exert anti-inflammatory effects[229]. In particular, the atypical chemokine receptor, CCRL2 (tentatively ACKR5) polarizes in favor of M2 phenotype[229]. Other GPCRs aiding polarizing to M2 phenotype, include lipoxinA4-activated FPR2, PGE2-receptors, and adenosine A2A/A2B-receptors[231–234]. GPCRs clearly critically control macrophage polarization and might well be employed to diminish macrophage-induced inflammation occurring in PH. The role of GPCRs in cardiac inflammation is clearly complex, and it should be mentioned that increasing recruitment of pro-angiogenic monocytes may be beneficial in ED, and is also under control of GPCRs[235–238].
GPCRS, WHICH MIGHT BECOME CLINICAL TARGETS IN PH
GPCRs activating cAMP-PKA axis in ECs or VSMCs, such as PGI2 and adenosine (A2BAR), generally induce vasodilation, are often anti-inflammatory and protective in PH. Secondly, GPCRs, such as for apelin, PGI2, opioids, which increase NO release from EC to promote vasodilation, are also usually protective. Thus, any signals increasing cAMP, cGMP, NO and inhibiting Ca2+ are usually protective[178,179]. By contrast, any GPCR signaling increasing Ca2+ in VSMC, or decreasing NO, cAMP, cGMP, or increasing inflammation, are usually pathogenic in PH. One very potent anti-inflammatory agent is adenosine, which exerts powerful anti-inflammatory effects acting at A2AAR, and clearly plays a protective role in PH[111,239]. New drugs (such as AEA061) are positive allosteric modulators of A2AAR, that activate receptors without binding to the normal agonist binding site, offer a therapeutic possibility of fewer side effects as they do not act at A1, A2B or A3ARs[239]. Activation of A2AAR without activating A1, A2B, and A3ARs has been an issue in developing anti-inflammatory therapies. Other potentially protective GPCRs include FPR2, an atypical chemokine receptor on macrophages, was reported to exert anti-inflammatory action[229,240]. Other protective receptors in PH include ET-1 receptor ETB[241], angiotensin II type 2 receptor[242], adiponectin-receptor[36,243], mas1 (a receptor for angiotensin 1–7)[244], and relaxin receptors[245,246]. ETB receptor is also protective in porto-pulmonary hypertension, a disease secondary to liver failure, but in which the same therapeutics, PGI2-cGMP-PDE-ET-1 receptor antagonist therapies are utilized[247,248].
GPCRs with pathogenic action, which could be antagonized such that the drugs would be protective could include the CaSR, calcium sensing receptor in EC[12,249], the succinate GPR91 on cardiac myocytes[205,206], thromboxane receptors[250], serotonin receptors[251], LTB4 receptors[252], shingosine-1-phosphate receptors[13,253–255] amongst others.
CONCLUSION
Research has highlighted many examples of pathological GPCR signaling, which can be targets for novel PH therapeutics. In PH pre-clinical studies many targets have been identified, but only few are druggable [Tables 1 and 2]. GPCRs, by contrast, represent good targets for pharmacological strategies and in all likelihood present one of the best opportunities for therapeutic intervention in PH. The heart alone is estimated to express some 200 different GPCRs, suggesting significantly better therapeutics based on targeting GPCRs are possible. The challenge is to devise the best pharmacological cocktail for the PH patient. At the moment, while much has been published with respect to GPCR action in PH, much more clearly awaits discovery.
Acknowledgments
Financial support and sponsorship
The study was supported by grants from National Institute of Health R01-HL-086783 (to Gerasimovskaya E).
Footnotes
Conflicts of interest
All authors declared that there are no conflicts of interest.
DECLARATIONS
Availability of data and materials
Not applicable.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
REFERENCES
- 1.Strange G, Playford D, Stewart S, Deague JA, Nelson H, Kent A, Gabbay E. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart 2012;98:1805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joppi R, Gerardi C, Bertele V, Garattini S. A disease looking for innovative drugs: the case of pulmonary arterial hypertension. Eur J Intern Med 2018;55:47–51. [DOI] [PubMed] [Google Scholar]
- 3.Thomsen W, Frazer J, Unett D. Functional assays for screening GPCR targets. Curr Opin Biotechnol 2005;16:655–65. [DOI] [PubMed] [Google Scholar]
- 4.Salazar NC, Chen J, Rockman HA. Cardiac GPCRs: GPCR signaling in healthy and failing hearts. Biochim Biophys Acta 2007;1768:1006–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Offermanns S, Simon MI. Organization of transmembrane signalling by heterotrimeric G proteins. Cancer Surv 1996;27:177–98. [PubMed] [Google Scholar]
- 6.Rajagopal S, Shenoy SK. GPCR desensitization: acute and prolonged phases. Cell Signal 2018;41:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kach J, Sethakorn N, Dulin NO. A finer tuning of G-protein signaling through regulated control of RGS proteins. Am J Physiol Heart Circ Physiol 2012;303:H19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan JL, Ravid S, Spudich JA. Control of nonmuscle myosins by phosphorylation. Annu Rev Biochem 1992;61:721–59. [DOI] [PubMed] [Google Scholar]
- 9.Ikebe M, Hartshorne DJ. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J Biol Chem 1985;260:10027–31. [PubMed] [Google Scholar]
- 10.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 1996;271:20246–9. [DOI] [PubMed] [Google Scholar]
- 11.Yamamura A Pathological function of Ca2+-sensing receptor in pulmonary arterial hypertension. J Smooth Muscle Res 2014;50:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith KA, Ayon RJ, Tang H, Makino A, Yuan JX. Calcium-sensing receptor regulates cytosolic [Ca (2+) ] and plays a major role in the development of pulmonary hypertension. Front Physiol 2016;7:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ota H, Beutz MA, Ito M, Abe K, Oka M, McMurtry IF. S1P(4) receptor mediates S1P-induced vasoconstriction in normotensive and hypertensive rat lungs. Pulm Circ 2011;1:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aiello RJ, Bourassa PA, Zhang Q, Dubins J, Goldberg DR, De Lombaert S, Humbert M, Guignabert C, Cavasin MA, McKinsey TA, Paralkar V. Tryptophan hydroxylase 1 inhibition impacts pulmonary vascular remodeling in two rat models of pulmonary hypertension. J Pharmacol Exp Ther 2017;360:267–79. [DOI] [PubMed] [Google Scholar]
- 15.Bhat L, Hawkinson J, Cantillon M, Reddy DG, Bhat SR, Laurent CE, Bouchard A, Biernat M, Salvail D. RP5063, a novel, multimodal, serotonin receptor modulator, prevents Sugen 5416-hypoxia-induced pulmonary arterial hypertension in rats. Eur J Pharmacol 2017;810:83–91. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Ren W, Warburton R, Toksoz D, Fanburg BL. Serotonin induces Rho/ROCK-dependent activation of Smads 1/5/8 in pulmonary artery smooth muscle cells. FASEB J 2009;23:2299–306. [DOI] [PubMed] [Google Scholar]
- 17.Mori MX, Itsuki K, Hase H, Sawamura S, Kurokawa T, Mori Y, Inoue R. Dynamics of receptor-operated Ca(2+) currents through TRPC channels controlled via the PI(4,5)P2-PLC signaling pathway. Front Pharmacol 2015;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong F, Ma L, Zou L, Meng K, Ji T, Zhang L, Zhang R, Jiao J. Alpha1-adrenergic receptor activation stimulates calcium entry and proliferation via TRPC6 channels in cultured human mesangial cells. Cell Physiol Biochem 2015;36:1928–38. [DOI] [PubMed] [Google Scholar]
- 19.Tanabe S, Kreutz B, Suzuki N, Kozasa T. Regulation of RGS-RhoGEFs by Galpha12 and Galpha13 proteins. Methods Enzymol 2004;390:285–94. [DOI] [PubMed] [Google Scholar]
- 20.Mahavadi S, Nalli A, Al-Shboul O, Murthy KS. Inhibition of MLC20 phosphorylation downstream of Ca2+ and RhoA: a novel mechanism involving phosphorylation of myosin phosphatase interacting protein (M-RIP) by PKG and stimulation of MLC phosphatase activity. Cell Biochem Biophys 2014;68:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horinouchi T, Higa T, Aoyagi H, Nishiya T, Terada K, Miwa S. Adenylate cyclase/cAMP/protein kinase A signaling pathway inhibits endothelin type A receptor-operated Ca(2)(+) entry mediated via transient receptor potential canonical 6 channels. J Pharmacol Exp Ther 2012;340:143–51. [DOI] [PubMed] [Google Scholar]
- 22.Nalli AD, Kumar DP, Al-Shboul O, Mahavadi S, Kuemmerle JF, Grider JR, Murthy KS. Regulation of Gbetagammai-dependent PLCbeta3 activity in smooth muscle: inhibitory phosphorylation of PLC-beta3 by PKA and PKG and stimulatory phosphorylation of Galphai-GTPase-activating protein RGS2 by PKG. Cell Biochem Biophys 2014;70:867–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Zhou H, Mahavadi S, Sriwai W, Murthy KS. Inhibition of Galphaq-dependent PLC-beta1 activity by PKG and PKA is mediated by phosphorylation of RGS4 and GRK2. Am J Physiol Cell Physiol 2007;292:C200–8. [DOI] [PubMed] [Google Scholar]
- 24.Zieba BJ, Artamonov MV, Jin L, Momotani K, Ho R, Franke AS, Neppl RL, Stevenson AS, Khromov AS, Chrzanowska-Wodnicka M, Somlyo AV. The cAMP-responsive Rap1 guanine nucleotide exchange factor, Epac, induces smooth muscle relaxation by downregulation of RhoA activity. J Biol Chem 2011;286:16681–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon MY, Kim HJ, Kim JG, Lee JY, Kim J, Kim SC, Choi IG, Kim PH, Park JB. Small GTPase Rap1 regulates cell migration through regulation of small GTPase RhoA activity in response to transforming growth factor-beta1. J Cell Physiol 2013;228:2119–26. [DOI] [PubMed] [Google Scholar]
- 26.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Aktdependent phosphorylation. Nature 1999;399:601–5. [DOI] [PubMed] [Google Scholar]
- 27.Nishida M, Watanabe K, Sato Y, Nakaya M, Kitajima N, Ide T, Inoue R, Kurose H. Phosphorylation of TRPC6 channels at Thr69 is required for anti-hypertrophic effects of phosphodiesterase 5 inhibition. J Biol Chem 2010;285:13244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murthy KS, Zhou H, Grider JR, Makhlouf GM. Inhibition of sustained smooth muscle contraction by PKA and PKG preferentially mediated by phosphorylation of RhoA. Am J Physiol Gastrointest Liver Physiol 2003;284:G1006–16. [DOI] [PubMed] [Google Scholar]
- 29.Rolli-Derkinderen M, Sauzeau V, Boyer L, Lemichez E, Baron C, Henrion D, Loirand G, Pacaud P. Phosphorylation of serine 188 protects RhoA from ubiquitin/proteasome-mediated degradation in vascular smooth muscle cells. Circ Res 2005;96:1152–60. [DOI] [PubMed] [Google Scholar]
- 30.Luykenaar KD, Welsh DG. Activators of the PKA and PKG pathways attenuate RhoA-mediated suppression of the KDR current in cerebral arteries. Am J Physiol Heart Circ Physiol 2007;292:H2654–63. [DOI] [PubMed] [Google Scholar]
- 31.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res 2007;100:309–27. [DOI] [PubMed] [Google Scholar]
- 32.Murthy KS. Contractile agonists attenuate cGMP levels by stimulating phosphorylation of cGMP-specific PDE5; an effect mediated by RhoA/PKC-dependent inhibition of protein phosphatase 1. Br J Pharmacol 2008;153:1214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo L, Zheng W, Lian G, Chen H, Li L, Xu C, Xie L. Combination treatment of adipose-derived stem cells and adiponectin attenuates pulmonary arterial hypertension in rats by inhibiting pulmonary arterial smooth muscle cell proliferation and regulating the AMPK/ BMP/Smad pathway. Int J Mol Med 2018;41:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li HH, Hsu HH, Chang GJ, Chen IC, Ho WJ, Hsu PC, Chen WJ, Pang JS, Huang CC, Lai YJ. Prostanoid EP4 agonist L-902,688 activates PPARgamma and attenuates pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2018;314:L349–L59. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa Y, Kishida K, Kihara S, Funahashi T, Shimomura I. Adiponectin ameliorates hypoxia-induced pulmonary arterial remodeling. Biochem Biophys Res Commun 2009;382:183–8. [DOI] [PubMed] [Google Scholar]
- 36.Isobe S, Kataoka M, Kawakami T, Fukuda K. Adiponectin in chronic thromboembolic pulmonary hypertension. Circ J 2018;82:1466–8. [DOI] [PubMed] [Google Scholar]
- 37.Telli G, Tel BC, Yersal N, Korkusuz P, Gumusel B. Effect of intermedin/adrenomedullin2 on the pulmonary vascular bed in hypoxiainduced pulmonary hypertensive rats. Life Sci 2018;192:62–7. [DOI] [PubMed] [Google Scholar]
- 38.Chawla S, Rahar B, Saxena S. S1P prophylaxis mitigates acute hypobaric hypoxia-induced molecular, biochemical, and metabolic disturbances: a preclinical report. IUBMB Life 2016;68:365–75. [DOI] [PubMed] [Google Scholar]
- 39.Harada-Shiba M, Takamisawa I, Miyata K, Ishii T, Nishiyama N, Itaka K, Kangawa K, Yoshihara F, Asada Y, Hatakeyama K, Nagaya N, Kataoka K. Intratracheal gene transfer of adrenomedullin using polyplex nanomicelles attenuates monocrotaline-induced pulmonary hypertension in rats. Mol Ther 2009;17:1180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burnouf C, Pruniaux MP. Recent advances in PDE4 inhibitors as immunoregulators and anti-inflammatory drugs. Curr Pharm Des 2002;8:1255–96. [DOI] [PubMed] [Google Scholar]
- 41.Tobin JV, Zimmer DP, Shea C, Germano P, Bernier SG, Liu G, Long K, Miyashiro J, Ranganath S, Jacobson S, Tang K, Im GJ, Sheppeck J, 2nd, Moore JD, Sykes K, Wakefield J, Sarno R, Banijamali AR, Profy AT, Milne GT, Currie MG, Masferrer JL Pharmacological characterization of IW-1973, a novel soluble guanylate cyclase stimulator with extensive tissue distribution, antihypertensive, anti-inflammatory, and antifibrotic effects in preclinical models of disease. J Pharmacol Exp Ther 2018;365:664–75. [DOI] [PubMed] [Google Scholar]
- 42.Stelzner TJ, O’Brien RF, Yanagisawa M, Sakurai T, Sato K, Webb S, Zamora M, McMurtry IF, Fisher JH. Increased lung endothelin-1 production in rats with idiopathic pulmonary hypertension. Am J Physiol 1992;262:L614–20. [DOI] [PubMed] [Google Scholar]
- 43.Humbert M, Labrune P, Sitbon O, Le Gall C, Callebert J, Herve P, Samuel D, Machado R, Trembath R, Drouet L, Launay JM, Simonneau G. Pulmonary arterial hypertension and type-I glycogen-storage disease: the serotonin hypothesis. Eur Respir J 2002;20:5965. [DOI] [PubMed] [Google Scholar]
- 44.Hood KY, Mair KM, Harvey AP, Montezano AC, Touyz RM, MacLean MR. Serotonin signaling through the 5-HT1B receptor and NADPH oxidase 1 in pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 2017;37:1361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 1992;327:70–5. [DOI] [PubMed] [Google Scholar]
- 46.Mahajan CN, Afolayan AJ, Eis A, Teng RJ, Konduri GG. Altered prostanoid metabolism contributes to impaired angiogenesis in persistent pulmonary hypertension in a fetal lamb model. Pediatr Res 2015;77:455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Elton TS, Chen YF, Oparil S. Increased endothelin receptor gene expression in hypoxic rat lung. Am J Physiol 1994;266:L553–60. [DOI] [PubMed] [Google Scholar]
- 48.Weir EK, Hong Z, Varghese A. The serotonin transporter: a vehicle to elucidate pulmonary hypertension? Circ Res 2004;94:1152–4. [DOI] [PubMed] [Google Scholar]
- 49.Wort SJ, Woods M, Warner TD, Evans TW, Mitchell JA. Cyclooxygenase-2 acts as an endogenous brake on endothelin-1 release by human pulmonary artery smooth muscle cells: implications for pulmonary hypertension. Mol Pharmacol 2002;62:1147–53. [DOI] [PubMed] [Google Scholar]
- 50.Chandra SM, Razavi H, Kim J, Agrawal R, Kundu RK, de Jesus Perez V, Zamanian RT, Quertermous T, Chun HJ. Disruption of the apelin-APJ system worsens hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol 2011;31:814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang P, Maguire JJ, Davenport AP. Apelin, Elabela/Toddler, and biased agonists as novel therapeutic agents in the cardiovascular system. Trends Pharmacol Sci 2015;36:560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang P, Read C, Kuc RE, Buonincontri G, Southwood M, Torella R, Upton PD, Crosby A, Sawiak SJ, Carpenter TA, Glen RC, Morrell NW, Maguire JJ, Davenport AP. Elabela/Toddler is an endogenous agonist of the apelin apj receptor in the adult cardiovascular system, and exogenous administration of the peptide compensates for the downregulation of its expression in pulmonary arterial hypertension. Circulation 2017;135:1160–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fediuk J, Gutsol A, Nolette N, Dakshinamurti S. Thromboxane-induced actin polymerization in hypoxic pulmonary artery is independent of Rho. Am J Physiol Lung Cell Mol Physiol 2012;302:L13–26. [DOI] [PubMed] [Google Scholar]
- 54.Hinton M, Gutsol A, Dakshinamurti S. Thromboxane hypersensitivity in hypoxic pulmonary artery myocytes: altered TP receptor localization and kinetics. Am J Physiol Lung Cell Mol Physiol 2007;292:L654–63. [DOI] [PubMed] [Google Scholar]
- 55.Sikarwar AS, Hinton M, Santhosh KT, Chelikani P, Dakshinamurti S. Palmitoylation of Galphaq determines its association with the thromboxane receptor in hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol 2014;50:135–43. [DOI] [PubMed] [Google Scholar]
- 56.Santhosh KT, Elkhateeb O, Nolette N, Outbih O, Halayko AJ, Dakshinamurti S. Milrinone attenuates thromboxane receptor-mediated hyperresponsiveness in hypoxic pulmonary arterial myocytes. Br J Pharmacol 2011;163:1223–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rondelet B, Van Beneden R, Kerbaul F, Motte S, Fesler P, McEntee K, Brimioulle S, Ketelslegers JM, Naeije R. Expression of the serotonin 1b receptor in experimental pulmonary hypertension. Eur Respir J 2003;22:408–12. [DOI] [PubMed] [Google Scholar]
- 58.Milara J, Gabarda E, Juan G, Ortiz JL, Guijarro R, Martorell M, Morcillo EJ, Cortijo J. Bosentan inhibits cigarette smoke-induced endothelin receptor expression in pulmonary arteries. Eur Respir J 2012;39:927–38. [DOI] [PubMed] [Google Scholar]
- 59.Falcetti E, Hall SM, Phillips PG, Patel J, Morrell NW, Haworth SG, Clapp LH. Smooth muscle proliferation and role of the prostacyclin (IP) receptor in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2010;182:1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuwano K, Hashino A, Asaki T, Hamamoto T, Yamada T, Okubo K, Kuwabara K. 2-[4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino] butoxy]-N-(methylsulfonyl)acetam ide (NS-304), an orally available and long-acting prostacyclin receptor agonist prodrug. J Pharmacol Exp Ther 2007;322:1181–8. [DOI] [PubMed] [Google Scholar]
- 61.Schermuly RT, Pullamsetti SS, Breitenbach SC, Weissmann N, Ghofrani HA, Grimminger F, Nilius SM, Schror K, Kirchrath JM, Seeger W, Rose F. Iloprost-induced desensitization of the prostacyclin receptor in isolated rabbit lungs. Respir Res 2007;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gatfield J, Menyhart K, Wanner D, Gnerre C, Monnier L, Morrison K, Hess P, Iglarz M, Clozel M, Nayler O. Selexipag active metabolite ACT-333679 displays strong anticontractile and antiremodeling effects but low beta-arrestin recruitment and desensitization potential. J Pharmacol Exp Ther 2017;362:186–99. [DOI] [PubMed] [Google Scholar]
- 63.Chen CH, Lin H, Hsu YH, Sue YM, Cheng TH, Chan P, Chen TH. The protective effect of prostacyclin on adriamycin-induced apoptosis in rat renal tubular cells. Eur J Pharmacol 2006;529:8–15. [DOI] [PubMed] [Google Scholar]
- 64.Chen HH, Chen TW, Lin H. Prostacyclin-induced peroxisome proliferator-activated receptor-alpha translocation attenuates NF-kappaB and TNF-alpha activation after renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 2009;297:F1109–18. [DOI] [PubMed] [Google Scholar]
- 65.Kimura TE, Duggirala A, Smith MC, White S, Sala-Newby GB, Newby AC, Bond M. The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP. J Mol Cell Cardiol 2016;90:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh S, Simpson RL, Bennett RG. Relaxin activates peroxisome proliferator-activated receptor gamma (PPARgamma) through a pathway involving PPARgamma coactivator 1alpha (PGC1alpha). J Biol Chem 2015;290:950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia J, Yang L, Dong L, Niu M, Zhang S, Yang Z, Wumaier G, Li Y, Wei X, Gong Y, Zhu N, Li S. Cefminox, a dual agonist of prostacyclin receptor and peroxisome proliferator-activated receptor-gamma identified by virtual screening, has therapeutic efficacy against hypoxia-induced pulmonary hypertension in rats. Front Pharmacol 2018;9:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falcetti E, Flavell DM, Staels B, Tinker A, Haworth SG, Clapp LH. IP receptor-dependent activation of PPARgamma by stable prostacyclin analogues. Biochem Biophys Res Commun 2007;360:821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Idris-Khodja N, Ouerd S, Trindade M, Gornitsky J, Rehman A, Barhoumi T, Offermanns S, Gonzalez FJ, Neves MF, Paradis P, Schiffrin EL. Vascular smooth muscle cell peroxisome proliferator-activated receptor gamma protects against endothelin-1-induced oxidative stress and inflammation. J Hypertens 2017;35:1390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrington LS, Moreno L, Reed A, Wort SJ, Desvergne B, Garland C, Zhao L, Mitchell JA. The PPARbeta/delta agonist GW0742 relaxes pulmonary vessels and limits right heart hypertrophy in rats with hypoxia-induced pulmonary hypertension. PLoS One 2010;5:e9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nemenoff R, Meyer AM, Hudish TM, Mozer AB, Snee A, Narumiya S, Stearman RS, Winn RA, Weiser-Evans M, Geraci MW, Keith RL. Prostacyclin prevents murine lung cancer independent of the membrane receptor by activation of peroxisomal proliferator-activated receptor gamma. Cancer Prev Res (Phila) 2008;1:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee KS, Kim SR, Park SJ, Park HS, Min KH, Jin SM, Lee MK, Kim UH, Lee YC. Peroxisome proliferator activated receptor-gamma modulates reactive oxygen species generation and activation of nuclear factor-kappaB and hypoxia-inducible factor 1alpha in allergic airway disease of mice. J Allergy Clin Immunol 2006;118:120–7. [DOI] [PubMed] [Google Scholar]
- 73.Wang J, Fu X, Yang K, Jiang Q, Chen Y, Jia J, Duan X, Wang EW, He J, Ran P, Zhong N, Semenza GL, Lu W. Hypoxia inducible factor-1-dependent up-regulation of BMP4 mediates hypoxia-induced increase of TRPC expression in PASMCs. Cardiovasc Res 2015;107:108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 2006;98:1528–37. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Lu W, Yang K, Wang Y, Zhang J, Jia J, Yun X, Tian L, Chen Y, Jiang Q, Zhang B, Chen X, Wang J. Peroxisome proliferator-activated receptor gamma inhibits pulmonary hypertension targeting store-operated calcium entry. J Mol Med (Berl) 2015;93:327–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen T, Zhou Q, Tang H, Bozkanat M, Yuan JX, Raj JU, Zhou G. miR-17/20 Controls prolyl hydroxylase 2 (PHD2)/hypoxia-inducible factor 1 (HIF1) to regulate pulmonary artery smooth muscle cell proliferation. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gusan S, Anand-Srivastava MB. cAMP attenuates the enhanced expression of Gi proteins and hyperproliferation of vascular smooth muscle cells from SHR: role of ROS and ROS-mediated signaling. Am J Physiol Cell Physiol 2013;304:C1198–209. [DOI] [PubMed] [Google Scholar]
- 78.Dai Y, Luo W, Chang J. Rho kinase signaling and cardiac physiology. Curr Opin Physiol 2018;1:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 2007;100:923–9. [DOI] [PubMed] [Google Scholar]
- 80.Zhao L, Sebkhi A, Ali O, Wojciak-Stothard B, Mamanova L, Yang Q, Wharton J, Wilkins MR. Simvastatin and sildenafil combine to attenuate pulmonary hypertension. Eur Respir J 2009;34:948–57. [DOI] [PubMed] [Google Scholar]
- 81.Yao J, Xiong M, Tang B, Chen G, Liang M, Ma X, Wang Z, Wu Z. Simvastatin attenuates pulmonary vascular remodelling by downregulating matrix metalloproteinase-1 and −9 expression in a carotid artery-jugular vein shunt pulmonary hypertension model in rats. Eur J Cardiothorac Surg 2012;42:e121–7. [DOI] [PubMed] [Google Scholar]
- 82.Li X, Liu L, Tupper JC, Bannerman DD, Winn RK, Sebti SM, Hamilton AD, Harlan JM. Inhibition of protein geranylgeranylation and RhoA/RhoA kinase pathway induces apoptosis in human endothelial cells. J Biol Chem 2002;277:15309–16. [DOI] [PubMed] [Google Scholar]
- 83.Kawasaki Y, Yokobayashi E, Sakamoto K, Tenma E, Takaki H, Chiba Y, Otashiro T, Ishihara M, Yonezawa S, Sugiyama A, Natori Y. Angiostatin prevents IL-1beta-induced down-regulation of eNOS expression by inhibiting the NF-kappaB cascade. J Pharmacol Sci 2015;129:200–4. [DOI] [PubMed] [Google Scholar]
- 84.Niwano K, Arai M, Koitabashi N, Hara S, Watanabe A, Sekiguchi K, Tanaka T, Iso T, Kurabayashi M. Competitive binding of CREB and ATF2 to cAMP/ATF responsive element regulates eNOS gene expression in endothelial cells. Arterioscler Thromb Vasc Biol 2006;26:1036–42. [DOI] [PubMed] [Google Scholar]
- 85.Niwano K, Arai M, Tomaru K, Uchiyama T, Ohyama Y, Kurabayashi M. Transcriptional stimulation of the eNOS gene by the stable prostacyclin analogue beraprost is mediated through cAMP-responsive element in vascular endothelial cells: close link between PGI2 signal and NO pathways. Circ Res 2003;93:523–30. [DOI] [PubMed] [Google Scholar]
- 86.Steven S, Daiber A, Dopheide JF, Munzel T, Espinola-Klein C. Peripheral artery disease, redox signaling, oxidative stress - Basic and clinical aspects. Redox Biol 2017;12:787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation 2003;107:3230–5. [DOI] [PubMed] [Google Scholar]
- 88.Paffett ML, Lucas SN, Campen MJ. Resveratrol reverses monocrotaline-induced pulmonary vascular and cardiac dysfunction: a potential role for atrogin-1 in smooth muscle. Vascul Pharmacol 2012;56:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maclean MR, Johnston ED, McCulloch KM, Pooley L, Houslay MD, Sweeney G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J Pharmacol Exp Ther 1997;283:619–24. [PubMed] [Google Scholar]
- 90.Amsellem V, Abid S, Poupel L, Parpaleix A, Rodero M, Gary-Bobo G, Latiri M, Dubois-Rande JL, Lipskaia L, Combadiere C, Adnot S. Roles for the CX3CL1/CX3CR1 and CCL2/CCR2 chemokine systems in hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol 2017;56:597–608. [DOI] [PubMed] [Google Scholar]
- 91.Balabanian K, Foussat A, Dorfmuller P, Durand-Gasselin I, Capel F, Bouchet-Delbos L, Portier A, Marfaing-Koka A, Krzysiek R, Rimaniol AC, Simonneau G, Emilie D, Humbert M. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med 2002;165:1419–25. [DOI] [PubMed] [Google Scholar]
- 92.Serag AR, Hazaa SM, Afifi IK, Ghoname NF, Serag AR. Regulated upon activation, normal T-cell expressed and secreted chemokine and interleukin-6 in rheumatic pulmonary hypertension, targets for therapeutic decisions. Eur J Cardiothorac Surg 2010;37:853–8. [DOI] [PubMed] [Google Scholar]
- 93.Launay JM, Herve P, Callebert J, Mallat Z, Collet C, Doly S, Belmer A, Diaz SL, Hatia S, Cote F, Humbert M, Maroteaux L. Serotonin 5-HT2B receptors are required for bone-marrow contribution to pulmonary arterial hypertension. Blood 2012;119:1772–80. [DOI] [PubMed] [Google Scholar]
- 94.Xu J, Morinaga H, Oh D, Li P, Chen A, Talukdar S, Mamane Y, Mancini JA, Nawrocki AR, Lazarowski E, Olefsky JM, Kim JJ. GPR105 ablation prevents inflammation and improves insulin sensitivity in mice with diet-induced obesity. J Immunol 2012;189:19929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gerasimovskaya EV, Ahmad S, White CW, Jones PL, Carpenter TC, Stenmark KR. Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. Signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J Biol Chem 2002;277:44638–50. [DOI] [PubMed] [Google Scholar]
- 96.Woodward HN, Anwar A, Riddle S, Taraseviciene-Stewart L, Fragoso M, Stenmark KR, Gerasimovskaya EV. PI3K, Rho, and ROCK play a key role in hypoxia-induced ATP release and ATP-stimulated angiogenic responses in pulmonary artery vasa vasorum endothelial cells. Am J Physiol Lung Cell Mol Physiol 2009;297:L954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol 2001;38:900–8. [DOI] [PubMed] [Google Scholar]
- 98.Tackett BC, Sun H, Mei Y, Maynard JP, Cheruvu S, Mani A, Hernandez-Garcia A, Vigneswaran N, Karpen SJ, Thevananther S. P2Y2 purinergic receptor activation is essential for efficient hepatocyte proliferation in response to partial hepatectomy. Am J Physiol Gastrointest Liver Physiol 2014;307:G1073–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burnstock G, Knight GE. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal 2018;14:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature 2014;509:310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stachon P, Peikert A, Michel NA, Hergeth S, Marchini T, Wolf D, Dufner B, Hoppe N, Ayata CK, Grimm M, Cicko S, Schulte L, Reinohl J, von zur Muhlen C, Bode C, Idzko M, Zirlik A. P2Y6 deficiency limits vascular inflammation and atherosclerosis in mice. Arterioscler Thromb Vasc Biol 2014;34:2237–45. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Z, Wang Z, Ren H, Yue M, Huang K, Gu H, Liu M, Du B, Qian M. P2Y(6) agonist uridine 5’-diphosphate promotes host defense against bacterial infection via monocyte chemoattractant protein-1-mediated monocytes/macrophages recruitment. J Immunol 2011;186:5376–87. [DOI] [PubMed] [Google Scholar]
- 103.Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, Bult H, Robaye B. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol 2008;74:777–84. [DOI] [PubMed] [Google Scholar]
- 104.Visovatti SH, Hyman MC, Goonewardena SN, Anyanwu AC, Kanthi Y, Robichaud P, Wang J, Petrovic-Djergovic D, Rattan R, Burant CF, Pinsky DJ. Purinergic dysregulation in pulmonary hypertension. Am J Physiol Heart Circ Physiol 2016;311:H286–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kylhammar D, Bune LT, Radegran G. P2Y(1) and P2Y(1)(2) receptors in hypoxia- and adenosine diphosphate-induced pulmonary vasoconstriction in vivo in the pig. Eur J Appl Physiol 2014;114:1995–2006. [DOI] [PubMed] [Google Scholar]
- 106.Nijmeh H, Balasubramaniam V, Burns N, Ahmad A, Stenmark KR, Gerasimovskaya EV. High proliferative potential endothelial colonyforming cells contribute to hypoxia-induced pulmonary artery vasa vasorum neovascularization. Am J Physiol Lung Cell Mol Physiol 2014;306:L661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karmouty-Quintana H, Zhong H, Acero L, Weng T, Melicoff E, West JD, Hemnes A, Grenz A, Eltzschig HK, Blackwell TS, Xia Y, Johnston RA, Zeng D, Belardinelli L, Blackburn MR. The A2B adenosine receptor modulates pulmonary hypertension associated with interstitial lung disease. FASEB J 2012;26:2546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karmouty-Quintana H, Weng T, Garcia-Morales LJ, Chen NY, Pedroza M, Zhong H, Molina JG, Bunge R, Bruckner BA, Xia Y, Johnston RA, Loebe M, Zeng D, Seethamraju H, Belardinelli L, Blackburn MR. Adenosine A2B receptor and hyaluronan modulate pulmonary hypertension associated with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2013;49:1038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karmouty-Quintana H, Philip K, Acero LF, Chen NY, Weng T, Molina JG, Luo F, Davies J, Le NB, Bunge I, Volcik KA, Le TT, Johnston RA, Xia Y, Eltzschig HK, Blackburn MR. Deletion of ADORA2B from myeloid cells dampens lung fibrosis and pulmonary hypertension. FASEB J 2015;29:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu MH, Gong YS, Su MS, Dai ZY, Dai SS, Bao SZ, Li N, Zheng RY, He JC, Chen JF, Wang XT. Absence of the adenosine A2A receptor confers pulmonary arterial hypertension and increased pulmonary vascular remodeling in mice. J Vasc Res 2011;48:171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alencar AK, Pereira SL, Montagnoli TL, Maia RC, Kummerle AE, Landgraf SS, Caruso-Neves C, Ferraz EB, Tesch R, Nascimento JH, de Sant’Anna CM, Fraga CA, Barreiro EJ, Sudo RT, Zapata-Sudo G. Beneficial effects of a novel agonist of the adenosine A2A receptor on monocrotaline-induced pulmonary hypertension in rats. Br J Pharmacol 2013;169:953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shang P, He ZY, Chen JF, Huang SY, Liu BH, Liu HX, Wang XT. Absence of the Adenosine A2A receptor confers pulmonary arterial hypertension through RhoA/ROCK signaling pathway in mice. J Cardiovasc Pharmacol 2015;66:569–75. [DOI] [PubMed] [Google Scholar]
- 113.Singh NK, Janjanam J, Rao GN. p115 RhoGEF activates the Rac1 GTPase signaling cascade in MCP1 chemokine-induced vascular smooth muscle cell migration and proliferation. J Biol Chem 2017;292:14080–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Norton CE, Broughton BR, Jernigan NL, Walker BR, Resta TC. Enhanced depolarization-induced pulmonary vasoconstriction following chronic hypoxia requires EGFR-dependent activation of NAD(P)H oxidase 2. Antioxid Redox Signal 2013;18:1777–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y, Xu X, Pan M, Jin T. ELMO1 directly interacts with gbetagamma subunit to transduce GPCR signaling to Rac1 activation in chemotaxis. J Cancer 2016;7:973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal 2013;19:1110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Y, Pagano PJ. Microvascular NADPH oxidase in health and disease. Free Radic Biol Med 2017;109:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rastogi R, Geng X, Li F, Ding Y. NOX activation by subunit interaction and underlying mechanisms in disease. Front Cell Neurosci 2016;10:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paulsen CE, Carroll KS. Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery. Chem Rev 2013;113:4633–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 1999;103:6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tan Q, Kerestes H, Percy MJ, Pietrofesa R, Chen L, Khurana TS, Christofidou-Solomidou M, Lappin TR, Lee FS. Erythrocytosis and pulmonary hypertension in a mouse model of human HIF2A gain of function mutation. J Biol Chem 2013;288:17134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang L, Xiong W, Li N, Liu H, He H, Du Y, Zhang Z, Liu Y. Estrogen stabilizes hypoxia-inducible factor 1alpha through G proteincoupled estrogen receptor 1 in eutopic endometrium of endometriosis. Fertil Steril 2017;107:439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li M, Liu Y, Jin F, Sun X, Li Z, Liu Y, Fang P, Shi H, Jiang X. Endothelin-1 induces hypoxia inducible factor 1alpha expression in pulmonary artery smooth muscle cells. FEBS Lett 2012;586:3888–93. [DOI] [PubMed] [Google Scholar]
- 124.De Francesco EM, Pellegrino M, Santolla MF, Lappano R, Ricchio E, Abonante S, Maggiolini M. GPER mediates activation of HIF1alpha/VEGF signaling by estrogens. Cancer Res 2014;74:4053–64. [DOI] [PubMed] [Google Scholar]
- 125.Ji R, Chou CL, Xu W, Chen XB, Woodward DF, Regan JW. EP1 prostanoid receptor coupling to G i/o up-regulates the expression of hypoxia-inducible factor-1 alpha through activation of a phosphoinositide-3 kinase signaling pathway. Mol Pharmacol 2010;77:102536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kasama H, Sakamoto Y, Kasamatsu A, Okamoto A, Koyama T, Minakawa Y, Ogawara K, Yokoe H, Shiiba M, Tanzawa H, Uzawa K. Adenosine A2b receptor promotes progression of human oral cancer. BMC Cancer 2015;15:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee SJ, No YR, Dang DT, Dang LH, Yang VW, Shim H, Yun CC. Regulation of hypoxia-inducible factor 1alpha (HIF-1alpha) by lysophosphatidic acid is dependent on interplay between p53 and Kruppel-like factor 5. J Biol Chem 2013;288:25244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee HY, Lee T, Lee N, Yang EG, Lee C, Lee J, Moon EY, Ha J, Park H. Src activates HIF-1alpha not through direct phosphorylation of HIF-1alpha specific prolyl-4 hydroxylase 2 but through activation of the NADPH oxidase/Rac pathway. Carcinogenesis 2011;32:70312. [DOI] [PubMed] [Google Scholar]
- 129.Du J, Xu R, Hu Z, Tian Y, Zhu Y, Gu L, Zhou L. PI3K and ERK-induced Rac1 activation mediates hypoxia-induced HIF-1alpha expression in MCF-7 breast cancer cells. PLoS One 2011;6:e25213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chang TC, Huang CJ, Tam K, Chen SF, Tan KT, Tsai MS, Lin TN, Shyue SK. Stabilization of hypoxia-inducible factor-1{alpha} by prostacyclin under prolonged hypoxia via reducing reactive oxygen species level in endothelial cells. J Biol Chem 2005;280:36567–74. [DOI] [PubMed] [Google Scholar]
- 131.Takabuchi S, Hirota K, Oda S, Nishi K, Oda T, Shingu K, Adachi T, Fukuda K. Opioid receptor stimulation does not affect cellular hypoxia-induced gene responses mediated by hypoxia-inducible factor 1 in cultured cell lines. J Anesth 2005;19:263–5. [DOI] [PubMed] [Google Scholar]
- 132.Griguer CE, Oliva CR, Kelley EE, Giles GI, Lancaster JR, Jr., Gillespie GY. Xanthine oxidase-dependent regulation of hypoxiainducible factor in cancer cells. Cancer Res 2006;66:2257–63. [DOI] [PubMed] [Google Scholar]
- 133.Xia L, Mo P, Huang W, Zhang L, Wang Y, Zhu H, Tian D, Liu J, Chen Z, Zhang Y, Chen Z, Hu H, Fan D, Nie Y, Wu K. The TNFalpha/ROS/HIF-1-induced upregulation of FoxMI expression promotes HCC proliferation and resistance to apoptosis. Carcinogenesis 2012;33:2250–9. [DOI] [PubMed] [Google Scholar]
- 134.Lee G, Won HS, Lee YM, Choi JW, Oh TI, Jang JH, Choi DK, Lim BO, Kim YJ, Park JW, Puigserver P, Lim JH. Oxidative dimerization of PHD2 is responsible for its inactivation and contributes to metabolic reprogramming via HIF-1alpha activation. Sci Rep 2016;6:18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fukai K, Nakamura A, Hoshino A, Nakanishi N, Okawa Y, Ariyoshi M, Kaimoto S, Uchihashi M, Ono K, Tateishi S, Ikeda K, Ogata T, Ueyama T, Matoba S. Pyk2 aggravates hypoxia-induced pulmonary hypertension by activating HIF-1alpha. Am J Physiol Heart Circ Physiol 2015;308:H951–9. [DOI] [PubMed] [Google Scholar]
- 136.Yang T, Li ZN, Chen G, Gu Q, Ni XH, Zhao ZH, Ye J, Meng XM, Liu ZH, Xiong CM, He JG. Increased levels of plasma CXC-chemokine ligand 10, 12 and 16 are associated with right ventricular function in patients with idiopathic pulmonary arterial hypertension. Heart Lung 2014;43:322–7. [DOI] [PubMed] [Google Scholar]
- 137.McCullagh BN, Costello CM, Li L, O’Connell C, Codd M, Lawrie A, Morton A, Kiely DG, Condliffe R, Elliot C, McLoughlin P, Gaine S. Elevated plasma CXCL12alpha is associated with a poorer prognosis in pulmonary arterial hypertension. PLoS One 2015;10:e0123709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kazimierczyk R, Blaszczak P, Jasiewicz M, Knapp M, Ptaszynska-Kopczynska K, Sobkowicz B, Waszkiewicz E, Grzywna R, Musial WJ, Kaminski KA. Increased platelet content of SDF-1alpha is associated with worse prognosis in patients with pulmonary arterial hypertension. Platelets 2018; doi: 10.1080/09537104.2018.1457780. [DOI] [PubMed] [Google Scholar]
- 139.Wei L, Zhang B, Cao W, Xing H, Yu X, Zhu D. Inhibition of CXCL12/CXCR4 suppresses pulmonary arterial smooth muscle cell proliferation and cell cycle progression via PI3K/Akt pathway under hypoxia. J Recept Signal Transduct Res 2015;35:329–39. [DOI] [PubMed] [Google Scholar]
- 140.Barst RJ. PDGF signaling in pulmonary arterial hypertension. J Clin Invest 2005;115:2691–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Y, Bai Y, Qin L, Zhang P, Yi T, Teesdale SA, Zhao L, Pober JS, Tellides G. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ Res 2007;101:560–9. [DOI] [PubMed] [Google Scholar]
- 142.Little PJ. GPCR responses in vascular smooth muscle can occur predominantly through dual transactivation of kinase receptors and not classical Galphaq protein signalling pathways. Life Sci 2013;92:951–6. [DOI] [PubMed] [Google Scholar]
- 143.Dahal BK, Heuchel R, Pullamsetti SS, Wilhelm J, Ghofrani HA, Weissmann N, Seeger W, Grimminger F, Schermuly RT. Hypoxic pulmonary hypertension in mice with constitutively active platelet-derived growth factor receptor-beta. Pulm Circ 2011;1:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Burch ML, Getachew R, Osman N, Febbraio MA, Little PJ. Thrombin-mediated proteoglycan synthesis utilizes both protein-tyrosine kinase and serine/threonine kinase receptor transactivation in vascular smooth muscle cells. J Biol Chem 2013;288:7410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McKean JS, Murray F, Gibson G, Shewan DA, Tucker SJ, Nixon GF. The cAMP-producing agonist beraprost inhibits human vascular smooth muscle cell migration via exchange protein directly activated by cAMP. Cardiovasc Res 2015;107:546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Viedt C, Vogel J, Athanasiou T, Shen W, Orth SR, Kubler W, Kreuzer J. Monocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-kappaB and activator protein-1. Arterioscler Thromb Vasc Biol 2002;22:914–20. [DOI] [PubMed] [Google Scholar]
- 147.Ashton AW, Cheng Y, Helisch A, Ware JA. Thromboxane A2 receptor agonists antagonize the proangiogenic effects of fibroblast growth factor-2: role of receptor internalization, thrombospondin-1, and alpha(v)beta3. Circ Res 2004;94:735–42. [DOI] [PubMed] [Google Scholar]
- 148.Gao Y, Yokota R, Tang S, Ashton AW, Ware JA. Reversal of angiogenesis in vitro, induction of apoptosis, and inhibition of AKT phosphorylation in endothelial cells by thromboxane A(2). Circ Res 2000;87:739–45. [DOI] [PubMed] [Google Scholar]
- 149.Ashton AW, Ware JA. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ Res 2004;95:372–9. [DOI] [PubMed] [Google Scholar]
- 150.Strassheim D, Riddle SR, Burke DL, Geraci MW, Stenmark KR. Prostacyclin inhibits IFN-gamma-stimulated cytokine expression by reduced recruitment of CBP/p300 to STAT1 in a SOCS-1-independent manner. J Immunol 2009;183:6981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ren J, Zhang Y, Cai H, Ma H, Zhao D, Zhang X, Li Z, Wang S, Wang J, Liu R, Li Y, Qian J, Wei H, Niu L, Liu Y, Xiao L, Ding M, Jiang S. Human GPR4 and the notch signaling pathway in endothelial cell tube formation. Mol Med Rep 2016;14:1235–40. [DOI] [PubMed] [Google Scholar]
- 152.Kerr BA, West XZ, Kim YW, Zhao Y, Tischenko M, Cull RM, Phares TW, Peng XD, Bernier-Latmani J, Petrova TV, Adams RH, Hay N, Naga Prasad SV, Byzova TV. Stability and function of adult vasculature is sustained by Akt/Jagged1 signalling axis in endothelium. Nat Commun 2016;7:10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lin CH, Lilly B. Notch signaling governs phenotypic modulation of smooth muscle cells. Vascul Pharmacol 2014;63:88–96. [DOI] [PubMed] [Google Scholar]
- 154.Chakravarti B, Yang J, Ahlers-Dannen KE, Luo Z, Flaherty HA, Meyerholz DK, Anderson ME, Fisher RA. Essentiality of regulator of G protein signaling 6 and oxidized Ca(2+)/calmodulin-dependent protein kinase II in Notch signaling and cardiovascular development. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sangphech N, Osborne BA, Palaga T. Notch signaling regulates the phosphorylation of Akt and survival of lipopolysaccharide-activated macrophages via regulator of G protein signaling 19 (RGS19). Immunobiology 2014;219:653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ma YX, Wu ZQ, Feng YJ, Xiao ZC, Qin XL, Ma QH. G protein coupled receptor 50 promotes self-renewal and neuronal differentiation of embryonic neural progenitor cells through regulation of notch and wnt/beta-catenin signalings. Biochem Biophys Res Commun 2015;458:836–42. [DOI] [PubMed] [Google Scholar]
- 157.Feng B, Li K, Zhong H, Ren G, Wang H, Shang Y, Bai M, Liang J, Wang X, Fan D. RhoE promotes metastasis in gastric cancer through a mechanism dependent on enhanced expression of CXCR4. PLoS One 2013;8:e81709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Collett GP, Goh XF, Linton EA, Redman CW, Sargent IL. RhoE is regulated by cyclic AMP and promotes fusion of human BeWo choriocarcinoma cells. PLoS One 2012;7:e30453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yue X, Lin X, Yang T, Yang X, Yi X, Jiang X, Li X, Li T, Guo J, Dai Y, Shi J, Wei L, Youker KA, Torre-Amione G, Yu Y, Andrade KC, Chang J. Rnd3/RhoE modulates hypoxia-inducible factor 1alpha/vascular endothelial growth factor signaling by stabilizing hypoxiainducible factor 1alpha and regulates responsive cardiac angiogenesis. Hypertension 2016;67:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Parker AR, Ayars AG, Altman MC, Henderson WR, Jr., Lipid mediators in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am 2016;36:749–63. [DOI] [PubMed] [Google Scholar]
- 161.Mitchell JA, Kirkby NS. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br J Pharmacol 2018; doi: 10.1111/bph.14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fink MP, O’Sullivan BP, Menconi MJ, Wollert PS, Wang H, Youssef ME, Fleisch JH. A novel leukotriene B4-receptor antagonist in endotoxin shock: a prospective, controlled trial in a porcine model. Crit Care Med 1993;21:1825–37. [DOI] [PubMed] [Google Scholar]
- 163.Qian J, Tian W, Jiang X, Tamosiuniene R, Sung YK, Shuffle EM, Tu AB, Valenzuela A, Jiang S, Zamanian RT, Fiorentino DF, Voelkel NF, Peters-Golden M, Stenmark KR, Chung L, Rabinovitch M, Nicolls MR. Leukotriene B4 activates pulmonary artery adventitial fibroblasts in pulmonary hypertension. Hypertension 2015;66:1227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ee MT, Kantores C, Ivanovska J, Wong MJ, Jain A, Jankov RP. Leukotriene B4 mediates macrophage influx and pulmonary hypertension in bleomycin-induced chronic neonatal lung injury. Am J Physiol Lung Cell Mol Physiol 2016;311:L292–302. [DOI] [PubMed] [Google Scholar]
- 165.Tian W, Jiang X, Tamosiuniene R, Sung YK, Qian J, Dhillon G, Gera L, Farkas L, Rabinovitch M, Zamanian RT, Inayathullah M, Fridlib M, Rajadas J, Peters-Golden M, Voelkel NF, Nicolls MR. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med 2013;5:200ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tabata T, Ono S, Song C, Noda M, Suzuki S, Tanita T, Fujimura S. Role of leukotriene B4 in monocrotaline-induced pulmonary hypertension. Nihon Kyobu Shikkan Gakkai Zasshi 1997;35:160–6. [PubMed] [Google Scholar]
- 167.Albertini M, Clement MG. In pigs, inhaled nitric oxide (NO) counterbalances PAF-induced pulmonary hypertension. Prostaglandins Leukot Essent Fatty Acids 1994;51:357–62. [DOI] [PubMed] [Google Scholar]
- 168.Ono S, Voelkel NF. PAF antagonists inhibit monocrotaline-induced lung injury and pulmonary hypertension. J Appl Physiol (1985) 1991;71:2483–92. [DOI] [PubMed] [Google Scholar]
- 169.Smallbone BW, Taylor NE, McDonald JW. Effects of L-652,731, a platelet-activating factor (PAF) receptor antagonist, on PAF- and complement-induced pulmonary hypertension in sheep. J Pharmacol Exp Ther 1987;242:1035–40. [PubMed] [Google Scholar]
- 170.Lu A, Zuo C, He Y, Chen G, Piao L, Zhang J, Xiao B, Shen Y, Tang J, Kong D, Alberti S, Chen D, Zuo S, Zhang Q, Yan S, Fei X, Yuan F, Zhou B, Duan S, Yu Y, Lazarus M, Su Y, Breyer RM, Funk CD, Yu Y. EP3 receptor deficiency attenuates pulmonary hypertension through suppression of Rho/TGF-beta1 signaling. J Clin Invest 2015;125:1228–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Toki S, Zhou W, Goleniewska K, Reiss S, Dulek DE, Newcomb DC, Lawson WE, Peebles RS, Jr. Endogenous PGI2 signaling through IP inhibits neutrophilic lung inflammation in LPS-induced acute lung injury mice model. Prostaglandins Other Lipid Mediat 2018;136:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhou W, Hashimoto K, Goleniewska K, O’Neal JF, Ji S, Blackwell TS, Fitzgerald GA, Egan KM, Geraci MW, Peebles RS, Jr. Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells. J Immunol 2007;178:702–10. [DOI] [PubMed] [Google Scholar]
- 173.Miyata M, Ueno Y, Sekine H, Ito O, Sakuma F, Koike H, Nishio S, Nishimaki T, Kasukawa R. Protective effect of beraprost sodium, a stable prostacyclin analogue, in development of monocrotaline-induced pulmonary hypertension. J Cardiovasc Pharmacol 1996;27:206. [DOI] [PubMed] [Google Scholar]
- 174.Pickworth J, Rothman A, Iremonger J, Casbolt H, Hopkinson K, Hickey PM, Gladson S, Shay S, Morrell NW, Francis SE, West JD, Lawrie A. Differential IL-1 signaling induced by BMPR2 deficiency drives pulmonary vascular remodeling. Pulm Circ 2017;7:768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Good RB, Gilbane AJ, Trinder SL, Denton CP, Coghlan G, Abraham DJ, Holmes AM. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am J Pathol 2015;185:1850–8. [DOI] [PubMed] [Google Scholar]
- 176.Nelin LD, White HA, Jin Y, Trittmann JK, Chen B, Liu Y. The Src family tyrosine kinases src and yes have differential effects on inflammation-induced apoptosis in human pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 2016;310:L880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yu XH, Tang ZB, Liu LJ, Qian H, Tang SL, Zhang DW, Tian GP, Tang CK. Apelin and its receptor APJ in cardiovascular diseases. Clin Chim Acta 2014;428:1–8. [DOI] [PubMed] [Google Scholar]
- 178.Wu Q, Wang HY, Li J, Zhou P, Wang QL, Zhao L, Fan R, Wang YM, Xu XZ, Yi DH, Yu SQ, Pei JM. Kappa-opioid receptor stimulation improves endothelial function in hypoxic pulmonary hypertension. PLoS One 2013;8:e60850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhou Y, Wang Y, Wang X, Tian X, Zhang S, Yang F, Guo H, Fan R, Feng N, Jia M, Gu X, Wang Y, Li J, Pei J. The protective effects of kappa-opioid receptor stimulation in hypoxic pulmonary hypertension involve inhibition of autophagy through the AMPK-MTOR pathway. Cell Physiol Biochem 2017;44:1965–79. [DOI] [PubMed] [Google Scholar]
- 180.Zhang J, Liu Q, Fang Z, Hu X, Huang F, Tang L, Zhou S. Hypoxia induces the proliferation of endothelial progenitor cells via upregulation of Apelin/APLNR/MAPK signaling. Mol Med Rep 2016;13:1801–6. [DOI] [PubMed] [Google Scholar]
- 181.Zhang H, Gong Y, Wang Z, Jiang L, Chen R, Fan X, Zhu H, Han L, Li X, Xiao J, Kong X. Apelin inhibits the proliferation and migration of rat PASMCs via the activation of PI3K/Akt/mTOR signal and the inhibition of autophagy under hypoxia. J Cell Mol Med 2014;18:542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang J, Liu Q, Hu X, Fang Z, Huang F, Tang L, Zhou S. Apelin/APJ signaling promotes hypoxia-induced proliferation of endothelial progenitor cells via phosphoinositide-3 kinase/Akt signaling. Mol Med Rep 2015;12:3829–34. [DOI] [PubMed] [Google Scholar]
- 183.Zheng H, Fu G, Dai T, Huang H. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J Cardiovasc Pharmacol 2007;50:274–80. [DOI] [PubMed] [Google Scholar]
- 184.Foresta C, De Toni L, Di Mambro A, Garolla A, Ferlin A, Zuccarello D. The PDE5 inhibitor sildenafil increases circulating endothelial progenitor cells and CXCR4 expression. J Sex Med 2009;6:369–72. [DOI] [PubMed] [Google Scholar]
- 185.Yaoita N, Shirakawa R, Fukumoto Y, Sugimura K, Miyata S, Miura Y, Nochioka K, Miura M, Tatebe S, Aoki T, Yamamoto S, Satoh K, Kimura T, Shimokawa H, Horiuchi H. Platelets are highly activated in patients of chronic thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc Biol 2014;34:2486–94. [DOI] [PubMed] [Google Scholar]
- 186.Diebold I, Djordjevic T, Hess J, Gorlach A. Rac-1 promotes pulmonary artery smooth muscle cell proliferation by upregulation of plasminogen activator inhibitor-1: role of NFkappaB-dependent hypoxia-inducible factor-1alpha transcription. Thromb Haemost 2008;100:1021–8. [PubMed] [Google Scholar]
- 187.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol 2007;293:H1883–91. [DOI] [PubMed] [Google Scholar]
- 188.Sun F, Lu Z, Zhang Y, Geng S, Xu M, Xu L, Huang Y, Zhuang P, Zhang Y. Stagedependent changes of beta2adrenergic receptor signaling in right ventricular remodeling in monocrotalineinduced pulmonary arterial hypertension. Int J Mol Med 2018;41:2493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang S, Wu J, You J, Shi H, Xue X, Huang J, Xu L, Jiang G, Yuan L, Gong X, Luo H, Ge J, Cui Z, Zou Y. HSF1 deficiency accelerates the transition from pressure overload-induced cardiac hypertrophy to heart failure through endothelial miR-195a-3p-mediated impairment of cardiac angiogenesis. J Mol Cell Cardiol 2018;118:193–207. [DOI] [PubMed] [Google Scholar]
- 190.Zhao Y, Yan M, Chen C, Gong W, Yin Z, Li H, Fan J, Zhang XA, Wang DW, Zuo H. MiR-124 aggravates failing hearts by suppressing CD151-facilitated angiogenesis in heart. Oncotarget 2018;9:14382–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liao X, Shen Y, Zhang R, Sugi K, Vasudevan NT, Alaiti MA, Sweet DR, Zhou L, Qing Y, Gerson SL, Fu C, Wynshaw-Boris A, Hu R, Schwartz MA, Fujioka H, Richardson B, Cameron MJ, Hayashi H, Stamler JS, Jain MK. Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc Natl Acad Sci U S A 2018;115:E4661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Renaud-Gabardos E, Tatin F, Hantelys F, Lebas B, Calise D, Kunduzova O, Masri B, Pujol F, Sicard P, Valet P, Roncalli J, Chaufour X, Garmy-Susini B, Parini A, Prats AC. Therapeutic benefit and gene network regulation by combined gene transfer of apelin, FGF2, and SERCA2a into ischemic heart. Mol Ther 2018;26:902–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dewachter C, Belhaj A, Rondelet B, Vercruyssen M, Schraufnagel DP, Remmelink M, Brimioulle S, Kerbaul F, Naeije R, Dewachter L. Myocardial inflammation in experimental acute right ventricular failure: effects of prostacyclin therapy. J Heart Lung Transplant 2015;34:1334–45. [DOI] [PubMed] [Google Scholar]
- 194.Wu L, Gao L, Zhang D, Yao R, Huang Z, Du B, Wang Z, Xiao L, Li P, Li Y, Liang C, Zhang Y. C1QTNF1 attenuates angiotensin IIinduced cardiac hypertrophy via activation of the AMPKa pathway. Free Radic Biol Med 2018;121:215–30. [DOI] [PubMed] [Google Scholar]
- 195.Chien PT, Lin CC, Hsiao LD, Yang CM. Induction of HO-1 by carbon monoxide releasing molecule-2 attenuates thrombin-induced COX-2 expression and hypertrophy in primary human cardiomyocytes. Toxicol Appl Pharmacol 2015;289:349–59. [DOI] [PubMed] [Google Scholar]
- 196.Ramos-Kuri M, Rapti K, Mehel H, Zhang S, Dhandapany PS, Liang L, Garcia-Carranca A, Bobe R, Fischmeister R, Adnot S, Lebeche D, Hajjar RJ, Lipskaia L, Chemaly ER. Dominant negative Ras attenuates pathological ventricular remodeling in pressure overload cardiac hypertrophy. Biochim Biophys Acta 2015;1853:2870–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cortes R, Rivera M, Rosello-Lleti E, Martinez-Dolz L, Almenar L, Azorin I, Lago F, Gonzalez-Juanatey JR, Portoles M. Differences in MEF2 and NFAT transcriptional pathways according to human heart failure aetiology. PLoS One 2012;7:e30915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Aguero J, Ishikawa K, Hadri L, Santos-Gallego C, Fish K, Hammoudi N, Chaanine A, Torquato S, Naim C, Ibanez B, Pereda D, GarciaAlvarez A, Fuster V, Sengupta PP, Leopold JA, Hajjar RJ. Characterization of right ventricular remodeling and failure in a chronic pulmonary hypertension model. Am J Physiol Heart Circ Physiol 2014;307:H1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huang J, Chen L, Yao Y, Tang C, Ding J, Fu C, Li H, Ma G. Pivotal role of regulator of G-protein signaling 12 in pathological cardiac hypertrophy. Hypertension 2016;67:1228–36. [DOI] [PubMed] [Google Scholar]
- 200.Ramadas N, Rajaraman B, Kuppuswamy AA, Vedantham S. Early growth response-1 (EGR-1) - a key player in myocardial cell injury. Cardiovasc Hematol Agents Med Chem 2014;12:66–71. [DOI] [PubMed] [Google Scholar]
- 201.van der Feen DE, Dickinson MG, Bartelds B, Borgdorff MA, Sietsma H, Levy M, Berger RM. Egr-1 identifies neointimal remodeling and relates to progression in human pulmonary arterial hypertension. J Heart Lung Transplant 2016;35:481–90. [DOI] [PubMed] [Google Scholar]
- 202.Dickinson MG, Kowalski PS, Bartelds B, Borgdorff MA, van der Feen D, Sietsma H, Molema G, Kamps JA, Berger RM. A critical role for Egr-1 during vascular remodelling in pulmonary arterial hypertension. Cardiovasc Res 2014;103:573–84. [DOI] [PubMed] [Google Scholar]
- 203.Lemon DD, Harrison BC, Horn TR, Stratton MS, Ferguson BS, Wempe MF, McKinsey TA. Promiscuous actions of small molecule inhibitors of the protein kinase D-class IIa HDAC axis in striated muscle. FEBS Lett 2015;589:1080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases C and D mediate agonistdependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol 2004;24:8374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang L, Yu D, Mo R, Zhang J, Hua H, Hu L, Feng Y, Wang S, Zhang WY, Yin N, Mo XM. The succinate receptor GPR91 is involved in pressure overload-induced ventricular hypertrophy. PLoS One 2016;11:e0147597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang L, Yu D, Fan HH, Feng Y, Hu L, Zhang WY, Zhou K, Mo XM. Triggering the succinate receptor GPR91 enhances pressure overload-induced right ventricular hypertrophy. Int J Clin Exp Pathol 2014;7:5415–28. [PMC free article] [PubMed] [Google Scholar]
- 207.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol 2009;297:H1806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2010;298:H16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nunn C, Zou MX, Sobiesiak AJ, Roy AA, Kirshenbaum LA, Chidiac P. RGS2 inhibits beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Cell Signal 2010;22:1231–9. [DOI] [PubMed] [Google Scholar]
- 210.Lee KN, Lu X, Nguyen C, Feng Q, Chidiac P. Cardiomyocyte specific overexpression of a 37 amino acid domain of regulator of G protein signalling 2 inhibits cardiac hypertrophy and improves function in response to pressure overload in mice. J Mol Cell Cardiol 2017;108:194–202. [DOI] [PubMed] [Google Scholar]
- 211.Sjogren B, Parra S, Atkins KB, Karaj B, Neubig RR. Digoxin-mediated upregulation of RGS2 protein protects against cardiac injury. J Pharmacol Exp Ther 2016;357:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Takimoto E, Koitabashi N, Hsu S, Ketner EA, Zhang M, Nagayama T, Bedja D, Gabrielson KL, Blanton R, Siderovski DP, Mendelsohn ME, Kass DA. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest 2009;119:408–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tokudome T, Kishimoto I, Horio T, Arai Y, Schwenke DO, Hino J, Okano I, Kawano Y, Kohno M, Miyazato M, Nakao K, Kangawa K. Regulator of G-protein signaling subtype 4 mediates antihypertrophic effect of locally secreted natriuretic peptides in the heart. Circulation 2008;117:2329–39. [DOI] [PubMed] [Google Scholar]
- 214.Yang J, Maity B, Huang J, Gao Z, Stewart A, Weiss RM, Anderson ME, Fisher RA. G-protein inactivator RGS6 mediates myocardial cell apoptosis and cardiomyopathy caused by doxorubicin. Cancer Res 2013;73:1662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Miao R, Lu Y, Xing X, Li Y, Huang Z, Zhong H, Huang Y, Chen AF, Tang X, Li H, Cai J, Yuan H. Regulator of G-protein signaling 10 negatively regulates cardiac remodeling by blocking mitogen-activated protein kinase-extracellular signal-regulated protein kinase 1/2 signaling. Hypertension 2016;67:86–98. [DOI] [PubMed] [Google Scholar]
- 216.Li Y, Tang XH, Li XH, Dai HJ, Miao RJ, Cai JJ, Huang ZJ, Chen AF, Xing XW, Lu Y, Yuan H. Regulator of G protein signalling 14 attenuates cardiac remodelling through the MEK-ERK1/2 signalling pathway. Basic Res Cardiol 2016;111:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Xiao Y, Liu Y, Liu J, Kang YJ. The association between myocardial fibrosis and depressed capillary density in rat model of left ventricular hypertrophy. Cardiovasc Toxicol 2018;18:304–1. [DOI] [PubMed] [Google Scholar]
- 218.Jabs M, Rose AJ, Lehmann LH, Taylor J, Moll I, Sijmonsma TP, Herberich SE, Sauer SW, Poschet G, Federico G, Mogler C, Weis EM, Augustin HG, Yan M, Gretz N, Schmid RM, Adams RH, Grone HJ, Hell R, Okun JG, Backs J, Nawroth PP, Herzig S, Fischer A. Inhibition of endothelial notch signaling impairs fatty acid transport and leads to metabolic and vascular remodeling of the adult heart. Circulation 2018;137:2592–608. [DOI] [PubMed] [Google Scholar]
- 219.Kumar R, Mickael C, Kassa B, Gebreab L, Robinson JC, Koyanagi DE, Sanders L, Barthel L, Meadows C, Fox D, Irwin D, Li M, McKeon BA, Riddle S, Dale Brown R, Morgan LE, Evans CM, Hernandez-Saavedra D, Bandeira A, Maloney JP, Bull TM, Janssen WJ, Stenmark KR, Tuder RM, Graham BB. TGF-beta activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat Commun 2017;8:15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gong H, An S, Sassmann A, Liu M, Mastej V, Mittal M, Zhang W, Hong Z, Offermanns S, Rehman J, Malik AB. PAR1 scaffolds TGFbetaRII to downregulate TGF-beta signaling and activate ESC differentiation to endothelial cells. Stem Cell Reports 2016;7:10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhou L, Chen H, Mao X, Qi H, Baker PN, Zhang H. G-protein-coupled receptor 30 mediates the effects of estrogen on endothelial cell tube formation in vitro. Int J Mol Med 2017;39:1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li Z, Cheng L, Liang H, Duan W, Hu J, Zhi W, Yang J, Liu Z, Zhao M, Liu J. GPER inhibits diabetes-mediated RhoA activation to prevent vascular endothelial dysfunction. Eur J Cell Biol 2016;95:100–13. [DOI] [PubMed] [Google Scholar]
- 223.Glembotski CC, Irons CE, Krown KA, Murray SF, Sprenkle AB, Sei CA. Myocardial alpha-thrombin receptor activation induces hypertrophy and increases atrial natriuretic factor gene expression. J Biol Chem 1993;268:20646–52. [PubMed] [Google Scholar]
- 224.Ide J, Aoki T, Ishivata S, Glusa E, Strukova SM. Proteinase-activated receptor agonists stimulate the increase in intracellular Ca2+ in cardiomyocytes and proliferation of cardiac fibroblasts from chick embryos. Bull Exp Biol Med 2007;144:760–3. [DOI] [PubMed] [Google Scholar]
- 225.Snead AN, Insel PA. Defining the cellular repertoire of GPCRs identifies a profibrotic role for the most highly expressed receptor, protease-activated receptor 1, in cardiac fibroblasts. FASEB J 2012;26:4540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nishida M, Sato Y, Uemura A, Narita Y, Tozaki-Saitoh H, Nakaya M, Ide T, Suzuki K, Inoue K, Nagao T, Kurose H. P2Y6 receptorGalpha12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J 2008;27:3104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Samuel CS, Du XJ, Bathgate RA, Summers RJ. ‘Relaxin’ the stiffened heart and arteries: the therapeutic potential for relaxin in the treatment of cardiovascular disease. Pharmacol Ther 2006;112:529–52. [DOI] [PubMed] [Google Scholar]
- 228.Chen Y, Yang S, Yao W, Zhu H, Xu X, Meng G, Zhang W. Prostacyclin analogue beraprost inhibits cardiac fibroblast proliferation depending on prostacyclin receptor activation through a TGF beta-Smad signal pathway. PLoS One 2014;9:e98483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Salvi V, Sozio F, Sozzani S, Del Prete A. Role of atypical chemokine receptors in microglial activation and polarization. Front Aging Neurosci 2017;9:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pullamsetti SS, Savai R. Macrophage regulation during vascular remodeling: implications for pulmonary hypertension therapy. Am J Respir Cell Mol Biol 2017;56:556–8. [DOI] [PubMed] [Google Scholar]
- 231.Li Y, Cai L, Wang H, Wu P, Gu W, Chen Y, Hao H, Tang K, Yi P, Liu M, Miao S, Ye D. Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene 2011;30:3887–99. [DOI] [PubMed] [Google Scholar]
- 232.Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr., Gause WC, Leibovich SJ, Hasko G. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J 2012;26:376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lin HH, Stacey M. G Protein-coupled receptors in macrophages. Microbiol Spectr 2016;4. [DOI] [PubMed] [Google Scholar]
- 234.Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. Pivotal advance: tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE(2) catabolism in myeloid cells. J Leukoc Biol 2010;88:839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kruger A, Mayer A, Roch T, Schulz C, Lendlein A, Jung F. Angiogenically stimulated alternative monocytes maintain their proangiogenic and non-inflammatory phenotype in long-term co-cultures with HUVEC. Clin Hemorheol Microcirc 2014;58:229–40. [DOI] [PubMed] [Google Scholar]
- 236.Presta M, Andres G, Leali D, Dell’Era P, Ronca R. Inflammatory cells and chemokines sustain FGF2-induced angiogenesis. Eur Cytokine Netw 2009;20:39–50. [DOI] [PubMed] [Google Scholar]
- 237.Sidibe A, Ropraz P, Jemelin S, Emre Y, Poittevin M, Pocard M, Bradfield PF, Imhof BA. Angiogenic factor-driven inflammation promotes extravasation of human proangiogenic monocytes to tumours. Nat Commun 2018;9:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dopheide JF, Geissler P, Rubrech J, Trumpp A, Zeller GC, Bock K, Dorweiler B, Dunschede F, Munzel T, Radsak MP, Espinola-Klein C. Inflammation is associated with a reduced number of pro-angiogenic Tie-2 monocytes and endothelial progenitor cells in patients with critical limb ischemia. Angiogenesis 2016;19:67–78. [DOI] [PubMed] [Google Scholar]
- 239.Welihinda AA, Amento EP. Positive allosteric modulation of the adenosine A2a receptor attenuates inflammation. J Inflamm (Lond) 2014;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dufton N, Hannon R, Brancaleone V, Dalli J, Patel HB, Gray M, D’Acquisto F, Buckingham JC, Perretti M, Flower RJ. Antiinflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol 2010;184:2611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ivy DD, McMurtry IF, Colvin K, Imamura M, Oka M, Lee DS, Gebb S, Jones PL. Development of occlusive neointimal lesions in distal pulmonary arteries of endothelin B receptor-deficient rats: a new model of severe pulmonary arterial hypertension. Circulation 2005;111:2988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cha SA, Park BM, Kim SH. Angiotensin-(1–9) ameliorates pulmonary arterial hypertension via angiotensin type II receptor. Korean J Physiol Pharmacol 2018;22:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Weng M, Raher MJ, Leyton P, Combs TP, Scherer PE, Bloch KD, Medoff BD. Adiponectin decreases pulmonary arterial remodeling in murine models of pulmonary hypertension. Am J Respir Cell Mol Biol 2011;45:340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hemnes AR, Rathinasabapathy A, Austin EA, Brittain EL, Carrier EJ, Chen X, Fessel JP, Fike CD, Fong P, Fortune N, Gerszten RE, Johnson JA, Kaplowitz M, Newman JH, Piana R, Pugh ME, Rice TW, Robbins IM, Wheeler L, Yu C, Loyd JE, West J. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur Respir J 2018;51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tozzi CA, Poiani GJ, McHugh NA, Shakarjian MP, Grove BH, Samuel CS, Unemori EN, Riley DJ. Recombinant human relaxin reduces hypoxic pulmonary hypertension in the rat. Pulm Pharmacol Ther 2005;18:346–53. [DOI] [PubMed] [Google Scholar]
- 246.Pintalhao M, Castro-Chaves P, Vasques-Novoa F, Goncalves F, Mendonca L, Fontes-Carvalho R, Lourenco P, Almeida P, Leite-Moreira A, Bettencourt P. Relaxin serum levels in acute heart failure are associated with pulmonary hypertension and right heart overload. Eur J Heart Fail 2017;19:218–25. [DOI] [PubMed] [Google Scholar]
- 247.Imamura M, Vitello AM, Limbird JN, Ivy DD, Fallon MB, Carter EP. Endothelin-B receptor overexpression prevents hypoxic pulmonary hypertension in cirrhotic rats. Chest 2005;128:580S–1S. [DOI] [PubMed] [Google Scholar]
- 248.Reichenberger F, Voswinckel R, Steveling E, Enke B, Kreckel A, Olschewski H, Grimminger F, Seeger W, Ghofrani HA. Sildenafil treatment for portopulmonary hypertension. Eur Respir J 2006;28:563–7. [DOI] [PubMed] [Google Scholar]
- 249.Xiao R, Su Y, Feng T, Sun M, Liu B, Zhang J, Lu Y, Li J, Wang T, Zhu L, Hu Q. Monocrotaline induces endothelial injury and pulmonary hypertension by targeting the extracellular calcium-sensing receptor. J Am Heart Assoc 2017;6:pii: e004865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.West JD, Voss BM, Pavliv L, de Caestecker M, Hemnes AR, Carrier EJ. Antagonism of the thromboxane-prostanoid receptor is cardioprotective against right ventricular pressure overload. Pulm Circ 2016;6:211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.West JD, Carrier EJ, Bloodworth NC, Schroer AK, Chen P, Ryzhova LM, Gladson S, Shay S, Hutcheson JD, Merryman WD. Serotonin 2B receptor antagonism prevents heritable pulmonary arterial hypertension. PLoS One 2016;11:e0148657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tian W, Jiang X, Sung YK, Qian J, Yuan K, Nicolls MR. Leukotrienes in pulmonary arterial hypertension. Immunol Res 2014;58:38793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pyne NJ, Pyne S. Sphingosine kinase 1: a potential therapeutic target in pulmonary arterial hypertension? Trends Mol Med 2017;23:78698. [DOI] [PubMed] [Google Scholar]
- 254.Sysol JR, Natarajan V, Machado RF. PDGF induces SphK1 expression via Egr-1 to promote pulmonary artery smooth muscle cell proliferation. Am J Physiol Cell Physiol 2016;310:C983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bhavanam NP, Failla A, Cho Y, Lockey RF, Kolliputi N. Commentary: the sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Front Pharmacol 2015;6:229. [DOI] [PMC free article] [PubMed] [Google Scholar]